Introduction
Circling mice (C57BL/6J-cir), an animal model of
deafness inherited in an autosomal recessive manner due to
spontaneous mutation of non-syndromic recessive deafness family
loci has pathogenomic lesions portraying spiral ganglion cell
degeneration in the cochlea, empty Rosenthal canal ascribing in
severe degeneration of the axons between hair cells and spiral
ganglion cells, and loss of organ of Corti (1). Around postnatal day 7, the circling
mice became hyperactive and exhibited head tossing, tail chasing
and circling behavior (1). These
characteristic behaviors were observed in the homozygote (cir/cir)
mice only.
Deafness is known to be associated with alterations
in the auditory pathways (2),
which may reflect the decrease in synaptic plasticity, marked
changes in neurotransmitters, including D-aspartate (3), glycine (4), gamma-amino butyric acid (5), N-Methyl-D-aspartic acid (NMDA),
including its receptors (e.g. NMDAR1) (6), and ion channels (7). Additionally, synapse associated
proteins, including protein kinases (8), calbindin (9), extracellular signal-related kinase
and stress-activated protein kinases (10), may affect auditory functions. Owing
to these changes, the imbalances between excitation and inhibition
signals critically affect the neuronal response (11) and tonotopic map profile (12).
Glycine is a major inhibitory neurotransmitter in
the brain-stem and acts through the strychnine-sensitive glycine
receptor (GlyR) (13), involved in
various fundamental physiological processes (14). Within the brainstem, glycinergic
neurotransmission is essential, particularly in the auditory nuclei
(15), for several aspects of
neuronal function (16) being
involved in basic function, including sound localization or lateral
inhibition (17). Lateral superior
olive (LSO), a major nucleus of superior olivary complex (SOC)
receives bilateral innervation and along with medial nucleus of the
trapezoid body (MNTB), medial superior olive (MSO) and superior
paraolivary nucleus (SPN), is an important part of hearing, since
it is at this level in the ascending auditory pathway where
binaural processing of sound localization cues initially occur
(18). MNTB provides inhibitory
input to LSO and imparts sensitivity to interaural intensity
differences in LSO cells (19).
Marked glycinergic input is also provided to the SPN by MNTB
(20), whose neurons display
offset responses to pure tones.
Alteration of the glycinergic synapses in the
auditory pathways due to hearing loss by cochlear ablation and
middle ear ossicle removal loss has been reported to induce
regulatory changes in the synaptic release and uptake of glycine in
the brain stem nuclei and GlyR binding (4,21,22).
Age-associated hearing loss is also associated with decreased
glycine-immunoreactive neurons and puncta (23), and changes in the GlyR subunits
(24). A decrease in the GlyR has
been reported to lead to improper functioning, particularly in the
brainstem region in specific SOC nuclei following deafness
(21,25). As glycine is implicated in the
deafness-associated decrease in the inhibition in the SOC,
understanding its distribution in a deafness model, including
circling mice, may further elucidate this.
Inhibitory glycinergic synapses in adult rodents at
MNTB-LSO synapses (21,26) release glutamate as the predominant
neurotransmitter from birth (27).
However, its release in the cir/cir mouse is sustained at a later
period of development (28). This
sustenance of the glutamatergic transmission in the later period of
developing the MNTB-LSO synapse in cir/cir in mature animals
(29) may also contribute to
changes in the distribution of GlyR in the brainstem region of the
cir/cir mice. Previous studies have suggested an activity-dependent
deafness-associated decrease in glycine in the SOC (21,24).
A previous study of GlyR immunoreactivity (IR) in circling mice was
performed in the LSO nuclei at p16 (28), however, no studies have been
reported in the SOC nuclei as a whole. Although GlyR IR appears in
all the SOC nuclei except MSO at p8 in rodents, mature staining is
obtained only following p21 (30).
Therefore, considering the sustenance of glutamatergic transmission
and deafness-associated GlyR decrease with the lack of studies of
GlyR IR in the adult circling mice, the present study used
immunohistochemical analysis to detect any possible
histopathological changes and to compare the GlyR IR, cell number
and the cell size in four SOC regions, LSO, SPN, MSO and MNTB,
between adult +/cir and cir/cir mice.
Materials and methods
Animal experimentation
The present study used 10 adult male mice (+/cir,
n=5; cir/cir, n=5; Orientbio, Inc., Daejeon, Korea). All animal
procedures were performed according to the NIH guidelines of animal
research (31) and were approved
by Dankook University Institutional Animal Care and Use Committee,
which adheres to the guidelines issued by the Institution of
Laboratory of Animal Resources.
Polymerase chain reaction (PCR)
PCR analysis was performed to differentiate between
the +/cir and cir/cir mice using genomic DNA obtained from mouse
tails. The genomic DNA was isolated, according to the
manufacturer's instructions (Bioneer, Daejeon, Korea). The cir/cir
mouse was identified by the absence of the tmie gene. PCR
was performed with primers designed to amplify the exon 1 coding
region of the tmie gene (forward: 5′-AGCTGTAGCTCTGAAATCT-3′
and reverse: 5′-TCTGGCAGAATGCATGGAGGCT-3′). A total of 100 ng
template DNA was used in a final reaction volume of 20 µl
[10 mM Tris-HCl (pH 9.0), 40 mM KCl, 1.5 mM MgCl2, 250
mM dNTP, 20 pmol each primer and 1 unit Taq DNA polymerase; Bioneer
Corporation, Daejeon, Korea]. PCR was performed in a thermal cycler
(C1000TM; Bio-Rad Laboratories, Singapore) in two stages. The first
stage consisted of four cycles of denaturation at 96°C for 5 min,
annealing at 59°C for 1 min and extension at 72°C for 1 min. The
second stage included 30 cycles of denaturation at 96°C for 1 min,
annealing at 59°C for 1 min and extension at 72°C for 1 min,
followed by a final extension at 72°C for 10 min. Electrophoresis
was performed at 93 V for 1 h at 25°C to identify the amplification
fragments.
Immunohistochemical procedure
Following perfusion, the brains were immediately
removed, post-fixed overnight in 4% paraformaldehyde and
cryoprotected by infiltration with a sucrose series (10, 20 and
30%) at 4°C. Serial coronal sections of 40 µm thickness were
obtained on a freezing sliding microtome (Leica Microsystems Ltd.,
Seoul, Korea) and were collected in 6-well plates.
Immunohistochemistry was performed using the free floating method,
as described previously (31). The
brain areas were identified based on the atlas of the mouse brain
by Paxinos and Franklin (32).
Briefly, coronal sections were incubated for 48 h at 4°C in rabbit
polyclonal antibodies against glycine receptor α1/2 (1:2,500; cat.
no. ab23809; Abcam, Cambridge, UK) diluted in blocking buffer,
containing 1% bovine serum albumin, 0.3% Triton X-100 and 1% normal
horse serum. To eliminate peroxidase activity, the sections were
treated with 10% hydrogen peroxide in phosphate-buffered saline
(PBS). The sections were incubated with biotinylated goat
anti-rabbit IgG antibody (cat. no. BA-1000; Vector Laboratories,
Inc., Burlingame, CA, USA) at a dilution of 1:250 for 1.5 h at room
temperature, followed by treatment with an avidin-biotin-peroxidase
complex (Vectastain ABC mouse Elite kit; Vector Laboratories,
Burlingame, CA, USA). Following three washes in PBS, the sections
were incubated with 3,3′-diaminobenzidine and hydrogen peroxide in
a distilled water solution for 5 min. The sections from each group
were stained together to minimize variability. Following additional
washes, the sections were mounted onto gelatin coated slides,
dehydrated in a solution of increasing ethanol, cleared in xylene
and cover slipped with mounting solution (SP15500; Thermo Fisher
Scientific, New Jersey, NJ, USA).
Image analysis
A BX51 microscope (Olympus, Tokyo, Japan) was used
for the analysis and images were captured using a digital camera
system (DP50; Olympus). Staining densities were determined using
NIH image program, version 1.44 (Scion Image; Scion Corporation,
Bethesda, MA, USA), as described previously (31). Cell counting was performed using a
manual cell counting and marking method, and measurement of cell
size (area) was performed using ImageJ version 1.44 software
(National Institute of Health, Bethesda, MD, USA). The analysis of
the slides was performed by an investigator in a blinded
manner.
Statistical analysis
Student's t-test was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference. The data are
expressed as the mean ± standard deviation. A comparison between
+/cir and cir/cir mice was performed for the LSO, SPN and MNTB
region from each of the 10 sections assessed in each group to
measure the GlyR IR, cell counting and cell area.
Results
Histopathological observations
Intense GlyR IR was observed in all the major nuclei
of the SOC in LSO, SPN, MSO and MNTB of +/cir (Fig. 1). GlyR IR was located in the soma,
as well as the neuropil, of the LSO region, while it was
predominantly located in the soma in the remaining nuclei (Fig. 1A, C and D). Intensely labeled
immunoreactive puncta surrounded the soma of these neurons, leaving
perikarya unlabeled (Fig. 2). The
puncta were restricted to the soma and were not present in the
dendrites or the neuropil. Mostly bipolar cells, with eccentric
nuclei, were observed in the LSO and MSO region, while the SPN
comprised bipolar, as well as a few multipolar neurons (Fig. 2I, K, M and O). The neuropil of the
SPN and MSO contained numerous GlyR immunoreactive fibers running
in between the stained cells (Fig. 2K
and M). MNTB exhibited a membrane localization staining pattern
with punctuate staining, however, dense patchy staining was
prominently visible within the soma on the lateral aspect of
MNTB.
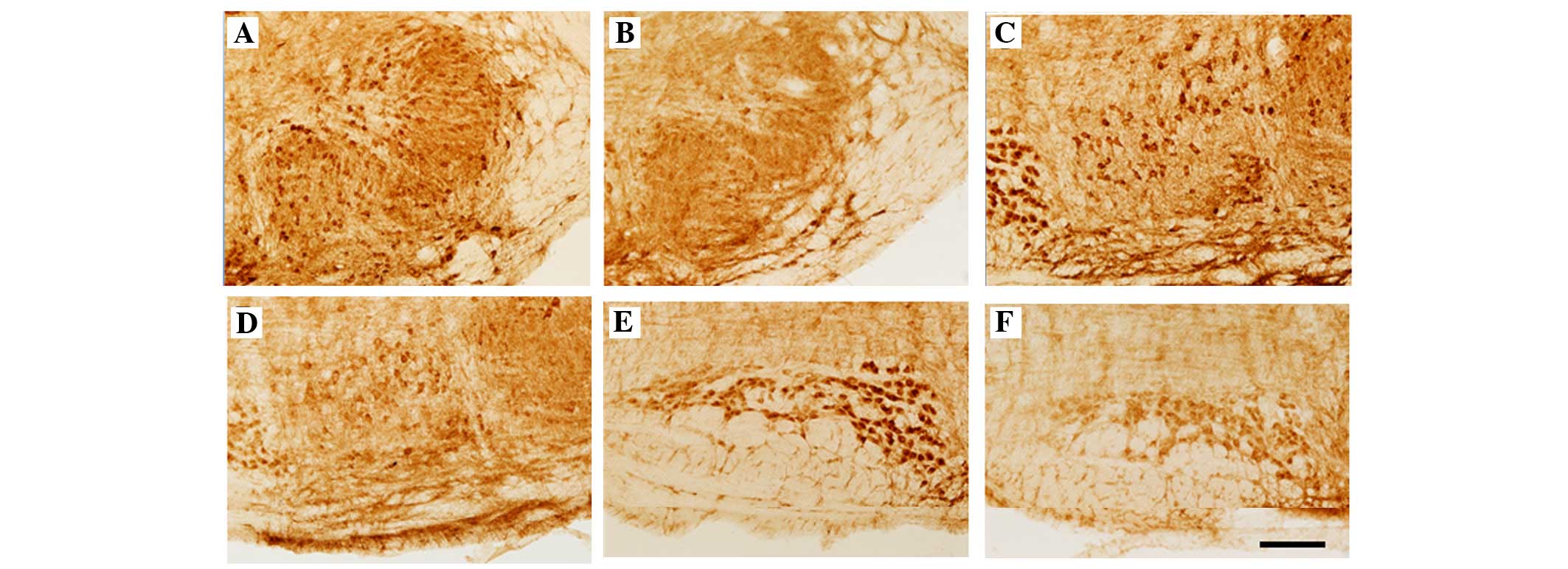 | Figure 1Immunohistochemical localization of
GlyRα1 and α2 IR in coronal sections through the SOC comprising (A
and B) LSO, (C and D) SPN and MSO, and (E and F) MNTB of
heterozygote (A, C and E) and homozygote (B, D and F) circling
mice. GlyR IR was observed in the neurons and neuropil of (A and B)
LSO, (C and D) SPN and MSO, and (E and F) MNTB of +/cir and cir/cir
mice. Compared with +/−, loss of GlyR IR was observed in all nuclei
and neuropil of the cir/cir mice. (Scale bar, 100 µm). SOC,
superior olivary complex; LSO, lateral superior olive; SPN,
superior paraolivary nucleus; MSO, medial superior olive; MNTB,
medial nucleus of the trapezoid body; GlyR, glycine receptor; IR,
immunoreactivity. |
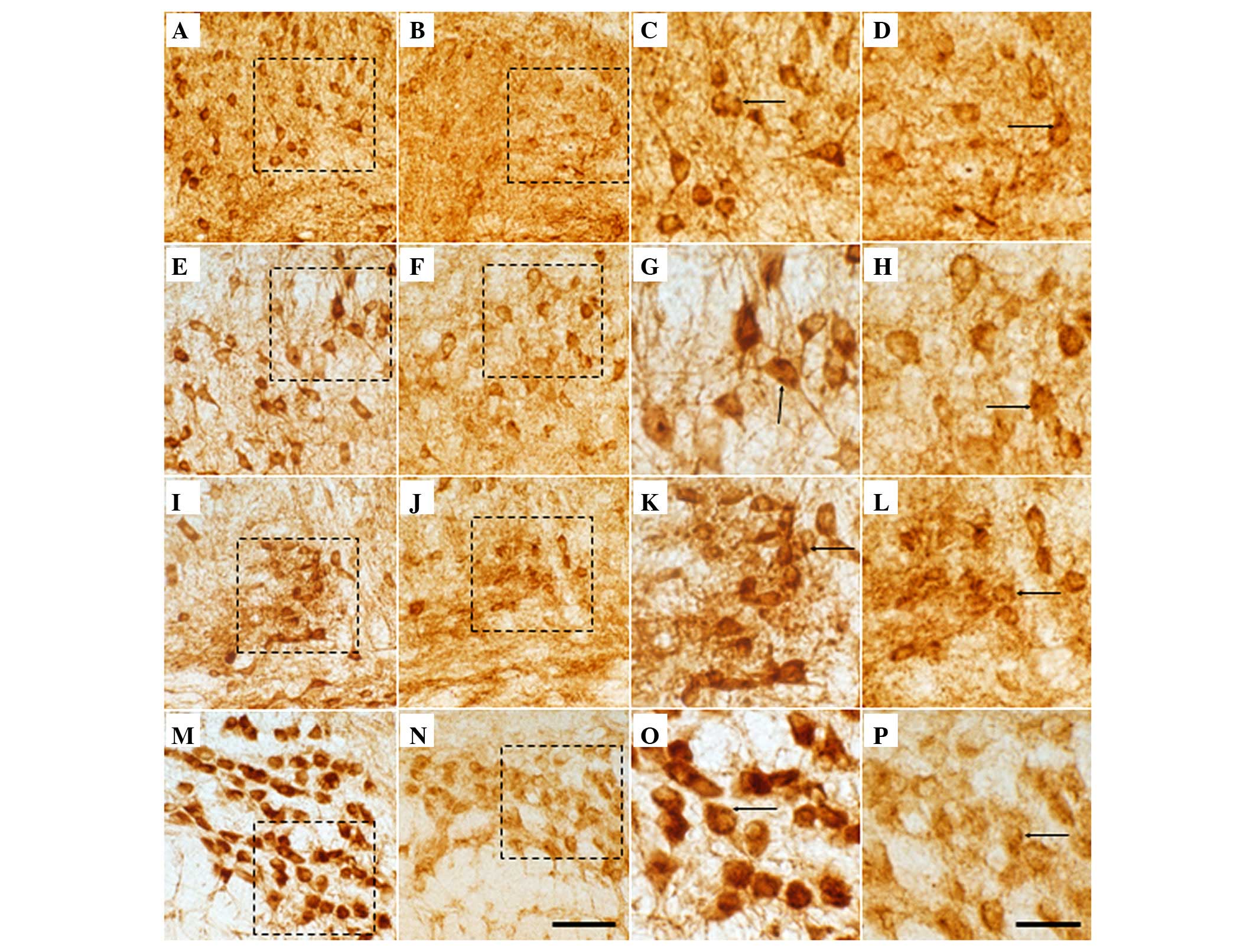 | Figure 2Magnified image of GlyRα1 and α2 IR in
coronal sections through the SOC comprising (A, B, I and J) LSO,
(C, D, K and L) SPN, (E, F, M and N) MSO and (G, H, O and P) MNTB
of +/cir (A, C, E, G, I, K, M and O) and cir/cir (B, D, F, H, J, K,
L and P) mice. Scattered highly GlyR IR was noted in all nuclei of
SOC. Puncta (arrows) possibly indicate presynaptic terminals. A
loss of GlyR immunoreactive cells was noted in the (B and J) LSO,
(D and K) SPN, (F and N) MSO and (H and P) MNTB of cir/cir mice. A
decrease in the size of the soma of cir/cir mice was observed,
compared with +/cir mice (Scale bar, A–H, 50 µm; I–P, 10
µm.). SOC, Superior Olivary Complex; LSO, Lateral Superior
Olive; SPN, Superior Paraolivary Nucleus; MSO, Medial Superior
Olive; MNTB, Medial Nucleus of the Trapezoid Body; IR,
immunoreactivity. |
Compared with +/cir, the cir/cir cells exhibited a
very prominent loss of GlyR IR in the soma, as well as the neuropil
(Fig. 1B, D and F). Prominent loss
of GlyR staining was evident in the neuropil of the LSO of cir/cir,
which was markedly more severe in the lateral limb of the LSO
compared with the medial limb, while the neurons of the LSO, SPN
and MNTB also revealed marked decrement in the GlyR IR. A marked
loss of staining was noted in the dendrites of the neurons of the
LSO and SPN region (Fig. 2J and
K). The GlyR immunoreactive fibers also appeared to be absent
in the SPN of the cir/cir cells. A decrease in cell number, as well
as cell size, was noted in the different nuclei of the SOC of the
cir/cir cells (Fig. 2J, K, L and
P).
IR analysis
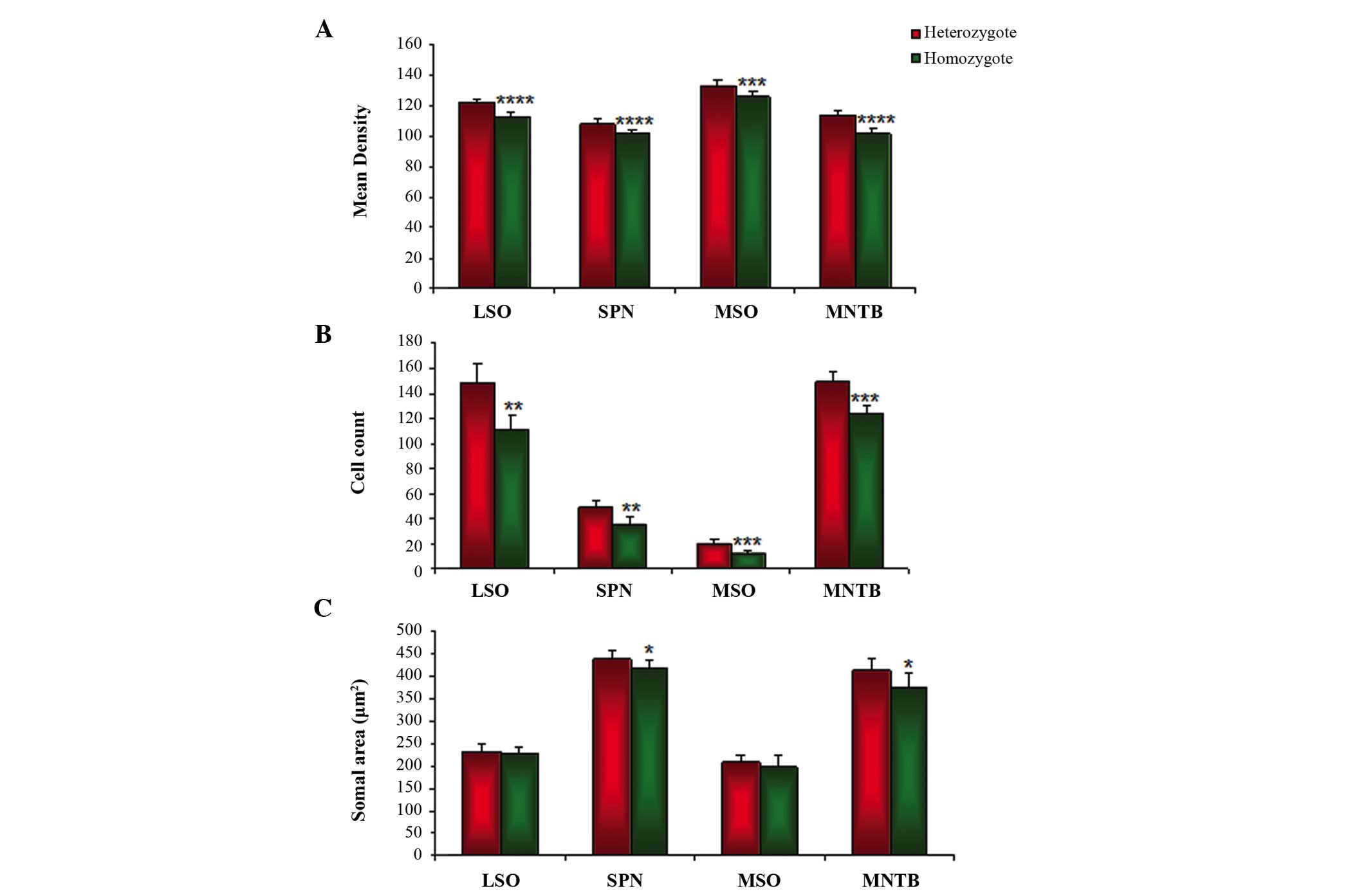
According to the relative density, the GlyR IR in
the SOC region was markedly decreased in all the major nuclei of
cir/cir, as compared with +/cir. The GlyR IR in the LSO region was
121.82±2.40 in +/cir, which was significantly decreased by 7.32% to
112.89±2.95 in cir/cir (P<0.0001; Fig. 3A). Compared with +/cir, a
significant decrement from 108.35±3.09 of 6.2% was noted in cir/cir
(101.54±2.74; P<0.0001; Fig.
3A). A significant decrease in the GlyR IR was also observed
from 133.21±4.15 in +/cir to 125.71±4.22 in cir/cir, which amounted
to 7.49% (P<0.001; Fig. 3A). A
significant 12.07% decrease to 101.63±3.46 in cir/cir from
113.70±4.08 in +/cir was demonstrated (P<0.0001).
Cell numbers
GlyR immunoreactive cells in the SOC region of +/cir
were compared with those from cir/cir. A trend of significant loss
of GlyR immunoreactive cells was noted in the cir/cir cells. In the
LSO region, a significant 25.30% decrease from 148.6±14.80 in +/cir
to 111±12.60 in cir/cir (P<0.005; Fig. 3B) was observed. A significant
26.63% loss of GlyR immunoreactive cells was also noted in the SPN
region, decreasing from 48.8±4.65 in +/cir to 35.8±5.71 in cir/cir
(P<0.005; Fig. 3B). MSO
exhibited a 39% loss, decreasing from 20±4.35 in +/cir to 12.2±1.92
in cir/cir (P<0.01). MNTB, by far, exhibited the least decrement
of 16.91% with 149±8.68 in +/cir and 123.8±7.19 in cir/cir
(P<0.001), although the IR loss was the most severe in MNTB
compared with the other SOC regions (Fig. 3B).
Cell size
The size of the cells present in each nuclei of the
SOC was compared between the +/cir and cir/cir groups. No
significant difference was observed in the LSO and MSO region of
cir/cir, although a trend of decrease was noted in each nuclei. A
2.12% decrement was observed in the LSO from 233.16±14.28 to
228.21±13.71 µm2 (Fig. 3C). Similarly, the MSO region also
exhibited a 4.94% decrement from 209.10±15.21 in +/cir to
198.76±25.85 µm2 in cir/cir (Fig. 3C). However, in the SPN, a
significant decrease of 4.85% was noted from 438.70±19.13 in +/cir
to 417.51±17.80 µm2 in cir/cir (P<0.01).
Similarly, principal cells in the MNTB region exhibited a
significant 9.44% decrement in the cir/cir as compared with +/cir
from 414.35±23.18 to 375.20±34.72 µm2,
respectively (P<0.01; Fig.
3C).
Discussion
The present study revealed a decrease in the GlyR
IR, as well as GlyR immunoreactive cell number and cell size in the
SOC nuclei of cir/cir when compared with +/cir. This is consistent
with previous reports of deafness-associated decreases in the Gly
immunoreactive cells in the SOC (25).
A decrease in the GlyR in cir/cir may be a
consequence of the decrease in the glycine levels, which may
possibly lower the immunocytochemical detection or it may be due to
a physical reduction in the number of the GlyR immunoreactive
cells, as well as the decrease in the cell size, as noted in the
present study. This may be the result of an activity-dependent
decrease following deafness, associated with decreased activity.
Pruning of glycinergic terminals in LSO and MSO (33) due to experience-dependent
plasticity is also possible, particularly due to sustenance of
glutamatergic transmission in cir/cir, although it has been
hypothesized to be a temporary phase (28). Readjustments in the location of
glycine immunoreactive terminals with terminals moving from soma to
dendrites has been previously reported (34), however the loss of dendritic
staining in the cir/cir firmly rejects this hypothesis.
Furthermore, even without the quantitative assessment of the GlyR
immunoreactive puncta in the present study, a qualitative decrement
was noted in all SOC nuclei. A qualitative and quantitative study
of glycine immunoreactive axo-somatic puncta may assist in
clarifying these issues.
Bilateral deafness, induced by intrascalar injection
of neomycin in rats, causes a significant decrease in glycine IR in
the nuclei of SOC (25). A
significant decrement in the number of glycine immunoreactive
puncta on the somata of principal cells was reported, which may be
due to transneuronal degeneration, as observed in the SOC following
cochlear damage (3,35). However, unilateral deafening
produced no changes in glycine release in LSO or MNTB, however,
this was noted in the cochlear nucleus (CN) (21). Bilateral deafness, as in cir/cir,
caused a significant decrease of GlyR in the SOC and CN
(unpublished data). This difference may be contributed to the
bilateral innervation received by the SOC nuclei from both
ipsilateral and contralateral CNs. Cochlear ablation also results
in a decrease in glycine release in the CNs (4,21,36),
suggesting that the decreased inhibition following deafness may be
associated with the decrease in glycine. Previous studies have
suggested deafness as a factor influencing the intracellular
pathways modulating neurotransmitters and their release.
The present study revealed a significant percentage
decrease of GlyR IR, as well as in cell number and cell size, in
the SOC. This decrement was higher for LSO and MNTB in all three
aspects, which is particularly important, taking into consideration
the importance of MNTB-LSO synapses. SOC uses glycine as major
inhibitory neurotransmitter with the majority of the glycinergic
pathway originating in the MNTB (37), while balance in the LSO between
glycinergic inhibition and glutamatergic excitation from the CN is
important for detection of interaural level differences, which
appears to be imbalanced in cir/cir due to sustained glutamatergic
transmission (28). Indeed,
deafness-associated changes in the potential regulators of
transmitter release has been reported in the auditory brainstem
(10,38) and is supported by the
immunohistochemical results of the present study.
The LSO receives excitatory projections from
ipsilateral and inhibitory from contralateral projections (39). Inhibitory projection arises from
homogeneous glycinergic nuclei, MNTB projects topographically along
the LSO frequency axis, while the excitatory projection emerges
from CN is glutamatergic whose tonotopic projection matches
perfectly with inhibitory projection from MNTB, allowing LSO
neurons to respond selectively to interaural level differences. The
inactivity of GlyR led to a significant decrease in the number of
cell surface receptor clusters on cultured spinal cord neurons
(40), suggesting that the loss of
the glycinergic inhibitor induces change/redistribution of
postsynaptic membrane GlyRs. Hearing loss leads to severe long term
weakening of glycinergic synaptic inhibition in the LSO,
contributing to the downregulation of synaptic release of glycine
in dorsal CN and downregulation of postsynaptic GlyR activity in
the LSO (5). Hence, a decrease in
the GlyR IR in the neurons and neuropil, and a decrease in cell
number and size, as noted in the cir/cir, may lead to a decrease in
inhibitory transmission leading to profound changes in the
functional properties in the SOC nuclei particularly at LSO and
MNTB. The data from electrophysiological experiments and electron
microscopy in adult circling mice may assist in further elucidating
the association between the GlyR and neural function associated
with deafness.
Acknowledgments
The present study was supported by the Basic Science
Research Program, through the National Research Foundation of Korea
funded by the Ministry of Education (grant no. NRF-2011-0011885)
and the Basic Science Research Program through the National
Research Foundation of Korea (NRF) funded by the Ministry of
Science, ICT & Future Planning (grant no.
NRF-2014R1A2A2A04003616).
References
|
1
|
Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT,
Lee TW, Lee JR, Kim HT, Suh JG, Kim TY and Ryoo ZY: Circling mouse:
Possible animal model for deafness. Comp Med. 51:550–554. 2001.
|
|
2
|
Syka J: Plastic changes in the central
auditory system after hearing loss, restoration of function and
during learning. Physiol Rev. 82:601–636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Potashner SJ, Suneja SK and Benson CG:
Regulation of D-aspartate release and uptake in adult brain stem
auditory nuclei after unilateral middle ear ossicle removal and
cochlear ablation. Exp Neurol. 148:222–235. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potashner SJ, Suneja SK and Benson CG:
Altered glycinergic synaptic activities in guinea pig brain stem
auditory nuclei after unilateral cochlear ablation. Hear Res.
147:125–136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milbrandt JC, Hunter C and Caspary DM:
Alterations of GABAA receptor subunit mRNA levels in the aging
Fischer 344 rat inferior colliculus. J Comp Neurol. 379:455–465.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakagawa H, Sato K, Shiraishi Y, Kuriyama
H and Altschuler RA: NMDAR1 isoforms in the rat superior olivary
complex and changes after unilateral cochlear ablation. Brain Res
Mol Brain Res. 77:246–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Hehn CA, Bhattacharjee A and Kaczmarek
LK: Loss of Kv3.1 tonotopicity and alterations in cAMP response
element-binding protein signaling in central auditory neurons of
hearing impaired mice. J Neurosci. 24:1936–1940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia MM, Edward R, Brennan GB and Harlan
RE: Deafferentation induced changes in protein kinase C expression
in the rat cochlear nucleus. Hear Res. 147:113–124. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Idrizbegovic E, Bogdanovic N and Canlon B:
Modulating calbindin and parvalbumin immunoreactivity in the
cochlear nucleus by moderate noise exposure in mice. A quantitative
study on the dorsal and posteroventral cochlear nucleus. Brain Res.
800:86–96. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suneja SK and Potashner SJ: ERK and SAPK
signaling in auditory brainstem neurons after unilateral cochlear
ablation. J Neurosci Res. 73:235–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bledsoe SC Jr, Nagase S, Miller JM and
Altschuler RA: Deafness induced plasticity in the mature central
auditory system. Neuroreport. 7:225–229. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagase S, Miller JM, Dupont J, Lim HH,
Sato K and Altschuler RA: Changes in cochlear electrical
stimulation induced Fos expression in the rat inferior colliculus
following deafness. Hear Res. 147:242–250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Legendre P: The glycinergic inhibitory
synapse. Cell Mol Life Sci. 58:760–793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caspery DM: Electrophysiological studies
of glycinergic mechanisms in auditory brainstem structures.
Ottersen OP and Storm-Mathisen J: Glycine Neurotransmission
Chichester: Wiley; pp. 453–483. 1990
|
|
15
|
Wenthold RJ, Huie D, Altschuler RA and
Reeks KA: Glycine immunoreactivity localized in the cochlear
nucleus and superior olivary complex. Neuroscience. 22:897–912.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wenthold RJ and Hunter C:
Immunocytochemistry of glycine and glycine receptors in the central
auditory system. Ottersen OP and Storm-Mathisen J: Glycine
Neurotransmission, Chichester: Wiley; pp. 391–416. 1990
|
|
17
|
Vater M, Habbicht H, Kössl M and Grothe B:
The functional role of GABA and glycine in monaural and binaural
processing in the inferior colliculus of horseshoe bats. J Comp
Physiol A. 171:541–553. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kavanagh GL and Kelly JB: Midline and
lateral feld sound localization in the ferret (MustelaPutorius):
Contribution of the superior olivary complex. J Neurophysiol.
67:1643–1658. 1992.PubMed/NCBI
|
|
19
|
O'Neill WE, Zettel ML, Whittemore KR and
Frisina RD: Calbindin D-28k immunoreactivity in the medial nucleus
of the trapezoid body declines with age in C57BL/6, but not
CBA/CaJ, mice. Hear Res. 112:158–166. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banks MI and Smith PH: Intracellular
recordings from neurobiotin-labeled cells in brain slices of the
rat medial nucleus of the trapezoid body. J Neurosci. 12:2819–2837.
1992.PubMed/NCBI
|
|
21
|
Suneja SK, Benson CG and Potashner SJ:
Glycine receptors in adult guinea pig brain stem auditory nuclei:
Regulation after unilateral cochlear ablation. Exp Neurol.
154:473–488. 1998. View Article : Google Scholar
|
|
22
|
Suneja SK, Potashner SJ and Benson CG:
Plastic changes in glycine and GABA release and uptake in adult
brain stem auditory nuclei after unilateral middle ear ossicle
removal and cochlear ablation. Exp Neurol. 151:273–288. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willott JF and Turner JG: Neural
plasticity in the mouse inferior colliculus: Relationship to
hearing loss, augmented acoustic stimulation and prepulse
inhibition. Hear Res. 147:275–281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krenning J, Hughes LF, Caspary DM and
Helfert RH: Age-related glycine receptor subunit changes in the
cochlear nucleus of Fischer-344 rats. Laryngoscope. 108:26–31.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buras ED, Holt AG, Griffith RD, Asako M
and Altschuler RA: Changes in glycine immunoreactivity in the rat
superior olivary complex following deafness. J Comp Neurol.
494:179–189. 2006. View Article : Google Scholar
|
|
26
|
Kandler K and Friauf E: Development of
glycinergic and glutamatergic synaptic transmission in the auditory
brainstem of perinatal rats. J Neurosci. 15:6890–6904.
1995.PubMed/NCBI
|
|
27
|
Gillespie DC, Kim G and Kandler K:
Inhibitory synapses in the developing auditory system are
glutamatergic. Nat Neurosci. 8:332–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong SH, Kim MJ and Ahn SC: Glutamatergic
transmission is sustained at a later period of development of
medial nucleus of the trapezoid body-lateral superior olive
synapses in circling mice. J Neurosci. 28:13003–13007. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwartz IR: The superior olivary complex
and lateral lemniscal nuclei. Webster B, Popper AN and Fay RR: The
Mammalian Auditory Pathways: Neuroanatomy New York: Springer; pp.
117–167. 1990
|
|
30
|
Friauf E, Hammerschmidt B and Kirsch J:
Development of adult-type inhibitory glycine receptors in the
central auditory system of rats. J Comp Neurol. 385:117–134. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maskey D, Kim M, Aryal B, Pradhan J, Choi
IY, Park KS, Son T, Hong SY, Kim SB, Kim HG and Kim MJ: Effect of
835 MHz radio-frequency radiation exposure on calcium binding
proteins in the hippocampus of the mouse brain. Brain Res.
1313:232–241. 2010. View Article : Google Scholar
|
|
32
|
Paxinos G and Franklin KBJ: The Mouse
Brain in Stereotaxic Coordinates. Second edition: San Diego:
Academic Press; 2001
|
|
33
|
Sanes DH and Friauf E: Development and
influence of inhibition in the lateral superior olivary nucleus.
Hear Res. 147:46–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kapfer C, Seidl AH, Schweizer H and Grothe
B: Experience-dependent refinement of inhibitory inputs to auditory
coincidence-detector neurons. Nat Neurosci. 5:247–253. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim J, Morest DK and Bohne BA:
Degeneration of axons in the brain stem of the chinchilla after
auditory overstimulation. Hear Res. 103:169–191. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asako M, Holt AG, Griffith RD, Buras ED
and Altschuler RA: Deafness-related decreases in
glycine-immunoreactive labeling in the rat cochlear nucleus. J
Neurosci Res. 81:102–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Spirou GA and Berrebi AS: Glycine
immunoreactivity in the lateral nucleus of the trapezoid body of
the cat. J Comp Neurol. 383:473–488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S and Oertel D: Neuronal circuits
associated with the output of the dorsal cochlear nucleus through
fusiform cells. J Neurophysiol. 71:914–930. 1994.PubMed/NCBI
|
|
39
|
Boudreau JC and Tsuchitani C: Cat superior
olive s-segment cell discharge to tonal stimulation. Contrib Sens
Physiol. 4:143–213. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lévi S, Vannier C and Triller A:
Strychnine-sensitive stabilization of postsynaptic glycine receptor
clusters. J Cell Sci. 111:335–345. 1998.PubMed/NCBI
|