Introduction
Aucklandiae Radix, the root of Aucklandia
(A.) lappa Decne, syn. Saussurea lappa C.B.
Clarke, which belongs to the family of Asteraceae, has been
officially documented as 'Mokhyang' in the Korean Herbal
Pharmacopoeia (1–4). A. lappa has been traditionally
used to treat anorexia, nausea and abdominal pain (5). Several biological properties of A.
lappa, have been confirmed by scientific studies, including its
anti-oxidative (6), anti-ulcer
(7), anti-cancer (8), anti-viral (9) and hepatoprotective effects (10). A recent study by our group reported
that A. lappa alleviates inflammatory chemokine production
in HaCaT cells and house dust mite-induced atopic-like dermatitis
in Nc/Nga mice (11). In addition,
it has been reported that sesquiterpene lactones from A.
lappa have anti-cancer (12,13),
anti-ulcer (14,15), and anti-inflammatory (16) effects. However, to the best of our
knowledge, the effects of active components of A. lappa
against atopic dermatitis have not yet been investigated. The
present study investigated the effects of the three sesquiterpene
lactones costunolide, dehydrocostus lactone and alantolactone on
the expression of Thelper type 2 chemokines, which are essential
factors in the development of atopic dermatitis, in HaCaT human
keratinocytes.
Materials and methods
Preparation of 70% methanolic
extract
The roots of A. lappa used in the present
study were purchased from HMAX (Jecheon, Korea) in July 2009. The
botanical identity of this sample was confirmed taxonomically by
Professor Je-Hyun Lee (Department of Herbology, College of Korean
Medicine, Dongguk University, Gyeongju, Republic of Korea). A
voucher specimen (no. 2009-KIOM62) has been deposited at the Herbal
Medicine Formulation Research Group (K-Herb Research Center, Korea
Institute of Oriental Medicine, Daejeon, Republic of Korea). Dried
roots of A. lappa (100 g) were extracted three times with
70% (v/v) methanol (1 l) (JT Baker, Phillipsburg, NJ, USA) for 90
min with heating under reflux. The extracted solution was filtered
through filter paper and the solvent was evaporated at 40°C using a
Büchi R-210 rotary evaporator (Büchi, Flawil, Switzerland) under
vacuum to dryness, followed by freeze-drying (PVTFD10R;
IlShinBioBase Co., Ltd., Dongducheon, Korea). The yield of the
freeze-dried 70% methanolic extract obtained was 28.57% (28.57
g).
High-performance liquid chromatography
(HPLC) analysis
Reference compounds costunolide, dehydrocostus
lactone and alantolactone were purchased from ChemFaces (Wuhan,
China). The purities of the three sesquiterpene lactones were
≥98.0% according to HPLC analysis. HPLC-grade solvents methanol,
acetonitrile and water were obtained from JT Baker. Glacial acetic
acid (analytical grade) was purchased from Merck KGaA (Darmstadt,
Germany). The sample was analyzed using a Shimadzu Prominence
LC-20A series HPLC apparatus (Shimadzu Co., Kyoto, Japan)
consisting of a solvent delivery unit (no. LC-20AT), on-line
degasser (no. DGU-20A3), column oven (no. CTO-20A), auto sample
injector (no. SIL-20AC) and PDA detector (no. SPD-M20A). Data were
collected and processed using LCsolution software (version 1.24;
Shimadzu Co.). The stationary phase used for the separation of the
compounds was a reverse-phase SunFire™ C18 analytical
column (Waters, Milford, MA, USA; 150×4.6 mm and 5 µm
particle size). The mobile phase was composed of water (A) and
acetonitrile (B) with isocratic elution (i.e., 40% A and 60% B).
The flow rate was 1.0 ml/min, the column temperature was maintained
at 35°C, and the injection volume was 10 µl. The detection
wavelength for quantification covered the range of 190–400 nm and
recorded at 225 nm. To prepare the stock solutions, reference
compounds were accurately weighed and dissolved in methanol to a
concentration of 1.0 mg/ml; the samples were stored below 4°C. The
concentration ranges of test samples for the generation of
calibration curves were 1.56–200.00, 2.34–300.00 and 0.23–30.00
µg/ml for costunolide, dehydrocostus lactone and
alantolactone, respectively. For HPLC analysis of the 70%
methanolic extract, 20 mg extracted solid was dissolved in 10 ml
70% methanol and dissolved by sonication for 30 min. The solution
was filtered through a 0.2-µm membrane filter (Woongki
Science, Seoul, Korea) prior to injection into the HPLC
instrument.
Cell culture
The HaCaT human keratinocyte cell line was obtained
from CLS Cell Lines Service GmbH (Eppelheim, Baden-Württemberg,
Germany). The HaCaT cells were cultured in Dulbecco's modified
Eagle's medium (DMEM, Gibco-BRL, Invitrogen Life Technologies,
Inc., Carlsbad, CA, USA) supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Gibco-BRL), penicillin (100 µg/ml;
Gibco-BRL) and streptomycin (100 µg/ml; Gibco-BRL) in an
incubator containing 5% CO2 at 37°C.
Cytotoxicity assay
Cell viability was evaluated using a Cell Counting
Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) following the
manufacturer's instructions. HaCaT cells (1×103
cells/well) were incubated in 96-well plates with costunolide or
dehydrocostus lactone at 0, 1.25, 2.5, 5 or 10 µM or with
alantolactone at 0, 0.625, 1.25, 2.5 or 5 µM for 24 h. CCK-8
reagent was added to each well and cells were incubated for an
additional 4 h. The absorbance was measured at 450 nm using a
Benchmark plus microplate reader (Bio-Rad Laboratories, Hercules,
CA, USA). The percentage of viable cells was calculated as follows:
Cell viability (%) = (mean absorbance in test wells/mean
absor-bance in untreated control well) ×100.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
according to the manufacturer's instructions (Invitrogen Life
Technologies, Inc.). HaCaT cells (1×106 cells/well) were
cultured to 80–90% confluency in 6-well plates. When the cells
reached confluence, the cells were washed and treated with
costunolide, dehydrocostus lactone or alantolactone in 1 ml
serum-free medium (Gibco-BRL) supplemented with tumor necrosis
factor (TNF)-α and interferon (IFN)-γ (each 10 ng/ml; R&D
Systems Inc., Minneapolis, MN, USA) for 24 h. Silymarin
(Sigma-Aldrich Inc., St. Louis, MO) was used as a positive control
drug. Total RNA (1 µg) was then converted into cDNA using an
iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc.) containing
oligo-dT primers. Diethylpyrocarbonate-treated water was added to a
final volume of 20 µl followed by incubation at 42°C for 30
min using a Bio-Rad iCycler apparatus (Bio-Rad Laboratories, Inc.).
For PCR amplification, the following gene-specific primers were
used: TARC forward, 5′-ACTGCTCCAGGGATGCCATCGTTTTT-3′ and reverse,
5′-ACAAGGGGATGGGATCTCCCTCACTG-3′; MDC forward,
5′-AGGACAGAGCATGGCTCGCCTACAGA′ and reverse,
5′-TAATGGCAGGGAGCTAGGGCTCCTGA-3′; RANTES forward,
5′-CCCCGTGCCGAGATCAAGGAGTATTT-3′ and reverse,
5′-CGTCCAGCCTGGGGAAGGTTTTTGTA-3′; IL-8 forward,
5′-GTGGCTCTCTTGGCAGCCTTCCTGAT-3′ and reverse,
5′-TCTCCACAACCCTCTGCACCCAGTTT-3′; and GAPDH forward,
5′-GTGATGGCATGGACTGTGGT-3′ and reverse, 5′-AAGGGTCATCATCTCTGCCC-3′.
The PCR reaction mixture contained 1 µl cDNA and 1.56
µl γTaq PCR master mix (cat. no. EBT-1014; Elpis Biotech,
Inc., Daejeon, Korea), which contained 1.5 mM MgCl2, 0.1
µM of each forward and reverse primer and 7.44 µl
water in a final volume of 10 µl. The thermocycling program
comprised initial denaturation at 94°C for 5 min, followed by 25
cycles of denaturation at 94°C for 30 sec, annealing at 64°C for 1
min, extension at 72°C for 1 min 30 sec for all of the chemokines,
and 25 cycles of denaturation at 94°C for 30 sec, annealing at 52°C
for 1 min, and extension at 72°C for 1 min 30 sec for GAPDH. A
final extension step was conducted at 72°C for 7 min. The amplified
products were separated by 1.5% agarose gel and visualized using
Loading STAR staining (A750; DYNE Bio, Seongnam, Korea). The
relative expression levels of TARC, MDC, RANTES and IL-8 mRNA were
normalized to those of GAPDH mRNA using a Chemi-Doc Band Analysis
system (Bio-Rad Laboratories, Inc.).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. All of the experiments were performed at least three
times. One-way analysis of variance was used to detect significant
differences between the control and treatment groups. Dunnett's
test was used for multiple comparisons using GraphPad InStat
version 3.10 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a significant difference between
values.
Results
Quantitative determination of three
components of A. lappa extract
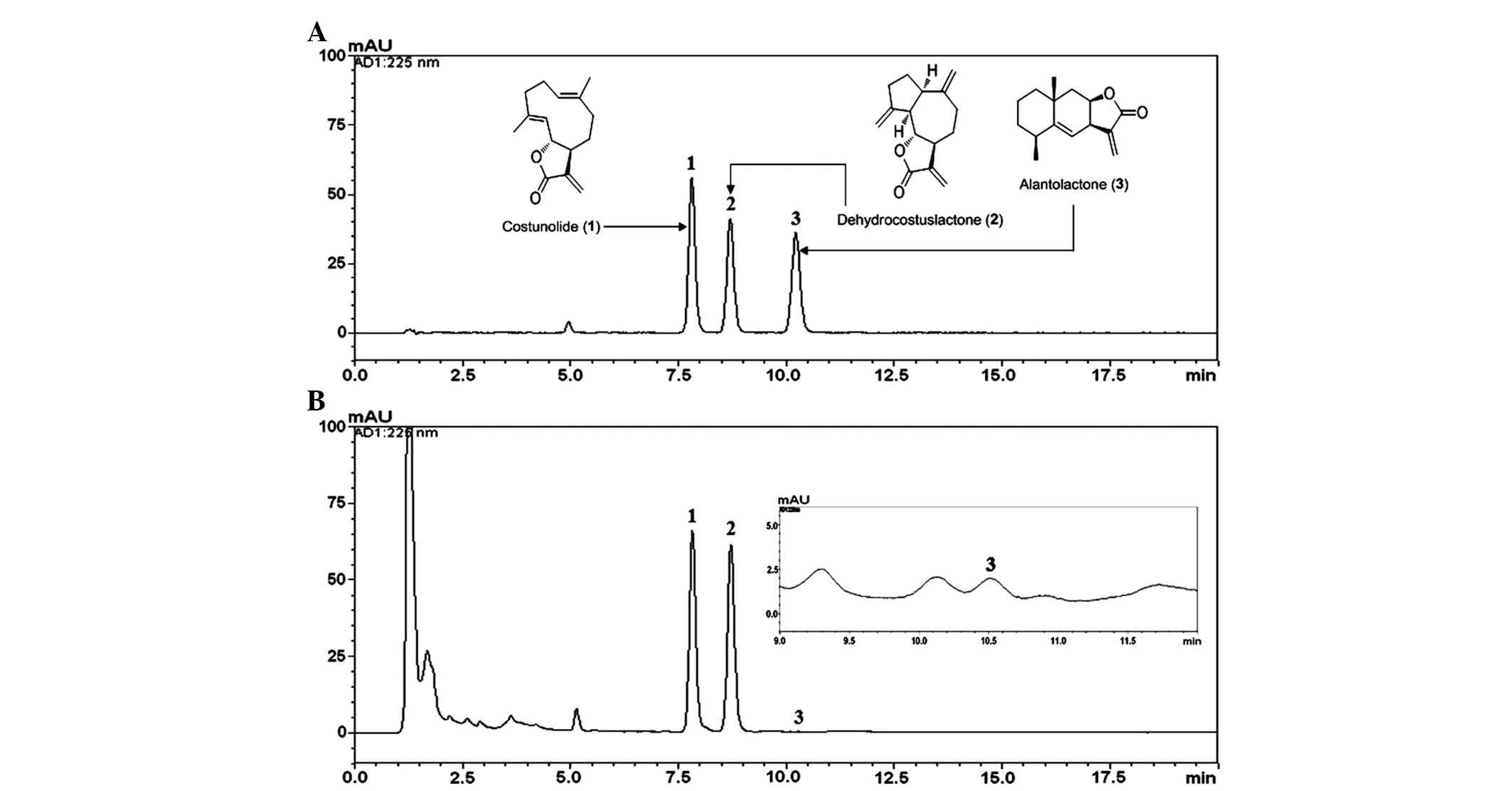
Each compound in the HPLC chromatogram was
identified by comparing the retention times and ultraviolet
absorption spectra with those of the reference standards. The
retention times of costunolide, dehydrocostus lactone and
alantolactone under the optimized conditions were 7.81, 8.70, and
10.22 min, respectively (Fig. 1).
Each regression equation (y=ax+b) was calculated
based on the ratio of peak area (y) and concentration
(x, µg/ml) of costunolide, dehydrocostus lactone and
alantolactone. Standard curves plotted for the three compounds
showed high linearity, with r2≥0.9999 in eight
different concentration ranges tested. The established analytical
HPLC method was applied for the simultaneous quantification of the
three compounds in the methanolic extract of A. lappa. The
amounts of costunolide, dehydrocostus lactone and alantolactone in
the extract were 17.32, 28.26, and 0.01 mg/g, respectively.
Cytotoxicity of A. lappa extract
components
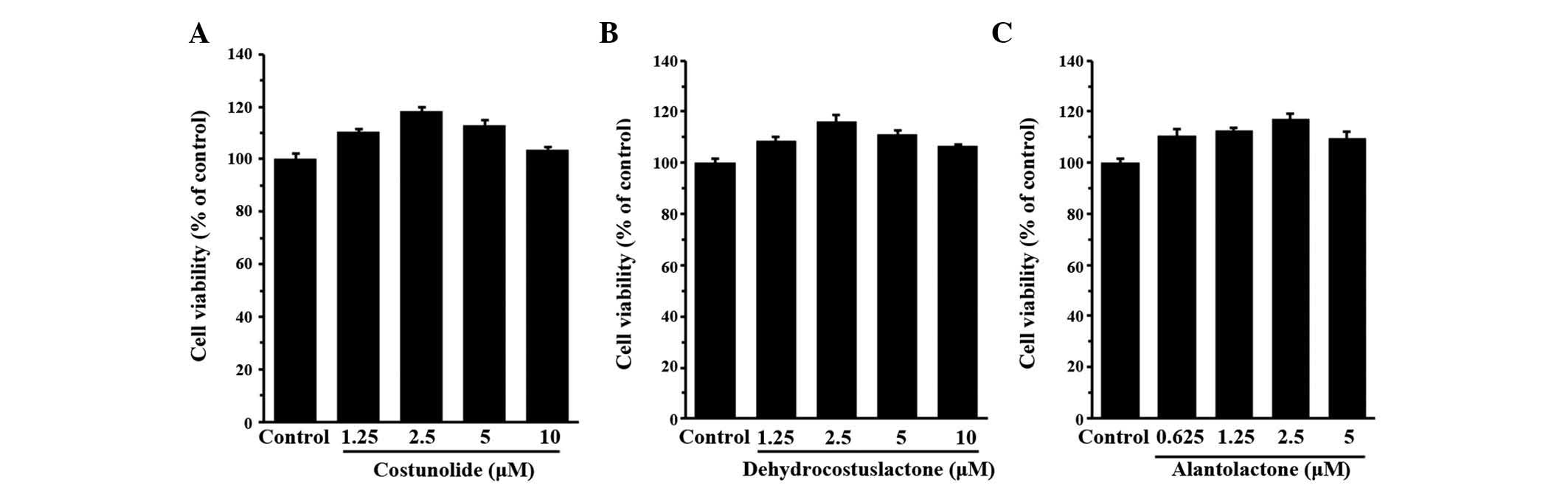
The present study assessed the cytotoxic effects of
the three components of the A. lappa extract on HaCaT cells
(Fig. 2). The non-toxic
concentrations of the three components were determined to be 10,
10, and 5 µM for costunolide, dehydrocostus lactone and
alantolactone, respectively. Non-toxic concentrations (>90%
compared with the control) of the three components were applied in
the biological assays.
Inhibitory effects of costunolide,
dehydrocostus lactone and alantolactone on the chemokine mRNA
levels in TNF-α- and IFN-γ-stimulated HaCaT cells
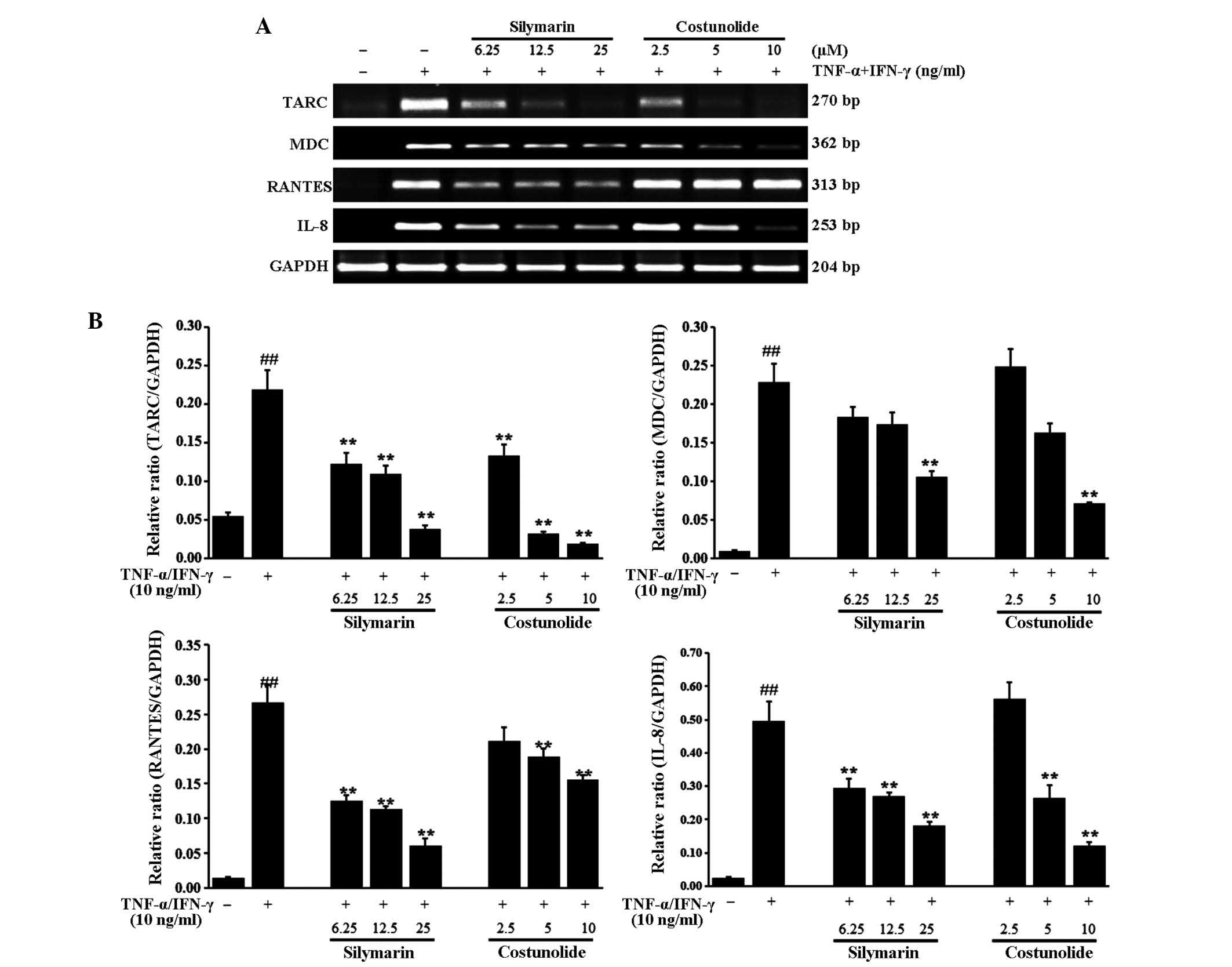
The effects of the three components of A.
lappa extract on mRNA levels of the Thelper cell type 2
cytokines TARC, MDC, RANTES and IL-8 were assessed in TNF-α and
IFN-γ-stimulated HaCaT cells. Stimulation with TNF-α and IFN-γ
significantly increased the expression of TARC, MDC, RANTES and
IL-8 mRNA in HaCaT cells (Figs.
3Figure 4–5). Treatment of the cells with compound
costunolide (Fig. 3),
dehydrocostus lactone (Fig. 4) and
alantolactone (Fig. 5)
significantly reduced the expression of TARC, MDC, RANTES and IL-8
mRNA in a dose-dependent manner. Silymarin, a positive control,
decreased the expression of TARC, MDC, RANTES and IL-8 mRNA
compared with that following stimulation with TNF-α and IFN-γ in a
dose-dependent manner.
Discussion
Chemokines have a pivotal role in immune responses
and inflammatory reactions. Various inflammatory cytokines
stimulate the production of chemokines and specific inflammatory
chemokines are found in the serum of patients with atopic
dermatitis (17). Among them,
TARC/CCL17, MDC/CCL22 and RANTES/CCL5 are typical inflammatory
chemokines that are predominantly expressed in various immune
cells, including lymphocytes, dendritic cells, keratinocytes and
eosinophils (18). Previous
studies showed that levels of these chemokines in serum and skin
lesions of patients with atopic dermatitis are elevated, suggesting
that chemokines produced by keratinocytes may be key molecules that
attract inflammatory lymphocytes to the skin (19). IL-8 is another important mediator
in atopic dermatitis and previous studies have demonstrated that
the amount of IL-8 is closely associated with the severity of
atopic skin lesions (20).
The present study investigated the inhibitory
effects of three components of A. lappa, including
costunolide, dehydrocostus lactone and alantolactone on chemokine
expression at the mRNA level in TNF-α and IFN-γ-stimulated HaCaT
cells. All three sesquiterpene lactones markedly reduced TNF-α and
IFN-γ-induced expression of TARC, MDC and IL-8 in a dose-dependent
manner. While costunolide and dehydrocostus lactone had weak
inhibitory effects on RANTES expression, alantolactone markedly
suppressed the mRNA expression of RANTES mRNA in TNF-α and
IFN-γ-stimulated HaCaT cells. These results indicated that these
three components of A. lappa may have activity against skin
inflammation through regulation of chemokine expression in
keratinocytes.
Several studies have demonstrated the
anti-inflammatory actions of sesquiterpene lactones from medicinal
herbs, includings Milleria quinqueflora (21), Tithonia diversifolia
(22), Ixeris dentate
(23) and Eupatorium
perfoliatum (24), suggesting
potential use of these herbs as anti-inflammatory agents.
Similarly, the present study reported the anti-inflammatory effects
of sesquiterpene lactones from A. lappa. Further
investigation is necessary to verify the results of the present
study and to evaluate the suitability of sesquiterpene lactones
from A. lappa for the treatment of skin inflammation,
including atopic dermatitis.
In conclusion, the present study demonstrated that
the costunolide, dehydrocostus lactone and alantolactone,
components of A. lappa, reduced the mRNA expression levels
of chemokines TARC, MDC, RANTES and IL-8 in TNF-α and
IFN-γ-stimulated HaCaT cells. These results indicated that the
three compounds may be the active components of A. lappa
that inhibit the production of chemokines. Further investigation is
required to elucidate the detailed mechanisms of action of the
sesquiterpene lactones, and the compounds should be subjected to
toxicological tests using an in vivo experimental model.
Acknowledgments
The present study was supported by a grant from the
Korea Institute of Oriental Medicine (Daejeon, Republic of Korea;
no. K14030).
References
|
1
|
Korea Food and Drug Administration: The
Korean herbal pharmacopoeia. Dongwon Munhwasa; Seoul: pp.
1322007
|
|
2
|
Kim H, Kang K, Choi G, Kim H, Jeong S and
Ju Y: A study on external and internal morphology and pattern
analysis in 4 kinds of Mok-Hyaeng Radix. Korean J Oriental Med.
12:117–130. 2006.
|
|
3
|
Yoon TS, Sung YY, Jang JY, Yang WK, Ji Y
and Kim HK: Anti-obesity activity of extract from Saussurea lappa.
Korean J Medicinal Crop Sci. 18:151–156. 2010.
|
|
4
|
Hasson SS, Al-Balushi MS, Alharthy K,
Al-Busaidi JZ, Aldaihani MS, Othman MS, Said EA, Habal O, Sallam
TA, Aljabri AA and Ahmedidris M: Evaluation of anti-resistant
activity of Auklandia (Saussurea lappa) root against some human
pathogens. Asian Pac J Trop Biomed. 3:557–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi JY, Na M, Hyun Hwang I, Ho Lee S,
Young Bae E, Yeon Kim B and Seog Ahn J: Isolation of betulinic
acid, its methyl ester and guaiane sesquiterpenoids with protein
tyrosine phosphatase 1B inhibitory activity from the roots of
Saussurea lappa C.B. Clarke. Molecules. 14:266–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saleem TS, Lokanath N, Prasanthi A,
Madhavi M, Mallika G and Vishnu MN: Aqueous extract of Saussurea
lappa root ameliorate oxidative myocardial injury induced by
isoproterenol in rats. J Adv Pharm Technol Res. 4:94–100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah NC: Herbal folk medicines in Northern
India. J Ethnopharmacol. 6:293–301. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim EJ, Hong JE, Lim SS, Kwon GT, Kim J,
Kim JS, Lee KW and Park JH: The hexane extract of Saussurea lappa
and its active principle, dehydrocostus lactone, inhibit prostate
cancer cell migration. J Med Food. 15:24–32. 2012. View Article : Google Scholar
|
|
9
|
Chen HC, Chou CK, Lee SD, Wang JC and Yeh
SF: Active compounds from Saussurea lappa Clarks that suppress
hepatitis B virus surface antigen gene expression in human hepatoma
cells. Antiviral Res. 27:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yaeesh S, Jamal Q, Shah AJ and Gilani AH:
Antihepatotoxic activity of Saussurea lappa extract on
D-galactosamine and lipopolysaccharide-induced hepatitis in mice.
Phytother Res. 24(Suppl 2): S229–S232. 2010. View Article : Google Scholar
|
|
11
|
Lim HS, Ha H, Lee MY, Jin SE, Jeong SJ,
Jeon WY, Shin NR, Sok DE and Shin HK: Saussurea lappa alleviates
inflammatory chemokine production in HaCaT cells and house dust
mite-induced atopic-like dermatitis in Nc/Nga mice. Food Chem
Toxicol. 63:212–220. 2014. View Article : Google Scholar
|
|
12
|
Rasul A, Khan M, Ali M, Li J and Li X:
Targeting apoptosis pathways in cancer with alantolactone and
isoalantolactone. Scientific World Journal. 2013:2485322013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohnishi M, Yoshimi N, Kawamori T, Ino N,
Hirose Y, Tanaka T, Yamahara J, Mirata H and Mori H: Inhibitory
effects of dietary protocatechuic acid and costunolide on
7,12-dimethylbenz[a] anthracene-induced hamster cheek pouch
carcinogenesis. Jpn J Cancer Res. 88:111–119. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshikawa M, Hatakeyama S, Inoue Y and
Yamahara J: Saussureamines A, B, C, D and E, new anti-ulcer
principles from Chinese Saussureae Radix. Chem Pharm Bull (Tokyo).
41:214–216. 1993. View Article : Google Scholar
|
|
15
|
Matsuda H, Kageura T, Inoue Y, Morikawa T
and Yoshikawa M: Absolute stereostructures and syntheses of
saussureamines A, B, C, D and E, amino acid-sesquiterpene
conjugates with gastroprotective effect, from the roots of
Saussurea lappa. Tetrahedron. 56:7763–7777. 2000. View Article : Google Scholar
|
|
16
|
Kassuya CA, Cremoneze A, Barros LF, Simas
AS, Lapa Fda R, Mello-Silva R, Stefanello ME and Zampronio AR:
Antipyretic and anti-inflammatory properties of the ethanolic
extract, dichloromethane fraction and costunolide from Magnolia
ovata (Magnoliaceae). J Ethnopharmacol. 124:369–376. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morita A, Kikuoka S, Korikawa T, Bito T,
Yamada H, Kanda M, Sasakura K, Tamaki M, Hirai K, Suzuki R and
Sugita K: Evaluation of human thymus and activation-regulated
chemokine concentration in blood using a new sandwich EKISA based
on monoclonal antibodies. Clin Chim Acta. 322:67–75. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saeki H and Tamaki K: Thymus and
activation regulated chemokine (TARC)/CCL17 and skin diseases. J
Dermatol Sci. 43:75–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pease JE: Targeting chemokine receptors in
allergic disease. Biochem J. 434:11–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casas C, Ribet V, Alvarez-Georges S,
Sibaud V, Guerrero D, Schmitt AM and Redoules D: Modulation of
interleukin-8 and staphylococcal flora by Avène hydrotherapy in
patients suffering from chronic inflammatory dermatoses. J Eur Acad
Dermatol Venereol. 25(Suppl 1): 19–23. 2011. View Article : Google Scholar
|
|
21
|
Castro V, Rüngeler P, Murillo R, Hernandez
E, Mora G, Pahl HL and Merfort I: Study of sesquiterpene lactones
from Milleria quinqueflora on their anti-inflammatory activity
using the transcription factor NF-kappaB as molecular target.
Phytochemistry. 53:257–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rüngeler P, Lyss G, Castro V, Mora G, Pahl
HL and Merfort I: Study of three sesquiterpene lactones from
Tithonia diversifolia on their anti-inflammatory activity using the
transcription factor NF-kappaB and enzymes of the arachidonic acid
pathway as targets. Planta Med. 64:588–593. 1998. View Article : Google Scholar
|
|
23
|
Kim SB, Kang OH, Joung DK, Mun SH, Seo YS,
Cha MR, Ryu SY, Shin DW and Kwon DY: Anti-inflammatory effects of
tectroside on UVB-induced HaCaT cells. Int J Mol Med. 31:1471–1476.
2013.PubMed/NCBI
|
|
24
|
Maas M, Deters AM and Hensel A:
Anti-inflammatory activity of Eupatorium perfoliatum L. extracts,
eupafolin, and dimeric guaianolide via iNOS inhibitory activity and
modulation of inflammation-related cytokines and chemokines. J
Ethnopharmacol. 137:371–381. 2011. View Article : Google Scholar : PubMed/NCBI
|