Introduction
Skin squamous cell carcinoma (SCC) occurs with a
higher rate of incidence compared with several other malignant skin
tumor types, and it occupies the largest percentage of the total
skin malignant tumors (routinely, 80–90%) (1,2). The
rate of incidence has increased on a year-by-year basis,
particularly among the elderly. The formation and development of
the tumor occurs progressively, and is governed by multistep
processes, involving the comprehensive action of internal and
external factors (3,4). In the last few years, research
efforts have intensified in investigating the regulation of the
disordering of fragmentation of cell proliferation, and the
association which exists between cell signal transmission and the
occurrence and development of tumors.
As two typical signaling molecules, which are
involved in signaling cascades, signal transducer and activator of
transcription 3 (STAT3) and mitogen-activated protein kinase (MAPK)
are involved in the physical processes of cell growth,
differentiation, division and development, and exert an important
role in the malignant transformation of cells (5–7).
STAT3 is an essential member of the STAT family of proteins. STAT3
is located in the cytoplasm in an unstimulated state, and upon
stimulation, the structural SH2 domain interacts with a
phosphorylated tyrosine residue, which itself is phosphorylated by
Janus kinase (JAK).
In providing the axis for several types of signaling
pathways, MAPK-cascaded activation exerts an important role in
receiving signals, which are transferred and carried by membrane
receptors and brought into the nucleus, which is an essential
process for numerous signaling pathways associated with cell
proliferation.
The overexpression of cyclin D1, which is one of the
regulatory factors involved in the cell cycle, is a characteristic
of numerous types of human primary tumor, and it is vitally
important for the prognostication and the diagnosis of tumors
(8,9).
To investigate whether the growth of skin SCC, as
with the majority of tumor types, comprises a cascade of molecular
abnormalities whereby the regulation of the proliferation of the
epidermal cells breaks down, and malignant transformation occurs
(10,11), immunohistochemical staining
techniques were used to assess the phosphorylation of STAT3 and
MAPK. Additionally, the protein levels of cyclin D1 were assessed,
measured against normal skin tissue as a control, and the
associations between phosphorylated (p-)STAT3, p-MAPK and cyclin D1
were investigated. The present study also aimed to explore the
mechanism underlying skin SCC and to identify novel means by which
an early diagnosis of the tumor may be accomplished.
Materials and methods
Patients and controls
Over the course of 5 years, samples of skin SCC were
collected from 30 patients who received surgical resection in The
First People's Hospital of Yancheng City (Yancheng, China), and who
had been diagnosed by pathological confirmation. Each case had
detailed clinical and pathological data, and no patient had
received preoperative chemotherapy or radiotherapy. The patients
with cancer included 20 males and 10 females, aged between 39 and
73 years (mean age, 53.9±11.6 years). According to Broders'
pathological grading criteria for skin SCC (12), eight cases were classified as grade
I, 12 as grade II and 10 as grade III. Normal tissue specimens were
collected by surgical resection from 10 individuals to serve as a
control group. These included five males and five females, aged
between 35 and 69 years (mean age, 49.5±10.4 years). No
statistically significant differences were detected in age or
gender between the two groups. All specimens were obtained
following informed patient consent and were approved by the Ethics
Committee of The First People's Hospital of Yancheng City
[Identification no. HMU (Ethics) 20121103].
Immunohistochemical staining
techniques
The EnVision™ staining immunohistochemical method
(Dako, Carpinteria, CA, USA) was used to detect the distribution of
p-STAT3, p-MAPK and cyclin D1. The immunohistochemical procedures
were performed strictly in accordance with the manufacturer's
instructions. The EnVision™ and 3,3′-diami-nobenzidine (DAB)
chromogenic reagent kits (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) were used for immunohistochemical staining. All slice
staining was performed under identical conditions. The tissue was
sliced to a diameter of 4 µm prior to dehydration at room
temperature for 60 min and dewaxing (placed in xylene for 10 min,
replacement of xylene, and soaking for 5 min in anhydrous alcohol,
5 min in 95% ethanol and 5 min in 75% ethanol), and the slices were
subsequently antigen-repaired using 0.01 mol/l citric acid (pH
6.0). Normal goat serum was dropped on to the slice and incubated
for 10 min at room temperature, and subsequently the corresponding
specific antibodies were added to the slice and incubated for 1.5 h
at room temperature. The following antibodies were used: p-STAT3
(cat. no. SC1409; Santa Cruz Biotechnology, Inc; 1:1,000) and
p-MAPK (cat. no. SC1312; Santa Cruz Biotechnology, Inc.; 1:1,000).
The slices were washed with phosphate-buffered saline (PBS) three
times (3 min/wash). The secondary antibody was dropped on to the
slice and incubated for 30 min at room temperature. The slices were
colored with DAB, the nuclei were stained using hematoxylin (Cell
Signaling Technology, Shanghai, China), and the slices were
subsequently dehydrated with gradient ethanol, cleared by xylene
(Dako North America, Inc., Carpinteria, CA, USA) and sealed with
natural gum (Cell Signaling Technology, Inc.). The staining of each
batch was accompanied by a positive control (with the known
positive section reagent, which was provided by Dako North America,
Inc.) and a negative control (where the corresponding specific
antibody was replaced with PBS).
Yellow- or tan-colored staining taken up by the
nucleus or tan-reactant particles, respectively, indicated a
positive result. Four independent experiments were performed for
random detection using an optical microscope (BH-2; Olympus,
Hamburg, Germany) at a high magnification (x200). According to the
degree of the positive staining and the percentage of tumor cells
present, the criteria for judgment were as follows: (−), no
expression identified (only a small quantity of cell shading was
present in <5% of the cells); (1+), a low level of expression
was identified (5–29% of the total cells were pale yellow,
positively identified cells); (2+), moderate expression was
identified (30–59% of the total cells were yellow, positively
identified cells); (3+), high expression was identified
(tancoloured, positively identified cells were present at a level
>60%).
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analyses. The χ2
test was used to compare the distribution of p-STAT3, p-MAPK and
cyclin D1 between normal and cancer tissues, and Spearman's rank
correlation coefficient analysis was used to analyze how the
distribution of p-STAT3, p-MAPK and cyclin D1 among the tissues was
associated. P<0.05 was considered to indicate a statistically
significant difference.
Results
Distribution of p-STAT3, p-MAPK and
cyclin D1 in the nuclei of SCC and normal skin as determined by the
dye staining pattern
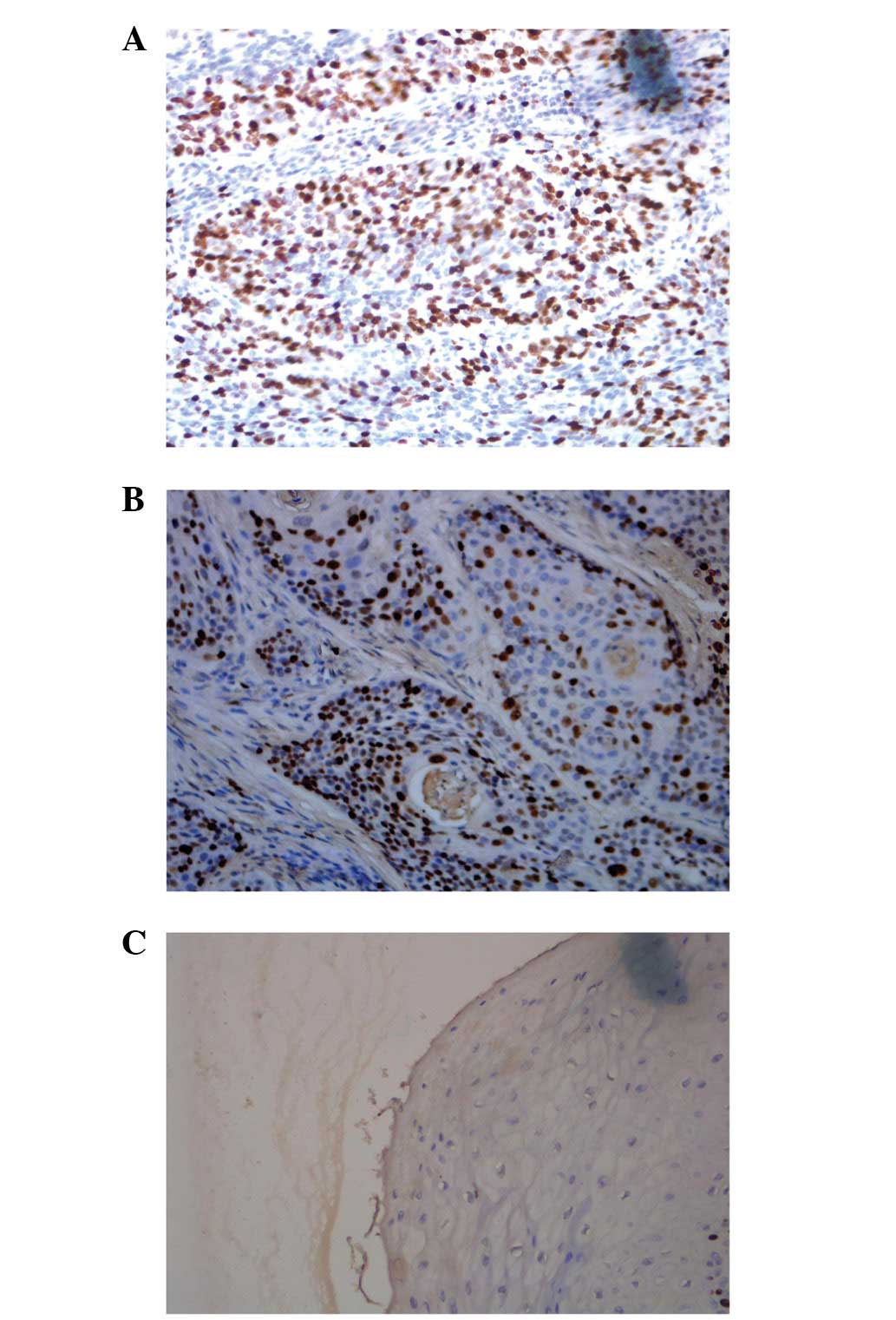
The positive result of p-STAT3 staining in the SCC
tissues of 86.7% of the patients (26/30) was significantly higher
compared with the normal skin tissue (0; P<0.05). Furthermore,
the staining intensity of p-STAT3 in patients with SCC at the
pathological grades II and III was significantly higher compared
with grade I (P<0.05; Fig.
1A–C; Table I). A positive
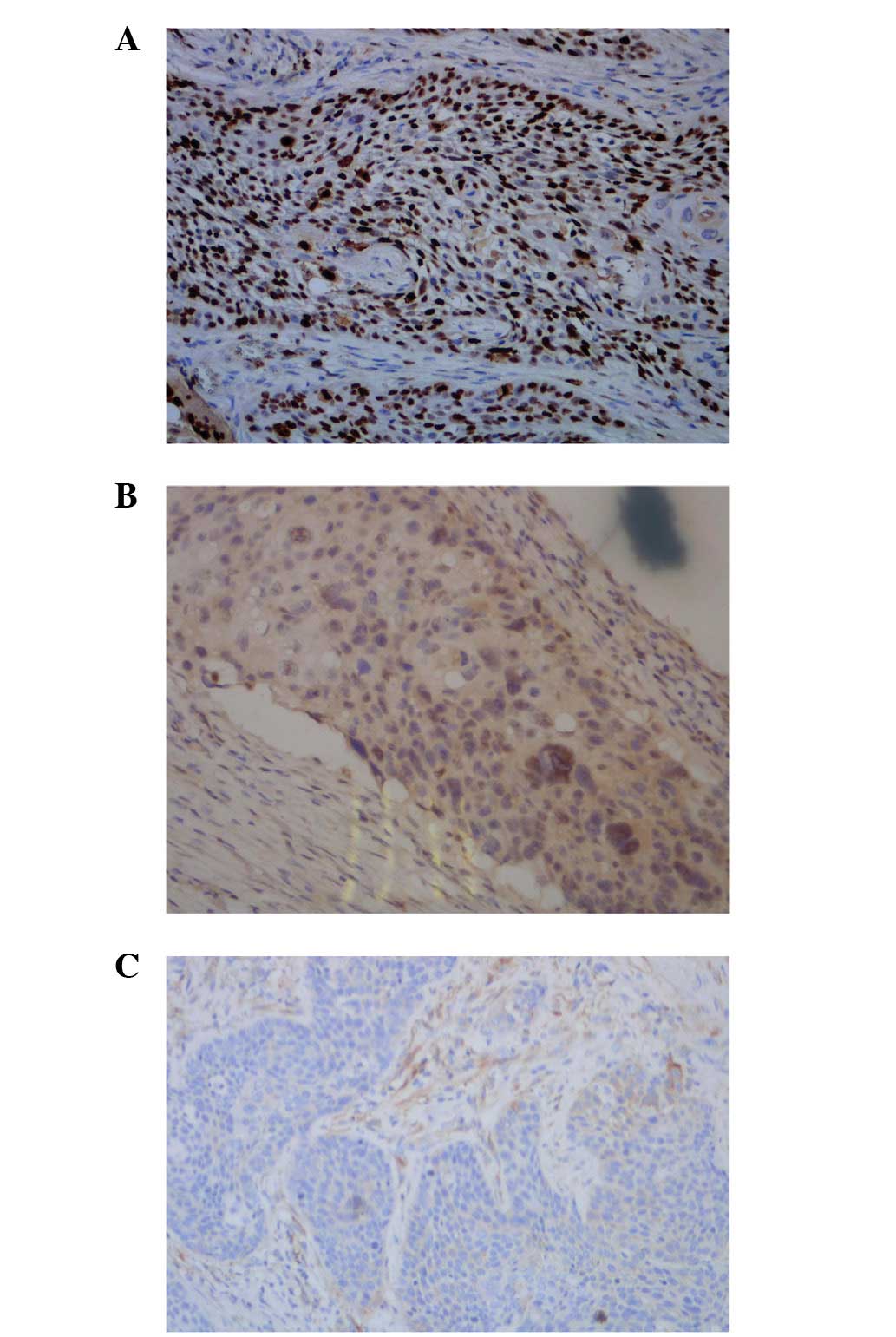
result was identified for p-MAPK in the SCC of 13.3%, although it
was revealed to be negative in normal skin tissues; in addition, no
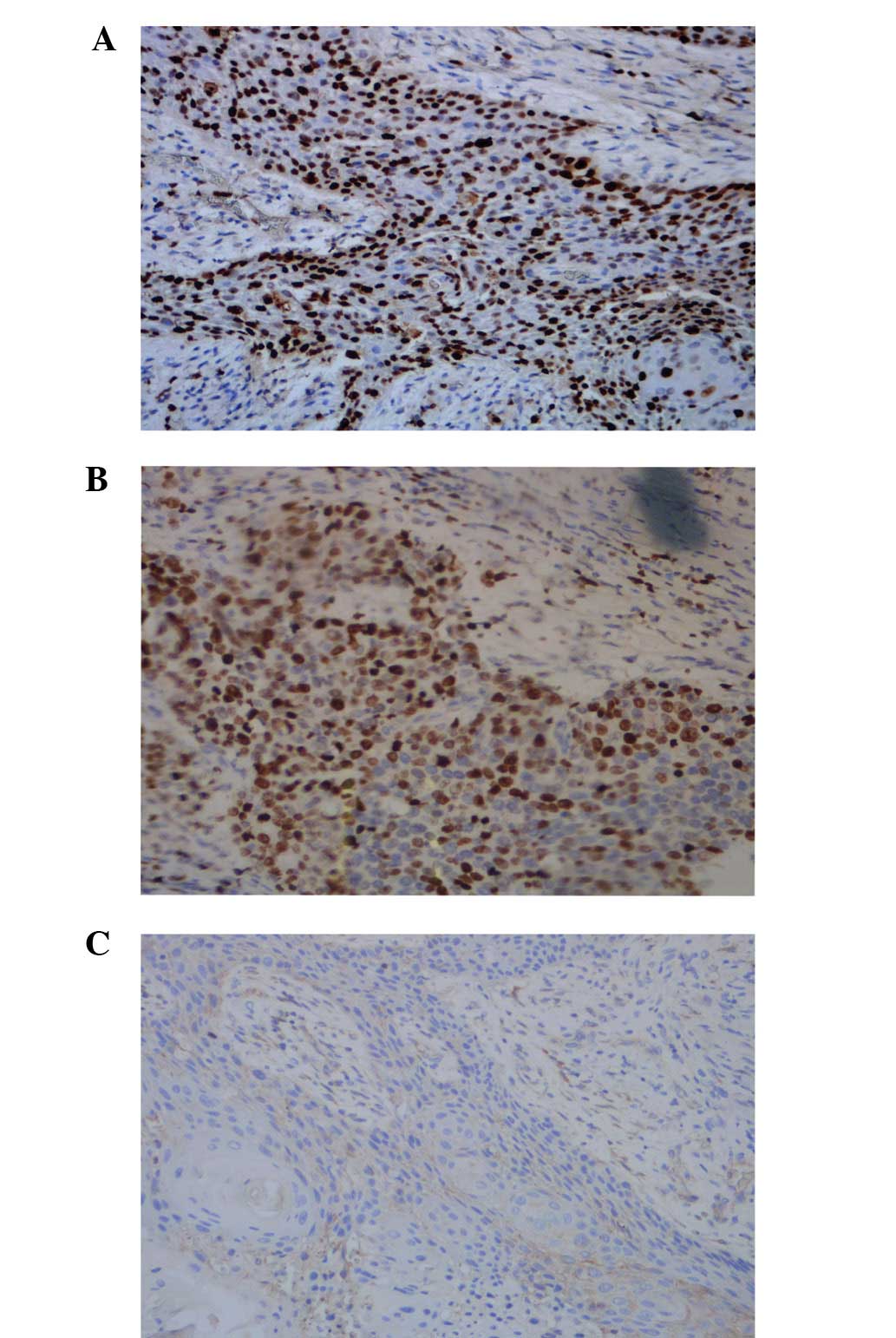
significant differences in the statistics were identified (Fig. 2A–C; Table II). Cyclin D1 protein was
scattered throughout the epidermis and cancerous tissue, and the
positive result of cyclin D1 in the SCC was determined to be 73.33%
(22/30 patients). Furthermore, the positive staining result of
cyclin D1 in the SCC at pathological grades II and III was
significantly higher compared with grade I (P<0.05; Fig. 3A–C; Table III).
 | Table IPositive staining results of EnVision™
immunohistochemistry for p-STAT-3 in SCC and normal skin. |
Table I
Positive staining results of EnVision™
immunohistochemistry for p-STAT-3 in SCC and normal skin.
| | p-STAT3 positive
| | | |
|---|
| Group | n | + | 2+ | 3+ | Negative (−) | PR (%) | SPR (%) |
|---|
| Normal | 10 | 0 | 0 | 0 | 10 | 0.0 | 0.0 |
| SCC | 30 | 4 | 9 | 13 | 4 | 86.7 | 43.3 |
| I | 8 | 1 | 2 | 2 | 3 | 62.5 | 25.0 |
| II | 12 | 1 | 5 | 5 | 1 | 91.7 | 41.7 |
| III | 10 | 2 | 2 | 6 | 0 | 100.0 | 60.0 |
 | Table IIPositive staining positive results of
EnVision™ immunohistochemistry for p-MAPK in the SCC and normal
skin. |
Table II
Positive staining positive results of
EnVision™ immunohistochemistry for p-MAPK in the SCC and normal
skin.
| | p-MAPK positive
| | | |
|---|
| Group | n | + | 2+ | 3+ | Negative (−) | PR (%) | SPR (%) |
|---|
| Normal | 10 | 0 | 0 | 0 | 10 | 0.00 | 0.00 |
| SCC | 30 | 1 | 2 | 1 | 26 | 13.33 | 3.33 |
| I | 8 | 0 | 0 | 0 | 8 | 0.00 | 0.00 |
| II | 12 | 1 | 1 | 0 | 10 | 16.67 | 0.00 |
| III | 10 | 0 | 1 | 1 | 8 | 20.00 | 10.00 |
 | Table IIIPositive staining positive results of
EnVision™ immunohistochemistry for cyclin D1 in the SCC and normal
skin. |
Table III
Positive staining positive results of
EnVision™ immunohistochemistry for cyclin D1 in the SCC and normal
skin.
| | Cyclin D1 positive
| | | |
|---|
| Group | n | + | 2+ | 3+ | Negative (−) | PR (%) | SPR (%) |
|---|
| Normal | 10 | 0 | 0 | 0 | 10 | 0.00 | 0.00 |
| SCC | 30 | 3 | 8 | 11 | 8 | 73.33 | 36.67 |
| I | 8 | 1 | 1 | 1 | 5 | 37.50 | 12.50 |
| II | 12 | 1 | 4 | 5 | 2 | 75.00 | 41.67 |
| III | 10 | 1 | 3 | 5 | 1 | 90.00 | 50.00 |
Degree of correlation among the p-STAT3,
p-MAPK and cyclin D1 proteins in SCC
To analyze the mutual associations among the
proteins, according to the expression of each antigen in SCC,
Spearman's correlation coefficient analysis was used. p-STAT3 and
cyclin D1 were positively correlated with the intensity of positive
staining (r=0.714; P<0.05), whereas the intensities of p-MAPK
and cyclin D1 bore no positive correlation with the positive
staining (r=0.234, P>0.05; Table
IV). Taken together, the results of the present study
demonstrated that p-STAT3 protein was abnormally increased in SCC,
however, no significant differences were observed in the protein
expression levels of p-MAPK between normal skin and SCC. The
positive rates of p-STAT3 and cyclinD1 were correlated with the
depth of tumor invasion
 | Table IVPercentage staining results of
p-STAT3, p-MAPK and cyclin D1 in skin squamous cell carcinoma. |
Table IV
Percentage staining results of
p-STAT3, p-MAPK and cyclin D1 in skin squamous cell carcinoma.
| Positive staining
result (%)
|
|---|
| Number | p-STAT3 | p-MAPK | Cyclin D1 |
|---|
| 1 | 50 | 5 | 5 |
| 2 | 45 | 8 | 5 |
| 3 | 85 | 10 | 2 |
| 4 | 45 | 30 | 35 |
| 5 | 23 | 12 | 20 |
| 6 | 85 | 10 | 6 |
| 7 | 45 | 65 | 8 |
| 8 | 65 | 15 | 60 |
| 9 | 53 | 4 | 3 |
| 10 | 75 | 8 | 57 |
| 11 | 50 | 7 | 5 |
| 12 | 75 | 4 | 3 |
| 13 | 49 | 5 | 3 |
| 14 | 4 | 10 | 60 |
| 15 | 45 | 8 | 30 |
| 16 | 60 | 20 | 60 |
| 17 | 58 | 35 | 30 |
| 18 | 90 | 8 | 5 |
| 19 | 50 | 8 | 4 |
| 20 | 82 | 5 | 5 |
| 21 | 40 | 75 | 15 |
| 22 | 90 | 2 | 5 |
| 23 | 18 | 90 | 50 |
| 24 | 42 | 30 | 15 |
| 25 | 80 | 10 | 10 |
| 26 | 45 | 10 | 55 |
| 27 | 50 | 70 | 8 |
| 28 | 90 | 5 | 0 |
| 29 | 42 | 12 | 30 |
| 30 | 82 | 28 | 6 |
Discussion
The occurrence and development of cancer is always
closely associated with abnormalities in cellular signal transfer
and regulation (13–15).
Several signaling pathways, which involve
carcinogenic tyrosine kinases, converge onto STAT3, including those
mediated by the epidermal growth factor receptor, interleukin-6/JAK
and Src, and overactivation of these pathways occur in diverse
types of cancer cells and tissues (16,17).
Following activation, STAT3 subsequently induces the anomalous
expression of pivotal genes, which are crucial for various cellular
activities, which consequently accelerate cell proliferation and a
vicious transformation of the cells, and inhibits the various
anticarcinogenic functions mediated by apoptosis; therefore, these
genes are termed oncogenes (18,19).
The present study revealed that, compared with normal skin tissues,
the positive results exhibited by the patients with SCC were
indicative of clearly increased levels of p-STAT3, and the protein
expression of STAT3 occurred in the nuclei in the case of SCC,
suggesting that the abnormal activation of the signaling cascade
mediated by STAT3 exerted an important role in the occurrence and
development of skin SCC. The staining intensity of p-STAT3 for
patients with skin SCC at the pathological grades II and III was
notably increased, which suggested that the overactivation of STAT3
may be closely associated with the invasive growth of skin SCC.
MAPK remains in a static state in unstimulated
cells, prior to being activated by the sequential activity of the
protein kinases, MAPK kinase and MAP kinase kinase (20,21),
which follows a sequential step-by-step phosphorylation process in
cells stimulated by growth factors, among other stimulatory agents.
Upon activation, MAPK is transferred into the nucleus and
subsequently activates a target oncogene to stimulate cellular
proliferation, and apoptosis is thereby inhibited. The results of
the present study demonstrated a positive result of 13.3% for MAPK
in skin SCC tissues, although this is statistically insignificant
compared with the normal skin tissues, suggesting that the MAPK
signal transduction pathway may not be the most important pathway
in skin SCC.
Previous studies have indicated that the
overexpression of cyclin D1 may result in a decrease in the G1
mitotic growth phase of the cells (22,23),
pushing them into the phase of synthesis, thereby completing the
duplication of DNA. The increase in the protein expression of
cyclin D1 is typically observed in certain primary malignant tumor
types, including those of parathyroid adenoma, neck squamous cell
carcinoma, breast cancer, esophageal cancer and hepatocellular
carcinoma (24–28). Due to the fact that the expression
of STAT3 and MAPK occurs upstream of the gene expression of cyclin
D1, the expression of p-STAT3 in skin SCC increased directly in
proportion with the positive intensity of cyclin D1. The results in
the present study also revealed that the positive result of cyclin
D1 in skin SCC was clearly higher compared with that in normal skin
tissues, and the staining intensity of cyclin D1 in the case of
skin SCC at pathological grades II and III was higher compared with
that in grade I. These results suggested that there may an
overexpression of cyclin D1 induced by the p-STAT3-mediated signal
transduction pathway, leading to an increased level of cell
proliferation in these tumor cells.
In conclusion, the present study has investigated
the extent in which p-STAT3, p-MAPK and cyclin D1 were stained
positively in skin SCC tissues compared with normal skin tissues.
The association among the staining patterns were explored, with a
view to characterizing a biological norm for diagnosing and
prognosticating patients with cutaneous SCC. By disrupting the
p-STAT3 signal transduction pathway in cutaneous SCC, the effect of
p-STAT3, which leads to the overexpression of cyclin D1, on
cutaneous SCC may be efficiently prevented. These results may be
useful for future strategies of clinical drug antitumor therapy,
and may provide the groundwork for a novel and pivotal method in
this field.
References
|
1
|
Kraljik N, Rosso M, Ageel A, Sepić T and
Gmajnić R: The incidence of skin squamous cell carcinoma in
Osijek-Baranja County - an epidemiological study. Coll Antropol.
35(Suppl 2): 77–80. 2011.
|
|
2
|
Wu J, Zhang JR and Qin J: Methylation of
E-cadherin and p14ARF gene promoters. Int J Clin Exp Med.
7:1808–1812. 2014.
|
|
3
|
Saladi RN, Nektalova T and Fox JL:
Induction of skin carcinogenicity by alcohol and ultraviolet light.
Clin Exp Dermatol. 35:7–11. 2010. View Article : Google Scholar
|
|
4
|
Gariboldi M, Peissel B, Fabbri A, Saran A,
Zaffaroni D, Falvella FS, Spinola M, Tanuma J, Pazzaglia S, Mancuso
MT, et al: SCCA2-like serpins mediate genetic predisposition to
skin tumors. Cancer Res. 63:1871–1875. 2003.PubMed/NCBI
|
|
5
|
Chen J, Chi M, Chen C and Zhang XD:
Obesity and melanoma: Exploring molecular links. J Cell Biochem.
114:1955–1961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riebe C, Pries R, Schroeder KN and
Wollenberg B: Phosphorylation of STAT3 in head and neck cancer
requires p38 MAPKinase, whereas phosphorylation of STAT1 occurs via
a different signaling pathway. Anticancer Res. 31:3819–3825.
2011.PubMed/NCBI
|
|
7
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elliman SJ, Howley BV, Mehta DS, Fearnhead
HO, Kemp DM and Barkley LR: Selective repression of the oncogene
cyclin D1 by the tumor suppressor miR-206 in cancers. Oncogenesis.
3:e1132014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Caffrey TC, Steele MM, Mohr A,
Singh PK, Radhakrishnan P, Kelly DL, Wen Y and Hollingsworth MA:
MUC1 regulates cyclin D1 gene expression through p120 catenin and
β-catenin. Oncogenesis. 3:e1072014. View Article : Google Scholar
|
|
10
|
Evert M, Calvisi DF, Evert K, De Murtas V,
Gasparetti G, Mattu S, Destefanis G, Ladu S, Zimmermann A, Delogu
S, et al: Thymoma viral oncogene homolog/mammalian target of
rapamycin activation induces a module of metabolic changes
contributing to growth in insulin-induced hepatocarcinogenesis.
Hepatology. 55:1473–1484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molven A, Søvik O, von der Lippe C, Steine
SJ, Njølstad PR, Houge G and Prescott TE: Molecular genetic
diagnostics in syndromes associated with the RAS/MAPK signalling
pathway. Tidsskr Nor Laegeforen. 129:2358–2361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montagner A, Delgado MB, Tallichet-Blanc
C, Chan JS, Sng MK, Mottaz H, Degueurce G, Lippi Y, Moret C,
Baruchet M, et al: Src is activated by the nuclear receptor
peroxisome proliferator-activated receptor β/δ in ultraviolet
radiation-induced skin cancer. EMBO Mol Med. 6:80–98. 2004.
View Article : Google Scholar
|
|
13
|
Kim GT, Lee SH and Kim YM: Quercetin
Regulates Sestrin 2-AMPK-mTOR Signaling Pathway and Induces
Apoptosis via Increased Intracellular ROS in HCT116 Colon Cancer
Cells. J Cancer Prev. 18:264–270. 2013. View Article : Google Scholar
|
|
14
|
Xu W, Yang Z, Zhou SF and Lu N:
Posttranslational regulation of phosphatase and tensin homolog
(PTEN) and its functional impact on cancer behaviors. Drug Des
Devel Ther. 8:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang N, Zhang C, Dill P, Panasyuk G, Pion
D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, et al:
Regulation of YAP by mTOR and autophagy reveals a therapeutic
target of tuberous sclerosis complex. J Exp Med. 211:2249–2263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Talbot JJ, Song X, Wang X, Rinschen MM,
Doerr N, LaRiviere WB, Schermer B, Pei YP, Torres VE and Weimbs T:
The cleaved cytoplasmic tail of polycystin-1 regulates
Src-dependent STAT3 activation. J Am Soc Nephrol. 25:1737–1748.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al:
Mutations in the EGFR kinase domain mediate STAT3 activation via
IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Zhang L, Chen G, Zhang J, Li Z, Lu
W, Liu M and Pang X: Small molecule 1′- acetoxychavicol acetate
suppresses breast tumor metastasis by regulating the SHP-
1/STAT3/MMPs signaling pathway. Breast Cancer Res Treat.
148:279–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snyder M, Huang J, Huang XY and Zhang JJA:
A signal transducer and activator of transcription 3 Nuclear Factor
κB (Stat3·NFκB) complex is necessary for the expression of fascin
in metastatic breast cancer cells in response to interleukin (IL)-6
and tumor necrosis factor (TNF)-γ. J Biol Chem. 289:30082–30089.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian H, Zhang D, Gao Z, Li H, Zhang B,
Zhang Q, Li L, Cheng Q, Pei D and Zheng J: MDA- 7/IL- 24 inhibits
Nrf2- mediated antioxidant response through activation of p38
pathway and inhibition of ERK pathway involved in cancer cell
apoptosis. Cancer Gene Ther. 21:416–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casimiro MC, Wang C, Li Z, Di Sante G,
Willmart NE, Addya S, Chen L, Liu Y, Lisanti MP and Pestell RG:
Cyclin D1 determines estrogen signaling in the mammary gland in
vivo. Mol Endocrinol. 9:1415–1428. 2013. View Article : Google Scholar
|
|
22
|
Pysz MA, Hao F, Hizli AA, Lum MA, Swetzig
WM, Black AR and Black JD: Differential regulation of cyclin D1
expression by protein kinase C α and ε signaling in intestinal
epithelial cells. J Biol Chem. 289:22268–22283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair SV, Ziaullah and Rupasinghe HP: Fatty
Acid Esters of Phloridzin Induce Apoptosis of Human Liver Cancer
Cells through Altered Gene Expression. PLoS One. 9:e1071492014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang YJ, Jiang W, Chen CJ, Lee CS, Kahn
SM, Santella RM and Weinstein IB: Amplification and overexpression
of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res
Commun. 29:1010–1016. 1993. View Article : Google Scholar
|
|
25
|
Yang Y, Zhao LH, Huang B, Wang RY, Yuan
SX, Tao QF, Xu Y, Sun HY, Lin C and Zhou WP: Pioglitazone, a PPARγ
agonist, inhibits growth and invasion of human hepatocellular
carcinoma via blockade of the rage signaling. Mol Carcinog.
2014.Epub ahead of print.
|
|
26
|
Shi QQ, Zuo GW, Feng ZQ, Zhao LC, Luo L,
You ZM, Li DY and Xia J: Effect of Trichostatin A on Anti HepG2
Liver Carcinoma Cells: Inhibition of HDAC Activity and Activation
of Wnt/β- Catenin Signaling. Asian Pac J Cancer Prev. 15:7849–7855.
2014. View Article : Google Scholar
|
|
27
|
Wang X, Liu H, Wang X, Zeng Z, Xie LQ, Sun
ZG and Wei MX: Preventive effect of Actinidia valvata Dunn extract
on N-methyl-N′-nitro-N-nitrosoguanidine-induced gastrointestinal
cancer in rats. Asian Pac J Cancer Prev. 15:6363–6367. 2014.
View Article : Google Scholar
|
|
28
|
Gopalakrishnan N, Saravanakumar M,
Madankumar P, Thiyagu M and Devaraj H: Colocalization of κ-catenin
with Notch intracellular domain in colon cancer: a possible role of
Notch1 signaling in activation of Cyclin D1-mediated cell
proliferation. Mol Cell Biochem. 396:281–293. 2014. View Article : Google Scholar : PubMed/NCBI
|