Introduction
Chitosan, a linear polysaccharide isolated from
shrimp and other crustacean shells, possesses low toxicicity, is
biodegradable and exhibits favorable biocompatible properties,
rendering it a promising material for biomedical applications
(1–3). Notably, several studies have
investigated the potential of chitosan for drug delivery,
bio-coating and the transferring of genetic materials (1–6).
However, one setback, which limits the use of chitosan, is its
insolubility under neutral conditions (3). Previously, a modified chitosan,
namely hydroxybutyl chitosan (HBC), has been developed and exhibits
advantages over the parental chitosan. HBC is prepared by the
conjugation of hydroxybutyl groups to the hydroxyl and amino
reactive sites of chitosan (7).
This change confers the polymer solubility under neutral
conditions, whilst maintaining low toxicity and retaining its
biodegradable and appreciable biocompatible properties (7–9).
Tissue factor (TF) is an essential cofactor in the
initiation of coagulation (10).
It is constitutively expressed and can be upregulated by various
stimuli, including platelet-derived growth factor (PDGF) in
vascular smooth muscle cells (11,12).
In response to cytokines and growth factors, endothelial cells,
monocytes and macrophages also produce TF (13–19).
Two forms of TF, membrane-bound and soluble TF, have been
identified (10,12,20–22).
TF has been implicated in the pathogenesis of cardiovascular
diseases, including hypertension, atherosclerosis, acute coronary
syndrome and restenosis following percutaneous coronary
intervention, by promoting thrombus formation and inducing the
migration and proliferation of vascular smooth muscle cells
(23–29). Therefore, targeting TF has been
suggested as an attractive strategy for the treatment of
cardiovascular diseases (30). In
the present study, the use of HBC for the transfer of siRNAs into
primary human umbilical vein vascular smooth muscle cells (HUVSMCs)
targeting TF was investigated, and the efficiency of HBC-mediated
siRNA transfer was examined, as were the effects of TF knockdown on
HUVSMC proliferation and apoptosis.
Materials and methods
siRNAs
All siRNAs were purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). A fluorescent
FAM-labeled siRNA was used to examine transfer efficiency. An siRNA
targeting human TF was designed, according to the published human
TF cDNA sequence (gene accession number: M16553.1; GenBank;
http://www.ncbi.nlm.nih.gov/nuccore/M16553.1). The
sequence of the antisense strand was 5′-AUU UGU AGU GCC UGA AGC
GCTT-3′. A scrambled RNA sequence was used as control.
Preparation of HBC/siRNA
nanoparticles
HBC/siRNA nanoparticles were prepared as follows:
The HBC solution (1 mg/ml) was made by dissolving HBC (molecular
weight: 130–160 kDa; degree of deacetylation: 86%) provided by the
Ocean University of China (Qingdao, China) in 0.2 M acetic acid (pH
5.5; Sigma-Aldrich, St. Louis, MO, USA). The siRNA (final
concentration, 20 µmol/l) was mixed with tripolyphosphate
(TPP) solution (0.5 mg/ml; Sigma-Aldrich) under magnetic stirring.
HBC/siRNA nanoparticles were then produced by slowly adding 1 ml of
the siRNA/TPP solution to 3 ml of the HBC solution with stirring
for 30 min. The loading efficiency of the siRNA within HBC was
determined as follows: The HBC/siRNA mixture, prepared as above,
was centrifuged at 13,000 × g for 15 min, following which the
supernatant was collected and the siRNA content in the supernatant
was determined by measuring the absorbance at 260 nm using a
spectrophotometer (UV-1100; Shimadzu Corporation, Kyoto, Japan).
The loading efficiency was calculated as a percentage, as follows:
Total siRNA used - free siRNA remaining in the supernatant) / total
siRNA used.
Cell culture and siRNA transfer
Primary HUVSMCs (Bai Li Biological Technology,
Shanghai, China) were maintained at 37°C in RPMI-1640 medium
(Gibco, Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 0.1 mg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were used between passages four and nine.
For siRNA transfer, the cells were detached with 0.25% trypsin
(Thermo Fisher Scientific, Inc.), seeded on 24-well plates
(1×105 cells/well) and incubated overnight at 37°C in
complete RPMI-1640 medium. Following replacing of the medium with
250 µl Opti-MEM serum-free medium (Gibco; Thermo Fisher
Scientific, Inc.), HBC/siRNA nanoparticle solution was added (siRNA
final concentration, 200 nM). The cells were incubated at 37°C for
4 h to allow for siRNA uptake. Following incubation, the medium
containing nanoparticles was replaced with fresh complete RPMI 1640
medium and the cells were maintained in culture and assayed at
different time points (24, 48 and 72 h), as indicated. A total of
24 h post-transfection, five high-power fields were randomly
selected under a fluorescence microscope (Nikon E600; Nikon
Corporation, Tokyo, Japan), and total fluorescent cells in each
field were counted. The percentage of average fluorescent cells
over average total cells from the five fields was used to determine
the transfection efficiency
TF protein measurement
As HUVSMCs express minimal basal TF, the cells
examined in the present study were treated with PDGF-BB to enhance
the production of TF protein. PDGF-BB (10 ng/ml, Sigma-Aldrich) was
added to the cell culture 24 h following HBC/siRNA transfection.
Inhibition of the PDGF-BB-induced expression of TF by TF-siRNA was
then determined 48 h following siRNA transfection. The levels of
soluble and cellular TF protein were measured using an
enzyme-linked immunosorbent assay (ELISA) and western blotting,
respectively. For the soluble TF assay, the cultured conditioned
medium was collected, centrifuged at 10,000 × g for 10 min and the
level of soluble TF in the medium was measured using a Human
F3/Tissue Factor ELISA kit (cat. no. RAB0642-1KT; Sigma-Aldrich),
according to the manufacturer's protocol. For western blotting,
total protein was extracted using radio-immunoprecipitation assay
lysis buffer (Sigma-Aldrich) and protein concentration was
determined using a bicinchoninic acid assay (Pierce Biotechnology,
Inc., Rockford, IL, USA), according to the manufacturer's protocol.
The proteins (50 µg) were separated by 10% SDS-PAGE (Thermo
Fisher Scientific, Inc.) and electrically transferred onto
nitrocellulose membrane (Thermo Fisher Scientific, Inc.). The
membrane was blocked with phosphate-buffered saline (pH 7.5) with
0.1% Tween-20 (PBST; Sigma-Aldrich) and 10% non-fat milk for 1 h at
room temperature, and incubated overnight at 4°C with mouse
anti-human TF monoclonal antibody (1:1,000; cat. no. ab17375;
Abcam, Cambridge, MA, USA), followed by three washes with PBST. The
membrane was then incubated with a horseradish peroxidase
(HRP)-conjugated goat anti-mouse secondary antibody (1:5,000; cat.
no. ab6789; Abcam) at room temperature for 1 h. Following three
washes with PBST, the specific TF band was visualized using an ECL
detection kit (Abcam). Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) on the same membrane was probed with a mouse anti-human
GAPDH monoclonal antibody (1:5,000; cat. no. ab9484; Abcam) as done
with TF. Densitometric analysis was performed, and relative
quantitation of TF normalized to GAPDH was determined using Image J
software (National Institutes of Health, Bethesda, MA, USA).
Cell proliferation assay
HUVSMC proliferation was assessed 48 h following the
addition of the HBC/siRNA nanoparticles using a Cell Counting Kit-8
(CCK-8; Sigma-Aldrich), according to the manufacturer's protocol.
Briefly, the culture medium was replaced with fresh complete RPMI
1640 medium (without phenol red) containing 10% CCK-8 reagent.
Following culture for 24 h, 200 µl of supernatant from each
well was transferred to a 96-well plate for the measurement of
optical density (OD) at 450 nm (OD450) using a
microplate reader (model 550; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Cell apoptosis assay
Apoptosis of the HUVSMCs was assayed using an
Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(Solarbio Science & Technology Co., Ltd., Beijing, China),
according to the manufacturer's protocol. Briefly, 72 h after siRNA
transfection, the cells were detached and resuspended in binding
buffer (Solarbio Science & Technology Co., Ltd.) at a density
of 1×106/ml. Subsequently, 100 µl of the cell
suspension was incubated with 5 µl Annexin V-FITC and 10
µl propidium iodide at 4°C for 15 min in the dark. Following
a single wash with PBS, the Annexin V-FITC-positive cells
(apoptotic cells) were analyzed using a FACSCalibur Flow Cytometer
(BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses of the differences were performed using one
way analysis of variance. Comparisons between two groups were
performed using the least-significant difference test. SPSS 17.0
(SPSS, Inc., Chicago, IL, USA) and PRISM software (GraphPad
Software, Inc., La Jolla, CA, USA) were used for statistical
analyses and plotting. P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation of HBC siRNA nanoparticles
and siRNA transfer
HBC and siRNA solutions were mixed and stirred to
form HBC/siRNA nanoparticles. TPP was used as a cross-linker to
further stabilize the nanoparticle. This method resulted in an
siRNA loading efficiency of 93.4±0.7% within HBC. Treatment of the
HUVSMCs with nanoparticles, which were formed by mixing HBC and
FAM-labeled siRNAs, showed green fluorescence in the transfected
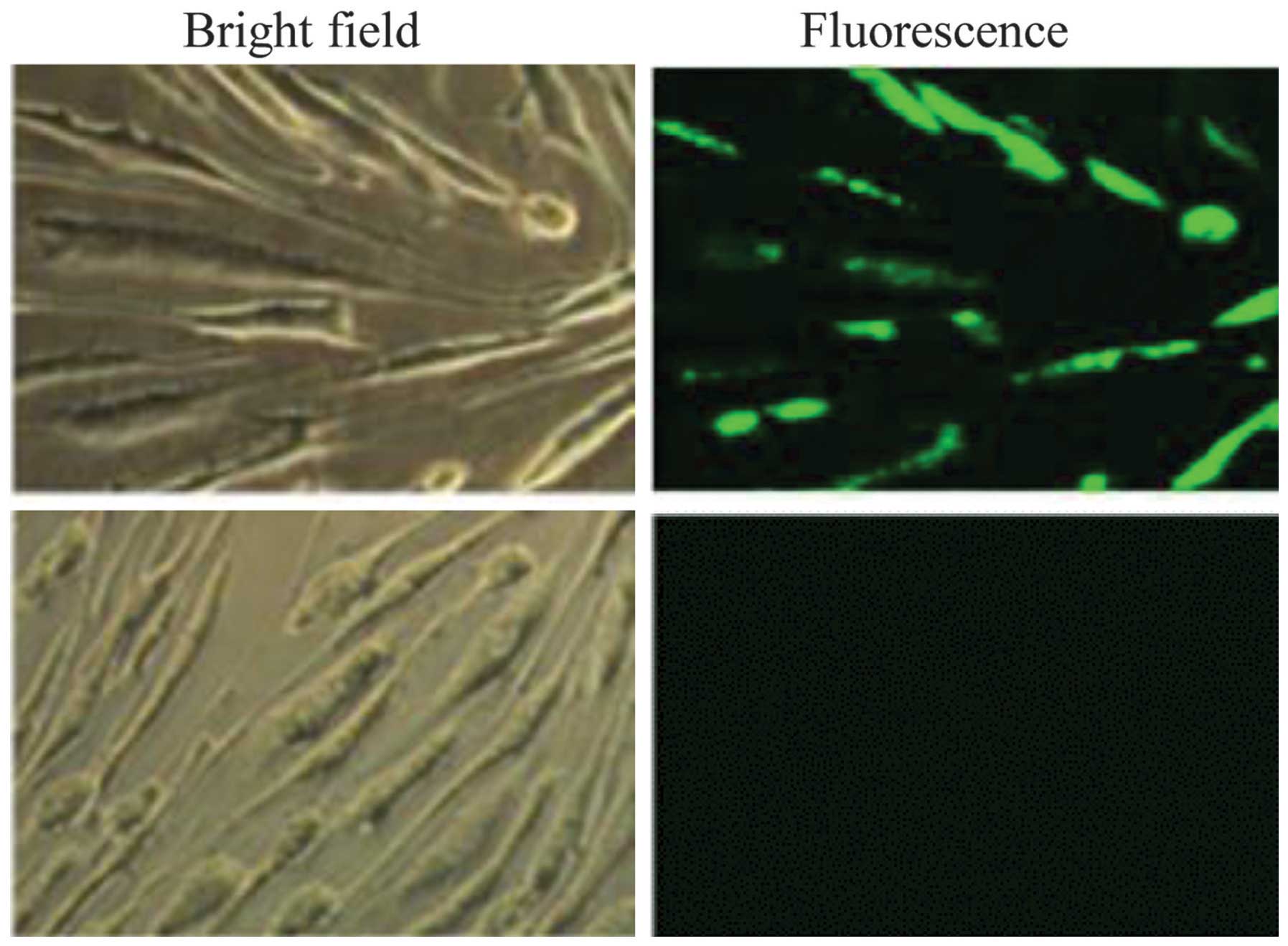
cells, as observed under fluorescence microscopy (Fig. 1). Counting of the numbers of
fluorescent and total cells revealed a 74±2.5% transfection
efficiency.
Knockdown of TF by HBC/TF-siRNA
nanoparticles in HUVSMCs
Inhibition of the PDGF-BB-induced expression of TF
by TF-siRNA in the HUVSMCs was determined 48 h following siRNA
transfection. The ELISA results showed that the level of soluble TF
in the TF-siRNA-transfected cells was 34.2±1.8 pg/ml, which was
significantly lower than the level in the non-transfected cells
(93.1±2.0 pg/ml; n=12; P<0.01). In the cells transfected with
scrambled siRNA, the level of soluble TF was 79.0±3.0 pg/ml, which
was not significantly different from that of the untreated cells
(Fig. 2A). Western blotting
revealed that the relative cellular level of TF in the
TF-siRNA-transfected cells was 38.5±2.6% of that in the
non-transfected cells (n=12; P<0.05). By contrast, scrambled
siRNA transfection caused no significant reduction in the level of
cellular TF (Fig. 2B and C).
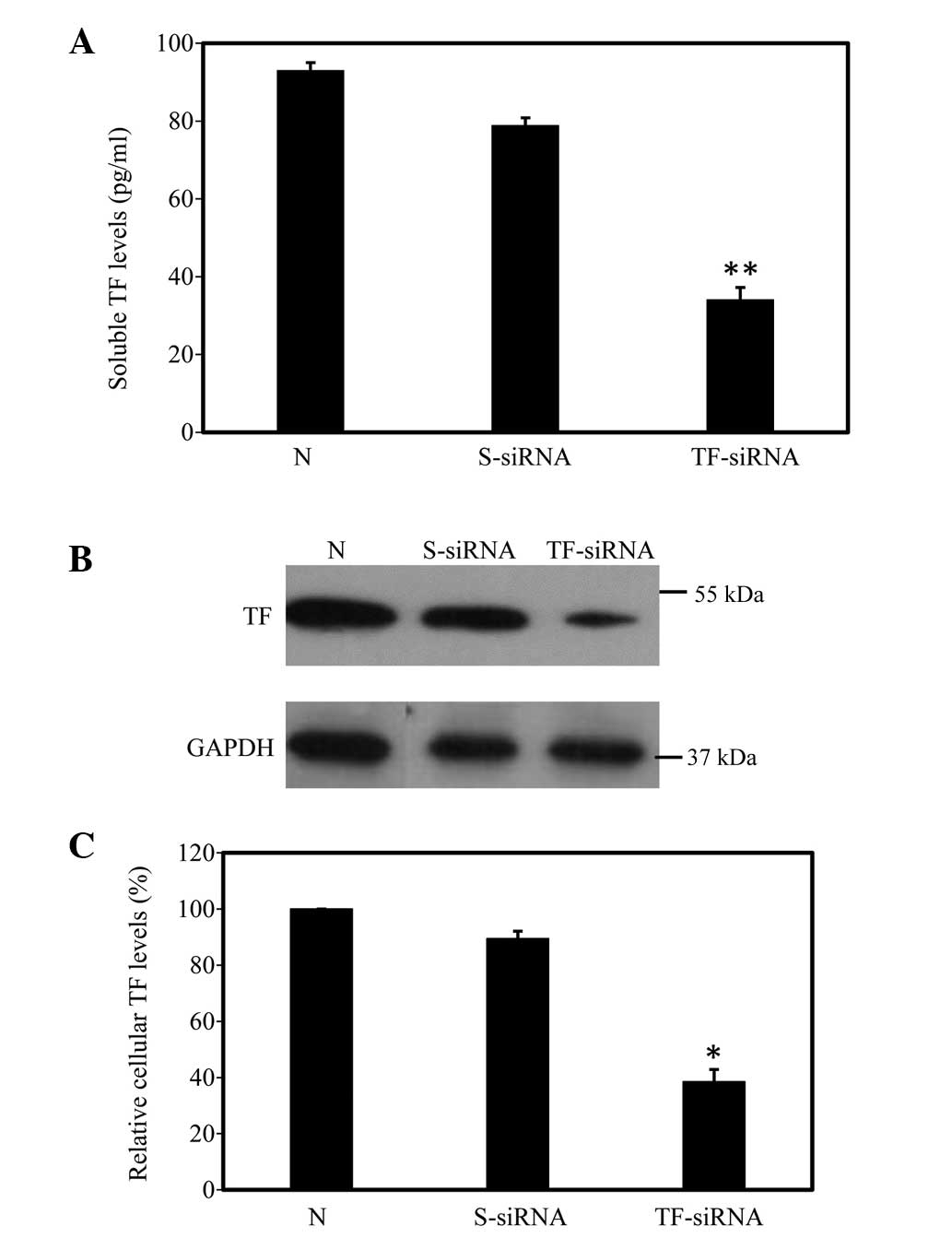 | Figure 2TF protein production is inhibited in
HUVSMCs following HBC/TF-siRNA transfection. (A) Enzyme-linked
immunosorbent assay results showed that the production of soluble
TF induced by PDGF-BB in HUVSMCs was significantly reduced in the
TF-siRNA cells, compared with the scramble siRNA cells. (B)
Representative western blot of cellular TF revealed that TF-siRNA,
but not scrambled-siRNA transfection, significantly suppressed the
protein expression of cellular TF induced by PDGF-BB. (C)
Summarized levels of cellular TF, normalized against GAPDH, were
plotted. The cellular levels of TF in the non-transfected cells
were arbitrarily set at 100%. Data are expressed as the mean ±
standard deviation. *P<0.05 and
**P<0.01, compared with the non-transfected cells.
TF, tissue factor; HUVSMC, human umbilical vein smooth muscle cell;
HBC, hydroxybutyl chitosan; PDGF-BB, platelet-derived growth
factor; N, non-transfected; S-siRNA, scrambled siRNA-transfected;
TF-siRNA, TF-siRNA-transfected. |
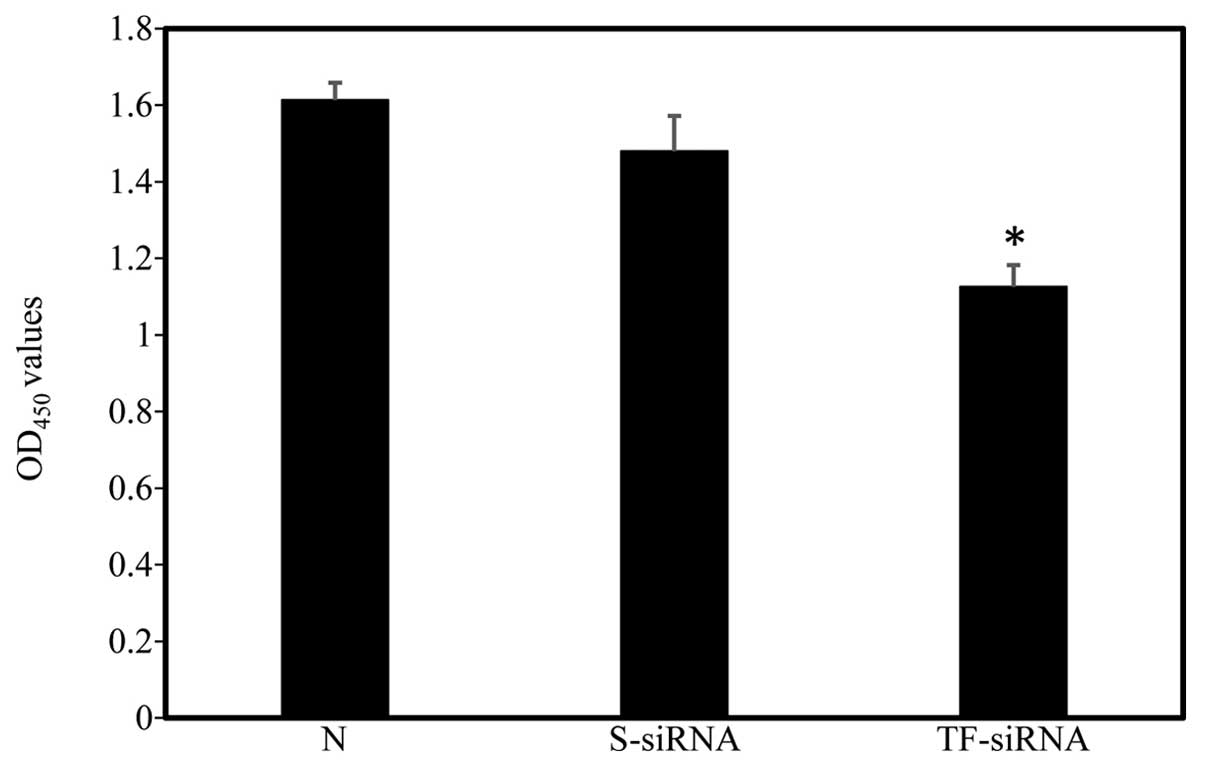
TF knockdown inhibits HUVSMC
proliferation
HUVSMC proliferation was measured 48 h following
siRNA transfection using a CCK-8 kit. No significant differences
were observed between the OD450 values of the
non-transfected and scrambled siRNA-treated HUVSMCs. By contrast,
TF-siRNA transfection significantly inhibited HUVMSC proliferation
(Fig. 3; n=4).
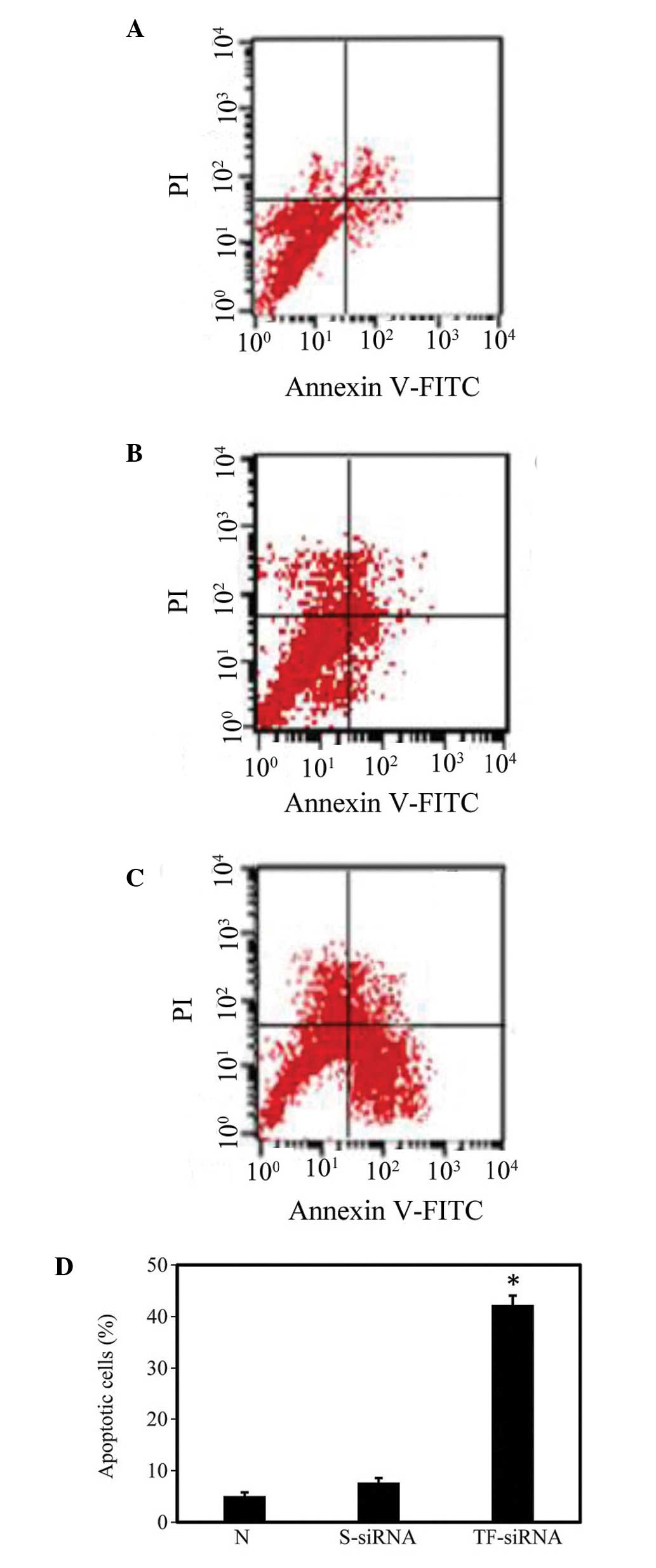
TF knockdown increases HUVSMC
apoptosis
HUVMSC apoptosis was assayed using Annexin V
staining and flow cytometric analysis 72 h following siRNA
delivery. As shown in Fig. 4, cell
apoptotic rates were 5.1±0.71 and 7.73±0.87% in the non-transfected
and HBC/scrambled siRNA nanoparticle-transfected cells,
respectively. Statistical analysis demonstrated no significant
difference between the levels of apoptosis in these two groups.
However, the apoptotic rate in the TF-knockdown HUVMSCs was
42.25±1.82%, which was significantly higher than those observed in
the non-transfected and scrambled siRNA-transfected cells
(P<0.05; n=4).
Discussion
Despite significant efforts that have been made to
improve chitosan as a non-viral gene delivery vector, the
application is significantly limited by its poor solubility under
physiological conditions (31).
HBC, a soluble derivative of chitosan under neutral conditions, has
been investigated as a scaffold for tissue engineering (32,33),
however its use as a gene delivery carrier remains to be fully
elucidated. In the present study, HBC was investigated as a vehicle
for siRNA delivery targeting TF in HUVSMCs. HBC/siRNA nanoparticles
were produced by mixing HBC and siRNA solutions with TPP as a
cross-linker. This resulted in >90% of the siRNAs being loaded
within HBC. Furthermore, treatment of the HUVSMCs with the
HBC/fluorescence-siRNA nanoparticles resulted in a transfection
efficiency of ~74%. These results validated that HBC was a
promising vehicle for siRNA delivery.
siRNA binds an mRNA sequence through
complementarity, which leads to the cleavage of target mRNA and,
eventually, the inhibition of gene expression (34). siRNA has been investigated
extensively for its therapeutic potential, for example in the use
of siRNAs to silence tumor genes, or genes in which overexpression
has been associated with disease (35,36).
TF is involved in thrombus formation and stimulates the migration
and proliferation of vascular smooth muscle cells, contributing to
the pathogenesis of cardiovascular diseases (23–29).
As a consequence, inhibition of the action of TF has been suggested
as an attractive therapeutic approach for cardiovascular diseases
(30). Following confirmation of
effective siRNA delivery with HBC, the present study assessed
whether HBC/TF-siRNA nanoparticles were able to inhibit the
expression of TF in HUVSMCs, and examined the effect of TF
knockdown on cell proliferation and apoptosis. The results showed
that HBC-mediated TF-siRNA transfer suppressed the production of
cellular and soluble TF, which led to the significant inhibition of
cell proliferation and increase of cell apoptosis.
In conclusion, the present study demonstrated that
HBC can be successfully applied for siRNA loading. HBC/siRNA
nanoparticles mediated high siRNA transfection efficiency in
HUVMSCs, and treatment of HUVSMCs with HBC/TF-siRNA nanoparticles
inhibited the protein expression of TF, which led to decreased cell
proliferation and enhanced cell apoptosis. These findings suggested
that HBC may be a promising vector for siRNA delivery, and that
in vivo HBC/siRNA nanoparticle delivery targeting TF is a
potential therapeutic option for the treatment of cardiovascular
diseases, warranting further investigation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant. no. 81200202); the Shandong
Province Natural Science Foundation, China (grant. no. ZR2010HM081)
and the China Postdoctoral Science Foundation (grant. no.
2012M511462).
References
|
1
|
Illum L: Chitosan and its use as a
pharmaceutical excipient. Pharm Res. 15:1326–1331. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felt O, Buri P and Gurny R: Chitosan: A
unique polysaccharide for drug delivery. Drug Dev Ind Pharm.
24:979–993. 1998. View Article : Google Scholar
|
|
3
|
Kumar MN, Muzzarelli RA, Muzzarelli C,
Sashiwa H and Domb AJ: Chitosan chemistry and pharmaceutical
perspectives. Chem Rev. 104:6017–6084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nafee N, Taetz S, Schneider M, Schaefer UF
and Lehr CM: Chitosan-coated PLGA nanoparticles for DNA/RNA
delivery: Effect of the formulation parameters on complexation and
transfection of antisense oligonucleotides. Nanomedicine.
3:173–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Köping-Höggård M, Tubulekas I, Guan H,
Edwards K, Nilsson M, Vårum KM and Artursson P: Chitosan as a
nonviral gene delivery system. Structure-property relationships and
characteristics compared with polyethylenimine in vitro and after
lung administration in vivo. Gene Ther. 8:1108–1121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katas H and Alpar HO: Development and
characterisation of chitosan nanoparticles for siRNA delivery. J
Control Release. 115:216–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang JM, Sun DD, Shin-Ya Y, Sieber AN,
Kostuik JP and Leong KW: Temperature-responsive hydroxybutyl
chitosan for the culture of mesenchymal stem cells and
intervertebral disk cells. Biomaterials. 27:406–418. 2006.
View Article : Google Scholar
|
|
8
|
Molinaro G, Leroux JC, Damas J and Adam A:
Biocompatibility of thermosensitive chitosan-based hydrogels: An in
vivo experimental approach to injectable biomaterials.
Biomaterials. 23:2717–2722. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei CZ, Hou CL, Gu QS, Jiang LX, Zhu B and
Sheng AL: A thermosensitive chitosan-based hydrogel barrier for
post-operative adhesions' prevention. Biomaterials. 30:5534–5540.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mackman N, Morrissey JH, Fowler B and
Edgington TS: Complete sequence of the human tissue factor gene, a
highly regulated cellular receptor that initiates the coagulation
protease cascade. Biochemistry. 28:1755–1762. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilcox JN, Smith KM, Schwartz SM and
Gordon D: Localization of tissue factor in the normal vessel wall
and in the atherosclerotic plaque. Proc Natl Acad Sci USA.
86:2839–2843. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schecter AD, Giesen PL, Taby O, Rosenfield
CL, Rossikhina M, Fyfe BS, Kohtz DS, Fallon JT, Nemerson Y and
Taubman MB: Tissue factor expression in human arterial smooth
muscle cells. TF is present in three cellular pools after growth
factor stimulation. J Clin Invest. 100:2276–2285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camera M, Giesen PL, Fallon J, Aufiero BM,
Taubman M, Tremoli E and Nemerson Y: Cooperation between VEGF and
TNF-alpha is necessary for exposure of active tissue factor on the
surface of human endothelial cells. Arterioscler Thromb Vasc Biol.
19:531–537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bavendiek U, Libby P, Kilbride M, Reynolds
R, Mackman N and Schönbeck U: Induction of tissue factor expression
in human endothelial cells by CD40 ligand is mediated via activator
protein 1, nuclear factor kappa B and Egr-1. J Biol Chem.
277:25032–25039. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawano H, Tsuji H, Nishimura H, Kimura S,
Yano S, Ukimura N, Kunieda Y, Yoshizumi M, Sugano T, Nakagawa K, et
al: Serotonin induces the expression of tissue factor and
plasminogen activator inhibitor-1 in cultured rat aortic
endothelial cells. Blood. 97:1697–1702. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cermak J, Key NS, Bach RR, Balla J, Jacob
HS and Vercellotti GM: C-reactive protein induces human peripheral
blood monocytes to synthesize tissue factor. Blood. 82:513–520.
1993.PubMed/NCBI
|
|
17
|
Mach F, Schönbeck U, Bonnefoy JY, Pober JS
and Libby P: Activation of monocyte/macrophage functions related to
acute atheroma complication by ligation of CD40: Induction of
collagenase, stromelysin and tissue factor. Circulation.
96:396–399. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He M, He X, Xie Q, Chen F and He S:
Angiotensin II induces the expression of tissue factor and its
mechanism in human monocytes. Thromb Res. 117:579–590. 2006.
View Article : Google Scholar
|
|
19
|
Wada H, Kaneko T, Wakita Y, Minamikawa K,
Nagaya S, Tamaki S, Deguchi K and Shirakawa S: Effect of
lipoproteins on tissue factor activity and PAI-II antigen in human
monocytes and macrophages. Int J Cardiol. 47(Suppl 1): S21–S25.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giesen PL, Rauch U, Bohrmann B, Kling D,
Roqué M, Fallon JT, Badimon JJ, Himber J, Riederer MA and Nemerson
Y: Blood-borne tissue factor: Another view of thrombosis. Proc Natl
Acad Sci USA. 96:2311–2315. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bogdanov VY, Balasubramanian V, Hathcock
J, Vele O, Lieb M and Nemerson Y: Alternatively spliced human
tissue factor: A circulating, soluble, thrombogenic protein. Nat
Med. 9:458–462. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szotowski B, Antoniak S, Poller W,
Schultheiss HP and Rauch U: Procoagulant soluble tissue factor is
released from endothelial cells in response to inflammatory
cytokines. Circ Res. 96:1233–1239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Day SM, Reeve JL, Pedersen B, Farris DM,
Myers DD, Im M, Wakefield TW, Mackman N and Fay WP: Macrovascular
thrombosis is driven by tissue factor derived primarily from the
blood vessel wall. Blood. 105:192–198. 2005. View Article : Google Scholar
|
|
24
|
Soejima H, Ogawa H, Yasue H, Kaikita K,
Takazoe K, Nishiyama K, Misumi K, Miyamoto S, Yoshimura M, Kugiyama
K, et al: Angiotensin-converting enzyme inhibition reduces monocyte
chemoattractant protein-1 and tissue factor levels in patients with
myocardial infarction. J Am Coll Cardiol. 34:983–988. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koh KK, Chung WJ, Ahn JY, Han SH, Kang WC,
Seo YH, Ahn TH, Choi IS and Shin EK: Angiotensin II type 1 receptor
blockers reduce tissue factor activity and plasminogen activator
inhibitor type-1 antigen in hypertensive patients: A randomized,
double-blind, placebo-controlled study. Atherosclerosis.
177:155–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mälarstig A, Tenno T, Johnston N,
Lagerqvist B, Axelsson T, Syvänen AC, Wallentin L and Siegbahn A:
Genetic variations in the tissue factor gene are associated with
clinical outcome in acute coronary syndrome and expression levels
in human monocytes. Arterioscler Thromb Vasc Biol. 25:2667–2672.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ott I, Koch W, von Beckerath N, de Waha R,
Malawaniec A, Mehilli J, Schömig A and Kastrati A: Tissue factor
promotor polymorphism-603 A/G is associated with myocardial
infarction. Atherosclerosis. 177:189–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pyo RT, Sato Y, Mackman N and Taubman MB:
Mice deficient in tissue factor demonstrate attenuated intimal
hyperplasia in response to vascular injury and decreased smooth
muscle cell migration. Thromb Haemost. 92:451–458. 2004.PubMed/NCBI
|
|
29
|
Giannarelli C, Alique M, Rodriguez DT,
Yang DK, Jeong D, Calcagno C, Hutter R, Millon A, Kovacic JC, Weber
T, et al: Alternatively spliced tissue factor promotes plaque
angiogenesis through the activation of hypoxia-inducible factor-1α
and vascular endothelial growth factor signaling. Circulation.
130:1274–1286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steffel J, Lüscher TF and Tanner FC:
Tissue factor in cardiovascular diseases: Molecular mechanisms and
clinical implications. Circulation. 113:722–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mao S, Sun W and Kissel T: Chitosan-based
formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev.
62:12–27. 2010. View Article : Google Scholar
|
|
32
|
Zhang K, Qian Y, Wang H, Fan L, Huang C,
Yin A and Mo X: Genipin-crosslinked silk fibroin/hydroxybutyl
chitosan nano-fibrous scaffolds for tissue-engineering application.
J Biomed Mater Res A. 95:870–881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen B, Dang J, Tan TL, Fang N, Chen WN,
Leong KW and Chan V: Dynamics of smooth muscle cell deadhesion from
thermosensitive hydroxybutyl chitosan. Biomaterials. 28:1503–1514.
2007. View Article : Google Scholar
|
|
34
|
Morris KV, Chan SW, Jacobsen SE and Looney
DJ: Small interfering RNA-induced transcriptional gene silencing in
human cells. Science. 305:1289–1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryther RC, Flynt AS, Phillips JA III and
Patton JG: SiRNA therapeutics: Big potential from small RNAs. Gene
Ther. 12:5–11. 2005. View Article : Google Scholar
|
|
36
|
Kanasty R, Dorkin JR, Vegas A and Anderson
D: Delivery materials for siRNA therapeutics. Nat Mater.
12:967–977. 2013. View
Article : Google Scholar : PubMed/NCBI
|