Introduction
Succinylcholine (SuCh) is currently used in the
clinic to achieve muscle relaxation, particularly when there is a
demand for rapid onset and elimination of contraction. Due to its
rapid onset, short half-life and reliably, SuCh treatment creates
excellent intubation conditions. At present, no non-depolarizing
relaxants that possess suitable pharmacodynamic characteristics for
replacing SuCh are available (1,2).
Therefore, SuCh is most frequently applied prior to urgent tracheal
intubation in the peri-operative period, at intensive care units
and emergency departments, and even outside the hospital during
emergency transportation of patients (3–6).
However, thermal injury and other forms of critical
tissue damage can cause denervation-associated changes in skeletal
muscles (7). Denervated skeletal
muscle displays two characteristic changes: An increase in the
number of nicotinic acetylcholine receptors (nAChRs) and de
novo expression of fetal-type nAChR (γ-AChR) (8). Upregulation of nAChRs results in
increased sensitivity to SuCh and resistance to non-depolarizing
relaxants (7–11). Certain studies reported that after
denervation, levels of nAChR and γ-AChR changed with time;
furthermore, the IC50 of d-tubocurarine (dTC)
correlated positively with nAChR and γ-AChR mRNA levels at
different time-points after denervation (12–14).
All of theses results indicated that the potency of muscle
relaxants may change with increasing time of skeletal muscle
denervation.
The aim of the present study was to assess the
changes in the potency of SuCh during the first month after
denervation in a mouse model. Furthermore, the present study
investigated whether the de novo expression of γ-AChR
contributed to the increased sensitivity of denervated skeletal
muscles to SuCh.
Materials and methods
Denervation
The present study was approved by the Animal Care
and Use Committee of Bengbu Medical School. Balb/C mice (35 days
old) were anesthetized with pentobarbital, (40 mg/kg
intraperitoneally). A sample of a few millimeters in size of the
right sciatic nerve was excised through a small (3–5 mm) incision
above the hip. The incision was sutured with a single stitch.
Animals were sacrificed at 1, 4, 7, 14, 21 and 28 days after
denervation by pentobarbital anesthesia and cervical dislocation.
Animals that had received surgery without incision of the sciatic
nerve were used as the innervated control (0 days after
denervation).
Isolation of muscle fibres
Single skeletal muscle cells from the flexor
digitorum brevis (FDB) muscle were obtained from the hindfeet of
the mice. The muscles were incubated for 3 h with Dulbecco's
modified Eagle's medium (DMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA) containing 10% fetal calf serum (FCS; Invitrogen
Life Technologies), 100 U/ml penicillin (Sigma-Aldrich, St. Louis,
MO, USA), 100 µg/ml streptomycin (Sigma-Aldrich) and 0.2%
collagenase 1A (Sigma-Aldrich) with agitation at 37°C.
Subsequently, flexor digitorum brevis (FDB) muscles were
disaggregated into single muscle cells by repetitive pipetting
using Pasteur pipettes of various tip sizes.
Expression of nAChR in human embryonic
kidney (HEK)293 cells
Expression plasmids Psp65α, Psp65β, Psp65δ, Psp64γ,
and Pbssk(+)ε, encoding complementary DNA-coding sequences for the
mouse muscle nAChR sub-units α, β, δ, γ and ε, respectively, were
provided by the Salk Institute for Biological Studies (La Jolla CA,
USA). These plasmids were sub-cloned into pcDNA3.1+ (Invitrogen
Life Technologies). HEK293 cells (Shanghai Institute of
Biochemistry and Cell Biology for Biological Sciences, Shanghai,
China) were cultured in DMEM supplemented with 10% FCS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in an
incubator containing 5% CO2. The HEK293 cells were
stably transfected with Lipofectamine™ 2000 (Invitrogen Life
Technologies) according to the manufacturer's instructions. After
transfection, positive cell clones were selected with G418
(Invitrogen Life Technologies). The transfected cells were then
incubated for 24 h prior to being subjected to the
electrophysiological assays.
Electrophysiology
FDB muscle cells or HEK293 cells were
voltage-clamped using a whole-cell patch clamp technique (15). All experiments were performed at
room temperature (20–24°C). Patch pipettes were pulled from
borosilicate glass using a Flaming Brown micropipette puller (P97;
Sutter Instrument Co., Novato, CA, USA), ranging from 1–2 MΩ. The
pipette electrode was filled with the following solution: 140 mM
KCl, 10 mM 4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid
(HEPES), 10 mM ethylene glycol tetraacetic acid, 1 mM
CaCl2 and 1 mM MgCl2; the pH was adjusted to
7.2 with KOH. The external solution contained 5 mM KCl, 140 mM
NaCl, 1 mM CaCl2, 1.25 mM MgCl2, 10 mM HEPES,
10 mM glucose and 0.5 µM atropine sulfate, and the pH was
adjusted to 7.4 with NaOH. The cells were voltage-clamped at -80 mV
in a whole-cell configuration after obtaining GΩ seals. A major
amount of the series resistance (60–80%) was compensated. Currents
were recorded with an EPC10 amplifier (HEKA Elektronik, Lambrecht,
Germany) and PatchMaster software (v2.15; HEKA Eletronik), sampled
at 10 kHz.
SuCh (Sigma-Aldrich) was dissolved in the external
solution and applied via a gravity-driven perfusion system.
Solutions and subsequent dilutions were prepared immediately prior
to the experiments.
Test solution applications containing various
concentrations of SuCh (1–500 µM) were applied for 10 sec to
the skeletal muscle cells obtained from mice sacrificed on days 0,
1, 4, 7, 14, 21 and 28 after denervation or to HEK293 cells for 5
sec, and the peak current was determined. The washout time between
each drug application was at least 60 sec in order to minimize the
amount of desensitization throughout the course of an experiment.
Currents were acquired from five skeletal muscle cells taken from
at least two mice or five HEK293 cells.
Statistical analysis
Data analysis was performed off-line using Origin 8
(OriginLab, Northampton, MA, USA) and GraphPad Prism 4 (Graphpad
Software, Inc., La Jolla, CA, USA). Concentration-response curves
were fitted to the four-parameter logistic equation by non-linear
regression analysis, and the EC50 was calculated as the
concentration of the agonist eliciting a half-maximal response.
Values are expressed as the mean ± standard deviation. Statistical
significance was assessed by one-way analysis of variance followed
by Tukey's test or unpaired two-tailed Student's t-tests. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
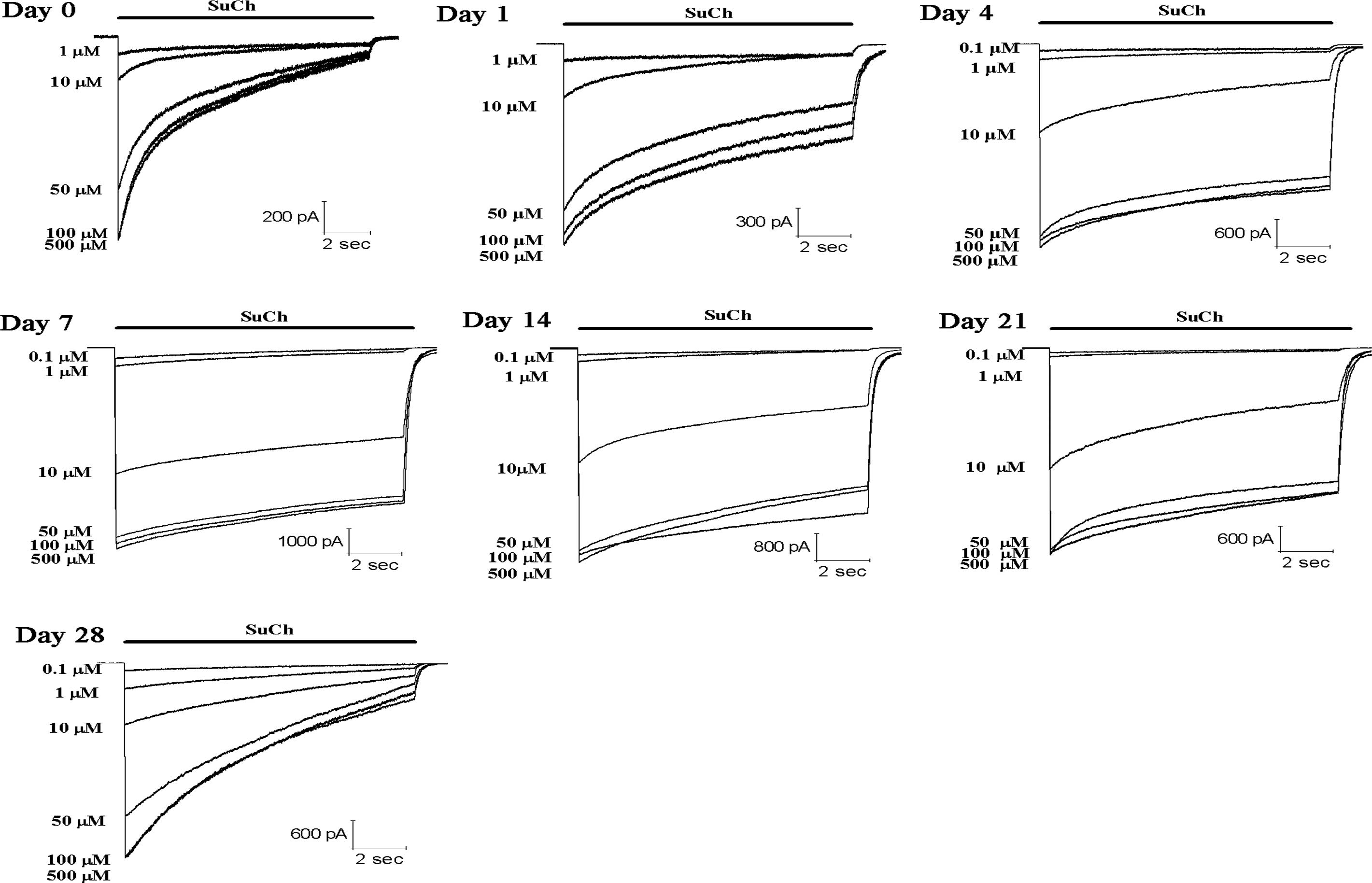
SuCh at various concentration (1–500 µM) was
applied for 10 sec to mouse skeletal muscle cells on days 0, 1, 4,
7, 14, 21, and 28 after denervation. SuCh elicited
concentration-dependent inward currents (Figs. 1 and 2). The data were fitted to a logistic
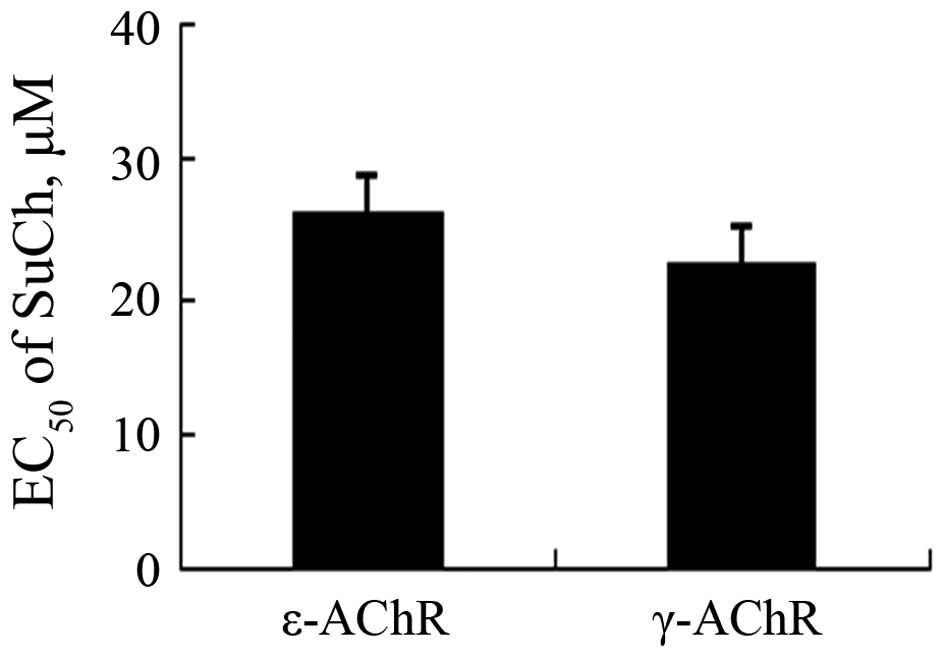
equation. The SuCh concentration producing 50% of the maximal
response (EC50) was 27.5 µM for the nAChR in the
innervated skeletal muscle (day 0 after denervation). Compared with
the EC50 value at day 0 after denervation, the
EC50 values were decreased by 20 (P>0.05), 56, 73,
66, 60 and 62% (P<0.05) on days 1, 4, 7, 14, 21 and 28 after
denervation, respectively (Fig.
3).
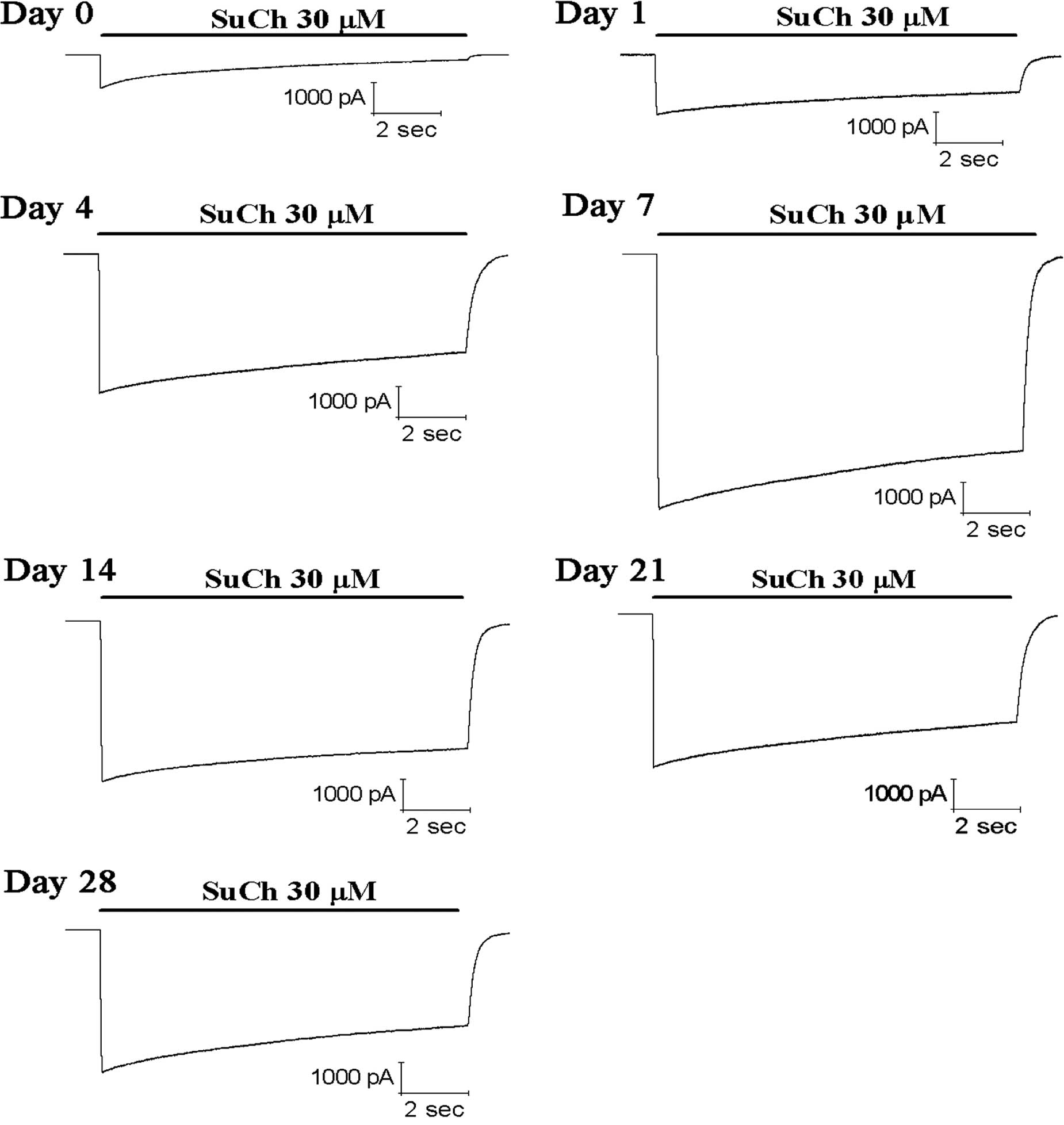
Application of 30 µM SuCh produced inward
currents with different amplitudes at the nAChRs of skeletal muscle
cells on days 0, 1, 4, 7, 14, 21 and 28 after denervation (Fig. 4). Compared with the current
responses on day 0 after dener-vation, the current responses
induced by 30 µM SuCh were increased by 1.9-, 4.6-, 9.4-,
7.1-, 5.2- and 5.1-fold (P<0.05) at days 1, 4, 7, 14, 21 and 28
after denervation, respectively (Fig.
5).
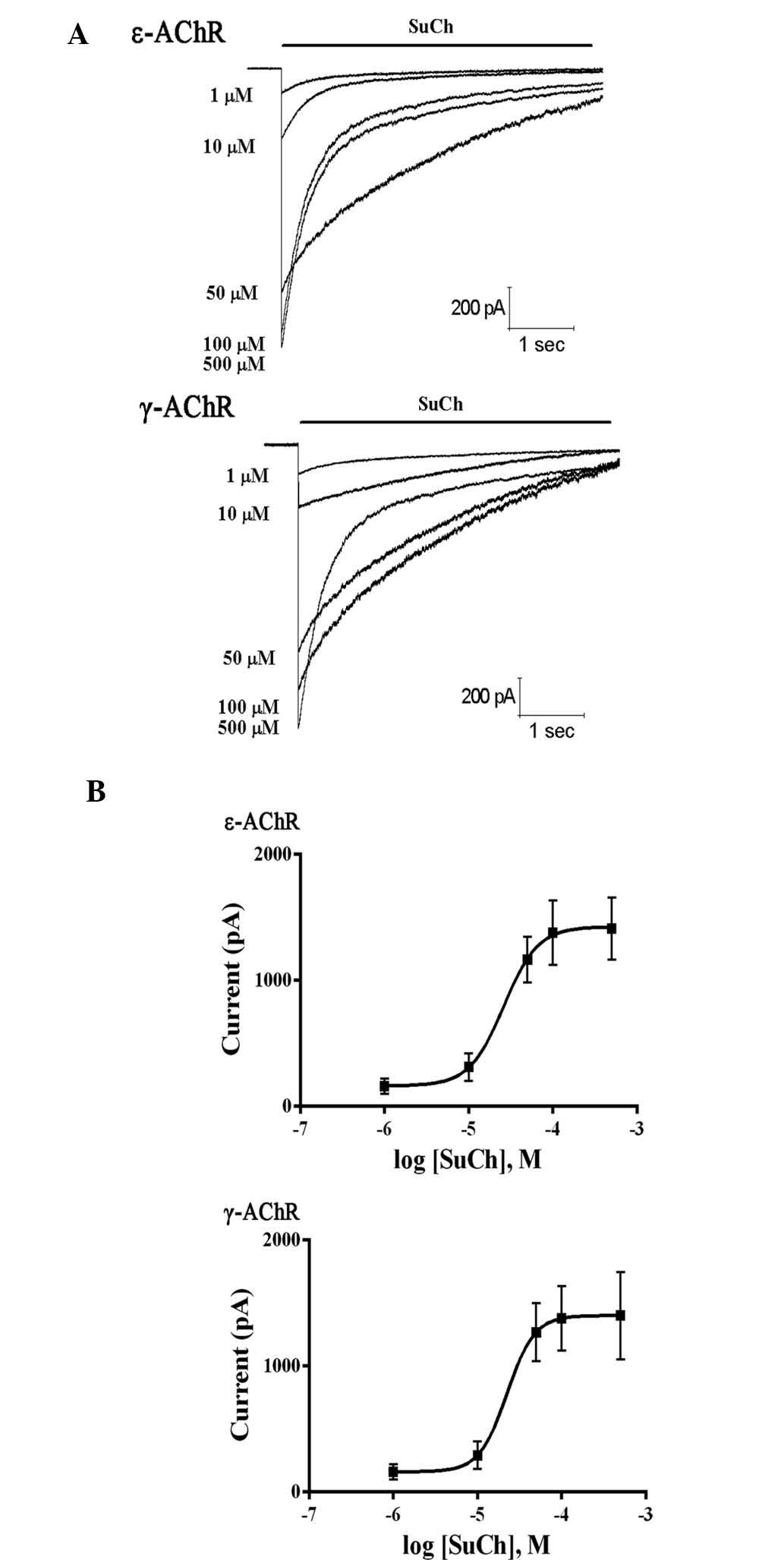
To further determine the effects of SuCh on nAChR
sub-types, HEK293 cells were stably transfected with plasmids
expressing either ε- or γ-AChR and then treated with SuCh. SuCh
elicited currents in the HEK293 cells in a concentration-dependent
manner (Fig. 6). However,
comparison of the EC50 values revealed that no
significant differences were present between the effects of SuCh on
currents in ε-AChR- and γ-AChR-expressing HEK293 cells (P>0.05)
(Fig. 7).
Discussion
The present study determined the effects of
short-term denervation on the sensitivity of skeletal muscles to
SuCh. As previous studies have indicated the amount of membrane
nAChR after denervation changed with time (7,8), the
present study further assessed whether the increased sensitivity to
SuCh after denervation was associated with the presence of
γ-AChR.
The present study generated a murine denervation
model according to methods of previous studies by our group
(7,8,11).
Innervated and denervated skeletal muscle cells were obtained at
various time-points after denervation. Compared with the values
obtained from innervated skeletal muscle cells, the potency of SuCh
significantly increased at day 4, peaked at day 7 and declined by
day 14. Ibebunjo and Martyn (12,16)
demonstrated that burn injuries induced increases in nAChRs at days
4, 7 and 14 in rat skeletal muscles. Furthermore, Adams et
al (14) reported that
denervation increased the expression of all sub-unit genes of
nAChRs during the first month of denervation. Furthermore, previous
studies by our group have confirmed that short-term muscle
denervation leads to a changing pattern of resistance to
non-depolarizing muscle relaxants that can be attributed to
upregulation of mature and immature nAChRs on the muscular membrane
(8,11).
The present study assessed the activated current
amplitude following application of 30 µM SuCh on nAChRs in
innervated and denervated skeletal muscle. Compared with that in
innervated skeletal muscle cells, the current amplitude after
denervation was significantly increased. The time-course of
increases in the current amplitude after denervation was similar to
that of the EC50 of SuCh. Consistent with the results of
the present study, a previous study on the denervation of a single
limb, a hyperkalemic response to SuCh occurred as early as on day
four after denervation (17).
Therefore, Gronert (18) suggested
that it is advisable to avoid the use of SuCh beyond 48–72 h of
denervation.
nAChRs include two sub-types: The adult form
(ε-AChR) composed of α2βδε sub-units and the fetal form
(γ-AChR) containing α2βδγ sub-units (7). Only ε-AChR is expressed in adult
muscle; however, denervation results in the de novo
expression of γ-AChR (19).
Ibebunjo (12) reported that
resistance to dTC is associated with the expression of γ-AChR. In
addition, a previous study by our group demonstrated that the
phenomenon of different magnitudes of resistance to
non-depolarizing muscle relaxants in denervated mouse skeletal
muscles is due to the different potencies of individual
non-depolarizing muscle relaxants with regard to nAChR sub-units
(7). The present study examined
the effects of SuCh on currents in HEK293 cells heterologously
expressing ε-AChR or γ-AChR. The results showed that SuCh had
equipotent effects on cells expressing either of the receptor
sub-types. Similarly, Yost and Winegar (20) reported that there were no
significant differences in the EC50 for SuCh on ε-AChR
and γ-AChR, and that ε-AChR had a six- to eight-fold higher
intrinsic activity for SuCh than γ-AChR. The results of the present
study therefore indicated that the changes in sensitivity of muscle
cells to SuCh after denervation are not associated with the de
novo expression of γ-AChR.
In conclusion, the present study found that
short-time denervation resulted an increased sensitivity of muscle
cells to SuCh, which is unlikely to be associated with the de
novo expression of γ-AChR. After denervation, the potency of
SuCh significantly increased by day 4, peaked at day 7, declined by
day 14 and remained constant until day 28. These results suggested
that it is advisable to avoid the use of SuCh at day 4 after
denervation and thereafter in order to prevent lethal hyperkalemic
responses.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 30571796).
References
|
1
|
Miller R: Will succinylcholine ever
disappear? Anesth Analg. 98:1674–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caldwell JE: The continuing search for a
succinylcholine replacement. Anesthesiology. 100:763–764. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laurin EG, Sakles JC, Panacek EA, Rantapaa
AA and Redd J: A comparison of succinylcholine and rocuronium for
rapid-sequence intubation of emergency department patients. Acad
Emerg Med. 7:1362–1369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marsch SC, Steiner L, Bucher E, Pargger H,
Schumann M, Aebi T, Hunziker PR and Siegemund M: Succinylcholine
versus rocuronium for rapid sequence intubation in intensive care:
A prospective, randomized controlled trial. Crit Care. 15:R1992011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norwood S, Myers MB and Butler TJ: The
safety of emergency neuromuscular blockade and orotracheal
intubation in the acutely injured trauma patient. J Am Coll Surg.
179:646–652. 1994.PubMed/NCBI
|
|
6
|
Ma OJ, Atchley RB, Hatley T, Green M,
Young J and Brady W: Intubation success rates improve for an air
medical program after implementing the use of neuromuscular
blocking agents. Am J Emerg Med. 16:125–127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Yang B, Xu YF, Yan T and Li ST:
Different magnitude of resistance to nondepolarizing muscle
relaxants in the denervated mouse skeletal mucle. Acta Pharmacol
Sin. 31:399–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Yang B, Han GW and Li ST: Potency
of nondepolarizing muscle relaxants at muscle-type acetylcholine
receptors in denervated mouse skeletal muscle. Acta Pharmacol Sin.
31:1541–1546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martyn JA, White DA, Gronert GA, Jaffe RS
and Ward JM: Up-and-down regulation of skeletal muscle
acetylcholine receptors. Effects on neuromuscular blockers.
Anesthesiology. 76:822–843. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yanez P and Martyn JA: Prolonged
d-tubocurarine infusion and/or immobilization cause upregulation of
acetylcholine receptors and hyperkalemia to succinylcholine in
rats. Anesthesiology. 84:384–391. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Liang QS and Cheng LR: Effects of
skeletal muscle denervation on potency of rocuronium. Asian
Biomedicine. 5:507–512. 2011.
|
|
12
|
Ibebunjo C and Martyn JA: Thermal injury
induces greater resistance to d-tubocurarine in local rather than
in distant muscles in the rat. Anesth Analg. 91:1243–1249.
2000.PubMed/NCBI
|
|
13
|
Ma J, Shen J, Garrett JP, Lee CA, Li Z,
Elsaidi GA, Ritting A, Hick J, Tan KH, Smith TL, et al: Gene
expression of myogenic regulatory factors, nicotinic acetylcholine
receptor subunits, and GAP-43 in skeletal muscle following
denervation in a rat model. J Orthop Res. 25:1498–1505. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams L, Carlson BM, Henderson L and
Goldman D: Adaptation of nicotinic acetylcholine receptor, myogenin
and MRF4 gene expression to long-term muscle denervation. J Cell
Biol. 131:1341–1349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamill OP, Marty A, Neher E, Sakmann B and
Sigworth FJ: Improved patch-clamp techniques for high-resolution
current recording from cells and cell-free membrane patches.
Pflugers Arch. 391:85–100. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ibebunjo C and Martyn J: Disparate
dysfunction of skeletal muscles located near and distant from burn
site in the rat. Muscle Nerve. 24:1283–1294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
John DA, Tobey RE, Homer LD and Rice CL:
Onset of succinylcholine-induced hyperkalemia following
denervation. Anesthesiology. 45:294–299. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gronert GA: Succinylcholine hyperkalemia
after burns. Anesthesiology. 91:320–322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witzemann V, Brenner HR and Sakmann B:
Neural factors regulate AChR subunit mRNAs at rat neuromuscular
synapses. J Cell Biol. 114:125–141. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yost CS and Winegar BD: Potency of
agonists and competitive antagonists on adult- and fetal-type
nicotinic acetylcholine receptors. Cell Mol Neurobiol. 17:35–50.
1997. View Article : Google Scholar : PubMed/NCBI
|