Introduction
Gene-environment interactions have been demonstrated
to contribute to the etiology of various mental disorders (1,2).
Although the genetic risk factors for different mental disorders
have previously been considered to be distinct, studies have
demonstrated that five major mental disorders: Autism spectrum
disorder, attention deficit-hyperactivity disorder, bipolar
disorder, major depressive disorder and schizophrenia, share
markedly overlapping genetic roots (3). Therefore, the identification of
candidate genes associated with mental disorders has increased in
prominence in modern psychiatric investigations.
MicroRNAs (miRNAs) are short RNA molecules, which
negatively regulate the stability and translation of mRNA targets
at the post-transcriptional level. miRNAs regulate target mRNA
expression by binding to the 3′-untranslated region through base
pairing, resulting in target mRNA cleavage or translational
inhibition (4–6). miRNAs are regarded as master
regulators of gene expression, in that a single miRNA may regulate
several hundred genes, and collectively miRNAs may regulate as much
as two thirds of the transcriptome (7). Notably, miRNAs regulate mRNA
translation locally in the axosomal and synaptodendritic
compartments, thereby contributing to the dynamic spatial
organization of axonal and dendritic structures and their
functions, and consequently, synaptic and neural plasticity
(8). Increasing evidence has
suggested that the dysregulation of miRNAs is associated with the
etiopathology of various neuropsychiatric disorders (9–13).
In particular, miRNAs have been found to be associated with
intellectual disabilities, including cognitive impairment and
cognitive retardation (14).
Although the etiopathology of mental disorders
remains unclear, disruptions across whole cellular networks and the
dysregulation of multiple signaling pathways, particularly those
contributing to synaptic plasticity, likely contribute to
pathogenesis. These disruptions are likely lead to aberrant
information processing in the circuits that regulate mood,
cognition and neurovegetative function, including sleep, appetite
and energy (15,16). As miRNAs are able to suppress the
translation of hundreds of target mRNAs, they are well-positioned
to target numerous cellular processes; therefore, therapeutic
strategies capable of affecting changes in cellular plasticity to
restore synaptic function and neuronal connectivity hold
significant potential (13).
Increasing evidence has implicated miR-30e in a number of diseases,
including heart failure, neoplasms, lymphoma, melanoma,
mesothelioma, aortic aneurysm, glioma, obesity, periodontal
diseases, lung diseases and, most notably, schizophrenia (17–19).
miR-30e regulates its target gene, ubiquitin-conjugating enzyme 9
(UBC9), in order to inhibit cell growth in carcinoma (20,21).
In addition, miR-30e has been identified as an immediate target
activated by the β-catenin/transcription factor 4 complex during
intestinal cell differentiation (22). Through bioinformatic analysis, our
previous study demonstrated that miR-30e may target a functional
network, comprising 10 interrelated genes, to regulate their levels
of expression in schizophrenia (19). Downregulation in the expression of
miR-30e has been demonstrated to promote neuronal survival in
long-lived calorie-restricted mice, suggesting that it may regulate
neuronal death (23). Our previous
investigations revealed that the expression of miR-30e is increased
in the peripheral blood leukocytes of patients with schizophrenia
(19), which was consistent with
the findings of other previous studies that observed abnormal
expression levels of miR-30e in the brain and peripheral blood
leukocytes of subjects with schizophrenia (24–26).
Rege et al (27)
demonstrated that miR-30e is upregulated in the frontal cortex and
striatum of certain neurodegenerative disorders, including
Huntington's disease. Furthermore, Banigan et al (28) revealed that exosomal miR-30e-5p is
significantly differentially expressed in patients with
schizophrenia and bipolar disorder.
Cerebralcare Granule® (CG; Tasly
Pharmaceutical Co., Ltd., Tianjin, China) is a Chinese herbal
medicine compound, which is used for the treatment of
cerebrovascular diseases. Compounds identified in CG include
rhynchophylline, genistein, ursolic acid, 2-alpha-hydroxyursolic
acid, naphthopyrones, alaternin, ferulic acid, ligustrazine,
L-tetrahydropalmatine, peoniflorin, rehmannioside and
methyleugenol, among which a substantial proportion exhibit
antioxidant properties (29). Wang
et al (30) identified six
active components in rat plasma following oral administration of
CG: Protocatechuic acid, chlorogenic acid, caffeic acid, ferulic
acid, rosmarinic acid and paeoniflorin, using liquid
chromatography-tandem mass spectrometry and pharmacokinetics. CG
inhibits the production of superoxide in the cerebral venular
endothelium and reduces albumin leakage across venules, as well as
attenuating bilateral common carotid artery occlusion-elicited
cerebral microcirculatory disturbance, hippocampal neuron injury
and blood-brain barrier disruption. In addition, CG protects the
brain from edema following ischemia and reperfusion injury
(29,30–33),
suggesting that it may alleviate cognitive impairment induced by
chronic cerebral hypoperfusion.
The present study aimed to investigate the effects
of CG on behavioral impairment in rats induced to continuously
overexpress miR-30e. Furthermore, the present study aimed to
investigate the molecular and cellular mechanisms underlying the
therapeutic effects of CG.
Materials and methods
Recombinant lentiviral vector production
and verification
Lentiviral vectors are useful for in vivo
investigations of the central nervous system (CNS), as they are
capable of maintaining expression for the life of an animal, when
injected into the brain (34–36).
In addition, as lentiviral vectors are replication-deficient and do
not leave the site of injection, they stably and safely deliver the
target gene into the CNS (37). In
the present study, vectors containing the target nucleotide
sequence were constructed. Rat miR-30e cDNA was amplified by
polymerase chain reaction (PCR) from the miR-30e-pLVX-IRES-Zs
Green1 vector (Shanghai SBO Medical Biotechnology Co., Ltd.,
Shanghai, China) using a Biosafer 9703 (Shenzhen Safer Science and
Technology Co., Ltd., Shenzhen, China). The following
oligonucleotide primers (Invitrogen Life Technologies, Carlsbad,
CA, USA) were used for the PCR isolation of miR-30e: Forward 5′-CAA
CAG AAG GCT CGA GCT GTT GGA GAA GTG GGC ATC-3′ and reverse 5′-ATT
CTG ATC AGG ATC CCT CCA AAC GAA GAG AGA CAGTC-3′, which carried
restriction sites for BamHI and XhoI, respectively.
The PCR cycling conditions were as follows: 98°C for 3 min,
followed by 30 cycles at 98°C for 10 sec, 55°C for 15 sec and 72°C
for 30 sec, and a final extension at 72°C for 10 min. The products
were subsequently stored at 4°C. The virus was generated by
transient co-transfection of the expression plasmid (10 µg),
the pseudotyping construct PMD2 G (10 µg; Shanghai SBO
Medical Biotechnology Co., Ltd.) and the packaging construct psPAX2
(10 µg; Shanghai SBO Medical Biotechnology Co., Ltd.) in a
75-mm plate containing 90% confluent 293T cells (ATCC, Manassas,
VA, USA), as previously described [Naldini et al (36); Rattiner et al (38) and Heldt et al (39)]. Following transfection for 12 h at
37°C, the medium was discarded and 10% Dulbecco's modified Eagle's
medium was added (Invitrogen Life Technologies). The medium was
collected 48 and 72 h post-transfection, cleared of debris by
low-speed centrifugation at 4,378 × g for 30 min at 4°C, and
filtered through 0.45-µm filters. High-titer stocks were
prepared by initial ultracentrifugation for 1 h at 296,000 × g at
4°C, and a secondary centrifugation at 96,360 × g for 30 min at
4°C. The viral pellets were resuspended in 1% bovine serum albumin
in phosphate-buffered saline and stored at −80°C. Green fluorescent
protein (GFP)-positive cells (pLVX-IRES-ZsGreen1 is able to express
GFP) were visualized using fluorescence microscopy (Leica
Microsystems Canada Inc., Richmond Hill, ON, Canada). The quantity
of p24 Gag conical virion capsid was measured using the QuickTiter™
Lentivirus Titer kit (Cell Biolabs, San Diego, CA, USA). Lentiviral
particle (LP) titers were assayed based on the manufacturer's
control titer of 1 ng p24 = 1.25×107 LPs. The yield
ranged between 1 and 25 µg/ml p24, corresponding to values
between 1.25×1010 and 0.3×1012 LPs/ml.
Animals and stereotaxic surgery
The present study was approved by the ethics
committee of the Laboratory Animal Facility Biomedical Analysis
Center, Tsinghua University (Beijing, China; 100084; 2012-LiuPZ-
mir30e). Male Sprague-Dawley (SD) rats (age, 4 weeks; average body
weight, 100±10 g) were bred at the Laboratory Animal Facility of
the Biomedical Analysis Center of Tsinghua University (Beijing,
China). The rats were housed (four animals per cage) with access to
food and water ad libitum, under a controlled 12-h/12-h
light-dark cycle (lights on at 7:00AM) at 22±2°C and 55±5%
humidity. All experimental protocols (permit no. 2012-LiuPZ-mir30e)
were approved by the Tsinghua University Laboratory Animal
Administration Committee, were performed according to the Tsinghua
University Guidelines for Animal Experimentation, and conformed to
the Guide for the Care and Use of Laboratory Animals (40) published by Tsinghua University.
The rats were intraperitoneally anesthetized with
10% chloral hydrate (2 mg/kg) and placed in a stereotaxic apparatus
(ST-51600; David Kopf Instruments, Tujunga, CA, USA). A 1 µl
aliquot of the lentivirus was injected into the hippocampal dentate
gyrus (DG) at a rate of 0.2 µl/min (UltramicropumpII; World
Precision Instruments, Inc., Sarasota, FL, USA). The injection
coordinates relative to the bregma were AP, −3.0; ML, ±2.6 and DV,
−3.0 [Paxinos and Franklin (41)]
and were based on previously described coordinates. The needle
remained in place for an additional 5 min and was then slowly
withdrawn. The rats were allowed to recover for 14 days, in order
to allow the virus to infect cells at the injection site and begin
producing miR-30e or GFP (used as an exogenous marker). The
lentivirus expressing GFP was used to control for surgery-induced
hippocampal damage, which may lead to abnormal behavior.
Theoretically, the lentivirus results in expression of miR-30e or
GFP for the remainder of the animal's life.
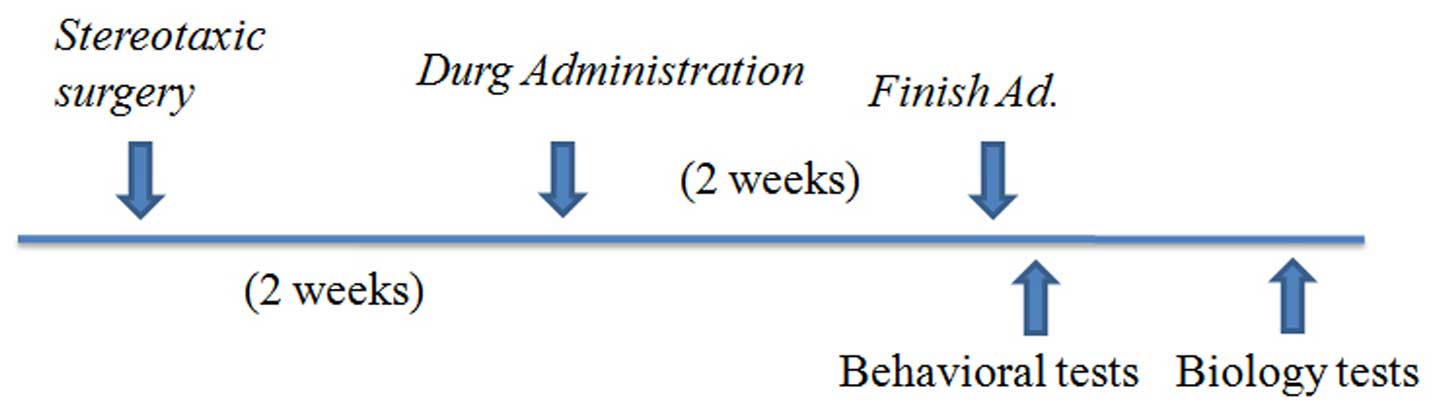
A total of 40 male SD rats (4-week-old; average body
weight, 100±10 g) were randomly divided into five groups, with
eight rats in each group: i) Control group; ii) lenti-GFP group;
iii) lenti-miR-30e group; iv) lenti-miR-30e plus CG (100 mg/kg, 14
days) group; and v) lenti-miR-30e plus fluoxetine (10 mg/kg, 14
days) group (Fig. 1). The optimal
concentration of CG for treatment of the rats was determined in
preliminary experiment, and was equivalent to a 6–7% concentration
in humans.
Drug administration
The CG used in the present study was produced by
Tasly Pharmaceutical Co., Ltd. A 50 mg/ml stock of CG (cat. no.
110306) solution was prepared using sterile water. Appropriate
quantities of CG were ground with a pestle and mortar to remove
lumps, and the resulting power was mixed with sterile water for
injection (50 mg/ml) once a day for 14 days.
Chronic administration was performed by dissolving
fluoxetine hydrochloride (10 ml/kg; Sigma-Aldrich, St. Louis, MO,
USA) in deionized water and dividing the solution into 14 equal
volumes (1 mg/ml), which were used for daily treatment over a
14-day period. The control rats received injections of deionized
water.
Behavioral testing open field test
The open field test assesses hyperactivity through
locomotion and anxious behavior. The open field box used in the
present study consisted of a square black box (60×60×25 cm)
consisting of plexiglass with an outlined center area. The center
area (30×30 cm) was demarcated with Tartan 1710 vinyl electrical
tape. Each rat was placed in the box for 10 min. The overall
activity of the rat in the box was measured using a videotracker
(Noldus version 8.0; ZS Dichuang, Beijing, China), and the duration
and distance travelled in the center area of the maze were
measured. This paradigm was based on the premise that rodents
naturally prefer to be close to a protective wall, rather than
being exposed to danger in the central area.
Water maze test
A modified version of the water maze task originally
developed by Morris (42) was used
in the present study to assess spatial learning and memory. The
maze consisted of a circular tank (180 cm in diameter) filled with
water at room temperature, which was made opaque by the addition of
non-toxic white paint. Extra-maze distal cues were positioned on
the walls around the tank. A 15-cm-wide circular platform was fixed
to the bottom of the tank and submerged 2 cm below the water
surface, in order to remain invisible to the animals.
In the version of the test assessing working memory,
the animals were subjected to two trials per day over six
consecutive days. In each trial, the rats were released from a
different position and had 60 sec to locate the platform and climb
onto it. The platform position was changed daily, but remained
constant throughout a given day. The rats were allowed an
inter-trial interval of 15 sec, during which they remained on the
platform. The latency in locating the platform was recorded.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Four weeks following treatment, four animals/group
were intraperitoneally injected with 10% chloral hydrate (Tianjin
Kermel Chemical Reagent Co., Ltd., Tianjin, China) at 4 ml/kg. The
chest was sectioned and opened following anesthesia. A fast
injection of normal saline was given through a catheter placed in
the left ventricle; at the same time, the right auricle was
sectioned and opened. When the liver turned white from bleeding, 4%
paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd., Beijing,
China) was infused until rigor mortis occurred. The brain was
removed and fixed in a solution containing 30% sucrose and 4%
paraformaldehyde. Once the brain sunk to the bottom of the
solution, 10 µm cryostat sections were prepared using a
freezing microtome (CM 1850; Leica Microsystems Inc., Buffalo
Grove, IL, USA). Apoptotic cells were identified using a TUNEL
assay (In Situ Cell Death Detection kit; TMR red; Roche
Diagnostics, Basel, Switzerland), according to the manufacturer's
instructions. For nuclear counterstaining, the sections were dyed
with DAPI (1:10,000) in PBS with Tween 20 (PBS-T) for 10 min, and
then briefly rinsed with PBS-T and PBS. The quantification of
TUNEL-positive cells on images of the hippocampal DG sections were
performed manually. A minimum of four sections (10 µm apart)
were quantified per animal.
Western blotting
The brains were removed from the cranium, the
hippocampus was rapidly dissected four weeks following treatment,
frozen in liquid nitrogen, and stored at −80°C prior to further
analysis. Extracts for western blot analysis were prepared by
homogenizing the tissues in ice-cold extraction buffer (Cancer
Type, Beijing, China) consisting of 75 mM β-glycerophosphate, 20 mM
MOPS, 15 mM EGTA, 2 mM EDTA, 1 mM NaVO4, 1 mM
phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin (pH 7.2) and then
sonicated on ice. Insoluble material was removed by centrifugation
at 12,000 × g at 4°C for 30 min. Protein concentrations were
determined using a protein assay kit (Bio-Rad Laboratories Inc.,
Hercules, CA, USA). Total protein (10 µg) was separated by
10% SDS-PAGE (Cancer Type) and transferred onto nitrocellulose
membranes (Sartorius AG, Goettingen, Germany). Subsequently, the
membranes were incubated with anti-B-cell lymphoma 2 (BCL-2;
1:1,000; mouse monoclonal; cat. no. 15071; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-UBC9 (1:1,000; rabbit
monoclonal; cat. no. 47861; Cell Signaling Technology, Inc.), and
anti-GAPDH (1:1,000; mouse monoclonal; cat. no. 14433; Cancer Type)
primary antibodies at 2–8°C overnight in 5% non-fat dried milk. The
membranes were then incubated with secondary antibodies, including
goat anti-mouse for Bcl-2 and GAPDH (cat. no. ZF-0512) and goat
anti-rabbit for UBC9 (cat. no. ZF-0511) (1:5,000; Cancer Type) for
2 h at room temperature. The blots were visualized using enhanced
chemiluminescence detection, following which images were captured
and protein expression levels were quantified using ImageJ software
(1.38e/Java 1.5.0_09; National Institutes of Health, Bethesda, MD,
USA).
Immunohistochemistry
Following removal and fixing of the brains of the
rats, 10 µm cryostat sections were prepared and
immunohistochemistry was performed using BCL-2 and UBC9 antibodies,
according to the manufacturer's instructions. The primary antibody
was replaced with normal serum in negative controls.
For quantitative immunohistochemistry, three rats
were selected from each group, and one in every four samples was
selected from a continuous series of the prepared hippocampal
tissue sections, which were processed immunohistochemically. In
total, 15 sections were observed under a ×20 objective (Leica
DM3000; Leica Microsystem, Wetzlar, Germany), and positively
stained cells were randomly selected from each section. ImageJ
software (National Institute of Health) was used to determine the
mean cytoplasmic grey scale and to assess the staining
intensity.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Data between two groups were compared using Student's
t-test. Results were analyzed using repeated measures analysis of
variance (ANOVA), followed by one-way ANOVA and a post-hoc Fisher's
protected least significant difference (PLSD) test. The number of
TUNEL-positive cells were analyzed using one-way ANOVA, followed by
a post-hoc Fisher's PLSD test. Statistical analyses were performed
using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
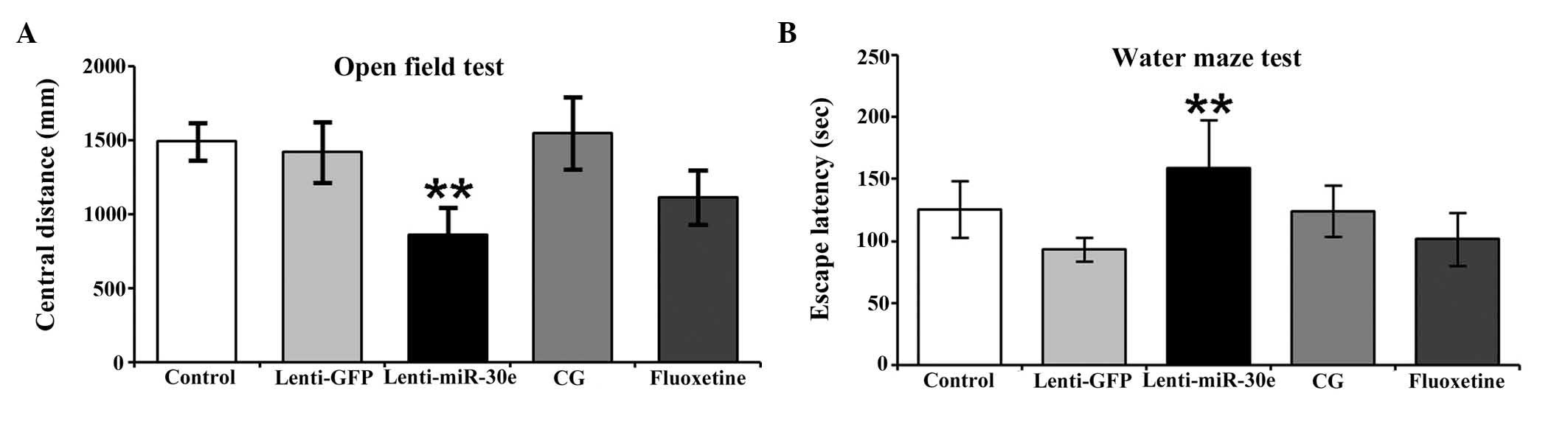
Open field test
The lenti-miR-30e rats exhibited a high degree of
cognitive impairment, as well as signs of anxiety, hyperactivity,
depression and schizophrenia. Administration of fluoxetine
significantly improved the cognitive abilities of the experimental
rats. Notably, administration of CG exerted a more marked
ameliorating effect on cognitive abilities, compared with
fluoxetine. Furthermore, the CG-treated rats exhibited marginally
improved cognitive performance, compared with the control group. A
significant difference in cognitive performance was observed
between the miR-30e group and the control group (P<0.01), and a
significant difference was also observed between the CG group and
the miR-30e group (P<0.01). There was a difference in
performance between the CG group and the fluoxetine group, however,
it was not significant (P>0.05). No significant difference was
observed between the CG group and the control group (P>0.05;
Fig. 2A).
Morris water maze test
The lenti-miR-30e rats exhibited increased signs of
working memory impairment. The group treated with CG exhibited no
significant difference in behavior, compared with the control
group, whereas the rats in the CG group exhibited a marginal
improvement in performance, compared with the rats in the
fluoxetine group. These results suggested that CG robustly
alleviated cognitive impairment in rats exhibiting miR-30e
overexpression. Significant differences were observed between the
miR-30e group and the control group (P<0.01), and between the CG
group and the miR-30e group (P<0.01). No significant difference
was observed between the CG group and the control or fluoxetine
groups (P>0.05; Fig. 2B).
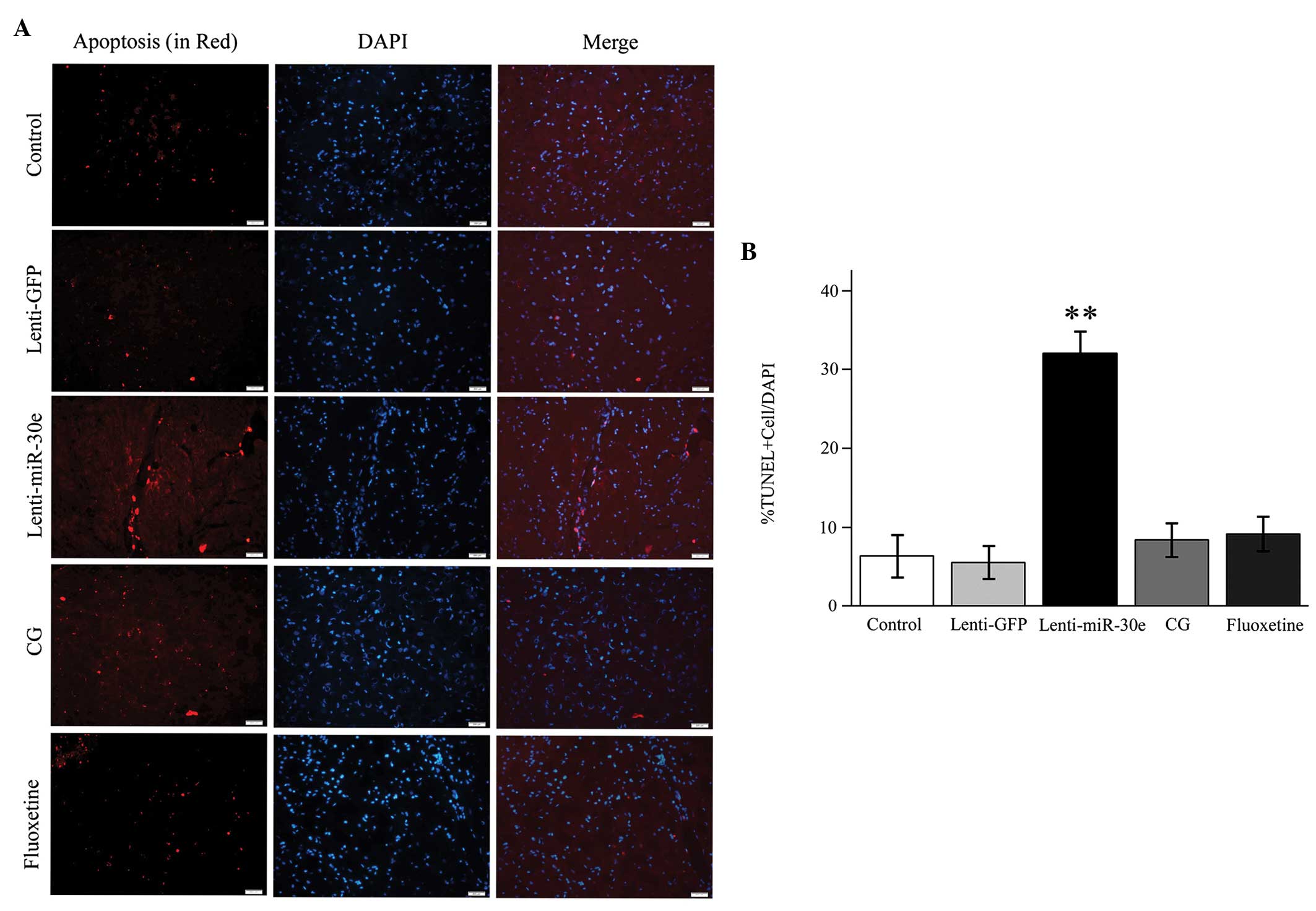
TUNEL assay
In the lenti-miR-30e rats, the overexpression of
miR-30e induced apoptosis of the neuronal cells in the DG of the
hippocampus, whereas treatment with CG significantly inhibited the
apoptotic process. Notably, CG exhibited similar anti-apoptotic
effects to those of fluoxetine (Fig.
3).
Protein expression levels of BCL-2 and
UBC9
The protein expression levels of BCL-2 and UBC9 were
significantly reduced in the lenti-miR-30e rats. Following
treatment with either fluoxetine or CG, the protein expression
levels of BCL-2 and UBC9 returned to normal in the hippocampus, and
were not significantly different from those in the control group.
There was a significant difference in the protein expression levels
between the CG group and the miR-30e group, with higher expression
levels in the former (P<0.01). However, no significant
differences were observed between the CG group and the control or
fluoxetine groups (P>0.05; Fig.
4A).
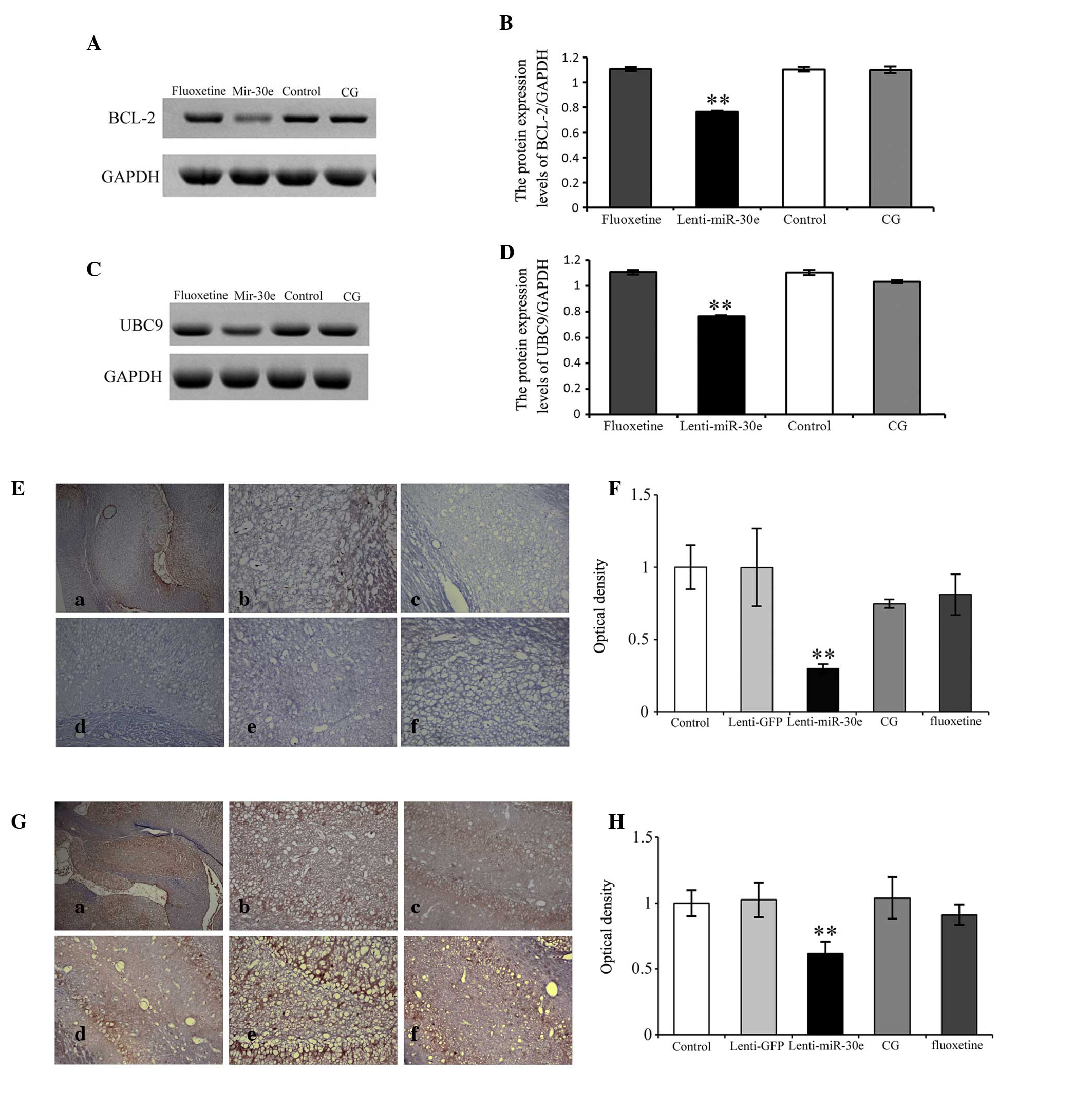 | Figure 4Western blotting to detect the
protein expression levels of (A and B) BCL-2) and (C and D) UBC9 in
the hippocampus of lenti-miR-30e rats. The protein expression
levels were decreased in the lenti-miR-30e rats, compared with the
controls, however there was no difference between the CG and
control groups. Following staining with (E and F) BCL-2 and (G and
H) UBC9, few positively-stained hippocampal neurons were identified
in the lenti-miR-30e rats; however, numerous positively-stained
hippocampal neurons were observed in the CG and control groups, and
staining was dark. (a) Hippocampus image (original magnification,
×4); (b) normal control group (original magnification, ×20); (c)
lenti-GFP group (original magnification, ×20); (d) lenti-miR-30e
group (original magnification, ×20); (e) lenti-miR-30e+fluoxetine
(10 mg/kg, 14 days) group (original magnification, ×20); and (f)
lenti-miR-30e+CG (100 mg/kg, 14 days) group (original
magnification, ×20). Scale bar=50 mm. Data are presented as the
mean ± standard error of the mean. **P<0.01, vs.
control. CG, Cerebralcare Granule®; BCL-2, B-cell
lymphoma-2; UBC9, ubiquitin-conjugating enzyme 9; GFP, green
fluorescent protein. |
BCL-2 and UBC9 immunohistochemical
analysis
The immunohistochemistry results obtained in the
present study were consistent with the results of the western blot
analysis. The protein expression levels of BCL-2 and UBC9 were
significantly reduced in the hippocampus of the lenti-miR-30e rats.
Following treatment with either fluoxetine or CG, the protein
expression levels of BCL-2 and UBC9 in the hippocampus of the
lenti-miR-30e rats returned to normal, and were not significantly
different from those in the control group. There was a significant
difference between the CG group and the miR-30e group, with higher
expression levels in the former (P<0.01). However, there was no
significant difference between the CG group and the control or
fluoxetine groups (P>0.05; Fig.
4B).
Discussion
Mental disorders are disabling diseases, which
frequently afflict individuals throughout their entire life. In
addition, the pathogenetic mechanisms remain to be fully elucidated
and current clinical therapies are only partially effective.
The present study aimed to determine whether miR-30e
is important in the initiation and progression of neuropsychiatric
disorders. The results demonstrated that the overexpression of
miR-30e induced behavioral abnormalities and cognitive impairment
in the rat model, resulting in signs of anxiety, hyperactivity and
schizophrenia. The present study also aimed to assess the clinical
efficacy of CG on mental disorders, and to assess the degree to
which it attenuates cognitive disabilities in the rat model
overexpressing miR-30e. Furthermore, the molecular mechanisms
underlying the therapeutic effects of CG were evaluated. The
overall aim of these investigations was to identify novel
therapeutic strategies for the treatment of mental disorders.
Genome-wide association studies of schizophrenia
(43) have suggested that only a
very few single-nucleotide polymorphisms (SNPs) are located in
exons, and that the majority are detected in introns, suggesting
that gene regulatory mechanisms may be key in the pathogenesis of
schizophrenia. miRNAs represent the predominant gene regulatory
factor in multicellular genomes, and are important in a wide range
of biological processes, including developmental timing, growth
control and differentiation (44,45).
Previous studies (46,47) have suggested that there are
abnormalities in miRNA expression levels in patients with
schizophrenia. For example, abnormal expression levels of DGCR8
exhibits marked correlation with the pathogenesis of DiGeorge's
syndrome, and the protein product of this gene is closely
associated with miRNA processing. Notably, approximately one in
four patients with DiGeorge's syndrome develop schizophrenia
(48). Autopsy investigations have
reported that the expression of miRNAs in certain parts of the
brain in patients with schizophrenia is significantly different
from that in healthy controls (26,49,50).
Studies in molecular genetics have also suggested that genetic
alterations in miRNAs are associated with the onset of
schizophrenia (51,52). Juhila et al (53) investigated miRNA expression in
various brain regions, including the prefrontal cortex, hippocampus
and hypothalamus, and identified inverse correlations between miRNA
and mRNA pathways. In this case, as expression of a miRNA
increased, expression of its target mRNA decreased.
Previous studies have reported that miR-30e has a
relatively lower level of expression in the adult hippocampus,
compared with other tissues, including the immune system, even
lower levels of expression in the adult midbrain and frontal
cortex, and almost no expression in the cerebellum. miRNAs have
been found to possess differential expression regulation in various
brain regions. For example, acute stress increases the expression
levels of let-7a, miR-9 and miR-26a/b in the mouse frontal cortex,
but not in the hippocampus (54).
Previous studies have demonstrated that the depletion of miRNAs in
the cerebral cortex and hippocampus, via genetic inactivation of
Dicer following the onset of forebrain neurogenesis, profoundly
impairs the morphological and proliferative characteristics of
neural stem and progenitor cells. The cytoarchitecture and
self-renewal potential of radial glial (RG) cells located within
the cerebral cortex and the hippocampus are profoundly altered,
thereby causing a significant disruption of the normal development
of the dorsal sub-ventricular zone and the DG. This effect has been
attributed to the high-temperature requirement A serine peptidase 1
(HtrA1) gene product, whose overexpression in the developing
forebrain mimics certain features of the Dicer phenotype (55). miR-30e was identified as a
post-transcriptional negative regulator of HtrA1 by binding to its
3′-untranslated region, and in vivo overexpression of
miR-30e in the Dicer forebrain rescues RG proliferation defects
(56). This suggests that miR-30e
may inhibit neuronal cell proliferation and promote neuronal
apoptosis.
In the present study, a novel rat model of mental
illness was constructed using targeted miRNA gene transfection. The
lenti-miR-30e rats, which exhibited miR-30e overexpression in the
hippocampus, exhibited features of mental disease, including
anxiety, hyperactivity and signs of schizophrenic behavior. The
hippocampus was selected for the site of miR-30e injection, as it
is closely associated with schizophrenia, depression and cognitive
impairment. In 2010, our previous study demonstrated that SNPs in
the miR-30e precursor are associated with schizophrenia (19) and depression (57), and are a clinical indicator of
cognitive ability and P300 latency (57). Therefore, the hippocampus was
considered an ideal target for investigating the role of miR-30e in
schizophrenia. In addition, the expression levels of miR-30e in the
hippocampus of patients with schizophrenia and depression remained
to be elucidated, particularly as living brain tissues cannot be
acquired from patients with mental illness. Furthermore, autopsy is
not reliable, as patients are subject to the effects of long-term
medication prior to mortality. The present study hypothesized that
miR-30e levels are elevated in pathological conditions, including
schizophrenia and depression, and a rat model overexpressing
miR-30e in the hippocampus.
The present study aimed to examine the effects of
miR-30e overexpression on the cognitive ability of rats, which may
provide insight into the role of miR-30e in the pathogenesis and
development of schizophrenia. The present study also aimed to
examine the therapeutic effect of CG on cognitive impairment in
these rats, and determine how it compares to other medicine
intervention in its therapeutic efficacy.
The lenti-miR-30e rats exhibited increased signs of
anxiety, hyperactivity and schizophrenia, resulting in a severe
impairment in cognitive abilities, confirmed using open field and
water maze tests. Treatment of the rat model with CG attenuated the
cognitive impairment to control levels. The present study also
demonstrated that miR-30e overexpression lead to increased neuronal
apoptosis and decreased protein expression levels of BCL-2 and
UBC9, and that treatment with CG increased the expression levels of
these proteins, thereby inhibiting the apoptosis of neuronal cells.
CG had a therapeutic effect similar to fluoxetine. Therefore, CG
may have similar therapeutic effects to dopamine, serotonin and/or
fluoxetine in the treatment of neuropsychiatric disorders.
Furthermore, the natural ingredients in the herbal medicine offer
potential in examining the pharmaceutical properties of herbal
medicines as alternatives in the treatment of mental illness,
although their toxicity requires careful characterization in the
future.
The mechanisms underlying the ameliorative effect of
CG on mental disorders and cognitive impairment may be associated
with its ability to inhibit the apoptosis of neuronal cells, which
may, in part, be attributed to its antioxidant properties. BCL-2 is
an inhibitor of apoptosis. The present study detected a significant
reduction in the protein expression levels of BCL-2 in the
hippocampus of the lenti-miR-30e rats, providing evidence that
miR-30e promoted the apoptotic process in the neuronal cells. These
findings are consistent with previous reports on the regulatory
role of miR-30e on the expression of BCL-2. The protein expression
of BCL-2 protein in the hippocampus of rats in the CG group was
markedly enhanced, compared with that in the lenti-miR-30e group,
resulting in levels similar to those in the control group.
UBC9 is a downstream target of miR-30e. The present
study demonstrated that, in the various groups, the expression of
UBC9 paralleled that of BCL-2, suggesting that miR-30e directly
regulated BCL-2 and UBC9, or that it directly regulated UBC9 and
indirectly regulated BCL-2, in order to affect neuronal
apoptosis.
In conclusion, the lenti-miR-30e rats, induced to
overexpress miR-30e in the DG of the hippocampus, exhibited signs
of cognitive impairment and anxious behavior. CG effectively
alleviated these symptoms, with a therapeutic effect similar to
that of fluoxetine. In addition, miR-30e overexpression induced
neuronal apoptosis, as revealed by a significant reduction in the
protein expression levels of BCL-2 and UBC9, whereas treatment with
CG markedly enhanced the expression levels of these proteins,
resulting in the inhibition of neuronal apoptosis.
Acknowledgments
The authors would like to thank Dr Tianmei Si for
proofreading the manuscript. The present study was supported by the
National Natural Science Foundation of China (grant. nos. 81000583
and 81271482), the China Postdoctoral Science Foundation (grant.
no. 2013M541207), the Clinical Scientific Research Program of Wu
Jieping Medical Funding (grant. no. 320.6750.1252), the Program for
New Century Excellent Talents in University (Mr. Yong Xu), the
Program for the Top Young Academic Leaders of Higher Learning
Institutions of Shanxi (Mr. Yong Xu) and the Doctoral Fund of
Shanxi Medical University (grant. no. 03201010).
References
|
1
|
Caspi A and Moffitt TE: Gene-environment
interactions in psychiatry: Joining forces with neuroscience. Nat
Rev Neurosci. 7:583–590. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuang MT, Bar JL, Stone WS and Faraone
SV: Gene-environment interactions in mental disorders. World
Psychiatry. 3:73–83. 2004.
|
|
3
|
Cross-Disorder Group of the Psychiatric
Genomics Consortium: Identification of risk loci with shared
effects on five major psychiatric disorders: A genome-wide
analysis. Lancet. 381:1371–1379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinez NJ and Gregory RI: MicroRNA gene
regulatory pathways in the establishment and maintenance of ESC
identity. Cell Stem Cell. 7:31–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olde Loohuis NF, Kos A, Martens GJ, Van
Bokhoven H, Nadif Kasri N and Aschrafi A: MicroRNA networks direct
neuronal development and plasticity. Cell Mol Life Sci. 69:89–102.
2012. View Article : Google Scholar :
|
|
9
|
Bravo JA and Dinan TG: MicroRNAs: A novel
therapeutic target for schizophrenia. Curr Pharm Des. 17:176–188.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dwivedi Y: Evidence demonstrating role of
microRNAs in the etiopathology of major depression. J Chem
Neuroanat. 42:142–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forero DA, van der Ven K, Callaerts P and
Del-Favero J: miRNA genes and the brain: Implications for
psychiatric disorders. Hum Mutat. 31:1195–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunsberger JG, Austin DR, Chen G and Manji
HK: MicroRNAs in mental health: From biological underpinnings to
potential therapies. Neuromolecular Med. 11:173–182. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller BH and Wahlestedt C: MicroRNA
dysregulation in psychiatric disease. Brain Res. 1338:89–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siew WH, Tan KL, Babaei MA, Cheah PS and
Ling KH: MicroRNAs and intellectual disability (ID) in Down
syndrome, X-linked ID, and Fragile X syndrome. Front Cell Neurosci.
7(41)2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brinton RD: Estrogen-induced plasticity
from cells to circuits: Predictions for cognitive function. Trends
Pharmacol Sci. 30:212–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Pehrson AL, Waller JA, Dale E,
Sanchez C and Gulinello M: A critical evaluation of the
activity-regulated cytoskeleton-associated protein (Arc/Arg3.1)'s
putative role in regulating dendritic plasticity, cognitive
processes, and mood in animal models of depression. Front Neurosci.
9(279)2015. View Article : Google Scholar
|
|
17
|
Perri R, Nares S, Zhang S, Barros SP and
Offenbacher S: MicroRNA modulation in obesity and periodontitis. J
Dent Res. 91:33–38. 2012. View Article : Google Scholar :
|
|
18
|
Markou A, Sourvinou I, Vorkas PA, Yousef
GM and Lianidou E: Clinical evaluation of microRNA expression
profiling in non small cell lung cancer. Lung Cancer. 81:388–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Li F, Zhang B, Zhang K, Zhang F,
Huang X, Sun N, Ren Y, Sui M and Liu P: MicroRNAs and target site
screening reveals a pre-microRNA-30e variant associated with
schizophrenia. Schizophr Res. 119:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao Y and Lönnerdal B: Beta-catenin/TCF4
transactivates miR-30e during intestinal cell differentiation. Cell
Mol Life Sci. 67:2969–2978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu F, Zhu S, Ding Y, Beck WT and Mo YY:
MicroRNA-mediated regulation of Ubc9 expression in cancer cells.
Clin Cancer Res. 15:1550–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Guan X, Guo F, Zhou J, Chang A,
Sun B, Cai Y, Ma Z, Dai C, Li X, et al: miR-30e reciprocally
regulates the differentiation of adipocytes and osteoblasts by
directly targeting low-density lipoprotein receptor-related protein
6. Cell Death Dis. 4:e8452013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khanna A, Muthusamy S, Liang R, Sarojini H
and Wang E: Gain of survival signaling by down-regulation of three
key miRNAs in brain of calorie-restricted mice. Aging (Albany NY).
3:223–236. 2011.
|
|
24
|
Gardiner E, Beveridge NJ, Wu JQ, Carr V,
Scott RJ, Tooney PA and Cairns MJ: Imprinted DLK1-DIO3 region of
14q32 defines a schizophrenia-associated miRNA signature in
peripheral blood mononuclear cells. Mol Psychiatry. 17:827–840.
2012. View Article : Google Scholar :
|
|
25
|
Mellios N, Huang HS, Baker SP, Galdzicka
M, Ginns E and Akbarian S: Molecular determinants of dysregulated
GABAergic gene expression in the prefrontal cortex of subjects with
schizophrenia. Biol Psychiatry. 65:1006–1014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perkins DO, Jeffries CD, Jarskog LF,
Thomson JM, Woods K, Newman MA, Parker JS, Jin J and Hammond SM:
microRNA expression in the prefrontal cortex of individuals with
schizophrenia and schizoaffective disorder. Genome Biol. 8:R272007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rege SD, Geetha T, Pondugula SR, Zizza CA,
Wernette CM and Babu JR: Noncoding RNAs in neurodegenerative
diseases. ISRN Neurol. 2013(375852)2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banigan MG, Kao PF, Kozubek JA, Winslow
AR, Medina J, Costa J, Schmitt A, Schneider A, Cabral H,
Cagsal-Getkin O, et al: Differential expression of exosomal
microRNAs in prefrontal cortices of schizophrenia and bipolar
disorder patients. PLoS One. 8:e488142013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu XS, Ma ZZ, Wang F, Hu BH, Wang CS, Liu
YY, Zhao XR, An LH, Chang X, Liao FL, et al: The antioxidant
Cerebralcare Granule attenuates cerebral microcirculatory
disturbance during ischemia-reperfusion injury. Shock. 32:201–209.
2009. View Article : Google Scholar
|
|
30
|
Wang F, Hu Q, Chen CH, Xu XS, Zhou CM,
Zhao YF, Hu BH, Chang X, Huang P, Yang L, et al: The protective
effect of Cerebralcare Granule® on brain edema, cerebral
microcirculatory disturbance, and neuron injury in a focal cerebral
ischemia rat model. Microcirculation. 19:260–272. 2012. View Article : Google Scholar
|
|
31
|
Huang P, Zhou CM, Qin-Hu, Liu YY, Hu BH,
Chang X, Zhao XR, Xu XS, Li Q, Wei XH, et al: Cerebralcare
Granule® attenuates blood-brain barrier disruption after
middle cerebral artery occlusion in rats. Exp Neurol. 237:453–463.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun K, Hu Q, Zhou CM, Xu XS, Wang F, Hu
BH, Zhao XY, Chang X, Chen CH, Huang P, et al: Cerebralcare
Granule, a Chinese herb compound preparation, improves cerebral
microcirculatory disorder and hippocampal CA1 neuron injury in
gerbils after ischemia-reperfusion. J Ethnopharmacol. 130:398–406.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong L, Zhang JJ, Sun D and Liu H:
Therapeutic benefit of Yangxue Qingnao Granule on cognitive
impairment induced by chronic cerebral hypoperfusion in rats. Chin
J Integr Med. 17:134–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abordo-Adesida E, Follenzi A, Barcia C,
Sciascia S, Castro MG, Naldini L and Lowenstein PR: Stability of
lentiviral vector-mediated transgene expression in the brain in the
presence of systemic antivector immune responses. Hum Gene Ther.
16:741–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyoshi H, Blömer U, Takahashi M, Gage FH
and Verma IM: Development of a self-inactivating lentivirus vector.
J Virol. 72:8150–8157. 1998.PubMed/NCBI
|
|
36
|
Naldini L, Blömer U, Gage FH, Trono D and
Verma IM: Efficient transfer, integration, and sustained long-term
expression of the transgene in adult rat brains injected with a
lentiviral vector. Proc Natl Acad Sci USA. 93:11382–11388. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zufferey R, Dull T, Mandel RJ, Bukovsky A,
Quiroz D, Naldini L and Trono D: Self-inactivating lentivirus
vector for safe and efficient in vivo gene delivery. J Virol.
72:9873–9880. 1998.PubMed/NCBI
|
|
38
|
Rattiner LM, Davis M, French CT and
Ressler KJ: Brain-derived neurotrophic factor and tyrosine kinase
receptor B involvement in amygdala-dependent fear conditioning. J
Neurosci. 24:4796–4806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heldt SA, Stanek L, Chhatwal JP and
Ressler KJ: Hippocampus-specific deletion of BDNF in adult mice
impairs spatial memory and extinction of aversive memories. Mol
Psychiatry. 12:656–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chenghong Zoology experimental guidance.
Tsinghua University; ISBN: 978-7-302-10763-72005
|
|
41
|
Paxinos G and Franklin KBJ: The mouse
brain in stereotaxic coordinates. San Diego: Academic Press;
2001
|
|
42
|
Morris RGM: Spatial localization does not
require the presence of local cues. Learn Motiv. 12:239–260. 1981.
View Article : Google Scholar
|
|
43
|
Ripke S, O'Dushlaine C, Chambert K, Moran
JL, Kähler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer
M, et al: Genome-wide association analysis identifies 13 new risk
loci for schizophrenia. Nat Genet. 45:1150–1159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nilsen TW: Mechanisms of microRNA-mediated
gene regulation in animal cells. Trends Genet. 23:243–249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Beveridge NJ, Tooney PA, Carroll AP,
Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I and Cairns MJ:
Dysregulation of miRNA 181b in the temporal cortex in
schizophrenia. Hum Mol Genet. 17:1156–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang F, Xu Y, Shugart YY, Yue W, Qi G,
Yuan G, Cheng Z, Yao J, Wang J, Wang G, et al: Converging evidence
implicates the abnormal microRNA system in schizophrenia. Schizophr
Bull. 41:728–735. 2015. View Article : Google Scholar
|
|
48
|
Murphy KC, Jones LA and Owen MJ: High
rates of schizophrenia in adults with velo-cardio-facial syndrome.
Arch Gen Psychiatry. 56:940–945. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Beveridge NJ, Gardiner E, Carroll AP,
Tooney PA and Cairns MJ: Schizophrenia is associated with an
increase in cortical microRNA biogenesis. Mol Psychiatry.
15:1176–1189. 2010. View Article : Google Scholar :
|
|
50
|
Beveridge NJ, Tooney PA, Carroll AP,
Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I and Cairns MJ:
Dysregulation of miRNA 181b in the temporal cortex in
schizophrenia. Hum Mol Genet. 17:1156–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Feng J, Sun G, Yan J, Noltner K, Li W,
Buzin CH, Longmate J, Heston LL, Rossi J and Sommer SS: Evidence
for X-chromosomal schizophrenia associated with microRNA
alterations. PLoS One. 4:e61212009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hansen T, Olsen L, Lindow M, Jakobsen KD,
Ullum H, Jonsson E, Andreassen OA, Djurovic S, Melle I, Agartz I,
et al: Brain expressed microRNAs implicated in schizophrenia
etiology. PLoS One. 2:e8732007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Juhila J, Sipilä T, Icay K, Nicorici D,
Ellonen P, Kallio A, Korpelainen E, Greco D and Hovatta I: MicroRNA
expression profiling reveals miRNA families regulating specific
biological pathways in mouse frontal cortex and hippocampus. PLoS
One. 6:e214952011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rinaldi A, Vincenti S, De Vito F, Bozzoni
I, Oliverio A, Presutti C, Fragapane P and Mele A: Stress induces
region specific alterations in microRNAs expression in mice. Behav
Brain Res. 208:265–269. 2010. View Article : Google Scholar
|
|
55
|
McLoughlin HS, Fineberg SK, Ghosh LL,
Tecedor L and Davidson BL: Dicer is required for proliferation,
viability, migration and differentiation in corticoneurogenesis.
Neuroscience. 223:285–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nigro A, Menon R, Bergamaschi A, Clovis
YM, Baldi A, Ehrmann M, Comi G, De Pietri Tonelli D, Farina C,
Martino G and Muzio L: MiR-30e and miR-181d control radial glia
cell proliferation via HtrA1 modulation. Cell Death Dis.
3:e3602012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu Y, Liu H, Li F, Sun N, Ren Y, Liu Z,
Cao X, Wang Y, Liu P and Zhang K: A polymorphism in the
microRNA-30e precursor associated with major depressive disorder
risk and P300 waveform. J Affect Disord. 127:332–336. 2010.
View Article : Google Scholar : PubMed/NCBI
|