Introduction
Tumor necrosis factor alpha (TNFα)-induced protein 1
(TNFAIP1) was originally identified as a gene whose
expression can be induced by TNFα in umbilical vein endothelial
cells (1). TNFAIP1 is a
highly conserved single-copy gene (1), implying that TNFAIP1 protein has an
important physiological role, which, however, has remained to be
sufficiently elucidated. Previous studies by our group have
demonstrated that TNFAIP1 interacts with proliferating cell nuclear
antigen and the small p50 sub-unit of DNA polymerase δ, implying
that it may be involved in DNA synthesis and DNA repair (2,3).
Another group reported that Ras homolog gene family, member B
(RhoB) induces apoptosis by interacting with TNFAIP1 via a c-Jun
N-terminal kinase-mediated signaling mechanism, suggesting that
TNFAIP1 is involved in apoptosis (4). Furthermore, TNFAIP1 is an adaptor for
cullin 3 to control RhoA degradation and regulate the structure of
the RhoA-associated actin cytoskeleton (5). In addition, the transcription levels
of TNFAIP1 were robustly induced in the brain of a
transgenic Caenorhabditis elegans model of Alzheimer's
disease (AD), indicating that TNFAIP1 may also be involved in AD
development (6). Therefore,
inhibition of TNFAIP1 expression may be beneficial for
neuronal cells under pathological conditions, for which the
elucidation of the transcriptional regulation mechanisms of
TNFAIP1 expression is required. Previous studies have shown
that transcription factor Sp1 is capable of controlling basal
TNFAIP1 expression by directly binding to the proximal promoter
region of human TNFAIP1 (7). However, the transcriptional
regulation mechanisms have largely remained elusive.
The present study aimed to determine the effects of
neuroprotective agents with activity against AD on the expression
of TNFAIP1 in SKNSH human neuroblastoma cells using
reverse-transcription polymerase chain reaction (RT-qPCR) analysis
and a luciferase reporter assay. As forskolin (8) and genistein (9) have been demonstrated to potently
stimulate cyclic adenosine monophosphate (cAMP) response
element-binding protein (CREB)-mediated transcription (8), the present study investigated the
role of transcription factor CREB in the regulation of
TNFAIP1. Further mechanistic studies were performed by
examining the effects of KG-501, a specific CREB inhibitor
(10), and overexpression of
wild-type (WT) CREB or CREB mutated at ser133a (CREBs133a) or at
its DNA-binding site (KCREB) on the expression of TNFAIP1
expression as well as on TNFAIP1 promoter activity in SKNSH
cells. Furthermore, the present study aimed to identify the
corresponding CREB binding sites in the promoter region of
TNFAIP1, which were verified using luciferase reporter and
chromatin immunoprecipitation (ChIP) assays. The present study
elucidated the regulatory mechanisms of TNFAIP1 by CREB,
which will be useful for future studies regarding the regulation of
TNFAIP1 and the development of TNFAIP1-targeted therapeutics
of neuronal disease.
Materials and methods
Materials
Lipofectamine™ 2000, Dulbecco's modified Eagle's
medium (DMEM), glutamine, penicillin and streptomycin were
purchased from Invitrogen Life Technologies, Inc., Carlsbad, CA,
USA). Fetal bovine serum (FBS) and 0.25% trypsin-EDTA were
purchased from Gibco-BRL (Invitrogen Life Technologies, Inc.).
Chemical compounds including dimethyl sulfoxide (DMSO), forskolin,
valproic acid, polydatin, genistein, KG-501 and mouse monoclonal
anti-β-actin antibody, were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Rabbit polyclonal anti-TNFAIP1 antibody, was
custom-made by Nanjing Chuanbo Bioch Co. Ltd (Nanjing, China)
according to the protocol of a previous study (3). Rabbit polyclonal anti-CREB antibody
was purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Cytomegalovirus plasmid (pCMV)-Myc and the CREB
dominant-negative vector set, including pCMV-CREB, pCMV-CREBs133a,
containing a mutation of the phosphorylatable serine 133, and
pCMV-KCREB, containing a mutation of the DNA binding domain, were
purchased from Clontech Inc. (Mountainview, CA, USA).
pGEM®-T Easy Vector System II, pGL3-Basic vector and the
Dual-Luciferase® Reporter Assay System were purchased
from Promega (Madison, WI, USA). LA Taq™ DNA Polymerase with GC
buffers was purchased from Takara (Otsu, Japan). The real-time PCR
primers for human TNFAIP1 and β-actin were purchased from Qiagen
GmbH (Hilden, Germany).
Reporter plasmid construction
The TNFAIP1 promoter region was previously
characterized by Liu et al (7). The proximal 5′ region of human
TNFAIP1 promoter region spanning from nucleotide −1087 to
nucleotide −139 (GenBank accession number, NM_021137) was amplified
by PCR using the forward primer P-948F
(5′-GGGGTACCCCACACATAACTGGCACTCA-3′) and the reverse primer P-948R
(5′-AAGCTTTCTCAGCAGCTGGGTGGCCA-3′) using the genomic DNA of SKNSH
cells as a template, and ligated into the pGL3-basic vector in the
KpnI/HindIII sites, denoted as P-948 (−1087/−139).
PCR was performed using a thermocycler (Eppendorf
Mastercycler® Nexus; Eppendorf, Hamburg, Germany) with
the following conditions: Initial denaturation of DNA, 94°C for 10
min; denaturation, 32 cycles of 95°C for 45 sec; annealing, 58°C
for 30 sec; extension, 72°C for 40 sec; and final extension, 72°C
for 5 min. The sequence of the promoter region of CRE from −1087 to
−139 bp was predicted by Jaspar (http://jaspar.genereg.net/). Deletion of the CRE1 and
CRE2 sites located at −285 and −425 bp was performed using
overlapping extension PCR, as described previously (11). In brief, in the first round, two
PCRs were performed in parallel using P-948 (−1087/−139) as a
template: One PCR was performed with the forward primer P-948F and
a reverse primer containing a CRE1-site deletion [Pr(p)CRE1mR
(5′-GAAGGTAGTGTAGGTAAACAGGCT-3′)] or a primer containing a
CRE2-site deletion [Pr(p)CRE2mR (5′-AGGTAGGCGGTCGGGGGACGCGGG-3′)];
the other PCR was performed with a forward primer containing a
CRE1-site mutation [Pr(p)CRE1mF (5′-TTTACCTACACTACCTTCCTGACTC-3′)]
or a forward primer containing a CRE2-site mutation [Pr(p) CRE2mF
(5′-CCCCCGACCGCCTACCTGCCGGCCC-3′)] and the reverse primer P-1152R.
In the second round, an equimolar mixture of the two PCR products
was used as template with P-1152F and P-1152R primers. The final
PCR products were then cloned into pGL3-Basic vector in the
KpnI/HindIII sites, denoted as P-948 (CRE1del) or
P-948 (CRE2del). The above-mentioned PCR amplifications were
performed using the Advantage 2 PCR kit (cat no. 639207; Clontech
Laboratories, Inc., Mountainview, CA, USA). All primers were
purchased from Sheng-Gong Technologies (Shanghai, China), and the
construct sequences were confirmed by sequencing performed by
Sheng-Gong Technologies.
Cell culture and transfection
SKNSH cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and routinely cultured in a
humidified atmosphere of 5% CO2 at 37°C in DMEM
supplemented with 10% fetal bovine serum, 0.3 mM glutamine and 50
U/ml penicillin/streptomycin. Transient transfections were
performed in cells at 70% confluence using Lipofectamine™ 2000
reagent according to the manufacturer's instructions. After
transfection for 4 h, the transfection medium was replaced with
DMEM.
Luciferase reporter assay
The luciferase assay was performed as previously
described (12). In brief, SKNSH
cells were transiently transfected with DNA constructs as well as
pRL-TK, which was used as control for transfection efficiency.
Cells were incubated for 24 h after transfection and were treated
with reagents, including DMSO, polydatin (10 µM), valproic
acid (10 µM), forskolin (10 µM) and genistein (10
µM) for another 24 h. The cells were finally harvested and
lysed using 1X passive lysis buffer, and lucif-erase activity was
assessed utilizing the Dual-Luciferase® Reporter Assay
System according to the manufacturer's instructions on a Veritas™
Microplate Luminometer (Turner BioSystems, Sunnyvale, CA, USA).
Activity was defined as the Firefly/Renilla ratio.
RT-PCR analysis
After treatment with drugs including DMSO (10
µM), forskolin (10 µM), valproic acid (10 µM),
polydatin (10 µM) or genistein (10 µM) for 24 h, the
SKNSH cells were harvested for extraction of total RNA using TRIzol
(Invitrogen Life Technologies, Inc.). Total mRNA was reversely
transcribed into cDNA using the Superscript system (Invitrogen Life
Technologies, Inc.). TNFAIP1 expression was analyzed by RT-PCR
using a SYBR green kit (cat no. 4367659; Applied Biosystems Life
Technologies, Foster City, CA, USA) according to the manufacturer's
instructions on an ABI 7500 detection system (Applied Biosystems
Life Technologies). The thermocycling conditions were as follows:
Initial denaturation of DNA, 94°C for 5 min; denaturation, 32
cycles of 95°C for 30 sec; annealing, 60°C for 30 sec; extension,
72°C for 30 sec; and final extension, 72°C for 5 min. The RNA
levels were determined using the 2−ΔΔCt method and
expressed as the fold-change of TNFAIP1 expression in forskolin,
valproic acid, polydatin or genistein-treated cells relative to
DMSO-treated cells after normalization to the housekeeping gene
β-actin.
Western blot analysis
Cell lysates were extracted from the SKNSH cells
using M-PER Mammalian Protein Extraction Reagent (Cell Signaling
Technology, Inc.) containing protease inhibitor (100X;
Sigma-Aldrich, St. Louis, MO, USA), and the concentration of total
proteins was determined by a Bicinchoninic Acid Protein assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). Protein (20
µg) extracted from SKNSH cells was separated by 4–12%
SDS-PAGE (Invitrogen Life Technologies, Inc.) and transferred onto
a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA) using
the iBlot® dry blotting system (Invitrogen Life
Technologies, Inc.). The membranes were blocked for 1 h at room
temperature in 5% non-fat dried milk in 10 mM phosphate-buffered
saline (PBS; pH 7.2). Immunoblotting was then performed at 4°C
overnight by incubation with the following primary antibodies:
Rabbit polyclonal TNFAIP1 antibody (1:1,000; custom-made; Nanjing
Chuanbo Biotech Co., Ltd., Nanjing, China), rabbit polyclonal CREB
antibody (1:1,000; cat no. 4820; Cell Signaling Technology, Inc.)
or mouse monoclonal β-actin antibody (1:5,000; cat no. A3854;
Sigma-Aldrich). Following incubation with species-appropriate,
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat no. M21001 for goat anti-mouse IgG-HRP; cat no. M21002 for goat
anti-rabbit IgG-HRP; Abmart, Shanghai, China) for 1 h at room
temperature, immunoreactive proteins were detected using enhanced
chemiluminescence (ECL detection system; Amersham Pharmacia
Biotech; GE Healthcare, Little Chalfont, UK) according to the
manufacturer's instructions and then exposed to film (X-Omat;
Eastman Kodak Co., Rochester, NY, USA). The intensity of the
chemiluminescence signal was quantified by densitometry using
ImageJ software version 1.49 (National Institutes of Health,
Bethesda, MD, USA).
ChIP assay
ChIP assays were performed using the EZChIP™ kit
(Upstate Biotechnology, EMD Millipore, Billerica, MA, USA)
according to the manufacturer's instructions as previously
described (12). In brief,
~1×106 SKNSH cells were cross-linked with 1%
formaldehyde and collected for sonication to shear the chromatin
with an average size of ~300 bp. Immunoprecipitation was then
performed with anti-CREB antibody or control rabbit polyclonal
immunoglobulin (Ig)G antibody. The DNA-protein complexes were then
reverse cross-linked at 65°C for 3 h. DNA from these samples was
subjected to PCR with the following conditions: DNA initial
denaturation, 94°C for 10 min; denaturation, 32 cycles of 95°C for
50 sec; annealing, 58°C for 30 sec; extension, 72°C for 50 sec; and
final extension, 72°C for 5 min. Primer sets used for amplifying a
285-bp fragment spanning the 519–234 bp region of the
TNFAIP1 promoter were Ch-F (5′-GAATTCCCACGTCTCTCCCC-3′) and
Ch-R (5′-ATGGGGGCTGTAAGTGCTTC-3′). 18s ribosomal (r)RNA PCR was
performed as a negative control. Primer sets used for amplifying a
233-bp DNA fragment corresponding to a region lacking CRE on the
human 18s rRNA gene promoter were h18sF
(5′-GTAACCCGTTGAACCCCATT-3′) and h18sR
(5′-CCATCCAATCGGTAGTAGCG-3′). The Agarose gel was prepared with
1.5% gel strength containing 1.0 µg/ml ethidium bromide
(Sheng-Gong Technologies). PCR products were subjected to agarose
gel electrophoresis (30 V for 1 h) using the gel electrophoresis
module (Beijing Liuyi Instrument Factory, Beijing, China).
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was performed using SPSS 18.0 software (International
Business Machines, Armonk, NY, USA). Results were analyzed by
either Student's t-test or one-way analysis of variance. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
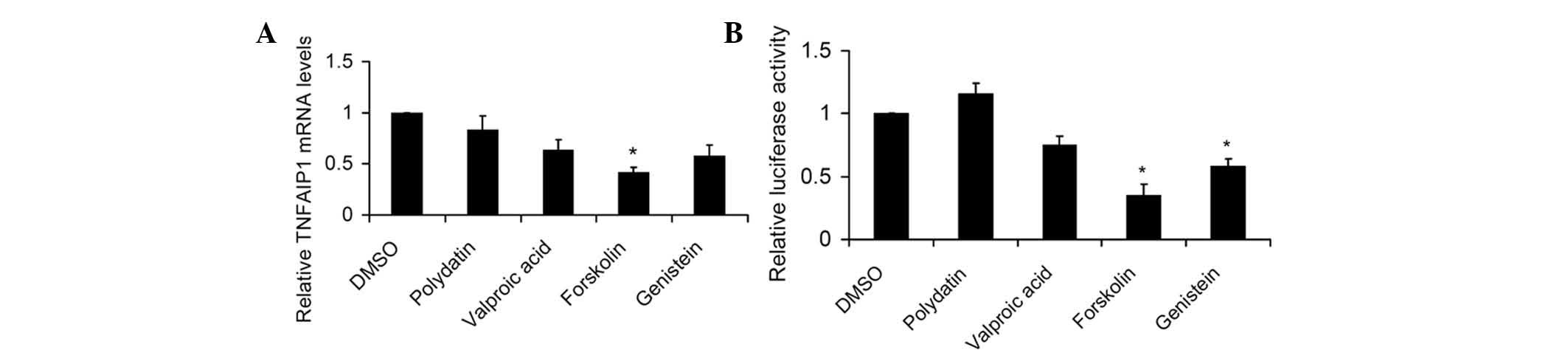
Forskolin inhibits mRNA expression of
TNFAIP1 and TNFAIP1 promoter activity in SKNSH cells
As TNFAIP1 was indicated to be involved in neuronal
damage associated with AD, the present study hypothesized that
neuroprotective agents with activity against AD may target TNFAIP1.
A group of chemical compounds, including valproic acid (13), polydatin (14), forskolin (15) and genistein (16) have been reported to exhibit
therapeutic potential in AD. The effects of these chemical
compounds on human TNFAIP1 transcription and promoter
activity in SKNSH cells were thus determined by RT-PCR and
luciferase assay, respectively. The results showed that forskolin
significantly inhibited human TNFAIP1 mRNA levels in SKNSH
cells (Fig. 1A). Similarly,
forskolin and genistein significantly attenuated the activity of
the P-948 (-1087/-139) TNFAIP1 promoter (Fig. 1B). Forskolin is a selective
activator of adenylate cyclase that potently increases
phosphorylation of CREB on its ser-133 site to stimulate
CREB-mediated transcription (17);
thus, CREB may be involved in the regulation of TNFAIP1 expression
by forskolin.
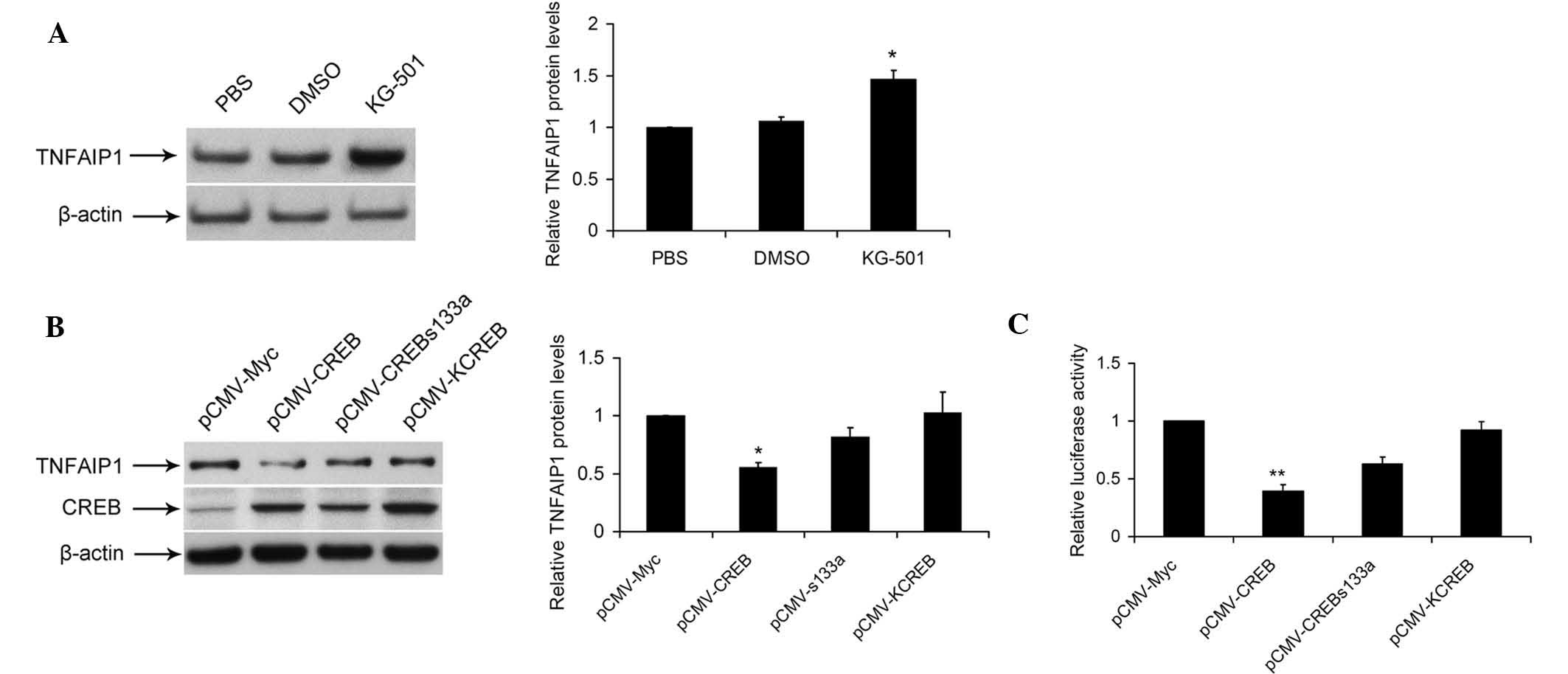
CREB acts as a negative transcriptional
regulator of TNFAIP1 expression
To examine the roles of CREB in TNFAIP1 regulation,
the present study used CREB inhibitor KG-501. The results showed
that KG-501 significantly increased the protein expression of
TNFAIP1 in SKNSH cells (Fig. 2A).
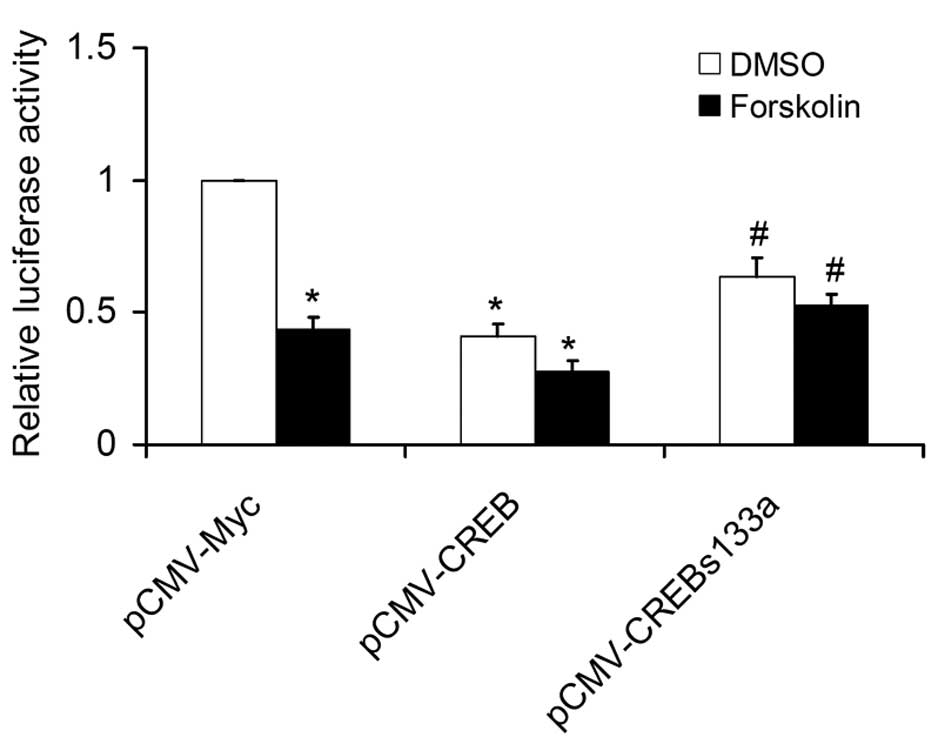
Next, the present study examined the effects of CREB and
dominant-mutant CREB vectors (KCREB and CREBs133a) on the protein
expression of TNFAIP1. As expected, overexpression of CREB resulted
in a decrease in TNFAIP1 protein expression, while KCREB or
CREBs133a had no significant effect (Fig. 2B). Furthermore, the present study
evaluated the effects of CREB on TNFAIP1 promoter activity.
SKNSH cells were transiently transfected with P-948 (-1087/-139)
and WT CREB expression vector or dominant-mutant CREB vectors
(KCREB and CREBs133a). WT CREB, but not the dominant-mutant CREB
vectors significantly reduced TNFAIP1 promoter activity
(Fig. 2C). In conclusion, these
results suggested that CREB negatively regulates TNFAIP1
expression.
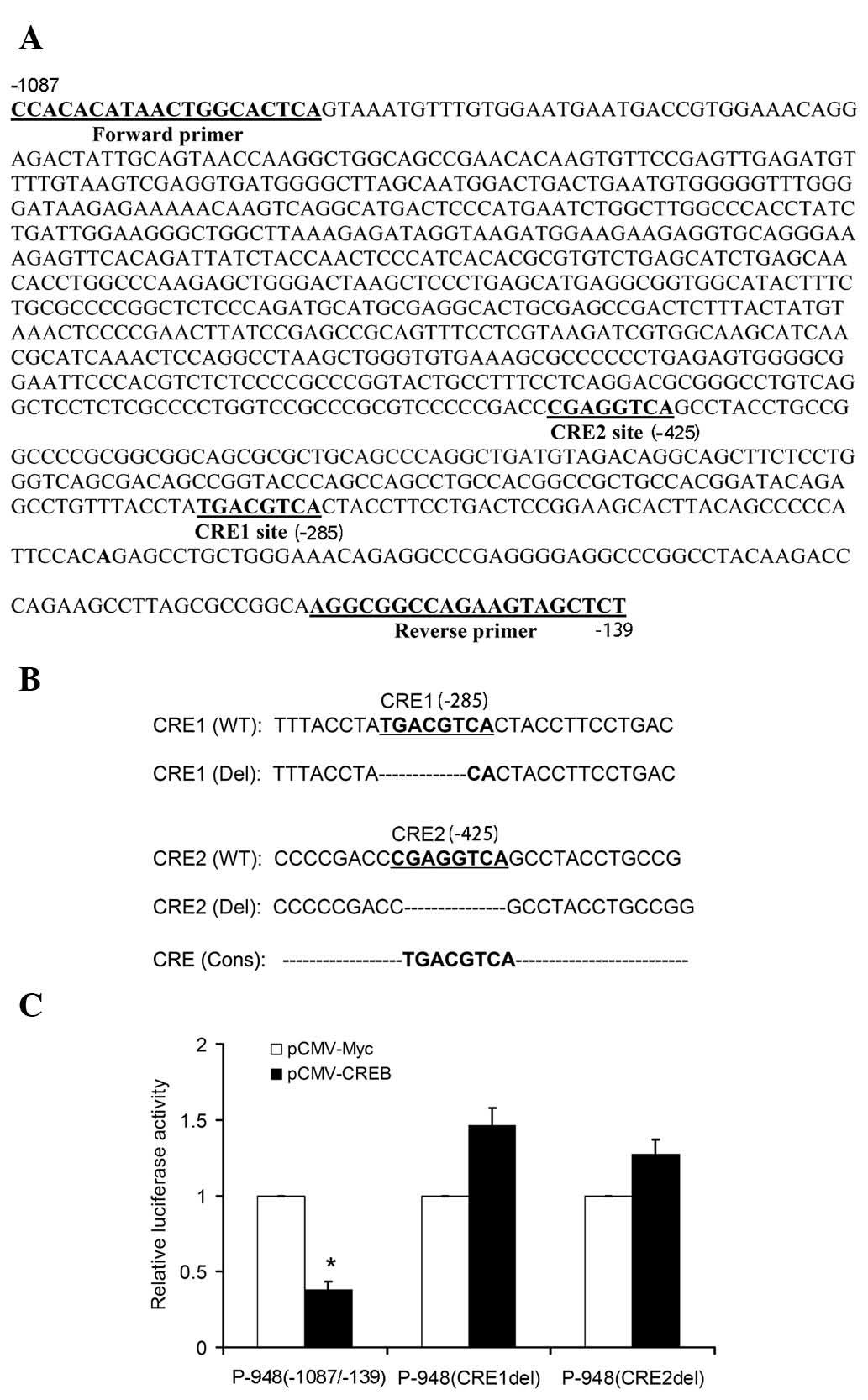
CREB suppresses TNFAIP1 promoter activity
through CREs
As CREB repressed TNFAIP1 promoter activity,
the present study further analyzed the potential CREs that are able
to bind to CREB at sites located in the region of −1087 to −139 by
using the bioinformatics transcription factor binding site
prediction tool Jaspar (http://jaspar.genereg.net/). Two putative cAMP
response elements, designated CRE1 and CRE2, were discovered at
−285 and −425 bp, respectively (Fig.
3A). To investigate whether these CRE sites mediate
CREB-decreased TNFAIP1 promoter activity, deleting mutations
of these two CRE sites were generated (Fig. 3B). SKNSH cells were co-transfected
with CREB expression vector or empty vector as well as P-948
(-1087/-139), P-984 (CRE1del) or P-984 (CRE2del). A luciferase
assay showed that CREB overexpression significantly decreased the
promoter activity of P-948 (−1087/−139), while CREB overexpression
did not cause inhibition of the promoter activity of P-948
(CRE1del) and P-948 (CRE2del; Fig.
3C). These results indicate that CRE1 and CRE2 are involved in
the suppression of TNFAIP1 expression by CREB.
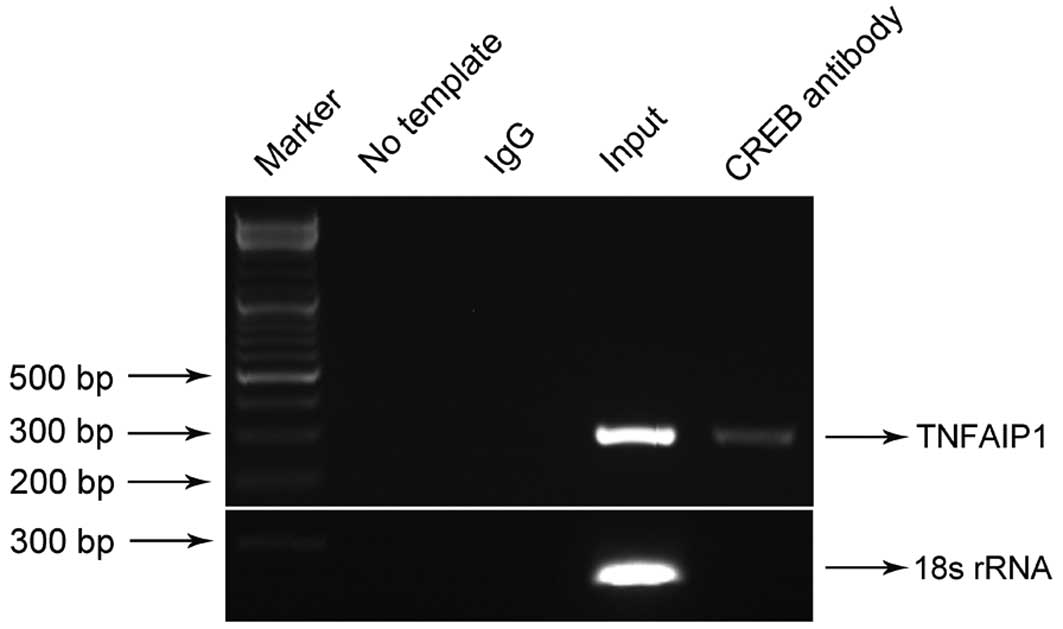
CREB is capable of binding to the TNFAIP1
promoter
The present study further investigated whether CREB
suppresses TNFAIP1 gene expression through directly binding
to the TNFAIP1 promoter. To test this, ChIP assays were
performed with CREB antibody or IgG controls in SKNSH cells. DNA
precipitated by ChIP was amplified by PCR using TNFAIP1
promoter-specific primers covering the two CRE sites from −519 bp
to −234 bp or 18s rRNA promoter primers lacking CRE sites. Analysis
of the PCR products by gel electrophoresis showed that CREB was
able to bind to the promoter region of TNFAIP1 covering two
CREs, but not to the 18s rRNA lacking CRE sites (Fig. 4). ChIP PCR therefore confirmed that
CREB binds to the TNFAIP1 promoter region in SKNSH
cells.
Forskolin decreases TNFAIP1 expression
mediated by CREB phosphorylation on ser133
The results of the present study suggested that CREB
is a negative regulator for TNFAIP1 expression. Therefore, it was
further investigated whether CREB is involved in forskolin-induced
inhibition of TNFAIP1 expression. In accordance with the
results shown in Figs. 1B and
2C, treatment with forskolin or
CREB overexpression significantly decreased TNFAIP1 promoter
activity, and forskolin combined with CREB overexpression further
reduced TNFAIP1 promoter activity (Fig. 5). However, although an identical
dose of forskolin was used, TNFAIP1 promoter activity was
partially restored in CREBs133a-transfected cells (Fig. 5). These results indicated that CREB
phosphorylation on ser133 is responsible for forskolin-induced
inhibition of TNFAIP1 expression.
Discussion
TNFAIP1 has been suggested to be an
apoptosis-associated protein (4)
and involved in the development of AD (6). Therefore, TNFAIP1 is likely to be
implicated in neuronal damage associated with neurodegenerative
disease. Indeed, this notion was confirmed by experiments performed
in our group, which demonstrated that inhibition of TNFAIP1
expression decreased Aβ-induced neuronal toxicity (unpublished
data). Therefore, inhibition of TNFAIP1 expression under
neuropathological conditions may have neuroprotective effects.
Elucidation of the transcriptional regulation mechanisms of
TNFAIP1 may aid in the development of therapeutics for
neuronal disease targeting TNFAIP1. The present study was the
first, to the best of our knowledge, to demonstrate that CREB is a
critical transcription factor for the negative regulation of
TNFAIP1 transcription in SKNSH cells.
Given that TNFAIP1 is implicated in the development
of AD and is induced in the generation of animal models of AD,
therapeutic drugs with activity against AD have been hypothesized
to inhibit TNFAIP1 expression. In the present study, a number of
well-known natural products, including polydatin, valproic acid,
forskolin and genistein, were selected for the evaluation of their
inhibitory effects on TNFAIP1 expression, as they have been
demonstrated to be potentially protective against AD (13–16).
The results showed that polydatin and valproic acid slightly
inhibited TNFAIP1 promoter activity. Of note, forskolin and
genistein significantly suppressed TNFAIP1 promoter
activity. To date, no study has indicated that polydatin is able to
activate CREB, whereas valproic acid (18), forskolin (8) and genistein (9) are known to potently stimulate the
phosphorylation of CREB at ser133 and consequently activate
CREB-mediated transcription (8).
These findings indicated that transcription factor CREB may be a
potential inhibitor of TNFAIP1 expression in neuronal cells.
Genistein is a known phytoestrogen with marked structural and
functional similarity with 17β-estradiol (19). A recent study suggested that
17β-estradiol is a negative regulator of TNFAIP1 in mouse
hippocampi (20). These findings
were consistent with the results of the present study,
demonstrating that TNFAIP1 expression was negatively regulated by
genistein.
CREB is a key transcription factor that is tightly
linked with neuronal cell survival and apoptosis (21,22).
Upon stimulation, CREB is phosphorylated at ser133 and subsequent
recruited to CRE (23,24). As expected, the present study
revealed that overexpression of WT CREB, but not mutated CREB
(CREBs133a and KCREB) significantly inhibited TNFAIP1
promoter activity as well as protein expression. In addition, the
present study demonstrated that WT CREB promoted forskolin-induced
inhibition of TNFAIP1 promoter activity, which was, however,
partially rescued by overexpression of CREBs133a. It has been
suggested that dominant-negative CREB interfered with the function
of the phosphorylated CREB, possibly via the formation of inactive
heterodimers (25). Thus, based on
the results of the present study, phosphorylation of CREB at ser133
is necessary for CREB-induced downregulation of TNFAIP1 and
activation of upstream signaling pathways leading to CREB
phosphorylation may thus intervene with TNFAIP1
expression.
As it was demonstrated that TNFAIP1 promoter
activity was inhibited by transcription factor CREB, the present
study further analyzed the CRE sites in the TNFAIP1 promoter
spanning from −1087 to −139 bp. Two CRE sites, CRE1 and CRE2, were
identified using bioinformatics analysis. In fact, a previous study
indicated that site-directed mutagenesis of the CRE1 site had no
effect on the basal TNFAIP1 promoter activity (7). However, the results of the present
study showed that a deleting mutation of CRE1 and CRE2 inhibited
the CREB-induced reduction of TNFAIP1 promoter activity. It
is possible that CREB binds to the CRE1 site upon stimulation, but
not under basal conditions. Although the ChIP assay of the present
study showed that CREB was able to bind to the TNFAIP1
promoter region from −519 to −234 bp covering the two CRE sites, is
not possible to conclude that CREB specifically binds to CRE1 or
CRE2 sites. Further study is required to determine whether CREB
specifically interacts with the CRE1 or the CRE2 site under basal
and pathological conditions.
In conclusion, the present study suggested that
forskolin suppressed the expression of pro-apoptotic protein
TNFAIP1 through activating neuronal survival mediator CREB. Recent
experiments performed in our group have shown that overexpression
of TNFAIP1 in neuronal cells induces significant neuronal apoptosis
(unpublished data). Therefore, the present study links neuronal
survival mediator CREB and apoptosis-associated protein TNFAIP1,
which is likely to be involved in the development of AD. The
present study thus provided a molecular basis for the intervention
of neuronal disorders by targeting TNFAIP1. Whether the expression
of TNFAIP1 is associated with neuropathological conditions will be
investigated in future studies.
Acknowledgments
The present study was supported in part by the
Cooperative Innovation Center of Engineering and New Products for
Developmental Biology of Hunan Province, the 973 Project of the
Ministry of Science and Technology of China (no. 2010CB529900), the
China Postdoctoral Science Foundation (no. 2014M552139), the
Research Fund for the Doctoral Program of Higher Education of China
(no. 20104306110005), the Hunan Postdoctoral Science Foundation
(no. 2012RS4016) and the Science and Technology Project of the
Provincial Department of Education (no. 2014RS4008).
Abbreviations:
|
CREB
|
cyclic adenosine monophosphate
responsive element binding protein
|
|
ChIP
|
chromatin immunoprecipitation
|
|
siRNA
|
small interfering RNA
|
|
WT
|
wild-type
|
|
Del
|
deletion
|
|
Cons
|
consensus
|
References
|
1
|
Wolf FW, Marks RM, Sarma V, Byers MG, Katz
RW, Shows TB and Dixit VM: Characterization of a novel tumor
necrosis factor-alpha-induced endothelial primary response gene. J
Biol Chem. 267:1317–1326. 1992.PubMed/NCBI
|
|
2
|
Zhou J, Hu X, Xiong X, Liu X, Liu Y, Ren
K, Jiang T, Hu X and Zhang J: Cloning of two rat PDIP1 related
genes and their interactions with proliferating cell nuclear
antigen. J Exp Zool A Comp Exp Biol. 303:227–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Liu N, Hu X, Zhang W, Wang T, Li
H, Zhang B, Xiang S, Zhou J and Zhang J: CK2 phosphorylates TNFAIP1
to affect its subcellular localization and interaction with PCNA.
Mol Biol Rep. 37:2967–2973. 2010. View Article : Google Scholar
|
|
4
|
Kim DM, Chung KS, Choi SJ, Jung YJ, Park
SK, Han GH, Ha JS, Song KB, Choi NS, Kim HM, et al: RhoB induces
apoptosis via direct interaction with TNFAIP1 in HeLa cells. Int J
Cancer. 125:2520–2527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Yang Z, Meng M, Zhao Y, Dong N,
Yan H, Liu L, Ding M, Peng HB and Shao F: Cullin mediates
degradation of RhoA through evolutionarily conserved BTB adaptors
to control actin cytoskeleton structure and cell movement. Mol
Cell. 35:841–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Link CD, Taft A, Kapulkin V, Duke K, Kim
S, Fei Q, Wood DE and Sahagan BG: Gene expression analysis in a
transgenic Caenorhabditis elegans Alzheimer's disease model.
Neurobiol Aging. 24:397–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu M, Sun Z, Zhou A, Li H, Yang L, Zhou
C, Liu R, Hu X, Zhou J, Xiang S and Zhang J: Functional
characterization of the promoter region of human TNFAIP1 gene. Mol
Biol Rep. 37:1699–1705. 2010. View Article : Google Scholar
|
|
8
|
Johannessen M, Delghandi MP, Seternes OM,
Johansen B and Moens U: Synergistic activation of CREB-mediated
transcription by forskolin and phorbol ester requires PKC and
depends on the glutamine-rich Q2 transactivation domain. Cell
Signal. 16:1187–1199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sárvári M, Szego EM, Barabás K, Jávor A,
Tóth S, Kovács Z and Abrahám IM: Genistein induces phosphorylation
of cAMP response element-binding protein in neonatal hypothalamus
in vivo. J Neuroendocrinol. 21:1024–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Best JL, Amezcua CA, Mayr B, Flechner L,
Murawsky CM, Emerson B, Zor T, Gardner KH and Montminy M:
Identification of small-molecule antagonists that inhibit an
activator: Coactivator interaction. Proc Natl Acad Sci USA.
101:17622–17627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu N, Yu Z, Li Y, Yuan J, Zhang J, Xiang
S and Wang X: Transcriptional regulation of mouse neuroglobin gene
by cyclic AMP responsive element binding protein (CREB) in N2a
cells. Neurosci Lett. 534:333–337. 2013. View Article : Google Scholar :
|
|
12
|
Liu N, Yu Z, Xiang S, Zhao S,
Tjarnlund-Wolf A, Xing C, Zhang J and Wang X: Transcriptional
regulation mechanisms of hypoxia-induced neuroglobin gene
expression. Biochem J. 443:153–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Zhang XJ, Li T, Li J, Tang Y and
Le W: Valproic acid reduces neuritic plaque formation and improves
learning deficits in APP(Swe)/PS1(A246E) transgenic mice via
preventing the prenatal hypoxia-induced down-regulation of
neprilysin. CNS Neurosci Ther. 20:209–217. 2014. View Article : Google Scholar
|
|
14
|
Li RP, Wang ZZ, Sun MX, Hou XL, Sun Y,
Deng ZF and Xiao K: Polydatin protects learning and memory
impairments in a rat model of vascular dementia. Phytomedicine.
19:677–681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vitolo OV, Sant'Angelo A, Costanzo V,
Battaglia F, Arancio O and Shelanski M: Amyloid beta-peptide
inhibition of the PKA/CREB pathway and long-term potentiation:
Reversibility by drugs that enhance cAMP signaling. Proc Natl Acad
Sci USA. 99:13217–13221. 2002. View Article : Google Scholar
|
|
16
|
Bagheri M, Joghataei MT, Mohseni S and
Roghani M: Genistein ameliorates learning and memory deficits in
amyloid β(1–40) rat model of Alzheimer's disease. Neurobiol Learn
Mem. 95:270–276. 2011. View Article : Google Scholar
|
|
17
|
Gonzalez GA and Montminy MR: Cyclic AMP
stimulates somatostatin gene transcription by phosphorylation of
CREB at serine 133. Cell. 59:675–680. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biermann J, Grieshaber P, Goebel U, Martin
G, Thanos S, Di Giovanni S and Lagrèze WA: Valproic acid-mediated
neuro-protection and regeneration in injured retinal ganglion
cells. Invest Ophthalmol Vis Sci. 51:526–534. 2010. View Article : Google Scholar
|
|
19
|
Lovekamp-Swan T, Glendenning M and
Schreihofer DA: A high soy diet reduces programmed cell death and
enhances bcl-xL expression in experimental stroke. Neuroscience.
148:644–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Yang L, Zhao Y, Zeng G, Wu Y, Chen
Y, Zhang J and Zeng Q: Estrogen is a novel regulator of Tnfaip1 in
mouse hippocampus. Int J Mol Med. 34:219–227. 2014.PubMed/NCBI
|
|
21
|
Walton M, Woodgate AM, Muravlev A, Xu R,
During MJ and Dragunow M: CREB phosphorylation promotes nerve cell
survival. J Neurochem. 73:1836–1842. 1999.PubMed/NCBI
|
|
22
|
Mabuchi T, Kitagawa K, Kuwabara K,
Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M and
Matsumoto M: Phosphorylation of cAMP response element-binding
protein in hippocampal neurons as a protective response after
exposure to glutamate in vitro and ischemia in vivo. J Neurosci.
21:9204–9213. 2001.PubMed/NCBI
|
|
23
|
Chrivia JC, Kwok RP, Lamb N, Hagiwara M,
Montminy MR and Goodman RH: Phosphorylated CREB binds specifically
to the nuclear protein CBP. Nature. 365:855–859. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parker D, Ferreri K, Nakajima T, LaMorte
VJ, Evans R, Koerber SC, Hoeger C and Montminy MR: Phosphorylation
of CREB at Ser-133 induces complex formation with CREB-binding
protein via a direct mechanism. Mol Cell Biol. 16:694–703. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson BE, Mochon E and Boxer LM:
Induction of bcl-2 expression by phosphorylated CREB proteins
during B-cell activation and rescue from apoptosis. Mol Cell Biol.
16:5546–5556. 1996. View Article : Google Scholar : PubMed/NCBI
|