Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease
and a significant health problem in southern China and
south-eastern Asia (1,2). The etiology of NPC includes
virological, genetic and environmental factors (3). NPC is radiation-sensitive and
chemo-sensitive (4); however for
patients who develop local recurrence, there is no efficient
systemic remedy. Distant metastasis is the leading cause of
mortality of advanced NPC patients (4,5).
Epithelial-mesenchymal transition (EMT), a process by which
epithelial cells lose epithelial markers and gain mesenchymal
markers, endows cancer cells with migratory and invasive properties
to initialize metastasis (6–8). In
NPC, amongst other cancer types, the EMT was reported to be a key
event involved in invasion and metastasis (9).
Forkhead box C1 (FoxC1), a member of the forkhead
transcription factor family, was reported to be a regulator of the
EMT (10). It is well known that
FoxC1 has an important role in embryonic and ocular development
(11,12). Recently, accumulating evidence
showed that FoxC1 was overexpressed and correlated with metastasis
and poor prognosis in several cancer types, including breast
cancer, hepatocellular carcinoma, pancreatic ductal adenocarcinoma,
Hodgkin lymphoma and lung cancer (13–17).
Indeed, FoxC1 induced EMT cells by downregulating E-cadherin to
promote cell migration and aggressiveness in breast cancer and
hepatocelluar carcinoma (10,18–20).
To date, no studies have investigated the
clinicopathological significance of FoxC1 and its role in the EMT
in NPC. The present study examined NPC tissues and NPC cell lines
as well as chronically inflamed nasopharyngeal tissue specimens and
an immortalized normal nasopharyngeal epithelial cell line for the
expression of FoxC1. In addition, a correlation study was performed
to assess the association of FoxC1 with clinicopathological
characteristics and the expression of mesenchymal markers in NPC.
Furthermore, the role of FoxC1's role in the EMT of NPC was
investigated in vitro.
Materials and methods
Tissue specimens
All NPC and chronically inflamed nasopharyngeal
tissue specimens were collected from the Affiliated Hospital of
Guilin Medical University (Guilin, China) between May 2011, and
February 2013. The study protocol was approved by the research
ethics committee of the Affiliated Hospital of Guilin Medical
University (Guilin, China) and written informed consent was
obtained from each patient. All samples were pathologically
re-assessed by two pathologists, and the percentage of tumor cells
was ≥70% in all NPC specimens. None of the patients had received
radiotherapy or chemotherapy prior to biopsy sampling (21). 93 NPC tissue specimens with
complete clinical data and 33 NP specimens were collected for
constructing tissue microarrays and subjected to further study. The
clinicopathological characteristics of the patients are listed in
Table I. Clinical staging was
performed according to the tumor-nodes-metastasis (TNM)
classification system of the International Union Against Cancer
staging system (22).
 | Table ICorrelation between
clinicopathological features and expression of FoxC1 in patients
with nasopharyngeal carcinoma. |
Table I
Correlation between
clinicopathological features and expression of FoxC1 in patients
with nasopharyngeal carcinoma.
| Variable | n | FoxC1 expression
| χ2 | P-value |
|---|
| Low (n, %) | High (n, %) |
|---|
| Gender | | | | 0.003 | 0.573 |
| Female | 23 | 13 (56.5) | 10 (43.5) | | |
| Male | 70 | 40 (57.1) | 30 (42.9) | | |
| Age (years) | | | | 2.155 | 0.104 |
| <50 | 50 | 25 (50.0) | 25 (50.0) | | |
| ≥50 | 43 | 28 (65.1) | 15 (34.9) | | |
| Histological
type | | | | 0.041 | 0.558 |
| DNKC | 10 | 6 (60.0) | 4 (40.0) | | |
| UDC | 83 | 47 (56.6) | 36 (43.4) | | |
| T
classification | | | | 30.233 | 0.000 |
| T1-T2 | 42 | 37 (88.1) | 5 (11.9) | | |
| T3-T4 | 51 | 16 (31.4) | 35 (68.6) | | |
| N
classification | | | | 6.012 | 0.012 |
| N0-N1 | 53 | 36 (67.9) | 17 (32.1) | | |
| N2-N3 | 40 | 17 (42.5) | 23 (57.5) | | |
| M
classification | | | | 7.091 | 0.009 |
| M0 | 80 | 50 (62.5) | 30 (37.5) | | |
| M1 | 13 | 3 (76.9) | 10 (23.1) | | |
| Clinical stage | | | | 5.641 | 0.015 |
| I–II | 23 | 18 (78.3) | 5 (21.7) | | |
| III–IV | 70 | 35 (50.0) | 35 (50.0) | | |
Tissue microarray and immunohistochemical
(IHC) analysis
NPC and chronically inflamed nasopharyngeal tissue
specimens were subjected to a tissue microarray study performed by
Pantomics Inc. (Richmond, VA, USA). Briefly, antigens in tissue
slices were retrieved by boiling in citrate buffer (0.01 M; pH 6.0)
and samples were stained with FoxC1 antibody (1:100; cat. no.
ab5079; goat polyclonal; Abcam, Cambridge, MA, USA) for 1 h at room
temperature. Incubation with horseradish peroxidase
(HRP)-conjugated secondary antibody and visualization with
diaminobenzidine (DAB) was performed using a MaxVision™ HRP-Polymer
IHC kit and a DAB kit from Maxim (Fuzhou, China) according to the
manufacturer's instructions (23).
Staining with vimentin antibody (1:50; cat. no. sc-6260; mouse
monoclonal; Santa Cruz Biotechnology, Inc, Dallas TX, USA),
Fibronectin antibody (1:50; cat. no. sc-18825; mouse monoclonal;
Santa Cruz Biotechnology, Inc.), N-cadherin antibody (1:100; cat.
no. sc-59987; mouse monoclonal; Santa Cruz Biotechnology, Inc.),
E-cadherin antibody (1:50; cat. no. sc-7870; rabbit polyclonal;
Santa Cruz Biotechnology, Inc.) and β-catenin antibody (1:100; cat.
no. 8478; rabbit polyclonal; Cell Signaling Technology, Danvers,
MA, USA) was performed in an identical manner.
Scoring of the IHC staining was performed by two
pathologists at Guilin Medical College (Guilin, China). Briefly,
each sample was assessed by adding up the scores for the intensity
and extent of staining. The intensity of staining was scored as
follows: 0, negative; 1, weak; 2, intermediate or 3, strong. The
extent of staining was scored based on the percentage of positive
tumor cells: 0, <5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4,
>76%. For vimentin, fibronectin and N-cadherin, each case was
finally classified as negative and positive, while for FoxC1,
E-cadherin and β-catenin, tissues were classified as low (final
score, 0–3) and high (final score, 4–7) (10).
Cell line and cell culture
The human NPC cell lines 5–8F, 6–10B, CNE1 and CNE2
as well as the NP69 human immortalized nasopharyngeal epithelial
cell line were obtained from the American Type Culture Collection
(Manassas, VA, USA). The 5–8F cell line has a high metastatic
potential, while that of the 6–10B cell line is low. The CNE1 cell
line is well-differentiated, while differentiation of CNE2 cells is
low. The NPC cell lines were cultured in RPMI-1640 (Gibco-BRL,
Invitrogen Life Technologies, Inc., Carlsbad, CA, USA) supplemented
with 10% heat-inactivated fetal bovine serum (FBS; Gibco-BRL) and
0.01% penicillin and streptomycin (Gibco-BRL). NP69 cells were
cultured in keratinocyte serum-free medium (Gibco-BRL) without any
supplementation. All cells were cultured at 37°C in a humidified
incubator in an atmosphere containing 5% CO2.
FoxC1 knockdown in CNE2 cells by RNA
interference
The human FoxC1 small interfering (si)RNA (cat. no.
sc-43766) and control siRNA-A (cat. no. sc-37007) were purchased
from Santa Cruz Biotechnology, Inc. At 24 h prior to transfection,
CNE2 cells were plated at a density of 5×104 cells/well
in a six-well plate. When the cells reached 50% confluency, they
were transfected with 200 pmol (80 µM) siRNA using
Lipofectamine 2000 (Invitrogen Life Technologies, Inc) according to
the manufacturer's instructions. The groups transfected with FoxC1
siRNA and control siRNA were named as siFoxC1 and siNC,
respectively. After 6 h of incubation, the cells were washed three
times with phosphate-buffered saline (PBS) and the medium was
replaced with RPMI-1640 supplemented with 10% FBS. After incubation
for 48 h, cells were subjected to western blot analysis and cell
motility assays.
Western blot analysis
Western blot analyses were performed using standard
methods. In brief, cell pallets were lysed in
radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of
Biotechnology, Haimen, China) with 1 mM phenylmethanesulfonyl
fluoride (Solarbio, Beijing, China). The cell lysate was
centrifuged at 10,000 × g for 10 min at 4°C and the supernatant was
transferred to a fresh micro-centrifuge tube. Protein concentration
was determined by the Bicinchoninic Acid Protein Assay Reagent kit
(cat. no. 23225; Pierce Biotechnology, Inc., Rockford, IL, USA) and
20 µg protein was loaded per lane. The proteins were separated by
10% SDS-PAGE and electrotransferred onto a 0.45-µm
poly-vinylidene difluoride membrane (Merck-Millipore, Billerica,
MA, USA). The membrane was blocked with 5% nonfat milk in 20 mM
Tris-HCl, 150 mM NaCl and 0.1% Tween-20 (pH 7.5) (TBST;
Sigma-Aldrich) for 1.5 h and incubated with the respective primary
antibodies overnight at 4°C. The dilution of FoxC1 antibody (cat.
no. 8758; rabbit polyclonal; Cell Signaling Technology), vimentin
antibody (cat. no. sc-6260; mouse monoclonal; Santa Cruz
Biotechnology, Inc.), fibronectin antibody (cat. no. sc-18825;
mouse monoclonal; Santa Cruz Biotechnology, Inc.), N-cadherin
antibody (cat. no. sc-59987; mouse monoclonal; Santa Cruz
Biotechnology, Inc.), E-cadherin antibody (cat. no. sc-7870; rabbit
polyclonal; Santa Cruz Biotechnology, Inc.), β-catenin antibody
(cat. no. 8478; rabbit polyclonal; Cell Signaling Technology) and
β-actin antibody (cat. no. sc-47778; mouse monoclonal; Santa Cruz
Biotechnology, Inc.) was 1:1,000. Subsequently, the membranes were
washed three times with TBST and incubated with HRP-conjugated
secondary antibodies goat anti-rabbit IgG (1:5,000; cat. no.
ZDR-5306; ZsBio, Beijing, China) and goat anti-mouse IgG (1:5,000;
cat. no. ZDR-5307; ZsBio) at room temperature for 1.5 h. The bands
were visualized using an ECL Plus Western Blot kit (cat. no.
sc-2048; Western Blotting Luminol Reagent; Santa Cruz
Biotechnology, Inc.). The ChemiDoc™ XRS+ system (Bio-Rad
Laboratories, Inc, Hercules, CA, USA) and X-OMAT BT film
(Carestream Health, Inc, Rochester, NY, USA) were used to capture
images of the blots. The intensity of the bands was quantified by
densitometry and normalized to that of β-actin (23). The gray values of all bands were
measured using Image-pro Plus 6.0 software (Media Cybernetics,
Rockville, MD, USA).
In vitro migration and invasion
assays
An 8-µm pore size Transwell insert (BD
Biosciences, Franklin Lakes, NJ, USA) was used to assess the
migratory and invasive ability of cells. At 48 h after
transfection, 5×104 CNE2 cells in serum-free RPMI-1640
were seeded into the upper chamber, which was lined with a
non-coated membrane, for Transwell migration assays. For invasion
assays, Transwell chambers were washed with serum-free medium and
200 mg/ml Matrigel (BD Biosciences) was added to the polycarbonate
membrane and dried overnight under sterile conditions.
Subsequently, 1×105 cells were seeded in the upper
chamber. These inserts were inserted in a 24-well plate with 500
µl RPMI-1640 containing 10% FBS. After incubation for 24 h,
the cells on the upper side of the basement membrane were removed
with a sterile cotton swab, and the cells that invaded to the lower
side of the basement membrane were stained with Giemsa
(Sigma-Aldrich). The cells which transgressed through the Transwell
polycarbonate membrane were counted under a microscope (BX51;
Olympus, Tokyo, Japan). Six random high-power fields were selected
for each sample, and the experiment was repeated three times.
Results were expressed as mean values of triplicate assays for each
experimental condition.
In vitro wound-healing assay
The wound healing assay was performed following a
procedure of a previous study (24). CNE2 cells were transfected with
siRNA as described above. After incubation for 48 h, the cells were
trypsinized (Gibco-BRL) and plated at 5×105 cells/ml
into six-well plates. After incubation overnight, line-shaped
wounds in the cell monolayer were generated using a pipette tip and
images were captured at 0, 24 and 48 h using and Olympus BX51
microscope (24). Experiments were
performed in triplicate and repeated three times.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Categorical data were analyzed using the χ2
test or Fisher's exact test. Quantitative data were analyzed by
using the Unpaired Student's t-test for comparing two groups and
one-way analysis of variance for multiple variant analysis.
P<0.05 was considered to indicate a statistically significant
difference between values and all analyses were performed with
using SPSS 19.0 (International Business Machines, Armonk, NY,
USA).
Results
FoxC1 is upregulated in NPC tissues and
cell lines and high expression of FoxC1 is associated with advanced
clinicopathological characteristics
To explore the role of FoxC1 in determining clinical
outcomes for NPC patients, the present study investigated the
expression of FoxC1 in a tissue microarray comprising 93 NPC and 33
chronically inflamed nasopharyngeal tissue specimens. IHC analysis
showed that FoxC1 was localized in the nucleus and cytoplasm. High
expression of FoxC1 was observed in 40 out of 93 (43.0%) NPC
tissues, but in only 4 out of 33 (12.1%) chronically inflamed
nasopharyngeal tissues (P<0.01) (Fig. 1A). Western blot analysis showed
that FoxC1 expression was high in NPC cell lines and low in the
NP69 immortalized normal human nasopharyngeal epithelial cell line
(Fig. 1B). High expression of
FoxC1 was significantly correlated with tumor size, lymph node
metastasis, distant metastasis and clinical stage (Table I).
High expression of FoxC1 is positively
correlated with vimentin, fibronectin and N-cadherin expression in
NPC tissues
Compared to cases with low FoxC1, mesenchymal
markers vimentin, fibronectin and N-cadherin were upregulated in
NPC tissues with high expression of FoxC1. However, the expression
levels of E-cadherin and β-catenin showed no significant difference
between the high FoxC1 group and the low FoxC1 group (Fig. 2A). Among the 40 cases with high
FoxC1 expression, the positive expression rate of vimentin,
fibronectin and N-cadherin was 29/40 (72.5%), 25/40 (62.5%) and
39/40 (97.5%), respectively, and the high expression rate of
E-cadherin and β-catenin was 26/40 (65.0%) and 24/40 (60.0%),
respectively. In the specimens with low FoxC1 expression, the
positive expression rate of vimentin, fibronectin and N-cadherin
was 22/53 (41.5%), 20/53 (37.7%) and 42/53 (79.3%), respectively,
and the high expression rate of E-cadherin and β-catenin was 34/53
(64.2%) and 39/53 (73.6%), respectively (Fig. 2B).
 | Figure 2High FoxC1 expression is associated
with mesenchymal and epithelial markers in nasopharyngeal
carcinoma. (A) Immunohistochemical staining of FoxC1 protein and
mesenchymal and epithelial markers, including vimentin,
fibronectin, N-cadherin, E-cadherin and β-catenin in nasopharyngeal
carcinoma. In case 1, FoxC1 expression was low and the cancer cells
were negative for vimentin, fibronectin and N-cadherin expression,
while being highly positive for E-cadherin and β-catenin
expression. In case 2, FoxC1 expression was high and tissue stained
positive for vimentin, fibronectin, N-cadherin, E-cadherin and
β-catenin. (B) According to statistical analysis, FoxC1 expression
was positively correlated with vimentin, fibronectin and N-cadherin
expression, but not with E-cadherin and β-catenin expression.
Values are expressed as the mean ± standard deviation. Fox,
forkhead box. |
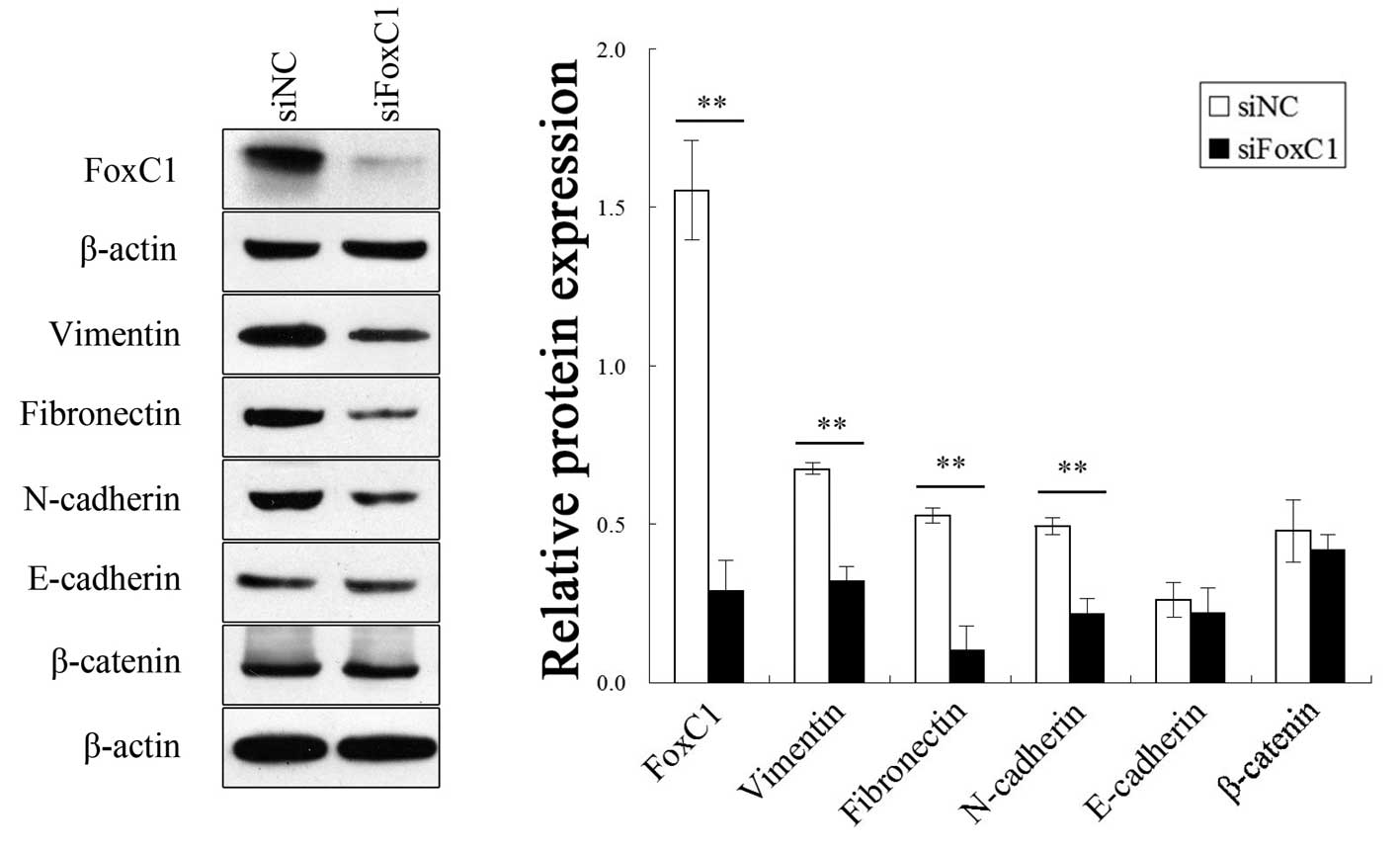
FoxC1 knockdown by siRNA decreases
vimentin, fibronectin and N-cadherin expression in CNE2 cells
After FoxC1 knockdown by siRNA, the mesenchymal
markers vimentin, fibronectin and N-cadherin were downregulated in
CNE2 cells. However, the expression of E-cadherin and β-catenin was
not significantly different between the knockdown group and the
control group (Fig. 3).
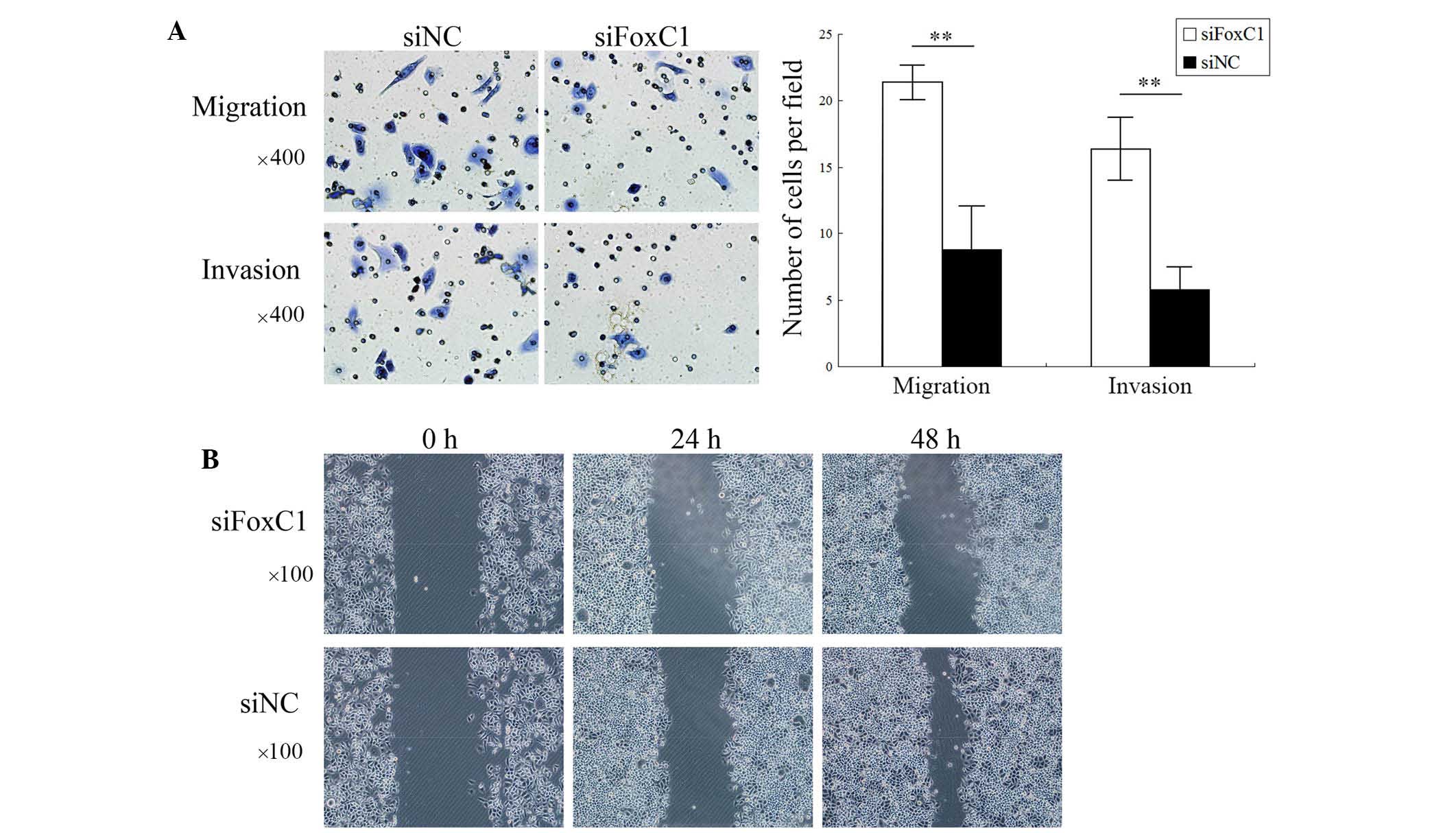
FoxC1 knockdown by siRNA reduces CNE2
cell migration and invasion in vitro
Transwell assays showed that the number of migrated
and invaded CNE2 cells was significantly decreased after FoxC1
knockdown by siRNA (Fig. 4A). As
shown in Fig. 4B, wound healing of
the CNE2 cell layer was inhibited after FoxC1 knockdown by siRNA.
These results demonstrated that the migratory and invasive
capabilities of NPC cells were deceased following downregulation of
FoxC1.
Discussion
The present study revealed that FoxC1 was
overexpressed in NPC tissues as compared with that in chronically
inflamed nasopharyngeal tissues. These results were consistent with
those obtained from cell lines, which showed high FoxC1 expression,
while the NP69 immortalized normal nasopharyngeal epithelial cell
showed low FoxC1 expression. Furthermore, high FoxC1 expression in
NPC tissues was correlated with aggressive phenotypes with large
tumor size, lymph node metastasis, distant metastasis and advanced
clinical stage. These results implied that FoxC1 is involved in the
development and progression in NPC. In addition, knockdown of FoxC1
significantly decreased the migratory, invasive and wound healing
capacities of CNE2 cells in vitro. The results from clinical
samples and cultured NPC cell lines supported the notion that FoxC1
contributes to aggressive phenotypes in NPC.
The EMT is a key event involved in tumor
invasiveness and metastasis and is regulated by several
transcription factors during tumor progression, including Twist,
Snai1, Slug, Goosecoid, zinc finger E-box binding homeobox 1 and
Smad-interacting protein 1 (25).
FoxC1 was recently reported to be a transcriptional factor involved
in the EMT (10). In the present
study, high FoxC1 was correlated with upregulated mesenchymal
markers (vimentin, fibronectin and N-cadherin) but not with
epithelial markers (E-cadherin and β-catenin) in NPC tissues as
well as in cell lines in vitro. Furthermore, the roles of
epithelial and mesenchymal markers in the motility of NPC cells
were verified by FoxC1 knockdown. Following downregulation of the
mesenchymal markers, the migratory and invasive capacities of CNE2
cells were inhibited in vitro.
In the process of EMT, epithelial cells lose their
cell-cell adhesion abilities, while cell-extracellular matrix
adhesion is enhanced (8).
Fibronectin is a high-molecular weight (~440 kDa) glycoprotein,
which binds to extracellular matrix components and is thereby
involved in cell adhesive and migratory processes (26). Fibronectin expression was reported
to be increased in lung cancer, particularly in non-small cell lung
carcinoma. The adhesion of lung carcinoma cells to fibronectin
enhances their tumorigenicity and confers resistance to
apoptosis-inducing chemotherapeutic agents (27,28).
N-cadherin is a calcium-dependent cell-cell adhesion glycoprotein,
which is abundant in cancer cells and facilitates transendothelial
migration (29). Vimentin is
responsible for maintaining cell shape, integrity of the cytoplasm
and stabilization of cytoskeletal interactions (30). Overexpression of vimentin was
observed in spindle-shaped NPC cells, which are thought to be
highly invasive (31). The results
of the present study showed that FoxC1 expression was positively
associated with the expression of fibronectin, N-cadherin and
vimentin, which all contributed to the migratory and invasive
properties of CNE2 cells.
E-cadherin is a component of an E-cadherin/catenin
adhesion complex located to adherens junctions, which are disrupted
in cancer. Downregulated E-cadherin in cancer, particularly in
advanced cases, leads to the disintegration of adherent junctions,
followed by release of β-catenin and its translocation to the
nucleus (8). However, the present
study showed that in NPC, FoxC1 was not associated with E-cadherin
and β-catenin expression. In hepatocellular carcinoma cells, it was
previously shown that FoxC1 transactivates the expression of Snai1,
a transcriptional inhibitor of E-cadherin, (10,32,33).
In human breast cancer, the expression of FoxC1 is also negatively
correlated with the expression of E-cadherin and β-catenin
(18). The underlying mechanism of
how FoxC1 regulates E-cadherin is still required to be elucidated.
The results of the present study implied that in NPC, FoxC1 is
involved in the EMT by increasing cell-extracellular matrix
adhesion rather than disrupting adherens junctions.
In conclusion, the present study showed that FoxC1
was overexpressed and involved in the process of EMT through
upregulating fibronectin, vimentin and N-cadherin, which
contributed to cell migration and invasion in human NPC. These data
suggested that FoxC1 is a potential promoter of the EMT and a
prospective therapeutic target in NPC.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Guangxi Province (grant no.
2011jjD40031).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang W, Li X, Jiang Q, Liu Z, Yang H, Wang
S, Xie S, Liu Q, Liu T, Huang J, et al: Transcriptional patterns,
biomarkers and pathways characterizing nasopharyngeal carcinoma of
Southern China. J Transl Med. 6(32)2008. View Article : Google Scholar
|
|
3
|
Chou J and Lin YC: Nasopharyngeal
carcinoma-review of the molecular mechanisms of tumorigenesis. Head
Neck. 30:946–963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang R, Wu F, Lu H, Wei B, Feng G, Li G,
Liu M, Yan H, Zhu J, Zhang Y and Hu K: Definitive
intensity-modulated radiation therapy for nasopharyngeal carcinoma:
Long-term outcome of a multicenter prospective study. J Cancer Res
Clin Oncol. 139:139–145. 2013. View Article : Google Scholar
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai LM, Lyu XM, Luo WR, Cui XF, Ye YF,
Yuan CC, Peng QX, Wu DH, Liu TF, Wang E, et al: EBV-miR-BART7-3p
promotes the EMT and metastasis of nasopharyngeal carcinoma cells
by suppressing the tumor suppressor PTEN. Oncogene. 34:2156–2166.
2015. View Article : Google Scholar
|
|
10
|
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen
Z, Zhang Y, Hu H, Fan D, Nie Y and Wu K: Overexpression of forkhead
box C1 promotes tumor metastasis and indicates poor prognosis in
hepatocellular carcinoma. Hepatology. 57:610–624. 2013. View Article : Google Scholar
|
|
11
|
Paylakhi SH, Moazzeni H, Yazdani S,
Rassouli P, Arefian E, Jaberi E, Arash EH, Gilani AS, Fan JB, April
C, et al: FOXC1 in human trabecular meshwork cells is involved in
regulatory pathway that includes miR-204, MEIS2 and ITGβ1. Exp Eye
Res. 111:112–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan AO, Aldahmesh MA, Mohamed JY and
Alkuraya FS: Congenital glaucoma with acquired peripheral
circumferential iris degeneration. J AAPOS. 17:105–107. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J,
Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, et al: FOXC1 is a
potential prognostic biomarker with functional significance in
basal-like breast cancer. Cancer Res. 70:3870–3876. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu ZY, Ding SM, Zhou L, Xie HY, Chen KJ,
Zhang W, Xing CY, Guo HJ and Zheng SS: FOXC1 contributes to
microvascular invasion in primary hepatocellular carcinoma via
regulating epithelial-mesenchymal transition. Int J Biol Sci.
8:1130–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Gu F, Liu CY, Wang RJ, Li J and Xu
JY: High level of FOXC1 expression is associated with poor
prognosis in pancreatic ductal adenocarcinoma. Tumour Biol.
34:853–858. 2013. View Article : Google Scholar
|
|
16
|
Nagel S, Meyer C, Kaufmann M, Drexler HG
and MacLeod RA: Deregulated FOX genes in Hodgkin lymphoma. Genes
Chromosomes. Cancer. 53:917–933. 2014.
|
|
17
|
Wei LX, Zhou RS, Xu HF, Wang JY and Yuan
MH: High expression of FOXC1 is associated with poor clinical
outcome in non-small cell lung cancer patients. Tumour Biol.
34:941–946. 2013. View Article : Google Scholar
|
|
18
|
Bloushtain-Qimron N, Yao J, Snyder EL,
Shipitsin M, Campbell LL, Mani SA, Hu M, Chen H, Ustyansky V,
Antosiewicz JE, et al: Cell type-specific DNA methylation patterns
in the human breast. Proc Natl Acad Sci USA. 105:14076–14081. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taube JH, Herschkowitz JI, Komurov K, Zhou
AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et
al: Core epithelial-to-mesenchymal transition interactome gene
expression signature is associated with claudin-low and
meta-plastic breast cancer subtypes. Proc Natl Acad Sci USA.
107:15449–15454. 2010. View Article : Google Scholar
|
|
20
|
Sizemore ST and Keri RA: The forkhead box
transcription factor FOXC1 promotes breast cancer invasion by
inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem.
287:24631–24640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei
RR, Zhang MY, Sun Y, Huang BJ, Chen M, et al: Prognostic value of a
microRNA signature in nasopharyngeal carcinoma: A microRNA
expression analysis. Lancet Oncol. 13:633–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th ed.
Springer; New York: 2009
|
|
23
|
Zhang XL, Huang CX, Zhang J, Inoue A, Zeng
SE and Xiao SJ: CtBP1 is involved in epithelial-mesenchymal
transition and is a potential therapeutic target for hepatocellular
carcinoma. Oncol Rep. 30:809–814. 2013.PubMed/NCBI
|
|
24
|
Thompson CC, Ashcroft FJ, Patel S, Saraga
G, Vimalachandran D, Prime W, Campbell F, Dodson A, Jenkins RE,
Lemoine NR, et al: Pancreatic cancer cells overexpress gelsolin
family-capping proteins, which contribute to their cell motility.
Gut. 56:95–106. 2007. View Article : Google Scholar
|
|
25
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams CM, Engler AJ, Slone RD, Galante
LL and Schwarzbauer JE: Fibronectin expression modulates mammary
epithelial cell proliferation during acinar differentiation. Cancer
Res. 68:3185–3192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han S, Khuri FR and Roman J: Fibronectin
stimulates non-small cell lung carcinoma cell growth through
activation of Akt/mammalian target of rapamycin/S6 kinase and
inactivation of LKB1/AMP-activated protein kinase signal pathways.
Cancer Res. 66:315–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramis-Conde I, Chaplain MA, Anderson AR
and Drasdo D: Multi-scale modelling of cancer cell intravasation:
The role of cadherins in metastasis. Phys Biol. 6(016008)2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogrodnik M, Salmonowicz H, Brown R,
Turkowska J, Średniawa W, Pattabiraman S, Amen T, Abraham AC,
Eichler N, Lyakhovetsky R and Kaganovich D: Dynamic JUNQ inclusion
bodies are asymmetrically inherited in mammalian cell lines through
the asymmetric partitioning of vimentin. Proc Natl Acad Sci USA.
111:8049–8054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Li X, Zhang W, Li W, Yi M, Yang J,
Zeng Z, Colvin Wanshura LE, McCarthy JB, Fan S, et al:
Oxidored-nitro domain containing protein 1 (NOR1) expression
suppresses slug/vimentin but not snail in nasopharyngeal carcinoma:
Inhibition of EMT in vitro and in vivo in mice. Cancer Lett.
348:109–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Batlle E, Sancho E, Franci C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|