Introduction
The mechanism underlying acute myocardial infarction
involves the occurrence of atherosclerosis, followed by
atherosclerotic plaque rupture, hemorrhage and thrombosis or
coronary artery spasm, leading to complete or incomplete coronary
occlusion (1). The recurrence and
mortality rates of acute myocardial infarction are high in China,
at ~10 and 16%, respectively. Following an initial myocardial
infarction, the mortality rate of second relapse increases to
>38% (2).
Inducible nitric oxide synthase (iNOS) is important
in angiogenesis and its generation. Nitric oxide generated by iNOS
catalysis may, in addition to inducing endothelial cell migration
and proliferation, mediate angiogenic action of growth factors,
including vascular endothelial growth factor, fibroblast growth
factor and transforming growth factor (3). Liu et al reported that
tirofiban reduces the size of the no-reflow and infarct of acute
myocardial infarction through suppression of iNOS activity
(4). Zaitone et al provided
evidence that rosuvastatin promotes angiogenesis in acute
myocardial infarction via the control of iNOS in rats (5).
Nuclear factor-κB (NF-κB) is present in almost all
cells, including neuronal and glial cells, as well as brain
endothelial cells and other cells, which can be combined
specifically with gene promoters or enhancer sequences of other
cells to promote transcription and expression, and are closely
associated to the inflammatory response, immune response, and the
proliferation, differentiation and apoptosis of cells (6,7). Sui
et al suggested that huperzine A ameliorates the damage
induced by acute myocardial infarction through inhibition of the
expression of the NF-κB subunit p65 in rats. Qiao et al
demonstrated that anesthetic preconditioning protects rat hearts
from ischemia/reperfusion (I/R) injury through the attenuation of
NF-κB activation (8).
Monocyte chemotactic protein-1 (MCP-1) promoter
sequence analysis has revealed that there is an activating
protein-1 (AP-1) binding site in the promoter region and NF-κB
binding sites, indicating that AP-1 activation is involved in
regulating the expression of MCP-1 (9). Interleukin-1β (IL-1β) induces the
expression of MCP-1 through the activation of NF-κB and AP-1, while
the proteasome inhibitor induces the expression of MCP-1 by the
AP-1 signaling pathway (10).
Valen et al reported that NF-κB and AP-1 regulate the
inflammatory genes in the hearts of patients with unstable angina
(11), and Xie et al
suggested that activation of the AP-1 transcription pathway may be
important in ventricular remodeling of myocardial infarction
(12).
Several studies (13–15)
have demonstrated the close association between heat shock protein
72 (HSP7) and myocardial protection, and the extent of HSP72
induction and myocardial protection intensity presents a positive
correlation (13). However, as the
induction of the endogenous expression of HSP72 requires sublethal
stress, it cannot be used clinically (11). Tanonaka et al suggested that
trandolapril increases tolerance against heat stress-induced
deterioration of acute myocardial infarction through HSP72 and
HSP73 (13), and Valen et
al reported that unstable angina activates myocardial
infarction through the activation of HSP72, and the suppression of
NF-κB and AP-1 (11).
Candesartan is an angiotensin II receptor
antagonist, belonging to diphenyl imidazole, with a similar effect
to losartan. The binding force of candesartan with angiotensin
receptor 1 is 80 times higher, compared with that of losartan
(16,17). Candesartan restores reperfusion
injury through HSP72 in hereditary insulin-resistant rats (18). The present study aimed to estimate
the potential effects of candesartan against acute myocardial
infarction, examine whether candesartan can treat acute myocardial
infarction and further investigate the mechanism underlying its
effects.
Materials and methods
Drug administration
The chemical structure of candesartan
(Sigma-Aldrich; purity 98%) is indicated in Fig. 1, which was dissolved in
physiological saline. The casein kinase (CK; cat. no. GA-PM0252MO;
GenAsia Biotech, Co., Ltd., Shanghai China), the MB isoenzyme of
creatine kinase (CK-MB; cat. no. E-EL-R1327c; Elabscience
Biotechnology Co., Ltd., Wuhan, China), lactate dehydrogenase (LDH;
cat. no. E-EL-R0338c, Elabscience Biotechnology Co., Ltd.) and
cardiac troponin T (cTnT; cat. no. E-EL-R0151c; Elabscience
Biotechnology Co., Ltd.) commercial kits, and iNOS (cat. no.
E-EL-R0520c; Elabscience Biotechnology Co., Ltd.), NF-κB p65 (cat.
no. ybA467Ge; Shanghai Yantuo Biotechnology) and MCP-1 (cat. no.
E-EL-R0633c, Elabscience Biotechnology Co., Ltd.) commercial enzyme
linked immunosorbent assay (ELISA) kits were purchased from
Beyotime Institute of Biotechnology (Nanjing, China). TRIzol
reagent was purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA). A PrimeScript RT reagent kit was purchase from Takara
Biotechnology, Co., Ltd. (Dalian, China). The Bicinchoninic Acid
protein assay and caspase-3 and caspase-9 activities colorimetric
kits were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China).
Animals
A total of 30 male Wistar rats (8-week-old; 280-300
g) were purchased from The Animal Center of Xi'an Jiaotong
University, and animal experiments were performed in accordance
with the Guide for the Care and Use of Laboratory Animals (19), and approved by the ethics committee
of the Xi'an Jiaotong University (Xi'an, China). These rats were
provided with access to water and rodent chow ad libitum,
and were housed under a 12 h/12 h light/dark cycle at 22–24°C.
Myocardial infarction induction and
experimental groups
Initially, all rats were anesthetized with sodium
pentobarbitone (40 mg/kg; Sigma-Aldrich) prior to surgery being
performed. The myocardial infarction rat model was induced by
ligating the left anterior descending coronary artery, as described
previously (20,21). The myocardial infarction rat model
was confirmed by regional cyanosis of the myocardial surface with
measurement of ST-segment elevation.
Briefly, the rats were divided into the following
three groups, one group per cage: Control group (n=10), comprising
normal rats administered with saline solution (0.1 ml/100 g);
vehicle group (n=10), comprising the myocardial infarction rat
model administered with saline solution (0.1 ml/100 g); candesartan
treatment group (n=10), comprising the myocardial infarction rat
model administered with candesartan 0.25 mg/kg for 2 weeks via the
caudal vein, on the basis of the previous report (18). Surgery was performed on the rats in
the myocardial infarction group by occlusion of the coronary artery
30 mins following the final administration.
Measurement of the activities of CK,
CK-MB, LDH and the level of cTnT
Following treatment with candesartan for 2 weeks,
whole bloods samples were collected from the rats in each group
following occlusion of the coronary artery for 6 h. Subsequently,
the supernatants were centrifuged at 12,000 g for 10 min at 4°C and
were stored for subsequent measurement at −20°C. According to the
manufacturer's instructions (Beyotime Institute of Biotechnology),
the activities of CK, CK-MB and LDH, and the level of cTnT in the
rats were determined using a suite of commercial kits according to
the manufacturer's instructions at 37°C.
Infarct size measurement
Following treatment with candesartan for 2 weeks,
three rats from each group were sacrificed using decollation, then
the hearts of the rats were immediately harvested and washed with
physiological saline. The hearts of the rats were immediately
washed with physiological saline, and the infarct size was
measured. The left ventricle was placed at −80°C for 10 min
following ligation of the coronary artery for 6 h. Infarct size was
measured following the addition of 1% 2,3,5-triphenyltetrazolium
chloride (1.5%; Sigma-Aldrich) at 37°C for 30 min in the dark
(22,23). The size of the infarct area was
measured by the volume and weight, as a percentage of the left
ventricle. The heart areas exhibiting a brick red color were
considered to be normal myocardium, whereas the areas without color
were considered to be ischemic heart muscles.
Western blot analysis of the protein
expression levels of iNOS and HSP-72
Following treatment with candesartan for 2 weeks,
cardiac cytosolic samples were collected from each group following
occlusion of the coronary artery for 6 h. The samples were
incubated with 100 µl tissue lysis buffer [1% (w/v) Triton,
0.1% SDS, 0.5% deoxycholate, 1 mmol/l EDTA, 20 mmol/l Tris (pH
7.4), 150 mmol/l NaCl, 10 mmol/l NaF; Beyotime Institute of
Biotechnology] for 20 min on ice. Subsequently, the supernatants
were centrifuged at 12,000 g for 10 min at 4°C and were stored for
subsequent measurement at −20°C. The protein concentrations were
determined using a Bicinchoninic Acid protein assay kit (Sangon
Biotech Co., Ltd.). An equal quantity of total protein (30
µg) was fractioned on 12% sodium dodecyl
sulfate-polyacrylamide gels (Beyotime Institute of Biotechnology),
and transferred electrophoretically onto polyvinylidene fluoride
membranes (0.22 mm; EMD Millipore, Billerica, MA, USA). The
membranes were blocked with phosphate-buffered saline (PBS) with 5%
non-fat milk to block nonspecific binding sites. Following
blocking, the membranes were incubated with monoclonal rabbit
anti-human anti-iNOS (1:1,500; cat. no. sc-49055; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), monoclonal rabbit
anti-HSP-72 (1:1,000; cat. no. sc-59570; Santa Cruz Biotechnology,
Inc.) and monoclonal rabbit anti-β-actin (1:3,000; cat. no.
D110007; Sango Bioscience, Beijing, China) overnight at 4°C. The
following day, the membrane was detected by incubation with sheep
anti-rabbit IgG (1:10,000; cat. no. C000944; Sango Bioscience),
conjugated with horseradish peroxidase at 37°C for 2 h. The
relative band intensity was determined using Chem Image 5500
software (UVP, Upland, CA, USA).
ELISA to determine the activities of iNOS
and NF-κB p65
Following treatment with candesartan for 2 weeks,
whole bloods samples were collected from the rats in each group,
following occlusion of the coronary artery for 6 h. Subsequently,
the supernatant were centrifuged at 12,000 × g for 10 min at 4°C
and were stored for subsequent measurement at −20°C. According to
the manufacturer's instructions (Beyotime Institute of
Biotechnology), the activities of iNOS and NF-κB p65 in the rats
were performed using a suite of commercial ELISA kits according to
the manufacturer's instructions at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses of the gene expression
levels of MCP-1 and AP-1
Following treatment with candesartan for 2 weeks,
cardiac cytosolic samples were collected from each group following
occlusion of the coronary artery for 6 h. Total RNA was obtained
from the myocardial samples using TRIzol reagent (Invitrogen Life
Technologies). RNA (1 µg) was reverse transcribed into cDNA
using a One Step PrimeScript miRNA cDNA Synthesis kit (Takara Bio,
Inc.), according to the manufacturer's instructions. The gene
expression levels of MCP-1 and AP-1 were measured by RT-qPCR using
a CFX96 Touch Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and a PrimeScript RT reagent kit, according to
manufacturer's instructions. The sequences of the AP-1 primers were
as follows: Forward 5′-AAC TGA AGC TCG CAC TCTCG-3′ and reverse
5′-TCA GCA CAG ATC TCC TTGGC-3′. The sequences of the MCP-1 primers
were as follows: Forward 5′-AAG TGT GAT GAC TCA GGT TTG CCC TGA-3′
and reverse 5′-AAG TGT GAT ATC TCA GGT TTG CCC TGA-3′. The GAPDH
primer sequences were as follows: Forward 5′-GCA CCG TCA AGG CTG
AGAAC-3′ and reverse 5′-TGG TGA AGA CGC CAG TGGA-3′. The reaction
was conducted with the following conditions: 95°C for 5 min, 40
cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec.
Measurement of caspase-3 and caspase-9
activities
Following treatment with candesartan for 2 weeks,
cardiac cytosolic samples were collected from the rats in each
group following occlusion of the coronary artery for 6 h. A total
of 50 µg protein was incubated in solution buffer
(Ac-DEVD-pNA for caspase-3; Ac-LEHD-pNA for caspase-9; BD
Biosciences, San Jose, CA, USA) at 37°C for 30 min in the dark.
According to the manufacturer's instructions (Sangon Biotech Co.,
Ltd.), the activities of caspase-3 and caspase-9 were measured at
an absorbance of 405 nm using a Bio-Rad 680 microplate reader
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA) and are presented as the mean ±
standard deviation. Statistical analyses were performed using
one-way analysis of variance, followed by Dunnett's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Candesartan suppresses the activities of
CK, CK-MB and LDH, and the level of cTnT in a rat model of acute
myocardial infarction
To determine the protective effects of candesartan
on the heart of the acute myocardial infarction rat model, the
present study examined the activities of CK, CK-MB and LDH, and the
level of cTnT in a rat model of acute myocardial infarction. The
measurements of serum levels of CK, CK-MB, LDH and cTnT in the
control group, vehicle group and candesartan treatment group (0.25
mg/kg) are summarized in Table I.
The activities of CK, CK-MB and LDH, and the level of cTnT were
significantly increased in the serum of the vehicle group, compared
with the control group. Pretreatment with candesartan at a dose of
0.25 mg/kg markedly reduced the activities of CK, CK-MB and LDH,
and the level of cTnT in the serum of the rat model of acute
myocardial infarction, compared with that in the vehicle group
(Table I).
 | Table ICandesartan suppresses the activities
of CK, CK-MB and LDH, and the level of cTnT in a rat model of
myocardial infarction. |
Table I
Candesartan suppresses the activities
of CK, CK-MB and LDH, and the level of cTnT in a rat model of
myocardial infarction.
| Group | CK (U/ml) | CK-MB (IU/l) | LDH (U/l) | cTnT (U/ml) |
|---|
| Control | 0.24±0.02 | 86.74±6.83 | 1711.14±353.58 | 0.08±0.03 |
| Vehicle | 0.91±0.05a | 205.39±9.32a |
5854.12±431.04a | 0.57±0.05a |
| CAN (0.25) | 0.43±0.03b | 112.38±7.56b |
3252.37±326.77b | 0.25±0.03b |
Candesartan suppresses infarct size in a
rat model of acute myocardial infarction
The infarct size in the vehicle group was
42.56±1.23%. Following treatment with candesartan (0.25 mg/kg), the
infarct size of the acute myocardial infarction group was
significantly reduced to 31.89±2.11%, compared with the vehicle
group (Fig. 2).
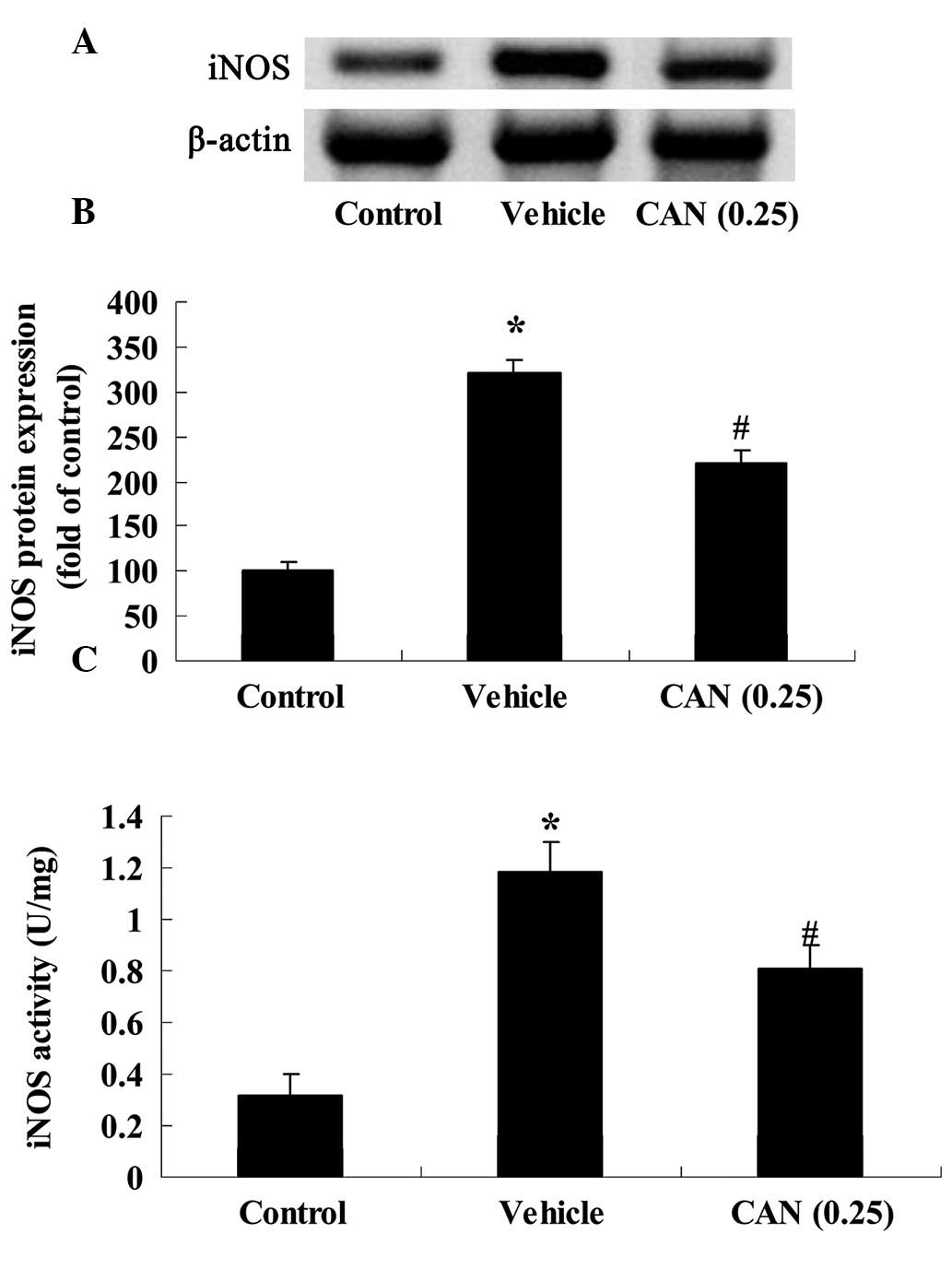
Candesartan inhibits the expression and
activity of iNOS in a rat model of acute myocardial infarction
To more directly determine whether candesartan (0.25
mg/kg) inhibited nitric oxide in the rat model of acute myocardial
infarction, the protein expression of iNOS in the rat model of
acute myocardial infarction was evaluated using western blot
analysis in the present study. The protein expression of iNOS in
the vehicle group was significantly increased in the rat model of
acute myocardial infarction, compared with the control group
(Fig. 3A and B). However,
following treatment with candesartan at a dose of 0.25 mg/kg, a
marked reduction in the protein expression of iNOS was noted in the
rat model of acute myocardial infarction, compared with that in the
vehicle group (Fig. 3A and B). In
addition, the activity of iNOS in the vehicle group was markedly
elevated, compared with the control group (Fig. 3C). In the candesartan treatment
groups (0.25 mg/kg), the activity of iNOS was significantly
decreased, compared with that in the control group (Fig. 3C).
Candesartan inhibits the activity of
NF-κB in a rat model of acute myocardial infarction
In order to corroborate the protection of
candesartan on NF-κB p65 activity in a rat model of acute
myocardial infarction, the activity of NF-κB p65 in the rat model
of acute myocardial infarction was determined using a commercial
ELISA kit. The results demonstrated that the activity of NF-κB p65
in the vehicle group increased, compared with the control group
(Fig. 4). Notably, treatment with
candesartan (0.25 mg/kg) reversed the effects of increased NF-κB
p65 activity, which were observed in the vehicle group (Fig. 4).
Candesartan inhibits the expression of
AP-1 in a rat model of acute myocardial infarction
The present study aimed to investigate whether
candesartan (0.25 mg/kg) had protective effects on the expression
of AP-1 in the rat model of acute myocardial infarction, The
present study examined the gene expression of AP-1 using RT-qPCR.
In the left ventricle, the gene expression of AP-1 in the vehicle
group was markedly elevated, compared with that in the control
group (Fig. 5). Ppretreatment with
candesartan (0.25 mg/kg) was observed to reduce the gen expression
of AP-1, compared with that in the vehicle group (Fig. 5).
Candesartan inhibits the expression of
MCP-1 in a rat model of acute myocardial infarction
The present study further analyzed the protective
effects of candesartan on the activity of MCP-1 in the rat model of
acute myocardial infarction, by determining the gene expression
levels of MCP-1 using RT-qPCR. In the vehicle group, the gene
expression of MCP-1 was remarkably increased, compared with that in
the control group (Fig. 6).
However, following treatment with candesartan (0.25 mg/kg) for 2
weeks, the gene expression of MCP-1 was decreased significantly,
compared with that in the vehicle group (Fig. 6).
Candesartan restores the expression of
HSP-72 in a rat model of acute myocardial infarction
To more directly determine whether candesartan
restores the expression of HSP-72 in a rat model of acute
myocardial infarction, the protein expression of HSP-72 was
determined using western blot analysis the present study. The
protein expression of HSP-72 in the vehicle group was markedly
decreased, compared with that in the control group (Fig. 7A and B). By contrast, pretreatment
with candesartan (0.25 mg/kg) increased the protein expression of
HSP-72, compared with that in the vehicle group (Fig. 7A and B).
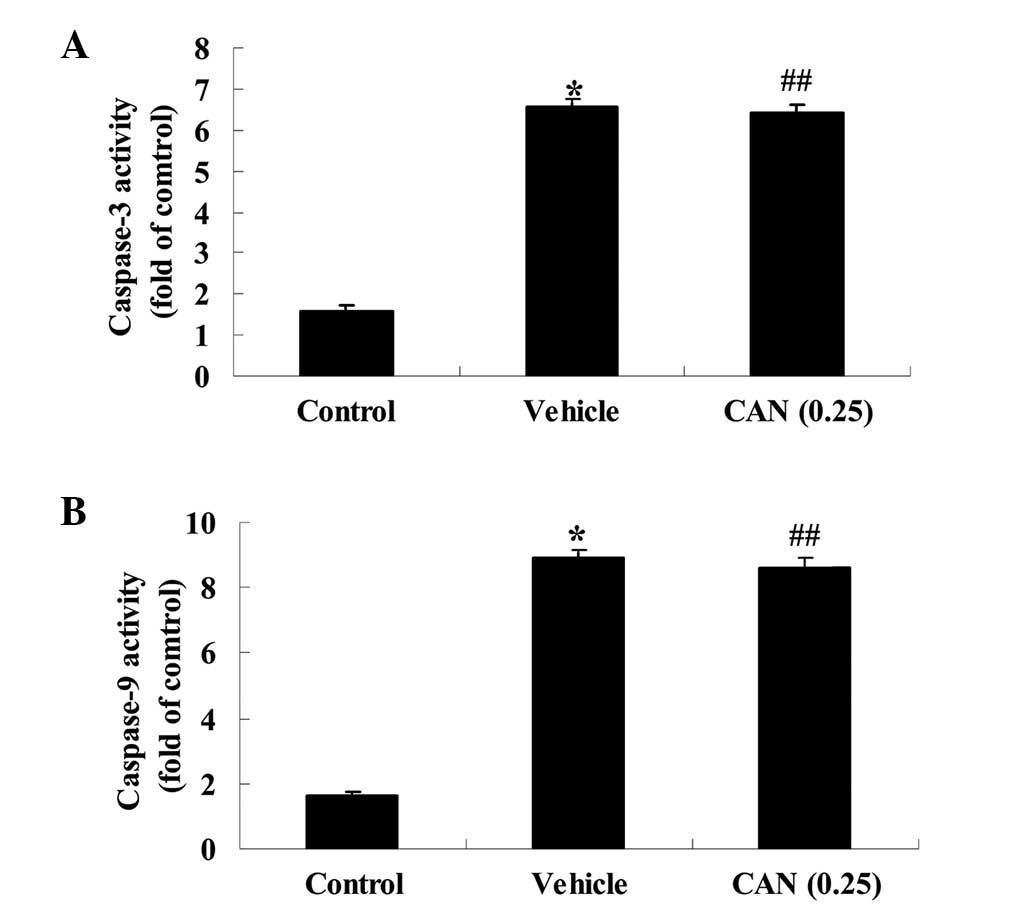
Candesartan alters the activities of
caspase-3 and caspase-9 in a rat model of acute myocardial
infarction
To investigate the mechanism of candesartan on acute
myocardial infarction, the activities of caspase-3 and caspase-9
were examined using colorimetric kits in the present study. The
activities of caspase-3 and caspase-9 in the vehicle group were
markedly increased, compared with those in the control group
(Fig. 8A and B). However, these
activities were not altered following treatment with candesartan
(0.25 mg/kg) in the candesartan treatment group, compared with the
vehicle group (Fig. 8A and B).
Discussion
With economic developments in society and the
improvement of living standards, more individuals are being found
to exhibit high blood pressure, high cholesterol and high blood
sugar, and patients with angina pectoris and myocardial infarction
are also following an increasing trend (24). The irreversible necrosis caused by
myocardial ischemic myocardial disease results in repeated episodes
of heart failure, which is the leading cause of mortality worldwide
(25). In the present study, the
activities of CK, CK-MB and LDH, and the level of cTnT were
observed to increase in the serum of a rat model of acute
myocardial infarction following candesartan (0.25 mg/kg) treatment.
In addition, the size of the acute myocardial infarction was
significantly reduced in these rats. Suzuki et al reported
that candesartan is more effective than angiotensin-converting
enzyme inhibitors in preventing left ventricular remodeling
following acute myocardial infarction (26).
There are two types of NOS for endothelial cells and
cardiomyocytes: Primary NOS of calcium/calmodulin-dependence and
iNOS of calcium/calmodulinnon-dependence (27). Endothelial cells synthesize a small
quantity of nitric oxide under physiological conditions by primary
NOS, which is important in the diffusion of nitric oxide into
vascular smooth muscle cells, vascular relaxation through cyclic
guanosine monophosphate pathway; entering the bloodstream to
inhibit the activation, adhesion and aggregation of platelets and
leukocytes; reducing the generation of superoxide radicals through
direct inhibition of reduced nicotinamide adenine dinucleotide
phosphate oxidase in neutrophils; and regulating the expression of
adhesion molecules in neutrophils and endothelial cells (28). Following acute myocardial
infarction, the basic function of coronary artery endothelial cells
and the release function of nitric oxide stimulation by
acetylcholine are impaired, and neutrophil-endothelial cell
adhesion is promoted. The present study found that the protein
expression and activity of iNOS were significantly reduced in a rat
model of acute myocardial infarction. Bian et al reported
the association between the effects of candesartan and NOS in
spontaneous hypertensive rats (29), and Miller et al identified
that candesartan attenuates diabetic retinal vascular pathology by
the reduction of iNOS (30).
NF-κB has a central regulatory role in a variety of
tissue and organ injuries (31).
As a nuclear transcription factor with multi-directional regulation
effect, NF-κB regulates the expression of a number of important
immune factors, and may be involved in the promotion of
inflammatory cytokine and chemokine generation, the proliferation
differentiation of fibroblasts, extracellular matrix crosslinking
and apoptosis, and is involved in the process of acute myocardial
infarction, promoting the progression of acute myocardial
infarction disease (7,32,33).
Therefore its role in the development of acute myocardial
infarction cannot be ignored. In the present study, candesartan was
observed to attenuate NF-κB p65 activity in the rat model of acute
myocardial infarction. Furthermore, Hadi et al reported that
candesartan significantly attenuates atherosclerotic lesions and
significantly reduces NF-κB activity in rabbits (34). In addition, Ishrat et al
demonstrated that candesartan reduces hemorrhage in rat embolic
stroke through a reduction in NF-κB activity (35).
MCP-1 is a chemokine of CC-type cells, involved as
signaling molecules. There are NF-κB and AP-1 binding sites in the
gene promoter site, which can be combined with the DNA binding
sequence of NF-κB, thus activating the transcription of the gene.
Experiments have demonstrated that MCP-1 can protect neonatal
myocardial cells of mice from hypoxic death, and its cytoprotective
and chemotaxis effects are achieved through different signaling
mechanisms. In the present study, the gene expression of MCP-1 was
decreased by candesartan treatment. Candesartan has also been
observed to inhibit LPS-induced gene expression through the
suppression of MCP-1 protein concentrations in human renal tubular
epithelial cells (36). Hadi et
al reported that candesartan retards the progression of
atherosclerosis via reducing MCP-1, NF-κβ and oxidative pathways
(34).
The AP-1 transcription factor is an important target
of intracellular c-Jun N-terminal kinase/stress-activated protein
kinase protein products, encoded by immediate early genes in the
nucleus, c-fos and c-Jun, from Fos and Jun family proteins
respectively, which form homogenous or heterogeneous dimers through
combination in a leucine zipper structure, to constitute the AP-1
transcription factor (11). The
results of the present study demonstrated that candesartan reduced
the gene expression of AP-1 in a rat model of acute myocardial
infarction. Tharaux et al indicated that AP-1-receptor
antagonism by candesartan activates the collagen I gene through the
mitogen-acitvated protein/extracellular-signal regulated kinase
pathway (37).
Previous studies have reported that HSP70 is an
important cardioprotective protein, which is involved in delayed
myocardial protection. (38,39)
It has been demonstrated that heat shock for 24 h can increase
myocardial tolerance to I/R injury, manifested by a reduction in
infarct size (18). There are two
types of method known to increase HSP72 content in cardiomyocytes.
The first is the induction of the endogenous expression of HSP72,
usually realized by heat stress at sublethal doses or by other
harmful stimulating factors, including ischemia, hypoxia, ethanol,
heavy metal salts and infection (40). The second is to guide exogenous
HSP70 genes, to increase the expression levels of cardiac HSP72 by
transgenic technology (11). The
present study demonstrated that candesartan increased the protein
expression of HSP-72 in a rat model of acute myocardial infarction.
Taniguchi et al also suggested that candesartan protects
against reperfusion injury in hereditary insulin-resistant rats
through recovery of the expression of HSP72 (18).
Previous studies have revealed that candesartan
improves peak systolic pressure, however, it does not alter the
level of apoptosis or the expression of caspase-3 (41–43).
Therefore, the present study examined the association between the
protective effect of candesartan on acute myocardial infarction and
the apoptosis of myocardial cells. In the present study,
candesartan had no affect on cell apoptosis in acute myocardial
infarction, or on the activities of caspase-3 and caspase-9 in the
rat model of acute myocardial infarction. These results indicated
that the protective effect of candesartan on acute myocardial
infarction may involve several pathways. In conclusion, the present
study demonstrated that the protective effect of candesartan
ameliorated acute myocardial infarction in rats and revealed that
its activity regulated iNOS, NF-κB, MCP-1, AP-1 and HSP72 in the
rat model of acute myocardial infarction. Further investigations
are required to clarify the other key signaling pathways of the
interaction between the protective effect of candesartan and acute
myocardial infarction.
Further investigations are required to clarify the
other signaling pathways underlying the interaction between the
protective effects of candesartan and acute myocardial infarction
for clinical application.
Acknowledgments
This study was supported by the Key Program of the
National Natural Science Foundation of China (grant no. 30830051),
the '13315' Scientific and Technological Innovation Project of
Shaanxi Province (grant no. 2008ZDKG-62) and the Scientific and
Technological Projects for Social Development of Shaanxi Province
(grant no. 2015SF024).
References
|
1
|
Zhong J, He Y, Chen W, Shui X, Chen C and
Lei W: Circulating microRNA-19a as a potential novel biomarker for
diagnosis of acute myocardial infarction. Int J Mol Sci.
15:20355–20364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao YJ, Fu XH, Ma XX, Wang DY, Dong QL,
Wang YB, Li W, Xing K, Gu XS and Jiang YF: Intracoronary fixed dose
of nitro-6prusside via thrombus aspiration catheter for the
prevention of the no-reflow phenomenon following primary
percutaneous coronary intervention in acute myocardial infarction.
Exp Ther Med. 6:479–484. 2013.PubMed/NCBI
|
|
3
|
Gui D, Li Y, Chen X, Gao D, Yang Y and Li
X: HIF1 signaling pathway involving iNOS, COX2 and caspase-9
mediates the neuroprotection provided by erythropoietin in the
retina of chronic ocular hypertension rats. Mol Med Rep.
11:1490–1496. 2015.
|
|
4
|
Liu X and Tao GZ: Effects of tirofiban on
the reperfusion-related no-reflow in rats with acute myocardial
infarction. J Geriatr Cardiol. 10:52–58. 2013.PubMed/NCBI
|
|
5
|
Zaitone SA and Abo-Gresha NM: Rosuvastatin
promotes angiogenesis and reverses isoproterenol-induced acute
myocardial infarction in rats: Role of iNOS and VEGF. Eur J
Pharmacol. 691:134–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiong HY, Ma TT, Wu BT, Lin Y and Tu ZG:
IL-12 regulates B7-H1 expression in ovarian cancer-associated
macrophages by effects on NF-kB signalling. Asian Pac J Cancer
Prev. 15:5767–5772. 2014. View Article : Google Scholar
|
|
7
|
Sui X and Gao C: Huperzine A ameliorates
damage induced by acute myocardial infarction in rats through
antioxidant, anti-apoptotic and anti-inflammatory mechanisms. Int J
Mol Med. 33:227–233. 2014.
|
|
8
|
Qiao S, Xie H, Wang C, Wu X, Liu H and Liu
C: Delayed anesthetic preconditioning protects against myocardial
infarction via activation of nuclear factor-kB and upregulation of
autophagy. J Anesth. 27:251–260. 2013. View Article : Google Scholar
|
|
9
|
Tang M, Wang Y, Han S, Guo S, Xu N and Guo
J: Endogenous PGE(2) induces MCP-1 expression via EP4/p38 MAPK
signaling in melanoma. Oncol Lett. 5:645–650. 2013.PubMed/NCBI
|
|
10
|
Martin T, Cardarelli PM, Parry GC, Felts
KA and Cobb RR: Cytokine induction of monocyte chemoattractant
protein-1 gene expression in human endothelial cells depends on the
cooperative action of NF-kappa B and AP-1. Eur J Immunol.
27:1091–1097. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valen G, Hansson GK, Dumitrescu A and
Vaage J: Unstable angina activates myocardial heat shock protein
72, endothelial nitric oxide synthase, and transcription factors
NFkappaB and AP-1. Cardiovasc Res. 47:49–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie SL, Wang JF, Nie RQ, Yuan WL, Li F and
Lin YQ: The expression and significance of activator protein-1 and
matrix metalloproteinases in the human heart post acute myocardial
infarction. Zhonghua Nei Ke Za Zhi. 48:205–207. 2009.In Chinese.
PubMed/NCBI
|
|
13
|
Tanonaka K, Toga W, Yoshida H, Furuhama K
and Takeo S: Effect of long-term treatment with trandolapril on
Hsp72 and Hsp73 induction of the failing heart following myocardial
infarction. Br J Pharmacol. 134:969–976. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marunouchi T, Araki M, Murata M, Takagi N
and Tanonaka K: Possible involvement of HSP90-HSF1 multichaperone
complex in impairment of HSP72 induction in the failing heart
following myocardial infarction in rats. J Pharmacol Sci.
123:336–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gholitabar S and Roshan VD: Effect of
treadmill exercise and Ferula gummosa on myocardial HSP72, vascular
function, and antioxidant defenses in spontaneously hypertensive
rats. Clin Exp Hypertens. 35:347–354. 2013. View Article : Google Scholar
|
|
16
|
Songur CM, Songur MO, Kocabeyoglu SS and
Basgut B: Effects of the AT1 receptor blocker candesartan on
myocardial ischemia/reperfusion in isolated rat hearts. Heart Surg
Forum. 17:E263–E268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jusufovic M, Sandset EC, Bath PM and Berge
E; Scandinavian Candesartan Acute Stroke Trial Study Group: Blood
pressure-lowering treatment with candesartan in patients with acute
hemorrhagic stroke. Stroke. 45:3440–3442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taniguchi Y, Takahashi N, Fukui A,
Nagano-Torigoe Y, Thuc LC, Teshima Y, Shinohara T, Wakisaka O, Ooie
T, Murozono Y, et al: Candesartan restored cardiac Hsp72 expression
and tolerance against reperfusion injury in hereditary
insulin-resistant rats. Cardiovasc Res. 92:439–448. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marx JO, Brice AK, Boston RC and Smith AL:
Incidence rates of spontaneous disease in laboratory mice used at a
large biomedical research institution. J Am Assoc Lab Anim Sci.
52:782–791. 2013.PubMed/NCBI
|
|
20
|
Faria Tde O, Baldo MP, Simões MR, Pereira
RB, Mill JG, Vassallo DV and Stefanon I: Body weight loss after
myocardial infarction in rats as a marker of early heart failure
development. Arch Med Res. 42:274–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng KW, Zhang T, Fu H, Liu GX and Wang
XM: Schisandrin B exerts anti-neuroinflammatory activity by
inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-kB
signaling pathway in lipopolysaccharide-induced microglia. Eur J
Pharmacol. 692:29–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova
MA, Johnson MH, Ergul A, Hill WD, Hess DC and Sazonova IY:
Sex-independent neuroprotection with minocycline after experimental
thrombo-embolic stroke. Exp Transl Stroke Med. 3:162011. View Article : Google Scholar
|
|
23
|
Hoda MN, Siddiqui S, Herberg S,
Periyasamy-Thandavan S, Bhatia K, Hafez SS, Johnson MH, Hill WD,
Ergul A, Fagan SC and Hess DC: Remote ischemic perconditioning is
effective alone and in combination with intravenous tissue-type
plasminogen activator in murine model of embolic stroke. Stroke.
43:2794–2799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv P, Zhou M, He J, Meng W, Ma X, Dong S,
Meng X, Zhao X, Wang X and He F: Circulating miR-208b and miR-34a
are associated with left ventricular remodeling after acute
myocardial infarction. Int J Mol Sci. 15:5774–5788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SR, Noh SJ, Pronto JR, Jeong YJ, Kim
HK, Song IS, Xu Z, Kwon HY, Kang SC, Sohn EH, et al: The critical
roles of zinc: Beyond impact on myocardial signaling. Korean J
Physiol Pharmacol. 19:389–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki H, Kusuyama T, Omori Y, Soda T,
Tsunoda F, Sato T, Shoji M, Iso Y, Kondo T, Koba S, et al:
Inhibitory effect of candesartan cilexetil on left ventricular
remodeling after myocardial infarction. Int Heart J. 47:715–725.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang P, Li C, Xiang Z and Jiao B:
Tanshinone IIA reduces the risk of Alzheimer's disease by
inhibiting iNOS, MMP-2 and NF-kBp65 transcription and translation
in the temporal lobes of rat models of Alzheimer's disease. Mol Med
Rep. 10:689–694. 2014.PubMed/NCBI
|
|
28
|
Lee KF, Chen JH, Teng CC, Shen CH, Hsieh
MC, Lu CC, Lee KC, Lee LY, Chen WP, Chen CC, et al: Protective
effects of Hericium erinaceus mycelium and its isolated erinacine A
against ischemia-injury-induced neuronal cell death via the
inhibition of iNOS/p38 MAPK and nitrotyrosine. Int J Mol Sci.
15:15073–15089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bian SH, Yu MY and Geng Q: The
relationship between the plasma concentration of urotension II (U
II) and NO, NOS in spontaneous hypertensive rats and influence of
candesartan. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 25:194–195.
2632009.In Chinese.
|
|
30
|
Miller AG, Tan G, Binger KJ, Pickering RJ,
Thomas MC, Nagaraj RH, Cooper ME and Wilkinson-Berka JL:
Candesartan attenuates diabetic retinal vascular pathology by
restoring glyoxalase-I function. Diabetes. 59:3208–3215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Ma W and Zheng W: Deguelin, a
novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest
and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol.
1:215–219. 2013.
|
|
32
|
Korkmaz S, Atmanli A, Li S, Radovits T,
Hegedűs P, Barnucz E, Hirschberg K, Loganathan S, Yoshikawa Y,
Yasui H, et al: Superiority of zinc complex of acetylsalicylic acid
to acetylsalicylic acid in preventing postischemic myocardial
dysfunction. Exp Biol Med (Maywood). 240:1247–1255. 2015.
View Article : Google Scholar
|
|
33
|
Basso C, Calabrese F, Angelini A, Carturan
E and Thiene G: Classification and histological,
immunohistochemical, and molecular diagnosis of inflammatory
myocardial disease. Heart Fail Rev. 18:673–681. 2013. View Article : Google Scholar
|
|
34
|
Hadi NR, Yousif NG, Abdulzahra MS,
Mohammad BI, Al-Amran FG, Majeed ML and Yousif MG: Role of NF-κβ
and oxidative pathways in atherosclerosis: Cross-talk between
dyslipidemia and candesartan. Cardiovasc Ther. 31:381–387. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishrat T, Pillai B, Ergul A, Hafez S and
Fagan SC: Candesartan reduces the hemorrhage associated with
delayed tissue plasminogen activator treatment in rat embolic
stroke. Neurochem Res. 38:2668–2677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao LQ, Huang JL, Yu Y, Lu Y, Fu LJ, Wang
JL, Wang YD and Yu C: Candesartan inhibits LPS-induced expression
increase of toll-like receptor 4 and downstream inflammatory
factors likely via angiotensin II type 1 receptor independent
pathway in human renal tubular epithelial cells. Sheng Li Xue Bao.
65:623–630. 2013.PubMed/NCBI
|
|
37
|
Tharaux PL, Chatziantoniou C, Fakhouri F
and Dussaule JC: Angiotensin II activates collagen I gene through a
mechanism involving the MAP/ER kinase pathway. Hypertension.
36:330–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Traister A, Walsh M, Aafaqi S, Lu M, Dai
X, Henkleman MR, Momen A, Zhou YQ, Husain M, Arab S, et al:
Mutation in integrin-linked kinase (ILK(R211A)) and heat-shock
protein 70 comprise a broadly cardioprotective complex. PLoS One.
8:e773312013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yadav HN, Singh M and Sharma PL:
Pharmacological inhibition of GSK-3β produces late phase of
cardioprotection in hyperlipidemic rat: Possible involvement of HSP
72. Mol Cell Biochem. 369:227–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Torrente MP and Shorter J: The metazoan
protein disaggregase and amyloid depolymerase system: Hsp110,
Hsp70, Hsp40, and small heat shock proteins. Prion. 7:457–463.
2013. View Article : Google Scholar
|
|
41
|
Chen C, Du P and Wang J: Paeoniflorin
ameliorates acute myocardial infarction of rats by inhibiting
inflammation and inducible nitric oxide synthase signaling
pathways. Mol Med Rep. 12:3937–3943. 2015.PubMed/NCBI
|
|
42
|
Nural-Guvener HF, Zakharova L, Nimlos J,
Popovic S, Mastroeni D and Gaballa MA: HDAC class I inhibitor,
Mocetinostat, reverses cardiac fibrosis in heart failure and
diminishes CD90+ cardiac myofibroblast activation. Fibrogenesis
Tissue Repair. 7:102014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moudgil R, Musat-Marcu S, Xu Y, Kumar D
and Jugdutt BI: Increased AT2R protein expression but not increased
apoptosis during cardioprotection induced by AT1R blockade. Can J
Cardiol. 18:873–883. 2002.PubMed/NCBI
|