Introduction
Ischemic stroke is the dominant subtype of stroke in
China, which leads to high rates of morbidity, disability and
mortality (1). Immediate
restoration or improvement of reduced regional cerebral blood
supply is critical for improving stroke outcomes and post-stroke
functional recovery (2).
Therefore, angiogenesis has become a focus in the field of ischemic
stroke investigation.
MicroRNAs (miRNAs), which are 21~23 nucleotide
non-protein-coding RNA molecules, have been identified as negative
regulators of gene expression in a post-transcriptional manner
(3). Several previous studies have
demonstrated that miRNAs are essential determinants of vascular
endothelial cell biology and angiogenesis, and contribute to stroke
pathogenesis (4–7). A previous study demonstrated that
glioma cells and angiogenic growth factors elevate the level of
miRNA (miR)-296 in primary human brain microvascular endothelial
cells in vitro (8).
Angiogenic growth factor-induced expression of miR-296 contributes
significantly to angiogenesis by directly targeting hepatocyte
growth factor-regulated tyrosine kinase substrate (HGS) mRNA, which
leads to reduced levels of HGS and HGS-mediated degradation of
vascular endothelial growth factor receptor 2 (VEGFR2) (8). Thus, the present study hypothesized
that miR-296 is involved in post-ischemic angiogenesis in cerebral
ischemia, and the role of miR-296 in angiogenesis following
cerebral ischemia was investigated.
Materials and methods
Ethical statement
The rats used in the present study were maintained
in a climate-controlled vivarium with a 12 h light-dark cycle with
free access to food and water. Each group consisted of six rats and
all treatments were performed under anesthesia. Efforts were made
to minimize pain and trauma to the animals. All procedures in the
present study were approved by the Ethics Committee of Xiangya
Hospital of Central South University (Changsha, China) (protocol
no. 34721).
Animal model and identification
A total of 24 male Sprague-Dawley (SD) rats
(weighing 250–300 g; aged 8–10 weeks) were obtained from the
Experimental Animal Center of Central South University (SCXK
2011-0003), and were randomly divided into four groups (n=6/group):
Baseline group, 1-day group, 3-day group and 7-day group. The
middle cerebral artery occlusion (MCAO)-induced focal cerebral
ischemia mouse model was conducted as previously described
(9). Following anesthetization
with 4% chloral hydrate (4 ml/kg via intraperitoneal injection;
Beijing Chemical Reagent Company, Beijing, China) the right common
carotid artery (CCA), the external carotid artery (ECA), and the
internal carotid artery were separated through a ventral midline
neck incision. The ECA was ligated and cut 4 mm distal from the
ECA-CCA branch. At ~2 mm distal from the ECA-CCA branch, a small
incision was made in the ECA. A 4-0 nylon filament with a silicone
resin-coated tip (0.23 mm diameter) was inserted through the
incision 18–20 mm into the internal carotid artery, and the wound
was closed. The sham-operated (control) animals were anesthetized
and underwent surgery without MCAO. The mice were subsequently
placed in a post-operative cage, and kept warm and undisturbed for
a minimum of 2 h observation.
Infarct area assessment
Identification of the ischemic area of the brain was
performed, according to the 2,3,5-triphenyl-2h-tetrazolium chloride
(TTC; Sigma-Aldrich, St. Louis, MO, USA) staining procedure. The
rats were sacrificed 24 h after the onset of MCAO by
intraperitoneal injection with 10% chloral hydrate (4 ml/kg),
followed by decapitation in order to harvest the brains. The rats
then received intracardial perfusion with saline (Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beiijng, China) and the brain
was removed and frozen for ~5 min at −80°C. The brains were
subsequently cut into 2 mm sections, which were incubated with 2%
TTC at 37°C for 20 min in the dark. Following staining with TTC,
the brain sections were fixed in 4% paraformaldehyde for 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the ischemic cortex
tissues using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), according the manufacturer's instructions. The
total RNA (2 µl) from each sample was added to 25 µl
reaction mixture, and cDNA was synthesized using a First Strand
cDNA Synthesis kit (Invitrogen Life Technologies). qPCR was
performed to determine the mRNA expression levels using an ABI
PRISM 7500 Sequence Detection system (Applied Biosystems Life
Technologies, Foster City, CA, USA) using SYBR green PCR Master Mix
(Applied Biosystems Life Technologies), according to the
manufacturer's instructions. The PCR mix consisted of: 25 µl
SYBR green PCR Master Mix, 0.5 µl cDNA, 2 µl primer
pair (5 pmol/ml each primer), 22.5 µl H2O. The
following primers were used: miR-296-RT, 5′-GTC GTA TCC AGT GCA GGG
TCC GAG GTA TTC GCACTG GAT ACG ACA CAG GA-3′; miR-296, forward
5′-GAA CTA GGG CCC CCC CTC AA-3′; miR-296, reverse 5′-GTG CAG GGT
CCG AGGT-3′; U6, forward 5′-CTC GCT TCG GCA GCA CA-3′; and U6,
reverse 5′-AAC GCT TCA CGA ATTTGCGT-3′ (Switchgear Genomics,
Shanghai, China). The PCR cycling conditions were as follows: 95°C
for 20 sec, followed by 40 cycles at 95°C for 3 sec and 60°C for 30
sec.
Western blot analysis
Each section of ischemic cortex tissue was titrated
and homogenized in lysis buffer (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.), the cells for western blotting were grown
for 48 h and lysed in radioimmunoprecipitation assay buffer
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.) containing a
protease inhibitor cocktail (Invitrogen Life Technologies). Equal
quantities of the extracted proteins were then separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Beijing
Dingguo Changsheng Biotechnology Co., Ltd.), followed by transfer
of the resolved proteins onto nitrocellulose membranes (Hunan
Honghui Reagent Co., Ltd., Changsha, China). The membranes were
blocked for 1 h at room temperature in 5% nonfat dry milk in
Tris-buffered saline with 1% Tween-20 (TBST; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) and incubated overnight
at 4°C with the following primary antibodies: Rabbit polyclonal
anti-Notch1 (cat. no. ab8925; 1:500), rabbit polyclonal anti-VEGFR2
(cat. no. ab11939; 1:1,000), rabbit polyclonal anti-VEGFA (cat. no.
ab51745; 1:1,000) (Abcam, Cambridge, MA, USA), rabbit polyclonal
anti-DLL4 (cat. no. 2589; 1:1,000), mouse monoclonal anti-HGS (cat.
no. 15087; 1:1,000) (Cell Signaling Technology, Inc., Danvers, MA,
USA), and mouse monoclonal anti-GAPDH (cat. no. sc-367714; 1:1,000)
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Subsequently,
following a standard washing cycle with TBST (5 min, 3 times),
membranes were incubated with a horseradish peroxidase-conjugated
bovine anti-goat (cat. no. sc-2350), anti-rabbit (cat. no. sc-2370)
and anti-mouse (cat. no. sc-2371) secondary antibodies (1:5,000)
for 1 h at room temperature and washed again with TBST (5 min, 3
times). GAPDH was used as a loading control for all experiments.
Quantification of the immunoreactivity corresponding to the bands
was performed using integrated optical density analysis using Gel
pro version 4.0 (Media Cybernetics, Inc., Rockville, MD, USA). All
western blot analyses were repeated a minimum of three times.
Immunohistochemistry
The ischemic brain tissues were fixed in 10%
neutralized formalin (Zhongtian Medical Equipment Co., Ltd., Wuhan,
China) and embedded in paraffin blocks (Zhongtian Medical Equipment
Co., Ltd.). Sections (5 µm) were then prepared for
immunohistochemical examination. Following deparaffinization and
rehydration, antigen retrieval was performed by boiling in 10
mmol/l citrate buffer (pH 6.0; Zhongtian Medical Equipment Co.,
Ltd.) for 10 min. Endogenous peroxidase activity was inhibited by
soaking for 30 min in 100% methanol (Hunan Honghui Reagent Co.,
Ltd.) containing 0.3% H2O2, then the sections
were blocked with 2% bovine serum albumin (Wuhan Boster Biological
Technology, Ltd.) in phosphate-buffered saline (PBS) for 30 min
prior to incubation with the mouse monoclonal CD105 antibody
(Thermo Fisher Scientific, Beijing, China) at a dilution of 1:150
overnight at 4°C. Following washing with PBS, the sections were
incubated in primary antibody enhancer (Thermo Fisher Scientific)
for 10 min and horseradish peroxidase polymer (cat. no. 87-8963;
Thermo Fisher Scientific) for 15 min at room temperature. Following
washing with PBS, the sections were incubated with diaminobenzidine
(Thermo Fisher Scientific) for 3 min at room temperature. The
sections were counterstained with hematoxylin (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) for 30 sec, and the staining
was evaluated by two individual observers, in a blinded-manner.
Assessment of microvessel density
Quantitative analysis of the microvessel density was
performed, as previously described (9). For the determination of microvessel
density, the three most vascular areas (hot spots) within a tissue
section were selected at a magnification of ×40, and were counted
under a light microscope (IX83; Olympus, Beijing, China) at a
magnification of ×100. The microvessel density was defined as the
number of CD105-positive vessels per optical field.
Endothelial cell culture
Human umbilical vein endothelial cell (HUVEC)-12
cells were obtained from the Cell Center of Central South
University. The cells were grown in Dulbecco's modified Eagle's
medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA), containing 10%
fetal bovine serum (Invitrogen Life Technologies), 100 mol/l
penicillin and 100 mg/l streptomycin (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.), and incubated at 37°C in a humidified
atmosphere containing 5% CO2.
Adenoviral transduction
The HUVEC-12 cells were seeded (2×105
cells/well) on six-well plates. On the day of transduction, the
cells were harvested and transduced with either the recombinant
AdV-Mir-296-green fluorescent protein (GFP) plasmid or with the
AdV-GFP plasmid as a control (Promega Corporation, Madison, WI,
USA). After 48 h, the cells were harvested and used for subsequent
analyses.
RT-qPCR
Total RNA was prepared from the HUVEC-12 cells using
TRIzol (Invitrogen Life Technologies), according to the
manufacturer's instructions. cDNA was synthesized using 15
µl total RNA and 2 µl oligo (dT) primer in a 25
µl total reaction volume. RT was performed by incubating the
mixture at 42°C for 60 min, and the reaction was terminated at 95°C
for 5 min. The following primers were used: HGS-564, forward 5′-CCA
CAA TGG CGA GTC TGA-3′ and reverse 5′-GAG GGC TGG TAG AGC ACA-3′;
VEGFA-427, forward 5′-CTA CTG CCA TCC AAT CGA GA-3′ and reverse
5′-CTT TCT CCG CTC TGA GCA A-3′; VEGFR-449, forward 5′-GGG ATT GAC
TTC AAC TGG-3′ and reverse 5′-ATG GGA TTG GTA AGG ATG-3′; DLL4-362,
forward 5′-CAG CAG GGA AGC CAT GAA-3′ and reverse 5′-CCG TGG CAA
TGA CAC ATT CA-3′; Notch1-452, forward 5′-CAA ACA TCC AGC AGC AGC
AA-3′ and reverse 5′-AAT GCG GGC GAT CTG GGA CT-3′; and GAPDH-452,
forward 5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse 5′-TCC ACC ACC
CTG TTG CTG TA-3′ (Invitrogen Life Technologies). The PCR products
were then electrophoresed on 1% agarose gels (Sigma-Aldrich) and
stained with ethidium bromide (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.).
Tube formation on Matrigel
Matrigel (50 µl of ~10.5 mg/ml; Solarbio
Science & Technology Co., Ltd., Shanghai, China) at 4°C was
used to coat each well of a 96-well plate and was allowed to
polymerize at 37°C for a minimum of 30 min, as described previously
(10). The HUVECs cells
(5×104) were added with 200 µl DMEM. Following
incubation for 24 h, cultures were visualized (magnification, ×400)
and images were captured using an inverted microscope (DIAPHOT-TMD;
Nikon Corporation, Tokyo, Japan).
Statistical analyses
All statistical analyses were performed using SPSS,
version 11.0 (SPSS, Inc., Chicago, IL, USA). Each set of
experiments was repeated three times. All continuous variables are
expressed as the mean ± standard deviation. The means between two
groups were compared using Student's t-test. Comparisons of means
among multiple groups were performed using one-way analyses of
variance followed by post-hoc pairwise comparisons using Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of the ischemic injury
model
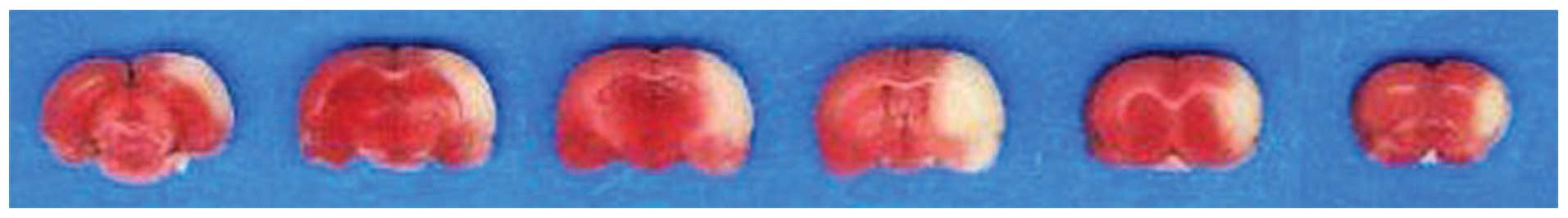
In the present study, cerebral ischemia was induced
in rats via MCAO. As shown in Fig.
1, the ischemic areas in the rat brain were stained white,
while the normal brain tissues were stained red by TTC staining,
which is reported to reflect neurological deficits in the rat brain
(11). This indicated that the rat
cerebral ischemic injury model had been successfully
established.
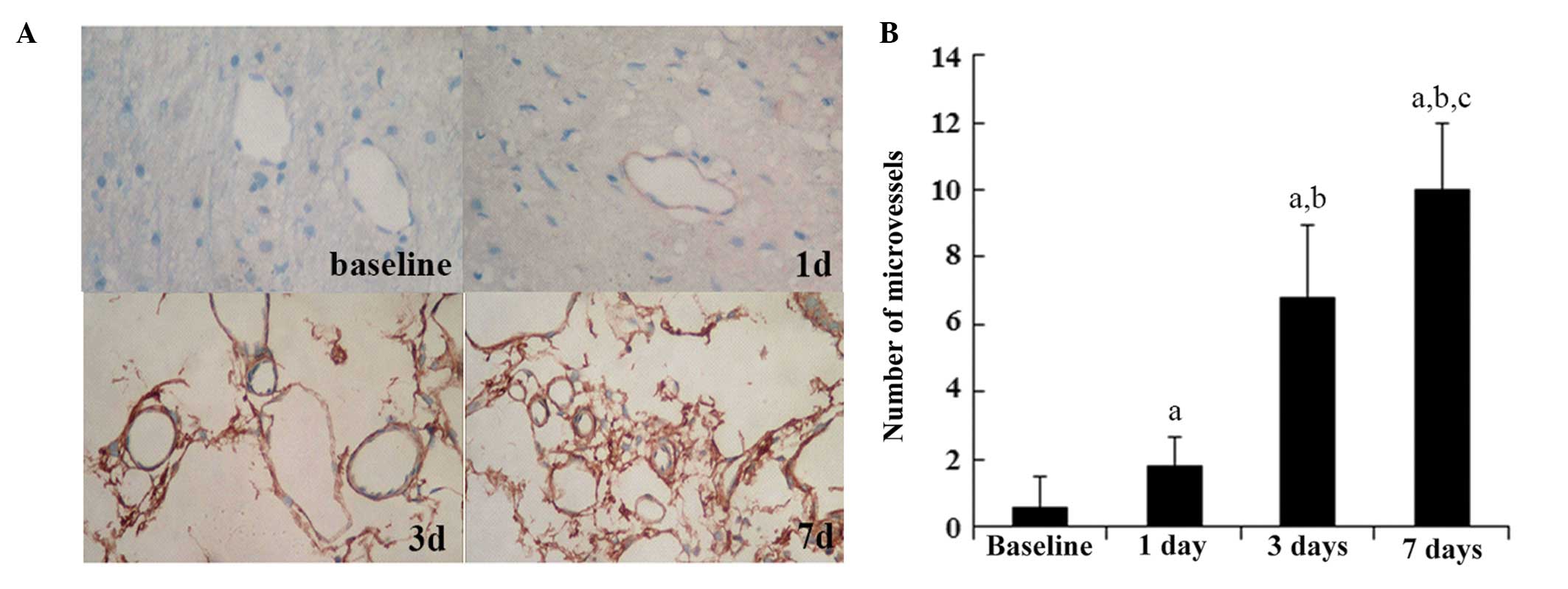
Angiogenesis increases over time in
response to ischemic injury
CD105 is a prominent feature of newly formed blood
vessels (12). To assess
ischemia-induced angiogenesis in ischemic brain areas,
immunohistochemical staining for CD105 was performed in the
ischemic rat brain cortex. As shown in Fig. 2, the number of CD105-stained
microvessels significantly increased over time following ischemic
injury. By contrast, the sham group did not exhibit significant
alterations in CD105 staining over time (data not shown). These
results indicated that angiogenesis was increased over time in the
cortex area following ischemic injury.
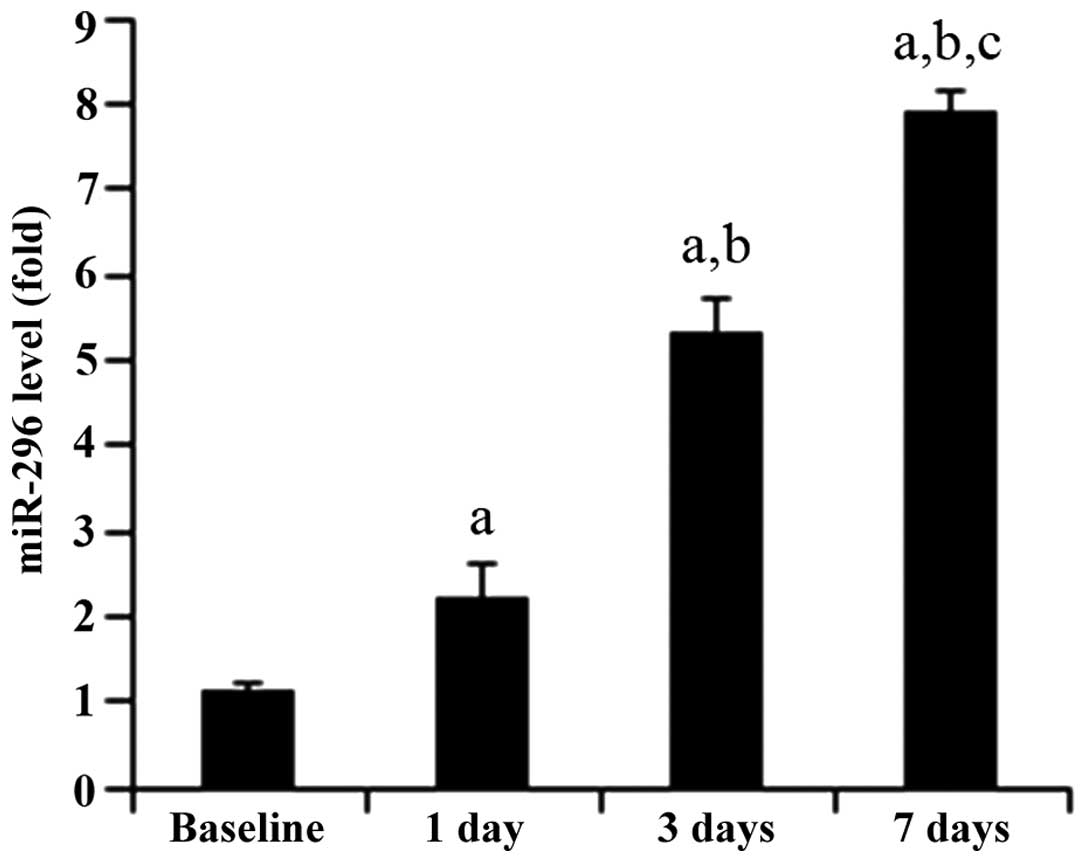
Expression of miR-296 increases over time
in the ischemic cortex
As shown in Fig. 3,
RT-qPCR revealed that the expression levels of miR-296
significantly increased over time in the ischemic brain. This
result suggested that miR-296 may be involved in the regulation of
post-ischemic angiogenesis.
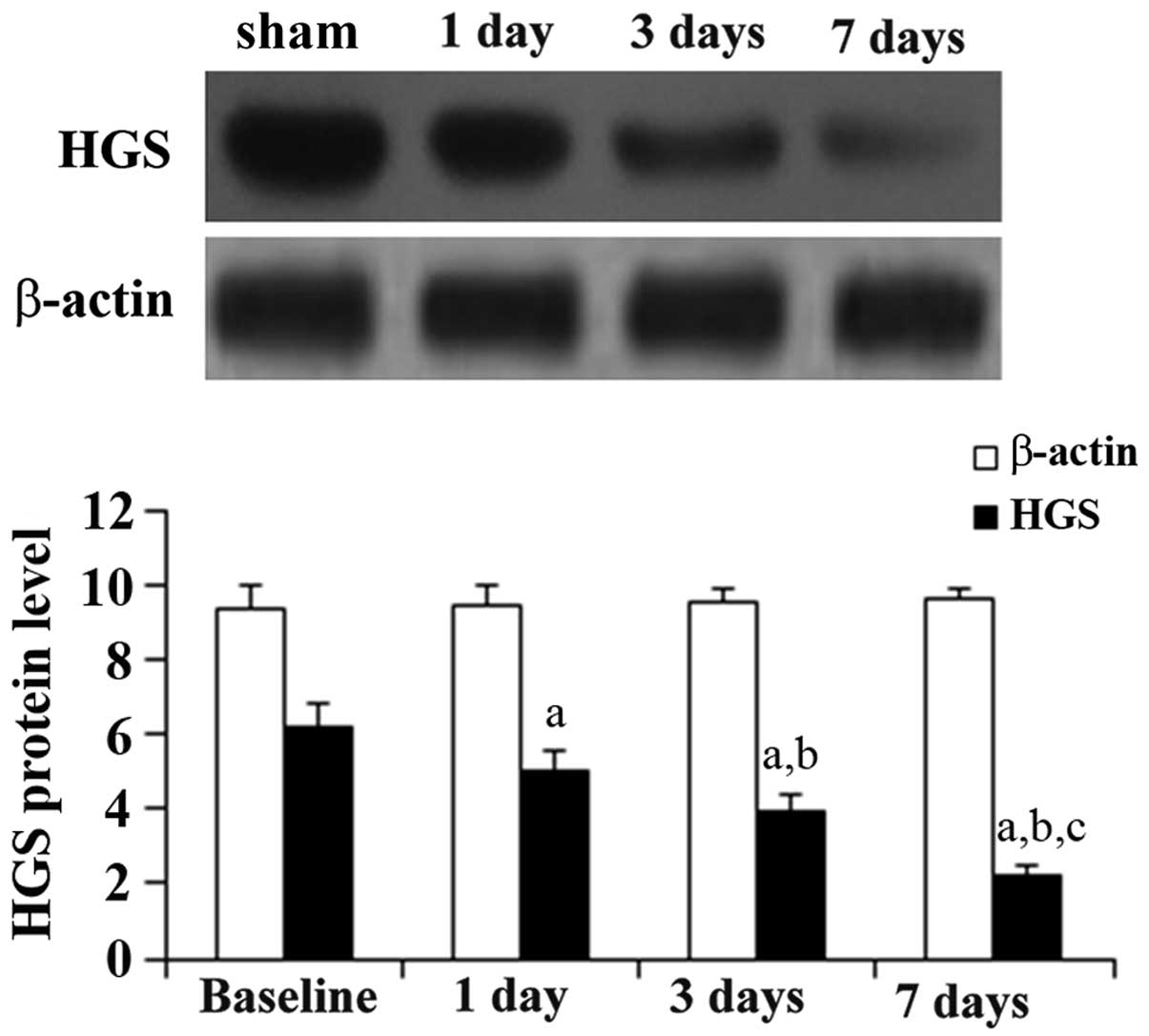
Expression of HGS reduces over time in
the ischemic cortex
To identify miR-296-regulated target genes involved
in angiogenesis, the protein levels of HGS following MCAO were
measured. As shown in Fig. 4,
western blot analyses demonstrated that the expression of HGS
reduced over time following the ischemic injury.
Overexpression of miR-296 enhances
angiogenesis in vitro
To examine the role of miR-296 in angiogenesis,
HUVECs were transduced with adenoviral miR-296 to stably
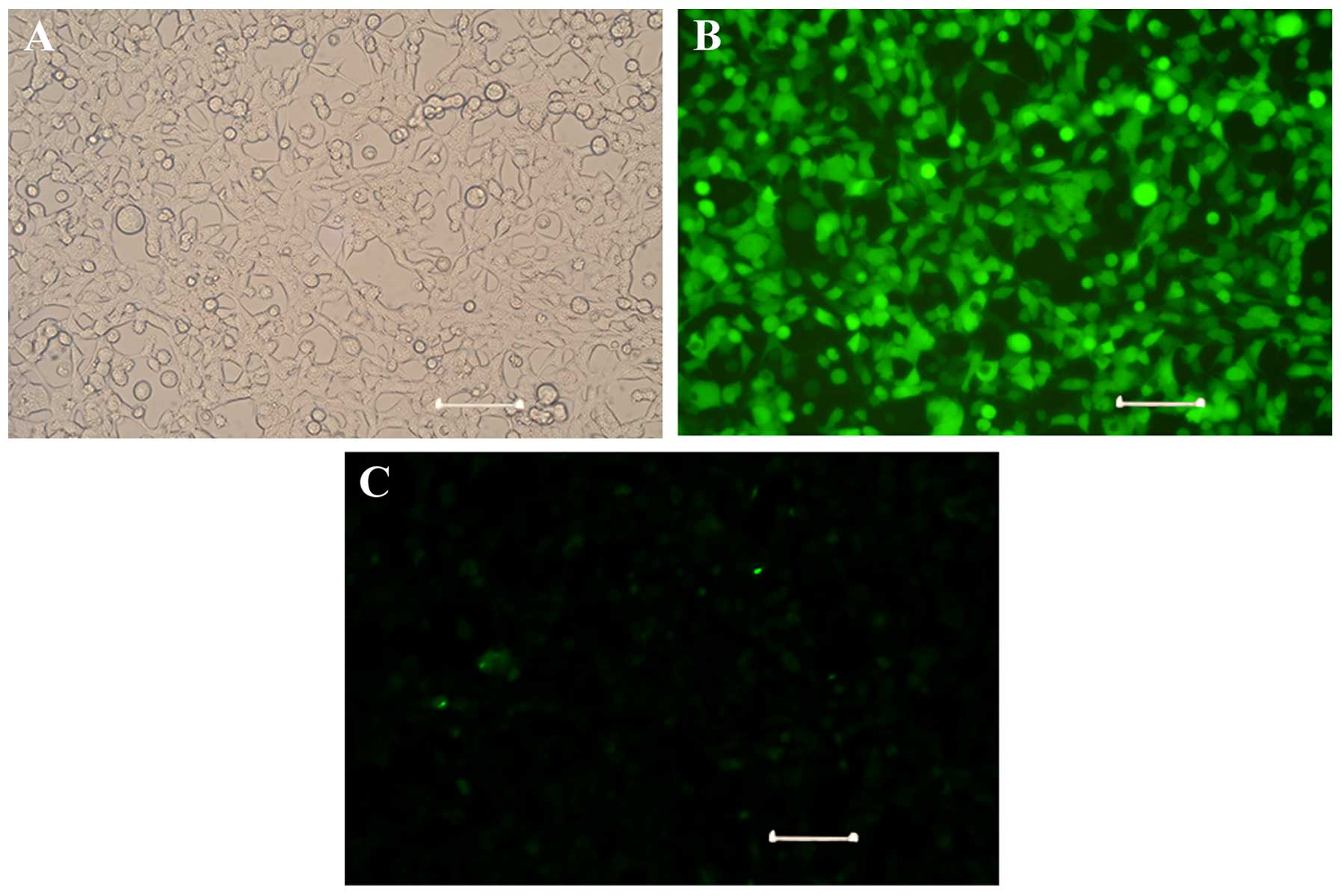
overexpress GFP-tagged miR-296. As shown in Fig. 5, >90% of the cells expressed GFP
compared with the control cells, indicating efficient adenoviral
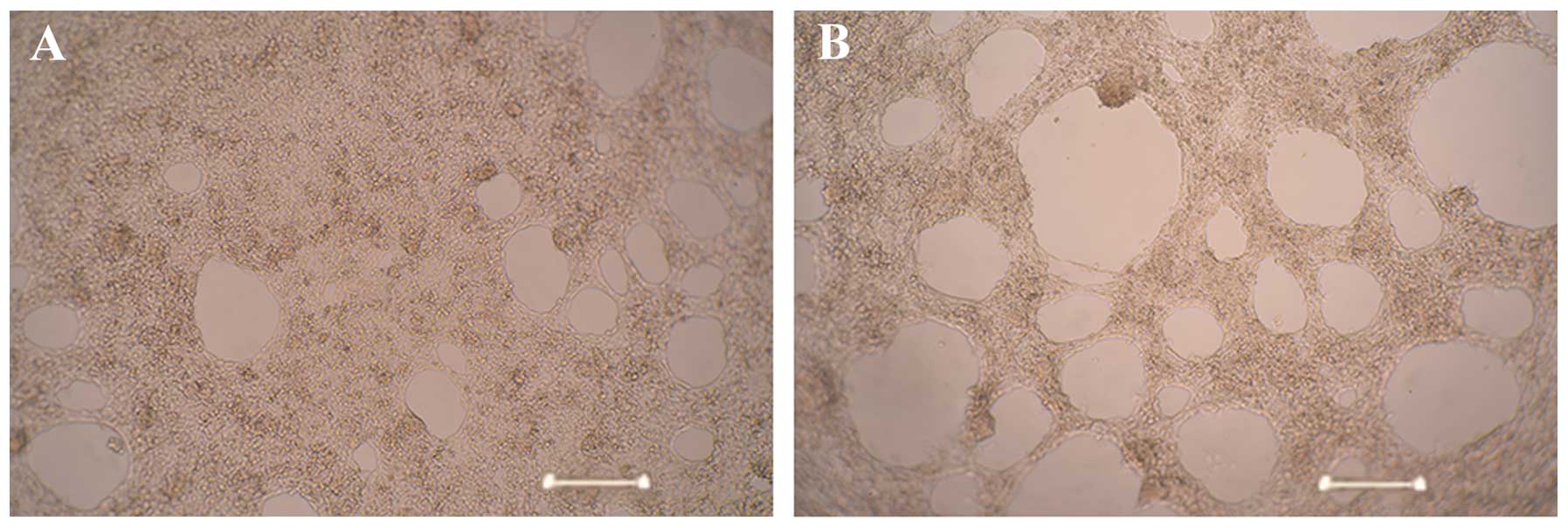
transduction. Tube formation assays demonstrated that the HUVECs
stably overexpressing miR-296 (AdV-Mir-296) formed significantly
more capillary-like structures (28±3.25), compared with the
untransduced control cells (12±2.05; P<0.05; Fig. 6). These results suggested a role
for miR-296 in promoting angiogenesis.
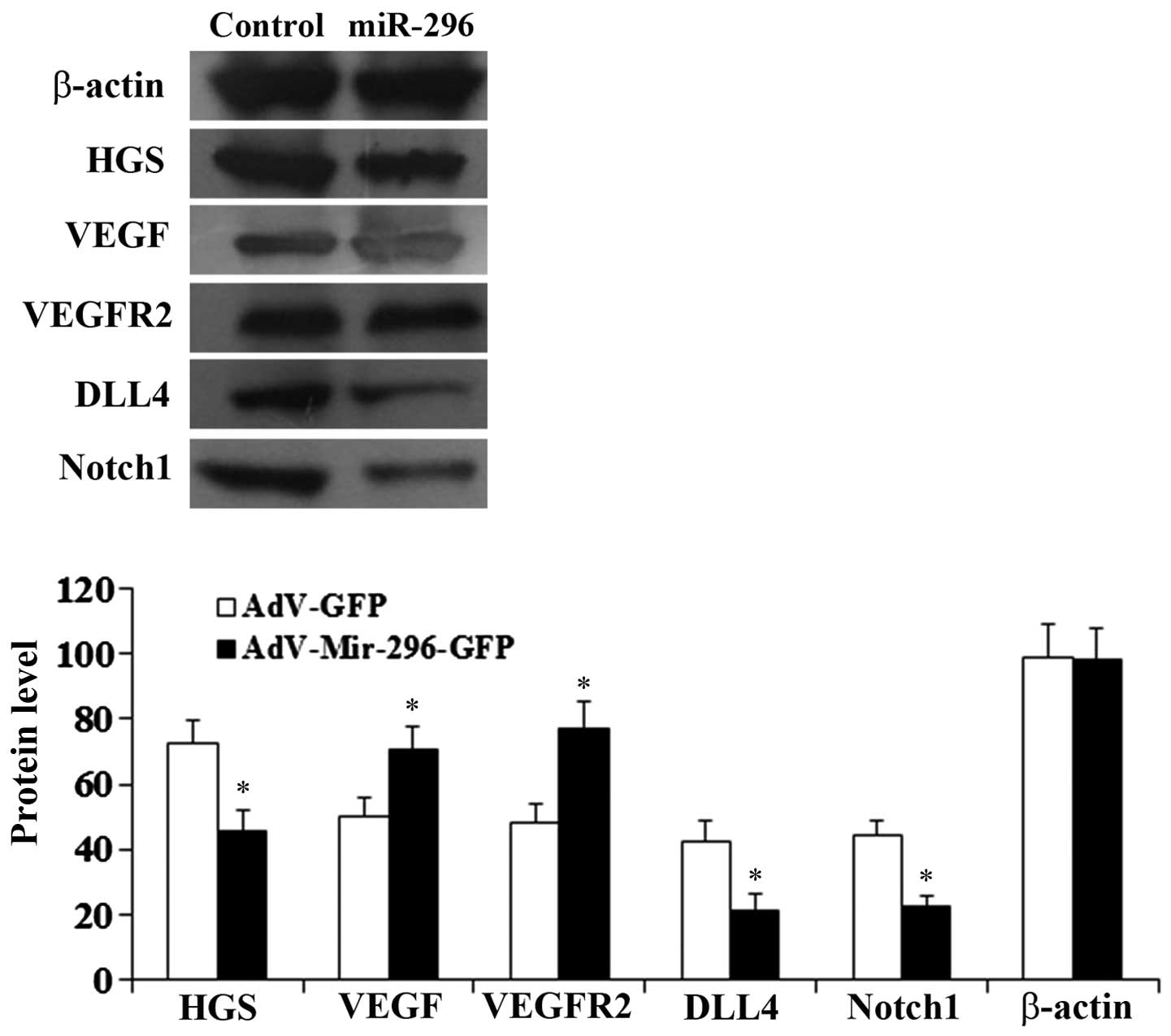
Mechanisms of angiogenesis are mediated
by miR-296
To examine the molecular mechanisms underlying
miR-296-induced angiogenesis, the expression of the components of
the VEGF/VEGFR and DLL4/Notch signaling pathways were examined. As
shown in Figs. 7 and 8, compared with the control HUVECs,
transduced with the empty adenoviral vector, the mRNA and protein
levels of DLL4, Notch1 and HGS were significantly reduced in the
HUEVCs stably overexpressing miR-296 (P<0.05). By contrast, the
mRNA and protein levels of VEGF and VEGFR2 were significantly
increased (P<0.05), compared with the control cells.
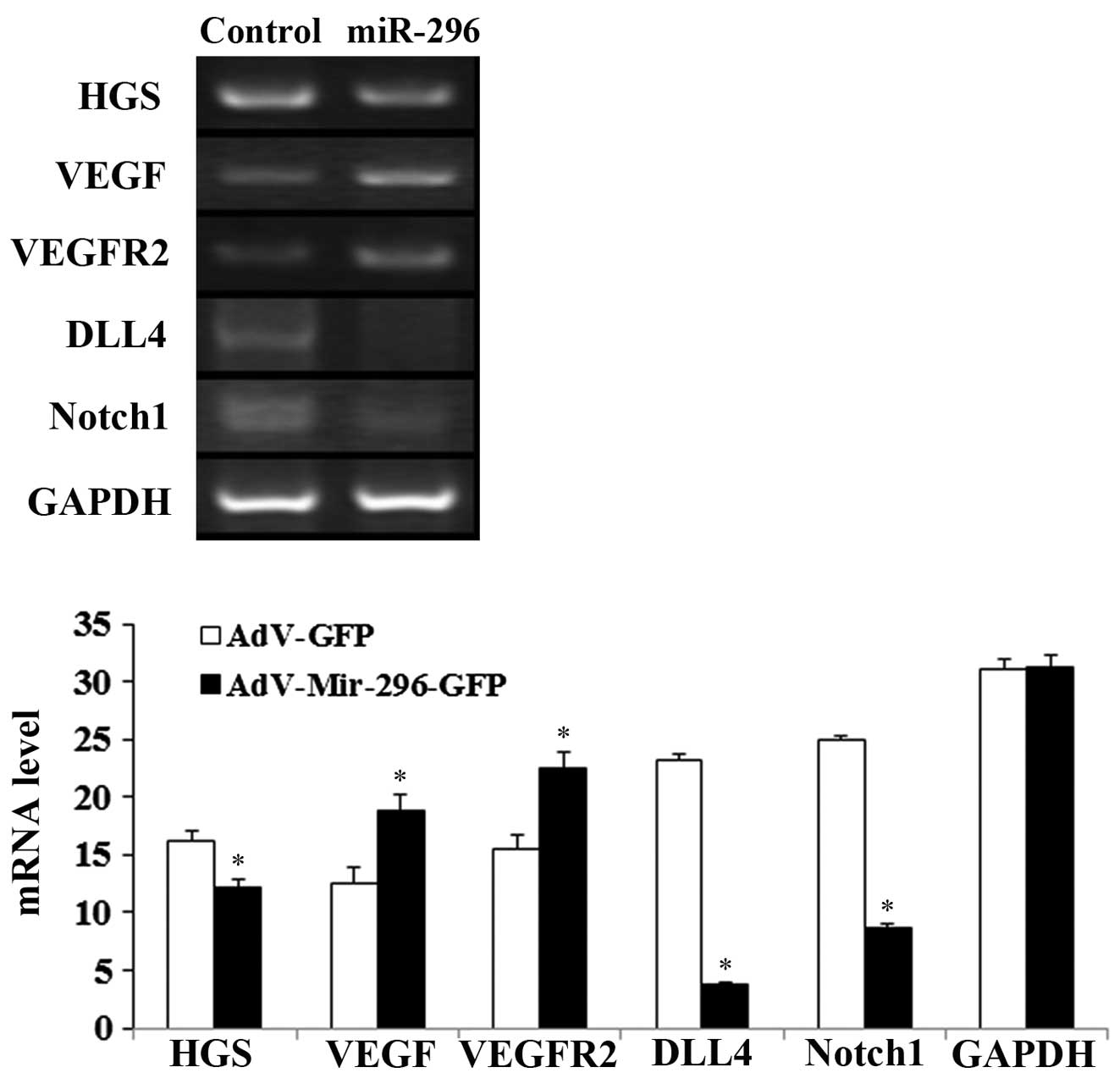 | Figure 7mRNA levels of the VEGF/VEGFR2 and
Notch signaling pathways. mRNA levels of VEGF, VEGFR2, Dll4, Notch1
and HGS in human umbilical vein endothelial cells transduced with
adenoviral miR-296 (AdV-Mir-296) or empty vector (control/AdV-GFP),
determined using reverse transcription-quantitative polymerase
chain reaction. GAPDH was an internal control.
*P<005, vs. control. VEGF, vascular endothelial
growth factor; VEGFR2, VEGF receptor 2; HGS, hepatocyte growth
factor-regulated tyrosine kinase substrate; miR, microRNA; GFP,
green fluorescent protein. |
Discussion
Ischemic stroke is a leading cause of disability,
with few effective treatments available to improve recovery
(13). Since degeneration of
neurons at the perimeter of the penumbra is reversible, the
formation of new blood vessels around the infarcted brain tissue
appears to be important in restoring adequate perfusion to the
ischemic penumbra (14).
Angiogenesis and vasculogenesis are fundamental processes during
formation of new blood vessels, and preclinical and clinical
studies have demonstrated that ischemic events in the brain
stimulate angiogenesis (15–18).
Krupiński et al (19)
demonstrated that the number of new vessels in ischemic penumbral
regions was correlated with increased survival rates in patients
with ischemic stroke, and previous studies have highlighted the
importance of angiogenesis in cerebral infarction (20–24).
Due to the variety of mechanisms involved in
post-ischemic angiogenesis, a broad understanding of the underlying
mechanisms at the cellular and molecular level is important. It has
been observed that microRNAs are key in regulating angiogenesis in
ischemic diseases (4–7). Endothelial-specific miR-126 has been
previously reported to affect the VEGF/VEGFR signaling pathway
(5). Although there are several
previous reports on miRNAs in cardiac ischemia (7,25),
few have focussed on cerebral ischemia (26). A previous study suggested that
miR-210 may regulate angiogenesis and the maturation of vasculature
in post-ischemic brain tissue (26). In the present study, brain
ischemia, induced by MCAO, led to the upregulation of miR-296 in
the ischemic cortex, in parallel with a downregulation in the
expression of HGS and an upregulation of angiogenesis. These
findings are in agreement with a previous study, which indicated
that HGS is a target for miR-296 (8). HGS reportedly mediates the
degradation of VEGFR2 (27). Thus,
miR-296 may increase angiogenesis by promoting VEGF/VEGFR2
signaling through decreasing HGS.
The VEGF and Notch signaling pathways are important
in angiogenesis (28–30). In the present study, overexpression
of miR-296 was observed to increase the expression levels of VEGF
and VEGFR2, and simultaneously reduce the expression levels of DLL4
and Notch1. This was supported by the results of the tube formation
assay, which demonstrated that HUVECs stably overexpressing miR-296
formed significantly more capillary-like structure, compared with
the untransduced control cells. These results also suggested that
there may be another target gene of miR-296, which downregulates
the expression of DLL4/Notch. Further investigations are required
in order to elucidate the underlying mechanisms.
In conclusion, the present study provided in
vivo and in vitro evidence suggesting that miR-296
promoted angiogenesis in the ischemic brain through upregulating
VEGF and downregulating Notch1 following cerebral ischemic injury.
Thus suggesting that miR-296 may promote angiogenesis following a
stroke.
Acknowledgments
This study was supported by the Fundamental Research
Funds for the Central Colleges of Central South University and the
Hunan Natural Science Foundation (grant no. 2013zzts094).
References
|
1
|
Wang Y, Liao X, Zhao X, et al: Using
recombinant tissue plasminogen activator to treat acute ischemic
stroke in China: Analysis of the results from the Chinese National
Stroke Registry (CNSR). Stroke. 42:1658–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jauch EC, Saver JL, Adams HP Jr, et al:
Guidelines for the early management of patients with acute ischemic
stroke: A guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke. 44:870–947.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C: MicroRNomics: A newly emerging
approach for disease biology. Physiol Genomics. 33:139–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang T, Weilang X, Liu Y, et al:
MicroRNAs and stroke: A brief summary of recent research progress.
J Mol Diagn Ther. 4:1–10. 2010.
|
|
5
|
Wang S, Aurora AB, Johnson BA, et al: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
et al: MicroRNA-210 modulates endothelial cell response to hypoxia
and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonauer A, Carmona G, Iwasaki M, et al:
MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Würdinger T, Tannous BA, Saydam O, et al:
miR-296 regulates growth factor receptor overexpression in
angiogenic endothelial cells. Cancer Cell. 14:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldmacher GV, Nasser R, Lee DY, et al:
Tracking transplanted bone marrow stem cells and their effects in
the rat MCAO stroke model. PLoS One. 8:e600492013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lobov IB, Renard RA, Papadopoulos N, et
al: Delta-like ligand 4 (Dll4) is induced by VEGF as a negative
regulator of angiogenic sprouting. Proc Natl Acad Sci USA.
104:3219–3124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bederson JB, Pitts LH, Germano SM, et al:
Evaluation of 2, 3, 5-triphenyltetrazolium chloride as a stain for
detection and quantification of experimental cerebral infarction in
rats. Stroke. 17:1304–1308. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fonsatti E, Altomonte M, Nicotra MR, et
al: Endoglin (CD105): A powerful therapeutic target on
tumor-associated angiogenetic blood vessels. Oncogene.
22:6557–6563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lloyd-Jones D, Adams RJ, Brown TM, et al:
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2010
update: A report from the American Heart Association. Circulation.
121:e46–e215. 2010. View Article : Google Scholar
|
|
14
|
Scheinowitz M: Therapeutic myocardial
angiogenesis: Past, present and future. Mol Cell Biochem.
264:75–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krupiński J, Kałuza J, Kumar P, Kumar S
and Wang JM: Role of angiogenesis in patients with cerebral
ischemic stroke. Stroke. 25:1794–1798. 1994. View Article : Google Scholar
|
|
16
|
Marti HJ, Bernaudin M, Bellail A, et al:
Hypoxia-induced vascular endothelial growth factor expression
precedes neovascularization after cerebral ischemia. Am J Pathol.
156:965–976. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamada Y, Gonda K, Takeda M, et al: In
vivo imaging of the molecular distribution of the VEGF receptor
during angiogenesis in a mouse model of ischemia. Blood.
118:e93–e100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hedhli N, Dobrucki LW, Kalinowski A, et
al: Endothelial-derived neuregulin is an important mediator of
ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res.
93:516–524. 2012. View Article : Google Scholar :
|
|
19
|
Krupiński J, Kaluza J, Kumar P, et al:
Some remarks on the growth-rate and angiogenesis of microvessels in
ischemic stroke. Morphometric and immunocytochemical studies. Patol
Pol. 44:203–209. 1993.
|
|
20
|
Sun Y, Jin K, Xie L, et al: VEGF-induced
neuroprotection, neurogenesis and angiogenesis after focal cerebral
ischemia. J Clin Invest. 111:1843–1851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harrigan MR, Ennis SR, Sullivan SE, et al:
Effects of intra-ventricular infusion of vascular endothelial
growth factor on cerebral blood flow, edema, and infarct volume.
Acta Neurochir (Wien). 145:49–53. 2003. View Article : Google Scholar
|
|
22
|
Navaratna D, Guo S, Arai K and Lo EH:
Mechanisms and targets for angiogenic therapy after stroke. Cell
Adhes Migr. 3:216–223. 2009. View Article : Google Scholar
|
|
23
|
Thurston G, Rudge JS, Ioffe E, et al:
Angiopoietin-1 protects the adult vasculature against plasma
leakage. Nat Med. 6:460–463. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gertz K, Priller J, Kronenberg G, et al:
Physical activity improves long-term stroke outcome via endothelial
nitric oxide synthase-dependent augmentation of neovascularization
and cerebral blood flow. Circ Res. 99:1132–1140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghosh G, Subramanian IV, Adhikari N, et
al: Hypoxia-induced microRNA-424 expression in human endothelial
cells regulates HIF-α isoforms and promotes angiogenesis. J Clin
Invest. 120:4141–4154. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lou Y-L, Guo F, Liu F, et al: miR-210
activates notch signaling pathway in angiogenesis induced by
cerebral ischemia. Mol Cell Biochem. 370:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ewan LC, Jopling HM, Jia H, et al:
Intrinsic tyrosine kinase activity is required for vascular
endothelial growth factor receptor 2 ubiquitination, sorting and
degradation in endothelial cells. Traffic. 7:1270–1282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmeliet P, De Smet F, Loges S and
Mazzone M: Branching morphogenesis and antiangiogenesis candidates:
Tip cells lead the way. Nat Rev Clin Oncol. 6:315–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gerhardt H, Golding M, Fruttiger M, et al:
VEGF guides angiogenic sprouting utilizing endothelial tip cell
filopodia. J Cell Biol. 161:1163–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siekmann AF and Lawson ND: Notch
signalling limits angiogenic cell behaviour in developing zebrafish
arteries. Nature. 445:781–784. 2007. View Article : Google Scholar : PubMed/NCBI
|