Introduction
White adipose tissue is innervated by sensory nerves
(1–5), however, the roles they exert in this
tissue remain to be fully elucidated (1,2,5). It
may be possible that these sensory innervations inform the central
nervous system of the correct size of fat stored in the peripheral
white adipose tissue. It was previously demonstrated that substance
P (SP) and calcitonin gene-related peptide are expressed in sensory
neurons (4,5).
SP is a conserved 11-amino-acid peptide (6) and is a member of the tachykinin
family of neurotransmitters (7).
Previous studies have defined the role of SP as a pain transmitter,
and it was demonstrated that SP and its specific receptor,
neurokinin 1 receptor (NK-1R), are expressed in nervous tissues
(8,9). However, a burgeoning body of evidence
has revealed that NK-1R is also expressed in a variety of
non-neuronal cell types including endothelial cells (10), monocytes (11), macrophages (11) and adipocytes (12). Therefore, novel roles identified
for SP in non-neuronal cells have been reported, including immune
modulation (13), mobilization of
bone-marrow-derived stem cells (14), wound healing (15,16),
and the regulation of insulin signaling (17).
Adipocyte dysfunction following the onset of insulin
resistance is associated with type 2 diabetes (18). These dysfunctions may contribute to
insulin resistance in the peripheral tissues, including adipose
tissue, through mechanisms including the release of non-esterified
fatty acids, glycerol, proinflammatory cytokines and proteins,
which induce the development of insulin resistance (19–21).
Notably, insulin resistance leads to a decrease in the uptake of
glucose and in the expression level of glucose transporter 4
(GLUT4) in adipose tissue (22,23).
Signaling pathways, which regulate energy
homeostasis, are associated with the development of insulin
resistance. The AMP-activated protein kinase (AMPK) is a key
protein associated with these signaling pathways (24). Indeed, AMPK is dysregulated in
animals and humans with type 2 diabetes, and its pharmacological
activation is one of the therapeutic targets which has been
identified for the treatment of this condition (25). The activation of AMPK following its
phosphorylation on residue Thr-172 occurs when intracellular ATP
levels decrease (26,27). Notably, AMPK promotes the
trans-localization of GLUT4 to the plasma membrane (28) and also increases the expression of
GLUT4 (29,30).
The level of SP varies under pathological
conditions, including type 2 diabetes. Previous studies
demonstrated that the level of SP in the serum from patients with
type 2 diabetes (31), in skin
biopsies from patients with types 1 and 2 diabetes (32), and in heart tissue from patients
with type 1 diabetes (33), is
markedly lower compared with the controls, although a previously
published study contradicted this evidence (34). Therefore, it is possible that the
decreased expression of SP in various different tissues from
patients with type 2 diabetes may be associated with pathological
features, including insulin resistance, through the regulation of
cellular functions in the peripheral tissues, including adipose
tissue. Although a previous study suggested that SP decreases the
insulin-mediated uptake of fatty acids in 3T3-L1 cells (12), the aim of the present study was to
investigate the role of SP in the process of lipid accumulation in
3T3-L1 cells during their differentiation into adipocytes in
response to a high concentration of glucose, under different medium
conditions and using different concentrations of SP.
Materials and methods
Cells and cell culture
The 3T3-L1 preadipocytes were purchased from
American Type Culture Collection (Manassas, VA, USA; cat. no.
CL-173) and the cells were maintained in Dulbecco's modified
Eagle's medium (DMEM; GE Healthcare Life Sciences, Little Chalfont,
UK) with 25 mM glucose, supplemented with 10% fetal bovine calf
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 U/ml penicillin/100 μg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). The cells were incubated at 37°C
in a humidified atmosphere, containing 5% CO2, and their
subcultures were performed at <70% confluence. The cells in
passage numbers P10 to P20 were used for subsequent
experiments.
Differentiation of 3T3-L1 preadipocytes
into adipocytes
The 3T3-L1 preadipocytes were cultured until they
reached 100% confluence under normal culture conditions. At 48 h
following the attainment of confluence, the cells were cultured
with DMEM containing 25 mM glucose and supplemented with 10%
heat-inactivated FBS, 5 μg/ml insulin, 5 μM
dexamethasone and 5 μM rosiglitazone (all from
Sigma-Aldrich, St. Louis, MO, USA) for 48 h. Subsequently, the
cells were incubated for 48 h with DMEM (25 mM glucose),
supplemented with 10% FBS and 5 μg/ml insulin. The medium
was subsequently exchanged with DMEM (25 mM glucose), supplemented
with 10% FBS on every other day for 4 days. If necessary, DMEM
containing a different concentration of glucose (5.5 mM) was used
for the differentiation of 3T3-L1 preadipocytes into
adipocytes.
Use of SP in experiments
SP was purchased from EMD Millipore (San Diego, CA,
USA; cat. no. 05-23-0600), and was prepared with 5% acetic acid
(Sigma-Aldrich). When required, SP was added to the 3T3-L1 cells at
various concentrations whenever the medium was exchanged.
5-Bromo-2′-deoxyuridine (BrdU)
incorporation assay
The 3T3-L1 cells were seeded onto fibronectin-coated
cover-slips (1 μg/ml) in 24-well plates at a density of
4×103 cells/well. The cells were initially incubated for
24 h with normal culture medium, prior to an incubation of 18–24 h
duration under conditions of serum starvation. The cells were
subsequently treated with SP for 48 h. For the final 6 h of the
incubation period, 20 μM BrdU (Sigma-Aldrich) was added to
the cells. The cells were subsequently prepared for the
immunocytochemical analysis using BrdU by fixation in 4%
paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA)
in phosphate-buffered saline (PBS) for 10 min on ice. The fixed
cells were incubated with 2 N HCl for 15 min at room temperature
and washed with PBS vigorously. Following permeabilization with
0.2% Triton X-100 (Affymetrix, Inc., Santa Clara, CA, USA), the
cells were treated with blocking solution (5% non-fat milk in PBS
with 0.1% Triton X-100) for 30 min at room temperature.
Subsequently, the cells were incubated with primary mouse
monoclonal anti-BrdU antibody (1:20, cat. no. #11-170-376-001;
Roche Diagnostics GmbH, Mannheim, Germany) for 1.5 h at room
temperature. Following three washes with 1% non-fat milk in PBS
with 0.1% Triton X-100, the secondary antibody, Invitrogen
Alexa-488 anti-mouse immunoglobulin G1 (Thermo Fisher Scientific,
Inc.), was added, and the cells were incubated for a further 45 min
at room temperature. Finally, the samples were mounted using
Invitrogen ProLong® Gold Antifade mounting solution with
4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific, Inc.), and
left to dry overnight prior to observation. Images were captured
using a fluorescence microscope (DMI4000; Leica, Solms, Germany),
and the total number of cells and BrdU-positive cells were
counted.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
SP (0, 10, 100 or 300 nM) was added to the 3T3-L1
cells on the initial day of differentiation, and the total RNA was
extracted from the cells on day 2 following the induction of
adipogenesis using the Invitrogen TRIzol™ reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Aliquots of 5 μg total RNA were used for single-strand cDNA
synthesis using the Invitrogen Superscript First-Strand cDNA
Synthesis system (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RT-qPCR was performed using the Invitrogen
Power SYBR Green PCR Master mix (Thermo Fisher Scientific, Inc.).
The ribosomal protein 36B4 gene from the mouse was used as an
endogenous control. The following primers were used to detect the
expression of peroxisome proliferator-activated receptor-γ
(PPAR-γ), adipocyte protein 2 (aP2) and 36B4 protein: PPAR-γ,
sense: 5′-CGC TGA TGC ACT GCC TAT GA-3′ and antisense: 5′-AGA CCT
CCA CAG AGC TGA TTCC-3′; aP2, sense: 5′-CAT GGC CAA GCC CAA CAT-3′
and antisense: 5′-CGC CAA GTT TGA AGG AAA TC-3′; 36B4, sense:
5′-GAA CAT CTC CCC CTT CTC CTT-3′ and antisense: 5′-GCA GGG CCT GCT
CTG TGAT-3′.
Oil Red O staining
To assess adipogenesis in the 3T3-L1 cells, Oil Red
O staining was performed on differentiated cells. Oil Red O
solution (0.3%; Sigma-Aldrich) was prepared by dissolving Oil Red O
in 60% isopropanol (Daejung Chemicals & Metals, Co., Ltd.,
Shiheung, Korea). The differentiated cells were fixed in 4%
paraformaldehyde (Electron Microscopy Sciences) in PBS for 10 min
at room temperature, and subsequently washed with PBS. The fixed
cells were incubated with Oil Red O solution for 30 min at room
temperature. Images of the stained cells were captured using a
light microscope (DMI4000; Leica, Solms, Germany). To further
quantify the Oil-Red O-stained lipid drops, the stained cells were
rinsed twice with 60% isopropanol and subsequently dried. The
stains were eluted with 1 ml isopropanol for 10 min at room
temperature and the optical density was measured at 490 nm using an
absorbance plate reader (Spectramax190; Molecular Devices; Thermo
Fisher Scientific, Inc.).
Western blotting
The cells were rinsed twice with ice-cold PBS and
lysed with 2X SDS loading buffer [100 mM Tris-HCl (pH 6.8), 4%
(w/v) SDS, 0.2% (w/v) bromophenol blue, 20% glycerol and 200 mM
β-mercaptoethanol]. Subsequently, the cell lysates were denatured
at 92°C for 10 min. The denatured protein samples were separated
using 10% SDS polyacrylamide gel electrophoresis and transferred
onto nitrocellulose membranes (Whatman, Dassel, Germany. Following
blocking with 5% non-fat milk in 20 mM Tris buffer, containing 0.1%
Tween-20 (TBS-T), the membranes were incubated with primary
antibody diluted with TBS-T buffer, including 5% non-fat milk,
overnight at 4°C. The following primary antibodies were used:
Rabbit monoclonal anti-phosphorylated AMPK (1:1,000, cat. no.
#2535; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
monoclonal anti-phosphorylated Akt (1:4,000, cat. no. #4060; Cell
Signaling Technology, Inc.,), rabbit polyclonal anti-GLUT4 (1:500,
cat. no. ab65976; Abcam, Cambridge, UK) and mouse monoclonal
anti-α-tubulin antibody (1:5,000, cat. no. T5618; Sigma-Aldrich).
Subsequently, the membranes were incubated with goat anti-rabbit
(1,5,000; cat. no. 7074; Cell Signaling Technology, Inc.) or goat
anti-mouse IgG (1:10,000; cat. no. 170-6516; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) horseradish peroxidase-conjugated
secondary antibodies at room temperature for 30 min. The target
proteins were visualized using an enhanced chemiluminescence
detection kit (EMD Millipore). The band densities were measured
using ImageJ software (NIH, Bethesda, MD, USA). If required, the
stripping of the membranes was performed using Restore™ Western
Blot Stripping buffer (Thermo Fisher Scientific, Inc.) for 15 min
at room temperature. The membranes were reblotted using an antibody
raised against α-tubulin.
Statistical analysis
The data are expressed as the mean ± standard
deviation or the mean ± standard error of the mean. An unpaired
Student's t-test was used to evaluate differences between the two
groups. All statistical analyses were performed using GraphPad
Prism version 5.01 software (GraphPad Software, Inc., San Diego,
CA, USA; http://www.graphpad.com). P<0.05 was
considered to indicate a statistically significant difference.
Results
SP causes no effect on the proliferation
of the 3T3-L1 preadipocytes
A previous study demonstrated that SP increases the
cellular proliferation of mesenteric preadipocytes (35). Therefore, whether or not SP
affected the proliferation of the 3T3-L1 preadipocytes was examined
using a BrdU incorporation assay. The 3T3-L1 preadipocytes were
revealed to express NK-1R, which is a receptor of SP (data not
shown) (12). As shown in Fig. 1, SP caused no effect on the
cellular proliferation of 3T3-L1 cells, irrespective of the
concentration of SP.
 | Figure 1Cellular proliferation of 3T3-L1
preadipocytes treated with SP. (A) BrdU incorporation assays were
performed to examine whether SP affected the proliferation of
3T3-L1 preadipocytes. The 3T3-L1 preadipocytes were treated with
various concentrations of SP (0, 0.1, 1, 10, 100, 300 or 600 nM;
denoted in Fig. 1A as SP 0, SP
0.1, SP 1, SP 10, SP 100, SP 300 and SP 600, respectively). The
BrdU staining is in green and nuclei are illustrated by the
staining in blue. (B) Graph illustrating the quantitative analysis
of the data (expressed as the mean ± standard deviation). SP,
substance P; BrdU, bromodeoxyuridine; DAPI,
4′,6-diamidino-2-phenylindole. |
SP causes no af fect on the dif
ferentiation of the 3T3-L1 preadipocytes
Whether SP regulated the differentiation of 3T3-L1
preadipocytes into adipocytes was subsequently examined. The 3T3-L1
cells were incubated with the differentiation medium, which
included 10% FBS, 5 μg/ml insulin, 5 μM dexamethasone
and 5 μM rosiglitazone (36), for 2 days. SP failed to affect the
expression level of PPAR-γ or aP2 (Fig. 2), which were previously used as
markers for differentiated adipocytes (37). Therefore, it is possible that SP
does not promote the differentiation of 3T3-L1 cells into
adipocytes.
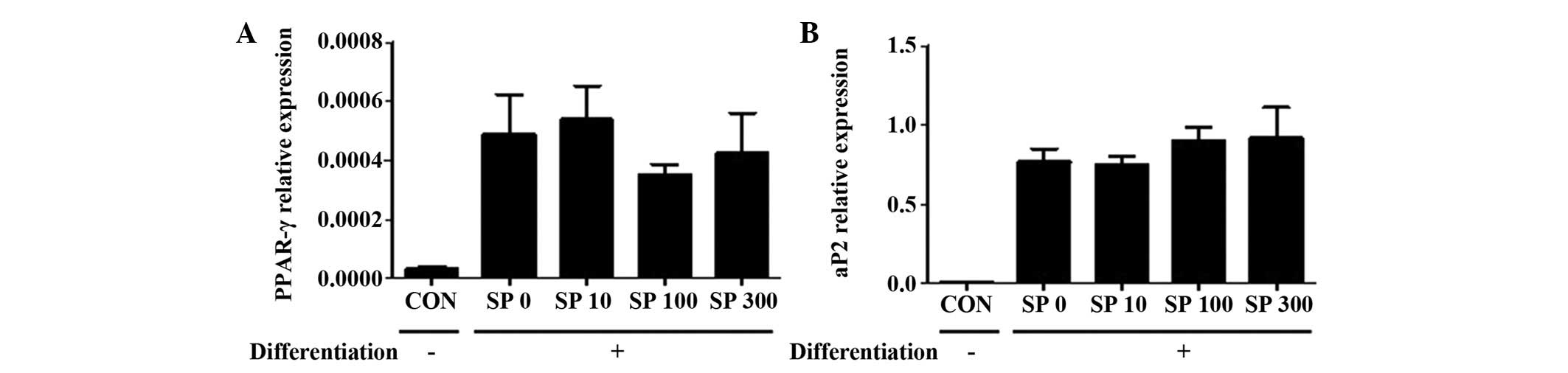 | Figure 2Effect of SP on the expression levels
of PPAR-γ and aP2 in the 3T3-L1 cells. Reverse
transcription-quantitative polymerase chain reaction was performed
to observe the expression levels of (A) PPAR-γ and (B) aP2 in
3T3-L1 cells, which were treated with different concentrations of
SP (0, 10, 100 or 300 nM; illustrated by the labels SP 0, SP 10, SP
100 and SP 300, respectively) under the differentiation medium
conditions for 2 days. Ribosomal protein 36B4 gene was used as an
internal control. Two independent experiments were performed and
the quantitative results are shown as the mean ± standard error of
the mean. aP2, adipocyte protein 2; CON, control; PPAR-γ,
peroxisome proliferator-activated receptor-γ; SP, substance P. |
SP upregulates the accumulation of lipids
in the 3T3-L1 preadipocytes
Although SP failed to promote the differentiation of
the 3T3-L1 cells, it was hypothesized that SP may affect the
accumulation of lipids in these cells. Therefore, the effects of SP
on the accumulation of lipids in the 3T3-L1 adipocytes were
analyzed using Oil Red O staining. SP was added to the 3T3-L1 cells
every other day during the differentiation of the cells into
adipocytes. Compared with the undifferentiated control cells, the
accumulation of lipids increased markedly in the differentiated
cells without SP treatment (Figs. 3A
and B). Notably, the treatment with SP increased the
accumulation of lipids in the 3T3-L1 adipocytes by ~0.3-fold
compared with the untreated differentiated 3T3-L1 cells (Figs. 3A and B), even though SP failed to
promote the differentiation of 3T3-L1 cells into adipocytes
(Fig. 2). In addition, an
SP-mediated increase in the accumulation of lipids was observed in
the differentiated 3T3-L1 adipocytes, which were cultured with
medium, containing a high concentration of glucose (25 mM),
however, not in the cells which were cultured in medium containing
a normal concentration of glucose (5 mM; Fig. 3C). These results suggested that SP
may increase the accumulation of lipids in adipocytes in a manner
which is dependent on the concentration of glucose.
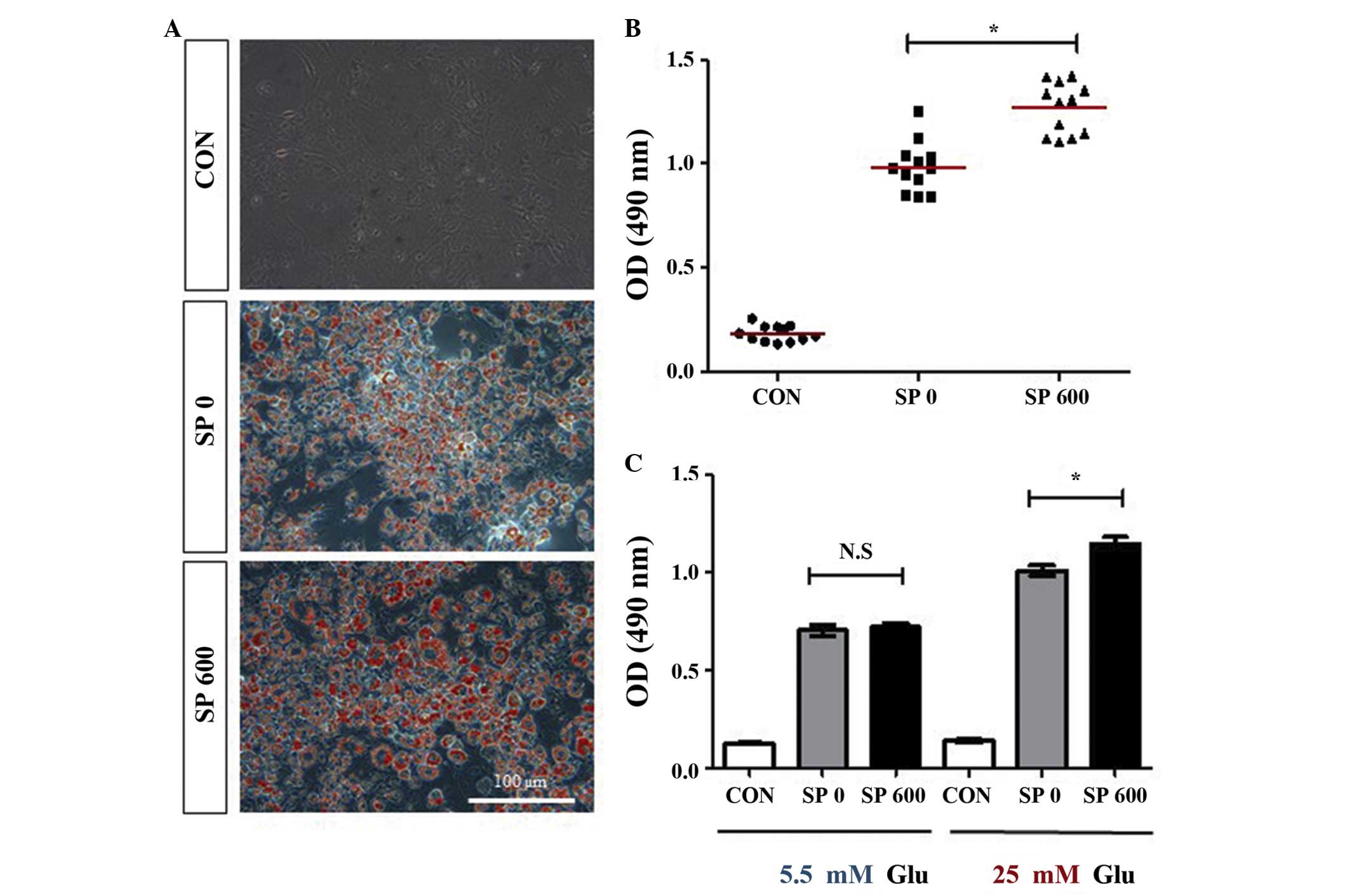 | Figure 3Effect of SP on lipid accumulation in
the 3T3-L1 adipocytes. (A and B) The 3T3-L1 cells were cultured in
the absence or presence of 600 nM SP (SP 0 or SP 600) under the
differentiation medium conditions for 8 days. The accumulation of
lipids was analyzed using Oil Red O staining of the 3T3-L1
adipocytes maintained in the presence of a high concentration of
glucose (25 mM). (A) Representative images are shown (scale bar,
100 μm), and (B) illustrates the quantification of the
results (the mean values are indicated by the red horizontal bars;
*P<0.0001, compared with CON). (C) The accumulation
of lipids was analyzed by Oil Red O staining of the 3T3-L1
adipocytes, which had differentiated in the absence or presence of
600 nM SP (SP 0 or SP 600) under the differentiation medium
conditions, including the presence of either 5 or 25 mM Glu, for 8
days. Three independent experiments were performed, and the
quantitative results are shown as the mean ± standard error of mean
[*P<0.003, compared with SP 0; N.S, not significant
(P>0.6)]. Undifferentiated 3T3-L1 cells were used as the control
in all the experiments. CON, control; SP, substance P; OD, optical
density; Glu, glucose. |
SP regulates the activity of AMPK and
Akt, and the expression levels of GLUT4
It is well known that AMPK is a key regulator in the
metabolism of glucose and fatty acids in various cell types,
including adipocytes (27,38). Therefore, whether SP regulated the
activity of AMPK in the 3T3-L1 cells was examined. Indeed, SP was
revealed to induce the activation of AMPK in a dose-dependent
manner (Fig. 4A). Notably,
however, the activity of Akt was downregulated by SP (Fig. 4B). These results are in very good
agreement with a previous report, which demonstrated that the
regulation of AMPK activity is associated with the regulation of
Akt activity (39). It is also
known that AMPK promotes the translocalization of GLUT4 to the
plasma membrane (28), and
furthermore, that AMPK increases the expression level of GLUT4
(29,30). It is noteworthy that SP increased
the expression level of GLUT4 in 3T3-L1 cells (Fig. 4C). Therefore, these results
suggested that SP may modulate glucose uptake in 3T3-L1 adipocytes,
and that this is associated with the activity of AMPK.
 | Figure 4Effect of SP on the protein
expression levels of p-AMPK, p-Akt and GLUT4 in the 3T3-L1
adipocytes. (A–C) The 3T3-L1 adipocytes were treated with various
concentrations of SP (0, 100, 300 or 600 nM; denoted by the labels
SP 0, SP 100, SP 300 and SP 600, respectively). Western blotting
was performed to observe the phosphorylation levels of (A) p-AMPK
and (B) p-Akt, and (C) the protein expression level of GLUT4.
α-Tubulin was used as an internal control. The numbers underlying
the gel in (C) indicate the protein expression values relative to
the control (SP 10). p-, phosphorylated; AMPK, AMP-activated
protein kinase; GLUT4, glucose transporter 4; SP, substance P. |
Discussion
The present study has demonstrated the ability of
the neurotransmitter SP to increase the accumulation of lipids in
3T3-L1 preadipocytes in the presence of a high concentration of
glucose, although not under normal glucose conditions. This
increase in the accumulation of lipids was associated with an
SP-mediated increase in the expression level of GLUT4 following the
activation of AMPK.
AMPK exerts an essential role in the regulation of
glucose uptake by adipocytes and muscle cells (30,40,41).
A previous report demonstrated that the activation of AMPK
increases glucose uptake, upregulates the expression of GLUT4 in
3T3-L1 adipocytes, and that the activation of AMPK is independent
of the insulin receptor-mediated signaling pathway (41). The present study also suggested
that the SP-mediated activation of AMPK increased glucose uptake by
means of an increased expression of GLUT4 in the 3T3-L1 adipocytes.
Although it remains to be fully elucidated whether insulin
signaling functions in association with SP in order to increase the
accumulation of lipids in 3T3-L1 adipocytes (the differentiation
medium used in this study contained insulin), SP was able to induce
this effect only under high glucose conditions. Notably, SP
decreased the activation of Akt in the adipocytes. Akt activity is
required for the differentiation of mouse embryonic fibroblasts and
3T3-L1 cells into adipocytes (42,43),
and the presence of the constitutively active mutation of Akt is
sufficient to induce the differentiation of the 3T3-L1 cells
(44). However, it is also known
that the insulin-mediated Akt signaling pathway is not a major
pathway for the induction of glucose uptake by adipocytes,
according to a previous report (41). Therefore, Akt was not be expected
to be involved in the SP-mediated accumulation of lipids following
glucose uptake. In the present study, the SP-mediated increases
observed in the accumulation of lipids and in the expression level
of GLUT4 may occur via AMPK activation, as the exercise-mediated
activation of AMPK was revealed to increase glucose uptake,
following the intracellular translocation of GLUT4 to the plasma
membrane (45,46).
Defects in the innervation of adipose tissues impair
the formation of blood vessels, damage the architecture of adipose
tissues and reduce the insulin-sensitivity, and the metabolism of
adipocytes (47). The defects in
innervation also include defects in the sensory neurons that
express SP, which may be involved in the regulation of cellular
functions in the adipose tissues. The present study suggested that
SP may rescue insulin-sensitivity and glucose tolerance in the
adipose tissue of patients with diabetes through the AMPK signaling
pathway. In addition, SP may be useful for the therapeutic
treatment of diabetes in order to control insulin resistance.
Acknowledgments
The present study was supported by the Korean Health
Technology R&D Project (Ministry of Health and Welfare,
Republic of Korea; no. HI13C1479), the Basic Science Research
Program through the National Research Foundation of Korea funded by
the Ministry of Education (no. NRF-2012R1A1A2042265) and the Bio
& Medical Technology Development Program of the NRF funded by
the Ministry of Science, ICT & Future Planning (no.
NRF-2012M3A9C6050485).
References
|
1
|
Bartness TJ and Bamshad M: Innervation of
mammalian white adipose tissue: Implications for the regulation of
total body fat. Am J Physiol. 275:R1399–R1411. 1998.PubMed/NCBI
|
|
2
|
Bartness TJ and Song CK: Thematic review
series: Adipocyte biology. Sympathetic and sensory innervation of
white adipose tissue. J Lipid Res. 48:1655–1672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fishman RB and Dark J: Sensory innervation
of white adipose tissue. Am J Physiol. 253:R942–R944.
1987.PubMed/NCBI
|
|
4
|
Giordano A, Morroni M, Santone G, Marchesi
GF and Cinti S: Tyrosine hydroxylase, neuropeptide Y, substance P,
calcitonin gene-related peptide and vasoactive intestinal peptide
in nerves of rat periovarian adipose tissue: An immunohistochemical
and ultrastructural investigation. J Neurocytol. 25:125–136. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi H and Bartness TJ: White adipose
tissue sensory nerve denervation mimics lipectomy-induced
compensatory increases in adiposity. Am J Physiol Regul Integr Comp
Physiol. 289:R514–R520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nicoll RA, Schenker C and Leeman SE:
Substance P as a transmitter candidate. Annu Rev Neurosci.
3:227–268. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maggi CA, Patacchini R, Rovero P and
Giachetti A: Tachykinin receptors and tachykinin receptor
antagonists. J Auton Pharmacol. 13:23–93. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantyh PW: Neurobiology of substance P and
the NK1 receptor. J Clin Psychiatry. 63(Suppl 11): 6–10. 2002.
|
|
9
|
De Felipe C, Herrero JF, O'Brien JA,
Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F and
Hunt SP: Altered nociception, analgesia and aggression in mice
lacking the receptor for substance P. Nature. 392:394–397. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quinlan KL, Song IS, Bunnett NW, Letran E,
Steinhoff M, Harten B, Olerud JE, Armstrong CA, Wright Caughman S
and Ansel JC: Neuropeptide regulation of human dermal microvascular
endothelial cell ICAM-1 expression and function. Am J Physiol.
275:C1580–C1590. 1998.PubMed/NCBI
|
|
11
|
Ho WZ, Lai JP, Zhu XH, Uvaydova M and
Douglas SD: Human monocytes and macrophages express substance P and
neurokinin-1 receptor. J Immunol. 159:5654–5660. 1997.
|
|
12
|
Miegueu P, St-Pierre DH, Lapointe M,
Poursharifi P, Lu H, Gupta A and Cianflone K: Substance P decreases
fat storage and increases adipocytokine production in 3T3-L1
adipocytes. Am J Physiol Gastrointest Liver Physiol. 304:G420–G427.
2013. View Article : Google Scholar
|
|
13
|
Jiang MH, Lim JE, Chi GF, Ahn W, Zhang M,
Chung E and Son Y: Substance P reduces apoptotic cell death
possibly by modulating the immune response at the early stage after
spinal cord injury. Neuroreport. 24:846–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn
W, Jiang MH, Kim JC and Son Y: A new role of substance P as an
injury-inducible messenger for mobilization of CD29(+) stromal-like
cells. Nat Med. 15:425–435. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kant V, Gopal A and Kumar D, Bag S, Kurade
NP, Kumar A, Tandan SK and Kumar D: Topically applied substance P
enhanced healing of open excision wound in rats. Eur J Pharmacol.
715:345–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Delgado AV, McManus AT and Chambers JP:
Exogenous administration of Substance P enhances wound healing in a
novel skin-injury model. Exp Biol Med (Maywood). 230:271–280.
2005.
|
|
17
|
Karagiannides I, Stavrakis D, Bakirtzi K,
Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, Bowe C, Bugni JM, Nuño
M, Lu B, et al: Substance P (SP)-neurokinin-1 receptor (NK-1R)
alters adipose tissue responses to high-fat diet and insulin
action. Endocrinology. 152:2197–2205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kahn SE, Hull RL and Utzschneider KM:
Mechanisms linking obesity to insulin resistance and type 2
diabetes. Nature. 444:840–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wellen KE and Hotamisligil GS:
Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scherer PE: Adipose tissue: From lipid
storage compartment to endocrine organ. Diabetes. 55:1537–1545.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koranyi L, James D, Mueckler M and Permutt
MA: Glucose transporter levels in spontaneously obese (db/db)
insulin-resistant mice. J Clin Invest. 85:962–967. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garvey WT, Maianu L, Huecksteadt TP,
Birnbaum MJ, Molina JM and Ciaraldi TP: Pretranslational
suppression of a glucose transporter protein causes insulin
resistance in adipocytes from patients with non-insulin-dependent
diabetes mellitus and obesity. J Clin Invest. 87:1072–1081. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coughlan KA, Valentine RJ, Ruderman NB and
Saha AK: AMPK activation: A therapeutic target for type 2 diabetes?
Diabetes Metab Syndr Obes. 7:241–253. 2014.PubMed/NCBI
|
|
25
|
Ruderman N and Prentki M: AMP kinase and
malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat Rev
Drug Discov. 3:340–351. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Motoshima H, Mahadev K, Stalker TJ,
Scalia R and Goldstein BJ: Involvement of AMP-activated protein
kinase in glucose uptake stimulated by the globular domain of
adiponectin in primary rat adipocytes. Diabetes. 52:1355–1363.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bolsoni-Lopes A, Festuccia WT, Chimin P,
Farias TS, Torres-Leal FL, Cruz MM, Andrade PB, Hirabara SM, Lima
FB and Alonso-Vale MI: Palmitoleic acid (n-7) increases white
adipocytes GLUT4 content and glucose uptake in association with
AMPK activation. Lipids Health Dis. 13(199)2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richter EA and Hargreaves M: Exercise,
GLUT4, and skeletal muscle glucose uptake. Physiol Rev.
93:993–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang LH, Zhou SX, Li RC, Zheng LR, Zhu JH,
Hu SJ and Sun YL: Serum levels of calcitonin gene-related peptide
and substance P are decreased in patients with diabetes mellitus
and coronary artery disease. J Int Med Res. 40:134–140. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lindberger M, Schröder HD, Schultzberg M,
Kristensson K, Persson A, Ostman J and Link H: Nerve fibre studies
in skin biopsies in peripheral neuropathies. I. Immunohistochemical
analysis of neuropeptides in diabetes mellitus. J Neurol Sci.
93:289–296. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song JX, Wang LH, Yao L, Xu C, Wei ZH and
Zheng LR: Impaired transient receptor potential vanilloid 1 in
streptozotocin-induced diabetic hearts. Int J Cardiol. 134:290–292.
2009. View Article : Google Scholar
|
|
34
|
Fu J, Liu B, Liu P, Liu L, Li G, Wu B and
Liu X: Substance P is associated with the development of obesity,
chronic inflammation and type 2 diabetes mellitus. Exp Clin
Endocrinol Diabetes. 119:177–181. 2011. View Article : Google Scholar
|
|
35
|
Gross K, Karagiannides I, Thomou T, Koon
HW, Bowe C, Kim H, Giorgadze N, Tchkonia T, Pirtskhalava T,
Kirkland JL, et al: Substance P promotes expansion of human
mesenteric preadipocytes through proliferative and antiapoptotic
pathways. Am J Physiol Gastrointest Liver Physiol. 296:G1012–G1019.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albrektsen T, Frederiksen KS, Holmes WE,
Boel E, Taylor K and Fleckner J: Novel genes regulated by the
insulin sensitizer rosiglitazone during adipocyte differentiation.
Diabetes. 51:1042–1051. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosen ED, Walkey CJ, Puigserver P and
Spiegelman BM: Transcriptional regulation of adipogenesis. Genes
Dev. 14:1293–1307. 2000.PubMed/NCBI
|
|
38
|
Towler MC and Hardie DG: AMP-activated
protein kinase in metabolic control and insulin signaling. Circ
Res. 100:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hahn-Windgassen A, Nogueira V, Chen CC,
Skeen JE, Sonenberg N and Hay N: Akt activates the mammalian target
of rapamycin by regulating cellular ATP level and AMPK activity. J
Biol Chem. 280:32081–32089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee WH, Lin RJ, Lin SY, Chen YC, Lin HM
and Liang YC: Osthole enhances glucose uptake through activation of
AMP-activated protein kinase in skeletal muscle cells. J Agric Food
Chem. 59:12874–12881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen Y, Honma N, Kobayashi K, Jia LN,
Hosono T, Shindo K, Ariga T and Seki T: Cinnamon extract enhances
glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing
LKB1-AMP-activated protein kinase signaling. PLoS One.
9:e878942014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu J and Liao K: Protein kinase B/AKT 1
plays a pivotal role in insulin-like growth factor-1 receptor
signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem.
279:35914–35922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen
A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman
KG, et al: Dwarfism, impaired skin development, skeletal muscle
atrophy, delayed bone development, and impeded adipogenesis in mice
lacking Akt1 and Akt2. Genes Dev. 17:1352–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kohn AD, Summers SA, Birnbaum MJ and Roth
RA: Expression of a constitutively active Akt Ser/Thr kinase in
3T3-L1 adipocytes stimulates glucose uptake and glucose transporter
4 translocation. J Biol Chem. 271:31372–31378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shepherd PR and Kahn BB: Glucose
transporters and insulin action - implications for insulin
resistance and diabetes mellitus. N Engl J Med. 341:248–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hayashi T, Hirshman MF, Kurth EJ, Winder
WW and Goodyear LJ: Evidence for 5′ AMP-activated protein kinase
mediation of the effect of muscle contraction on glucose transport.
Diabetes. 47:1369–1373. 1998.PubMed/NCBI
|
|
47
|
Ruschke K, Ebelt H, Klöting N, Boettger T,
Raum K, Blüher M and Braun T: Defective peripheral nerve
development is linked to abnormal architecture and metabolic
activity of adipose tissue in Nscl-2 mutant mice. PLoS One.
4:e55162009. View Article : Google Scholar : PubMed/NCBI
|


















