Introduction
Adult mesenchymal stem cells (MSCs) can be used as
an innovative tool in cell-based therapy for degenerative
disorders, chronic inflammatory and autoimmune diseases, and
allograft rejection. MSCs have been isolated from several different
tissues, including bone marrow, adipose, neuronal, amniotic,
placental and Wharton's jelly of the umbilical cord (1). Self-regeneration and multi-lineage
potential for differentiation are essential characteristics of MSCs
(2). The low expression levels of
co-stimulatory molecules, including CD80, CD86 and CD40, is another
important feature of MSCs. The fourth important feature is that
MSCs inhibit the activation, proliferation and function of immune
cells, including T-cells, B-cells, natural killer-cells and
antigen-presenting cells (3).
These features render MSCSs an attractive cell-based therapeutic
tool for developmental defects, degenerative diseases and
mesodermal tissue injury, including bone, cartilage and muscle
injury (4–11). However, the results of previous
clinical trials investigating MSCs have not been encouraging, and
one explanation for this is the recognition of MSCs in vivo
by the host immune system, leading to injury and a marked reduction
in activity (12,13).
Toll-like receptors (TLRs) are a family of
pathogen-associated pattern recognition receptors, which are
important in the induction of effective immune responses. The
conserved pathogen-derived components and endogenous ligands, also
termed 'danger signals' activate TLRs (14). Distinct microbial products from
bacteria, viruses and fungi are recognized by 11 TLR subfamilies in
human cells. Among these, TLR9 recognizes CpG-DNA from bacteria and
DNA viruses (15). MSCs express
TLRs and elicit a number of biological functions in MSCs.
Activation of TLR3 and TLR4 in MSCs isolated from adipose tissue
increases osteogenic differentiation without impairing the
immunogenic and immunosuppressive properties of MSCs (16). In addition, the migration of MSCs
increases under activation by TLR8 and TLR9 (17).
At present, the sourcing of MSCs for clinical trials
relies primarily on bone marrow, which is inherently limited by the
invasive collection procedure for bone marrow MSCs (BMMSCs), and by
their reduced self-renewal and proliferative abilities (18). Clinical trials have demonstrated
the safe and beneficial application of UCMSC treatment in graft,
vs. host-disease and systemic lupus erythematosus (2,19,20).
In the present study MSCs were isolated from umbilical tissue and
stimulated with the TLR9 agonist, CpG-oligodeoxynucleotide
(CpG-ODN), in order to determine whether activation of the TLR9
pathway affects the immune status and biological functions of
UCMSCs. Co-culturing UCMSCs with peripheral blood leukocytes (PBLs)
was used to measure the proliferation of PBLs following TLR9
activation. Flow cytometry was conducted to determine whether
activation of the TLR9 pathway could increase the expression of
CD80 and CD86. Antibody array and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) were
performed to assay the secretion of cytokines in the supernatant,
and to determine the mRNA expression levels of immune-associated
molecules in the presence of a TLR9 agonist.
Materials and methods
Isolation and culture of human
UCMSCs
UCMSCs were purchased from the Sichuan Umbilical
Cord Blood Stem Cell Bank (Chengdu, China). The cells were cultured
in an incubator at 37°C, with 5% CO2 and saturated humid
air, and the medium was replaced three times each week. Once the
cell density exceeded 70%, TrypLE Express (Gibco-BRL, Grand Island,
N Y, USA) was used to digest the cells, following which the cells
were subcultured. The present study was approved by the ethics
committee of West China Hospital, Sichuan University (Chengdu,
China).
Immunofluorescence assay
The cells were seeded onto coverslips and fixed with
neutral-buffered formalin for 15 min. The cells were then
permeabilized in 0.1% Triton X-100 for 10 min and incubated with
monoclonal antibodies against stage-specific embryonic antigen 4
(SSEA-4; cat. no. Ab16287; Abcam Cambridge, UK). Immunofluorescence
analysis was then performed and the immunostained cells were
visualized using a Bio-Rad A1S1 laser confocal microscope (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Stimulation of MSCs using TLR9
agonists
The TLR9 reagent, CpG (Hycult Biotech, Inc.,
Plymouth Meeting, PA, USA), was dissolved in DMSO at a storage
concentration of 25 mg/ml. This was added to the UCMSC culture
medium at 5 µg/ml, the final stimulation concentration,
following which the UCMSCs were then seeded into a 6-well plate at
1.5×105 concentration in 2 ml of the medium.
Co-cultivation with peripheral blood
leukocytes
Following the provision of informed consent, human
peripheral blood leukocytes (PBLs) were acquired from two healthy
donors (aged 25 and 26 years-old; male) and were labeled with
carboxyfluorescein succinimidyl ester. The UCMSCs were co-cultured
with the labeled PBLs (1:106) for 72 h at 37°C, and cells were
collected to detect the proliferation of PBLs using
fluorescence-activated cell sorting (FACS; FC500 Cytomics; Beckman
Coulter, Inc., Brea, CA, USA).
Flow cytometric analysis
To detect the surface markers of the MSCs, CD80,
CD86, HLA-E, CD90, CD59 and CD29 (eBioscience, San Diego, CA, USA)
were used. The UCMSCs treated with the TLR9 agonist and the control
group were collected following 72 h stimulation at room temperature
and incubated with specific antibodies for 30 min at room
temperature. The cells were then analyzed using flow cytometry,
gating cells according to fluorescence intensity, also performed in
the control group.
RT-qPCR
Total RNA was extracted from confluent UCMSCs using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA), and reverse-transcribed using a ReverTraAce qPCR RT kit
(FSQ-101; Toyobo, Co. Ltd., Kagoshima, Japan) to synthesize cDNA
under the following conditions: 65°C for 5 min, 37°C for 15 min and
98°C for 5 min. RealMaster mix (SYBR Green; FP202; Tiangen Biotech,
Co., Ltd., Beijing, China) was used to perform qPCR on 500 ng cDNA
under the following cycling conditions: 95°C for 30 sec, 40 cycles
at 95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec, followed
by a melt curve between 55 and 95°C in 0.5°C increments and 10 sec
intervals. Primers for IL-1β, IL-6, IL-8, L-10, IL-12, IL-16,
IFN-α, IFN-β, IFN-γ, TGF-β, TNF-α, iNOS, IP-10, MCP-1, MIP-1α, M I
P-1β, RANTES, MCP-3, P53, c-myc, cdc2, TCAM-1, Selectin-E, VCAM-1,
CCL21, CCL24, CCL26 and GAPDH were used. The primer sequences were
synthesized by Tsingke Biotech Company (Chengdu, China).
Antibody chip array
From the two groups of UCMSCs, the supernatant was
collected following 4 h stimulation, according to the
manufacturer's instructions of the RayBio Human Antibody Array C
Series 1000 (RayBiotech Norcross, GA, USA), and the expression of
proteins were analyzed.
Assessment of the effects of TLR
activation on proliferation and migration of human UCMSCs
According to the manufacturer's instructions, a
Cell-Light EdU Apollo 567 in vitro kit (RiboBio, Guangzhou,
China) was used to assess UCMSC proliferation. The immunostained
cells were visualized using a Bio-Rad A1S1 laser confocal
microscope (Bio-Rad Laboratories, Inc.). UCMSC migration was
examined using a Transwell assay/modified Boyden chamber. Briefly,
1×106 cells were seeded on the upper chamber in 1%
serum-free Dulbecco's modified Eagle's medium (DMEM; Invitrogen
Life Technologies), with or without the agonists. DMEM (100 ml)
supplemented with 10% fetal bovine serum (Invitrogen, Waltham, MA,
USA) was added to the lower chamber. Subsequently, non-migratory
cells were removed from the upper chamber, and the migratory cells
were visualized using a Leica inverted microscope (DM4000B) after
staining with crystal violet, and Leica Application Suite Advanced
Fluorescence software (Leica Microsystems, Inc., Buffalo Grove, IL,
USA).
Western blot analysis
The cells were harvested with 200 µl
radioimmunoprecipitation assay lysis buffer (25 mM Tris-HCl pH 7.6,
150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS),
supplemented with a protease inhibitor (Sigma-Aldrich, St. Louis,
MO, USA), following which the immunoreactive protein was detected
using electrochemilu-minescence on an X-ray film. Using ImageJ
1.48u software (National Institutes of Health, Bethesda, MD, USA),
the resulting autoradiographs were scanned and quantified. The
primary antibodies used for western blotting were as follows:
Rabbit monoclonal CD29 (1:1,000; cat. no. 1798-1; Epitomics,
Burlingame, CA, USA), rabbit monoclonal mitogen-activated protein
kinase (MAPK)-p38 (1:1,000; cat. no. 1544-1; Epitomics) rabbit
monoclonal β-catenin (1:5,000; cat. no. 1247-1; Epitomics) and
mouse monoclonal GAPDH (1:2,000; cat. no. ab8245; Abcam).
Evaluation of UCMSC differentiation in
vitro
To analyze UCMSC differentiation, conditioned media
specific for chondrocytes (cat. no. A10071-01; Gibco-BRL),
osteocytes (cat. no. A10072-01l; Gibco-BRL) and adipocytes (cat.
no. A10070-01; Gibco-BRL) were added to induce osteogenic and
adipogenic differentiation of hUCMSCs. The two groups were treated
with Oil Red O (Baso Diagnostics, Inc., Zhuhai, China) staining,
Alizarin red staining (MeiLianShengWu, Shanghai, China) and
safranine staining (Shanghai Kayon Biotechnology Co., Ltd.,
Shanghai, China) respectively after 3, 10 and 14 days to detect
adipogenesis, osteogenesis and chondrogenesis.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean and were analyzed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance was used for
multiple comparisons, followed by a Dunnett's test to analyze the
significance between two groups. P<0.05 was considered to
indicate a st atistically significant difference.
Results
TLR9 ligand-regulated secretion of
immunogenicity molecules
UCMSCs were stimulated by the TLR9-specific ligand
CpG in order to detect changes in immune-associated molecule
expression. From the UCMSCs treated with TLR9 agonist for varied
durations (4, 12, 24, 72 and 120 h), mRNA was isolated, which was
assayed using RT-qPCR. The data indicated an increase following
TLR9 exposure of several important expressed cytokines and
chemokines, including mucosa-associated epithelial chemokine,
thymus-expressed chemokine, Leuk, nuclear factor-κB, monocyte
chemoat-tractant protein 2, transforming growth factor (TGF-β) and
other important immune factors (Fig.
1A). In addition, TLR9 stimulation markedly inhibited the
expression of specific stem cell markers, and certain important
tumor-associated factors were also induced by CpG.
 | Figure 1Detection of variations in gene
expression and the expression of downstream signaling molecules and
relative protein levels. (A) Gene expression levels were assessed
using reverse transcription-quantitative polymerase chain reaction
and (B) protein levels were detected using western blot analysis.
The data are expressed as the mean ± standard error of the mean.
*P<0.05, vs. control. MSCs, mesenchymal stem cells; TLR9,
Toll-like receptor 9; MAPK, mitogen-activated protein kinase; TGF,
transforming growth factor; VEGF, vascular endothelial growth
factor; NF, nuclear factor; MEC, mucosa-associated epithelial
chemokine; TECK, thymus-expressed chemokine; MCP-2, monocyte
chemoattractant protein 2; IFN, interferon; IL, interleukin; TNF,
tumor necrosis factor. |
TLR9 ligand leads to activation of
downstream signal molecules
To examine downstream signaling capabilities, the
UCMSCs were stimulated between 4 and 120 h with TLR9 ligands and
the results were assessed using western blot analysis. Several
important kinase signal proteins, including β-catenin and CD29, as
well as two members of the MAPK family, P38, were assessed.
Treatment with the TLR9 agonist appeared to enhance the protein
level of CD29, particularly at 24 and 72 h post-stimulation
(Fig. 1A). Previous studies have
confirmed that phosphorylated signal transducer and activator of
transcription 3, as the downstream signal pathway of interleukin
(IL)-6 (16), MAPK-P38 and
β-catenin decreases at different intervals. In MAPK detection in
the present study, one important member, p38, exhibited no
significant variation following TLR9 stimulation. This observation
is significantly different from that of a previous study, which
reported that the protein level of MAPK is promoted by TLR agonist
treatment (17). The present study
also found that the protein level of β-catenin first decreased at
the 12- and 24-h intervals, but increased at 72 h (Fig. 1B). This suggested that treatment
with the TLR9 agonist treatment did not affect these pathways.
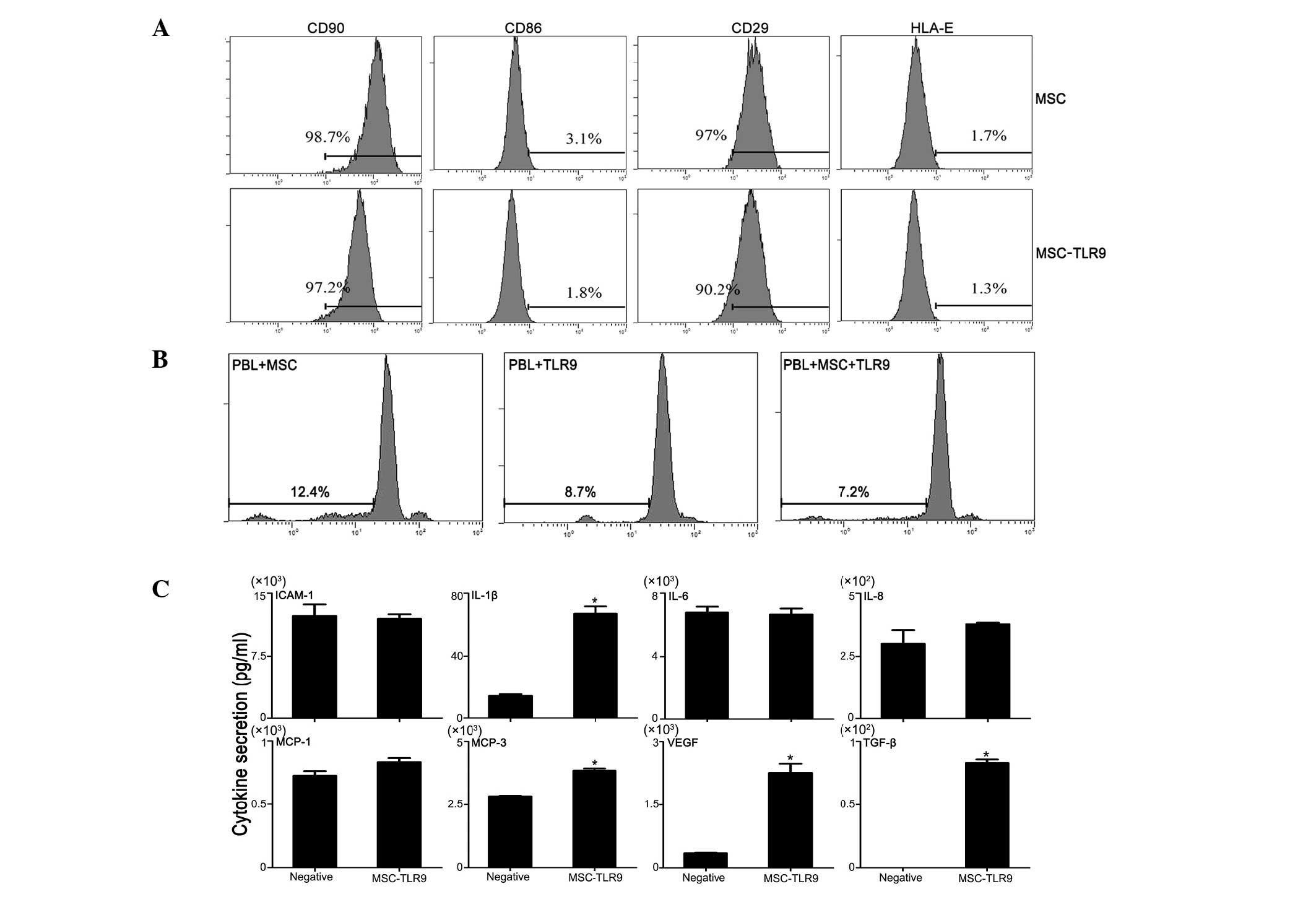
Proliferation of PBL in co-culture with
UCMSCs remains unchanged in the presence of TLR9 agonist
A number of ongoing clinical trials are based on the
observation that MSCs can inhibit immune response (21). Therefore, the present study
assessed whether this function of MSCs is compromised following
addition of the TLR9 agonist. Initially, human PBLs were labelled
with carboxyfluorescein succinimidyl ester (CFSE; Multiscience,
Hangzhou, China) and co-cultured with UCMSCs, with or without TLR9
activation. The proliferation of PBLs was measured 72 h after
co-culture, based on CFSE dilution. These experiments revealed that
PBL proliferation did not vary significantly in the presence of the
TLR9 agonist, compared with those without TLR9 ligands (Fig. 2A). The expression of co-stimulatory
molecules in the UCMSCs exhibited almost no change in response to
the TLR9 agonist (Fig. 2B).
Therefore, the present results suggested that one of the mechanisms
by which MSCs are not rejected or injured in vivo is their
contact with TLR9 ligands. Antibody measurement confirmed the
variation of secreted proteins (Fig.
2C), which were observed in Fig.
1.
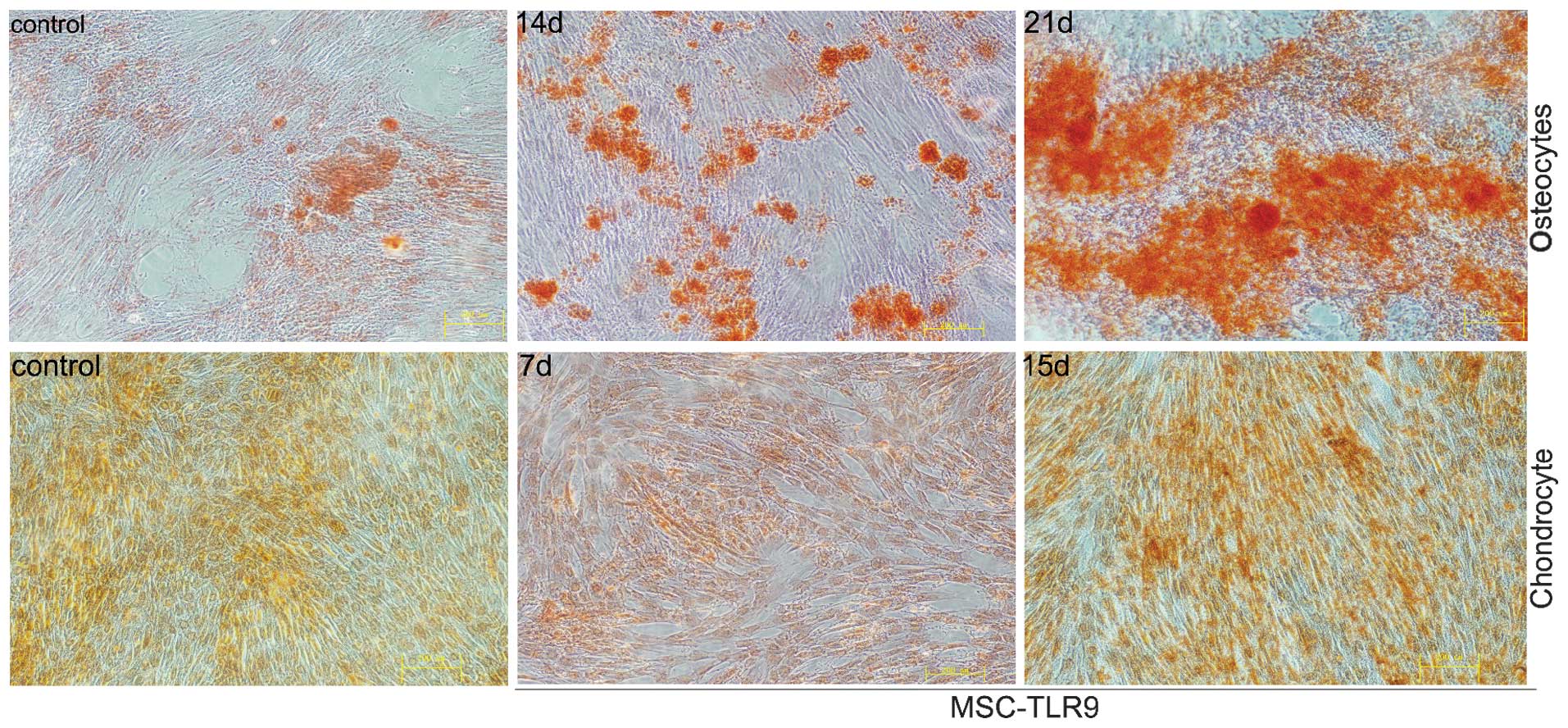
TLR9 ligand CpG-ODN enables osteoblast
differentiation of UCMSCs
To examine the effect of TLR9 on UCMSC
differentiation, TLR9 agonist was added to the culture medium, as
UCMSCs were induced by the conditioned medium to differentiate into
osteocytes, chondrocytes and adipocytes. Osteocyte differentiation
was detected, following supplementation of the cell culture media
with osteogenic inducing medium via Alizarin red staining.
Adipogenic differentiation, induced by adipogenic medium, appeared
under Oilred O staining. UCMSCs grown in culture with
chondrocyte-inducing media resulted in chondrogenic
differentiation, which was detected by safranine. As shown in
Fig. 3, the presence of the TLR9
agonist in the osteogenic medium markedly enhanced the level of
calcium deposition, compared with conditioned medium without CpG,
particularly 10 and 14 days after differentiation. The control
groups exhibited no marked differences in the presence of CpG in
adipogenic and chondrogenic media (data not shown).
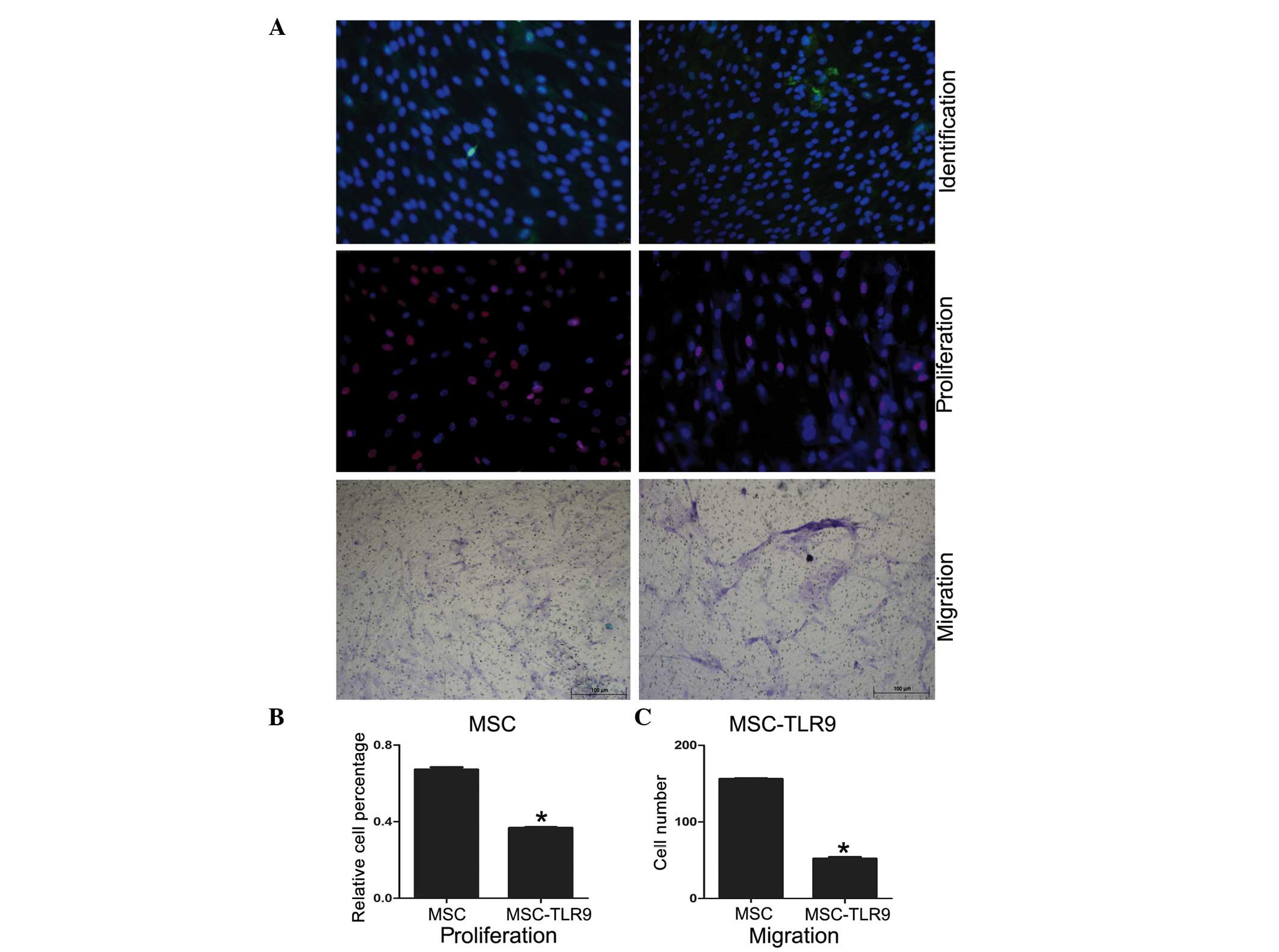
TLR9 activation inhibits hUCMSC
proliferation and migration
The Cell-Light EdU Apollo 567 In Vitro kit and the
transwell/modified Boyden chamber were used to investigate the
proliferation and migration of UCMSCs in the present study. The
data suggested that proliferation and migration were inhibited
following stimulation by the TLR9 ligands (Fig. 4).
Discussion
MSCs have multiple functions and are considered to
be important in prospective cell-based therapies. Understanding the
factors and mechanisms, which regulate their ability to
differentiate, self-renew, induce injury repair and suppress
ongoing immune responses are crucial, and may allow the
manipulation of MSCs for therapeutic use (22). However, several studies have
suggested detrimental outcomes (23). Observations from human and animal
studies have demonstrated the elimination of the majority of MSCs
within a few days of infusion, indicating unknown host mechanisms
of MSC recognition and rejection (24). Several other studies have also
confirmed that MSCs alter immune status and cause immune responses,
inducing the rejection and injury of MSCs (25–27).
TLRs are important in bridging innate and adaptive
immune systems by recognizing conserved components from invasive
pathogens (15). As UCMSCs have
attracted increased attention in MSC-based therapy, the molecular
details of the TLR agonist response are critical for improving
controlled and desirable clinical outcomes. During cell therapy,
engrafted MSCs may encounter molecular infectious agents, either in
the blood circulation or in peripheral tissues, resulting in TLR
activation. Numerous endogenous ligands of TLR, including heat
shock protein (HSP)-60, HSP-70, heparin sulfate, fbro-nectin extra
domain A, hyaluronan, oxidized LDL, uric acid, myeloid-associated
proteins 8 and 14, intracellular components of fragmented cells,
eosinophil-derived neurotoxin and human defensin-3 have been
identified (28). These endogenous
TLR ligands regulate the biological function of UCMSCs through
endogenous stimuli during inflammation and tissue repair in the
microenvironments of tissue injury and cell necrosis (29). An important aspect, which has not
been previously investigated in detail is the activation and
modulation of MSC activity by these danger signals.
Although a previous study indicated that TLRs have
no effect on the immunogenicity of BMMSCs, the immunoge-nicity of
TLRs in UCMSCs remains to be elucidated. The effect of the
activation of TLR9 on the function of MSCs has been confirmed in a
previous study (30). In the
present study, functional TLR9 ligands were expressed in UCMSCs,
and their activation by CpG-ODN regulated MSC function, including
cytokine release, multi-lineage differentiation, proliferation and
migration.
MSCs are important in migration, expansion,
apoptosis and immunomodulatory activities through the secretion of
numerous growth factors, cytokines and chemokines (31). The present study demonstrated that
stimulation of TLR9 ligands in UCMSCs elevated the secretion of
pro-inflammatory cytokines (IL-1β, IL-8, IL-9, interferon (IFN)-γ,
IFN-β, TGF-β and tumor necrosis factor-α), excluding IL-6 and
IL-12. Several important stem cell markers (Nanog, Nestin, OCT4 and
TP63) were also markedly inhibited upon TLR9 stimulation.
MSCs possess an increased tendency to enhance tumor
sphere formation and tumor initiation (1). Interactions between MSCs and tumor
cells involve MSC secretion of signaling molecules, including
vascular endothelial growth factor (VEGF), IL-6, phosphatase and
tensin homolog (PTEN) and CCLs, which stimulate various signaling
pathways, particularly those associated with cell growth and
apoptotic regulation in tumor cells (24). Similar to a previous study, the
present study demonstrated that, in the presence of TLR9, detection
of gene expression revealed upregulated secretion by
tumor-initiation genes (PTEN, proliferating cell nuclear antigen,
β-catenin and VEGF). The observation thatTLR9 activation-induced
the expression of pro-inflammatory factors led to the hypothesis
that exposure of UCMSCs to TLR9 agonists may increase the
immunogenicity of UCMSCs. Therefore, co-cultures of PBLs and UCMSCs
were performed in the present study to identify whether TLR9
increased the proliferation of PBLs. To measure the expression of
co-stimulatory molecules, CD80, CD86 and HLA-II, FACS was also
performed. The results revealed a negative effect of TLR9 on the
immune status of UCMSCs. One basic characteristic of UCMSCs are
their differentiation ability. As a previous study indicated, human
AD-MSCs osteogenic differentiation increases in response to LPS and
peptidoglycan activation, whereas CPG-ODN impairs differentiation
(29). The results of the present
study indicated that the presence of a TLR9 agonist enabled
osteogenic differentiation.
The results of the present study demonstrated for
the first time, to the best of our knowledge, TLR9 regulation of
immune status and biological function. Several paths of evidence
were pursued. Firstly, TLR9 was observed to increase the expression
of pro-inflammatory molecules and decrease the expression of stem
cell markers. Subsequently, the marked effects of TLR9 agonist
treatment on UCMSCs were observed to inhibit proliferation and
migration. Finally, the differentiation results confirmed that the
TLR9 agonist increased osteoblast differentiation capability. In
conclusion, the present study provided a novel observation of TLR9
mediating immune modulator response via UCMSCs, critical to
MSC-based therapy design. As UCMSCs are attracting more attention
as a replacement for BMMSCs, another important aspect of the
present study is that MSCs isolated from umbilical tissue were
used. Understanding how factors and mechanisms regulating
biological functions, including differentiation, self-renewal and
involvement in immune responses may allow the manipulation of
UCMSCs for clinical use.
The present study critically implicated TLR9 as a
vital modulator in UCMSC function, including differentiation,
proliferation and migration. Further characterization of
TLR9-activated UCMSCs using in vivo experimental systems is
required to establish the physiological role of TLRs in the
regulation of UCSMC function.
Acknowledgments
This study was supported by the National Natural
Scientific Foundations of China (grant no. 81200315) and the China
Postdoctoral Science Foundation Grants (grant nos. 2011M501413 and
2013T60855).
References
|
1
|
Phinney DG and Prockop DJ: Concise review:
mesenchymal stem/multipotent stromal cells: the state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesen-chymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom
HJ, Kim MG, Oh KH, Ahn C and Yang J: Immunosuppressive mechanisms
of embryonic stem cells and mesenchymal stem cells in alloimmune
response. Transpl Immunol. 25:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagaya N, Fujii T, Iwase T, Ohgushi H,
Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K and Kitamura S:
Intravenous administration of mesenchymal stem cells improves
cardiac function in rats with acute myocardial infarction through
angio-genesis and myogenesis. Am J Physiol Heart Circ Physiol.
287:H2670–H2676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tatebe M, Nakamura R, Kagami H, Okada K
and Ueda M: Differentiation of transplanted mesenchymal stem cells
in a large osteochondral defect in rabbit. Cytotherapy. 7:520–530.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Im GI, Kim DY, Shin JH, Hyun CW and Cho
WH: Repair of cartilage defect in the rabbit with cultured
mesenchymal stem cells from bone marrow. J Bone Joint Surg Br.
83:289–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bensaïd W, Oudina K, Viateau V, Potier E,
Bousson V, Blanchat C, Sedel L, Guillemin G and Petite H: De novo
reconstruction of functional bone by tissue engineering in the
metatarsal sheep model. Tissue Eng. 11:814–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris CT and Cooper LF: Comparison of
bone graft matrices for human mesenchymal stem cell-directed
osteogenesis. J Biomed Mater Res A. 68:747–755. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horwitz EM, Gordon PL, Koo WK, Marx JC,
Neel MD, McNall RY, Muul L and Hofmann T: Isolated allogeneic bone
marrow-derived mesenchymal cells engraft and stimulate growth in
children with osteogenesis imperfecta: Implications for cell
therapy of bone. Proc Natl Acad Sci USA. 99:8932–8937. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawada H, Fujita J, Kinjo K, Matsuzaki Y,
Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, et
al: Nonhematopoietic mesenchymal stem cells can be mobilized and
differentiate into cardiomyocytes after myocardial infarction.
Blood. 104:3581–3587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Damme A, Vanden Driessche T, Collen D
and Chuah MK: Bone marrow stromal cells as targets for gene
therapy. Curr Gene Ther. 2:195–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allison M: Genzyme back Osiris, despite
Prochymal flop. Nat Biotechnol. 27:966–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang XP, Sun Z, Miyagi Y, McDonald
Kinkaid H, Zhang L, Weisel RD and Li RK: Differentiation of
allogeneic mesen-chymal stem cells induces immunogenicity and
limits their long-term benefits for myocardial repair. Circulation.
122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lombardo E, DelaRosa O, Mancheño-Corvo P,
Menta R, Ramírez C and Büscher D: Toll-like receptor-mediated
signaling in human adipose-derived stem cells: Implications for
immunogenicity and immunosuppressive potential. Tissue Eng Part A.
15:1579–1589. 2009. View Article : Google Scholar
|
|
17
|
Cassatella MA, Mosna F, Micheletti A, Lisi
V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G and
Krampera M: Toll-like receptor-3-activated human mesenchymal
stromal cells significantly prolong the survival and function of
neutrophils. Stem Cells. 29:1001–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Liu J, Manuchehrabadi N, Weir MD,
Zhu Z and Xu HH: Umbilical cord and bone marrow mesenchymal stem
cell seeding on macroporous calcium phosphate for bone regeneration
in rat cranial defects. Biomaterials. 34:9917–9925. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fong CY, Chak LL, Biswas A, Tan JH,
Gauthaman K, Chan WK and Bongso A: Human Wharton's jelly stem cells
have unique transcriptome profiles compared to human embryonic stem
cells and other mesenchymal stem cells. Stem Cell Rev. 7:1–16.
2011. View Article : Google Scholar
|
|
20
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Developmental Committee of the European Group for Blood
and Marrow Transplantation: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586
|
|
21
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pevsner-Fischer M, Morad V, Cohen-Sfady M,
Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR and Zipori D:
Toll-like receptors and their ligands control mesenchymal stem cell
functions. Blood. 109:1422–1432. 2007. View Article : Google Scholar
|
|
23
|
Shi Y, Su J, Roberts AI, Shou P, Rabson AB
and Ren G: How mesenchymal stem cells interact with tissue immune
responses. Trends Immunol. 33:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaplan JM, Youd ME and Lodie TA:
Immunomodulatory activity of mesenchymal stem cells. Curr Stem Cell
Res Ther. 6:297–316. 2011. View Article : Google Scholar
|
|
25
|
Spaggiari GM, Capobianco A, Becchetti S,
Mingari MC and Moretta L: Mesenchymal stem cell-natural killer cell
interactions: Evidence that activated NK cells are capable of
killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell
proliferation. Blood. 107:1484–1490. 2006. View Article : Google Scholar
|
|
26
|
Li Y and Lin F: Mesenchymal stem cells are
injured by complement after their contact with serum. Blood.
120:3436–3443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nauta AJ, Westerhuis G, Kruisselbrink AB,
Lurvink EG, Willemze R and Fibbe WE: Donor-derived mesenchymal stem
cells are immunogenic in an allogeneic host and stimulate donor
graft rejection in a nonmyeloablative setting. Blood.
108:2114–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torsvik A and Bjerkvig R: Mesenchymal stem
cell signaling in cancer progression. Cancer Treat Rev. 39:180–188.
2013. View Article : Google Scholar
|
|
30
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee
IH, Lin WS, Wu CH, Lin WY and Cheng SM: Mesenchymal stem cells from
human umbilical cord express preferentially secreted factors
related to neuroprotection, neurogenesis, and angiogenesis. PLoS
One. 8:e726042013. View Article : Google Scholar : PubMed/NCBI
|