Introduction
Inflammation and immune response are likely to be
closely associated with the occurrence and development of type 2
diabetes mellitus (T2DM) and diabetic nephropathy (DN). A study
reported that monocytes from T2DM patients had a pro-inflammatory
profile with a marked capacity for the expression of inflammatory
cytokines (1). 1,25-dihydroxy
vitamin D3 (VD3) is the active metabolite of vitamin D3, and its
role in the prevention and treatment of diabetes is well known.
Despite the classical role of regulating the metabolism of calcium
and phosphorus in the body, VD3 can also regulate the
differentiation and proliferation of numerous cell types (2); furthermore, a previous study
demonstrated that vitamin D has anti-inflammatory and
immunomodulatory effects (3).
Previous in vitro studies have found that
vitamin D3 and its derivatives can downregulate inflammatory
factors secreted by monocytes (4)
and have a hyperresponsiveness to ligands, showing a time- and
concentration dependence (5,6). The
correlation between vitamin D and diabetes mellitus (DM),
particularly T2DM, has become the focus of current research
(7).
A large number of in vivo and in vitro
studies, as well as observational and interventional studies, have
gradually clarified the association between vitamin D deficiency
and the Toll-like receptor (TLR)/nuclear factor-κB (NF-κB)
signaling pathway. Thirteen members in the human TLR family have
been identified, among which TLR4 has been most thoroughly studied.
TLR4 is mainly expressed in lymphoid tissues, monocytes and
macrophages, as well as T and B lymphocytes (8). Devaraj et al (9) and Dasu et al (10) found that the expression of TLR4 was
increased in peripheral blood mononuclear cells (PBMC) from type 1
diabetes mellitus (T1DM) and T2DM patients, and that this increase
was correlated with the inflammatory response (9,10).
Verma et al (11) found that VD3 can upregulate the
levels of TLR10 in THP-1 cells, while downregulating the levels of
TLR2, -4 and -5, which was in accordance with a study by Sadeghi
et al (12). Gu et
al (13) reported that VD3
downregulated pulmonary TLR4 expression in asthmatic BALB/c mice.
In addition, Li et al (14)
demonstrated that vitamin D had a protective role in
diabetes-induced vascular injury and reduced TLR4 and NF-κB
expression in diabetic rats.
A previous study by our group showed that endotoxins
in T2DM and DN patients were increased, and monocytes where
dysfunctional when they grew in an endotoxin-containing
microenvironment (15–17). As an inflammatory stimulant,
lipopolysaccharide (LPS) can activate mononuclear macrophages and
promote the expression of cytokines, and it was used in previous
studies as a replacement for endotoxin. LPS is a ligand of TLR4,
causing inflammatory cytokine disorders by producing interleukin
(IL)-6, IL-15, IL-18 and IL-10 through reaction with TLR4 located
on the cell surface (15–17). Interleukin-15 (IL-15) is a soluble
and abundant cytokine, which is most highly expressed in monocytes
and macrophages. Alleva et al (18) reported that IL-15 acts on
mononuclear macrophages in an autocrine-like manner as a monokine,
and that mononuclear macrophages are highly sensitive to changes in
the levels of IL-15. High concentrations of IL-15 (10–1,000 ng/ml)
led to increased production of pro-inflammatory cytokines,
including IL-1, IL-6 and tumor necrosis factor α, by LPS-activated
mononuclear macrophages, whereas low concentrations of IL-15
(<1.0 ng/ml) selectively inhibited the production of
pro-inflammatory cytokines.
A previous study by our group has demonstrated that
VD3 alleviates inflammation, and that this anti-inflammatory effect
may be associated with signal transducer and activator of
transcription 5 (STAT5)-vitamin D receptor (VDR) crosstalk in
monocytes (19). However, whether
the anti-inflammatory effects of VD3 in monocytes from patients
with T2DM and DN with uremia are associated with the TLR/NF-κB
signaling pathway has remained elusive.
The present study examined the expression of TLR4
and NF-κB in monocytes incubated in serum from patients with T2DM
or undialyzed DN uremia as well as the effect of VD3 on these. The
inflammatory effects of the above genes in T2DM and DN uremia and
the possible mechanism for the anti-inflammatory effects of VD3
were also investigated.
Subjects and methods
Patients
From May 2009 to June 2011, 20 subjects (10 males
and 10 females) were selected from the medical examination center
at the First Affiliated Hospital of Chongqing Medical University
(Chongqing, China) for the control group. The control subjects were
volunteers who were physically healthy after examination and
without a family history of diabetes. Simultaneously, patients from
the Department of Nephrology and Endocrinology of Chonqing Medical
University were selected to constitute the T2DM group (28 cases,
including 15 males and 13 females) and the DN uremia group (30
cases, including 16 males and 14 females). The diagnosis of
diabetes was performed according to the 1999 World Health
Organization (WHO) diabetes diagnosis and classification standards
(20). The serum levels of 25(OH)
VD were 21~29 ng/ml and the levels of glutamic acid decarboxylase
antibodies (GADA) were <1.0. The inclusion criteria were set as
fasting blood glucose <7.0 mmol/l and glycosylated hemoglobin
(HbAlc) <6.5%. The diagnosis of DN was consistent with the
diagnostic criteria of chronic renal failure (uremia): Serum
creatinine (Scr) ≥707 µmol/l or endogenous creatinine
clearance <10 ml/min, and patients were in a stable state
(Table I). The exclusion criteria
were as follows: Type 1 diabetes mellitus or secondary diabetes
mellitus; acute infectious diseases; cardiac or hepatic
dysfunction; acute complications of diabetes or infections in the
last 3 months; pregnancy or lactation; other connective tissue
diseases or autoimmune diseases; tumors; cardiovascular or
cerebrovascular event; asthma; and intake of drugs affecting the
study. All selected subjects were unrelated individuals of Chinese
Han ethnicity.
 | Table IPatient characteristics at baseline in
control, T2DM and DN uremia groups. |
Table I
Patient characteristics at baseline in
control, T2DM and DN uremia groups.
| Characteristic | Control | T2DM | DN uremia | P-value |
|---|
| Male/female | 10/10 | 15/13 | 16/14 | 0.845 |
| Age (years) | 49±6 | 52±8 | 53±7 | 0.249 |
| Diabetes duration
(years) | | 4.4±1.9 | 4.5±1.6 | |
| Body mass index
(kg/m2) | 23.1±1.9 | 22.4±3.1 | 23.6±4.2 | 0.066 |
| HBA1c (%) | 5.6±0.5 | 5.8±0.6 | 5.7±0.6 | 0.413 |
| Fasting glucose
(mmol/l) | 5.5±0.9 | 5.9±0.8 | 5.8±0.7 | 0.182 |
| SBP (mmHg) | 134±6 | 136±9 | 140±9a,b | 0.036 |
| DBP (mmHg) | 76±9 | 76±8 | 80±10 | 0.194 |
| TG (mmol/l) | 1.6±0.5 | 1.9±0.7 | 1.5±0.5 | 0.394 |
| TC (mmol/l) | 4.2±0.6 | 4.2±0.8 | 4.6±0.9 | 0.063 |
| LDL-c (mmol/l) | 2.6±0.6 | 2.2±0.6a | 2.6±0.6 | 0.034 |
| HDL-c (mmol/l) | 1.2±0.5 | 1.1±0.2 | 1.1±0.5 | 0.777 |
| BUN (mmol/l) | 5.6±0.7 | 5.7±1.5 | 17.6±4.3c,d | <0.01 |
| SCr
(µmol/l) | 69.0±9.1 | 68.1±14.6 | 740.3±130.4c,d | <0.01 |
| WBC
(×109/l) | 5.9±0.7 | 5.4±1.4 | 5.8±1.0 | 0.183 |
| N
(×109/l) | 3.0±0.4 | 2.9±0.6 | 3.3±0.6 | 0.051 |
| M
(×109/l) | 0.3±0.1 | 0.3±0.1 | 0.3±0.1 | 0.873 |
| 24 h UALB
(mg/l) | 17.2±2.6 | 19.5±5.3 |
1719.3±373.1c,d | <0.01 |
| 25 (OH)VD
(ng/ml) | 45.1±7.2 | 24.8±2.6c | 23.9±2.6c | <0.01 |
| GADA | 0.3±0.1 | 0.4±0.1 | 0.4±0.1 | 0.188 |
| CRP (mg/l) | 1.7±0.7 | 3.3±1.6c | 5.4±2.8c,d | <0.01 |
| ET (EU/ml) | 0.03±0.01 | 0.10±0.05c | 0.28±0.13c,d | <0.01 |
Informed consent was obtained from the patients and
was approved by the ethics committee of the First Affiliated
Hospital of Chongqing Medical University.
Preparation and inactivation of serum,
VD3, LPS and human recombinant IL-15
Morning fasting venous blood from subjects was
collected and analyzed in the laboratory of the First Affiliated
Hospital of Chongqing Medical University, for determination of
parameters associated with blood, liver and kidney function, as
well as blood lipids and other blood components. After standing for
30 min at room temperature, 2 ml of each blood sample was
centrifuged at a low temperature at 3,000 g at −4°C for 15 min. The
upper serum was collected, filtered and sterilized through
0.22-µm microporous membranes, and separately filled into
Eppendorf tubes to be stored at −80°C for later use. A VD3 stock
solution (10−4 diluted to 10−5,
10−6, and 10−7 mol/l in the preliminary
experiment, respectively; Sigma-Aldrich, St. Louis, MO, USA) was
prepared in 95% ethanol, and filtered and sterilized through
0.22-µm microporous membranes (Millipore Millex needle-type
filters; EMD Millipore, Billerica, MA, USA). It was stored in
Eppendorf tubes at −80°C for further use. An LPS stock solution (10
mg/ml; Sigma-Aldrich) was prepared in serum-free culture media and
stored in Eppendorf tubes at −20°C. Human recombinant IL-15
(Peprotech, Rocky Hill, NJ, USA) was diluted with
phosphate-buffered saline to 50 ng/ml and stored in Eppendorf tubes
at −20°C.
Grouping and monocyte culture
The THP-1 cell line was provided by the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China). Prior to
intervention, THP-1 cells in the logarithmic growth phase were
selected and, after the culture solution was aspirated, the cells
were re-suspended in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) culture medium, containing 2% fetal bovine
serum and benzylpenicillin and streptomycin 100 IU/ml; (GE
Healthcare Life Sciences, Logan, UT, USA) and counted. Cells were
incubated in six-well culture plates at 5×105 cells/ml.
Following culture at 37°C in a 5.0% CO2 incubator for 24
h, serum from individual subjects (three) from the three groups
were added to the cells at a concentration of 5.0%, with a total of
three wells used for each experimental condition. Following culture
with or without VD3 (10−7 mol/l) for 48 h, LPS and human
recombinant IL-15 were added at final concentrations of 1
µg/ml and 100 ng/ml, respectively, followed by incubation
for another 4 h. The concentrations of serum, VD3, LPS and human
recombinant IL-15 were optimized in preliminary experiments with
the conditions used by previous studies as guidelines (21,22).
Monocytes and supernatants were then collected for subsequent
analyses.
THP-1 monocyte cell viability assay
Trypan blue and the MTT method were used to detect
the influence of serum, LPS, human recombinant IL-15 and VD3 on the
cell viability. Under all experimental conditions, the cell
viability was >95%.
Reverse transcription quantitative
polymerase chain reaction analysis (RT-qPCR)
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to extract total cellular RNA followed by determination of
purity and content of RNA. The RNA was reversely transcribed into
cDNA.
According to the mRNA sequences from GenBank,
primers were synthesized by Takara Biotechnology Co., Ltd. (Dalian,
China) and β-actin (132 bp) was set as an internal reference. The
sequences of the primers were as follows: TLR4 upstream,
5′-CGGTCCTCAGTGTGCTTGTAGTA-3′ and downstream,
5′-CATTCCTTACCCAGTCCTCATCC-3′, with a 162-bp amplified fragment;
TLR9 upstream, 5′-AGATGGAGGGGAGAAGGTCTG-3′ and downstream,
5′-CAAGGTGAAGTTGAGGGTGCT-3′, with a 112-bp amplified fragment;
IL-15 upstream, 5′-GCAATGAAGTGCTTTCTCTTGGA-3′ and downstream,
5′-TTTTCCTCCAGTTCCTCACATTC-3′, with a 167-bp amplified fragment;
β-actin upstream, 5′-CCACGAAACTACCTTCAACTCC-3′ and downstream,
5′-GTGATCTCCTTCTGCATCCTGT-3′, with a 132-bp amplified fragment. The
reaction conditions were: Pre-denaturation for 5 min at 94°C,
followed by 35 cycles of denaturation for 30 sec at 94°C, annealing
for 30 sec at 57°C, extension for 30 sec at 72°C and a final
extension step of 5 min at 72°C.
β-Actin (as the internal reference), together with
the target genes, were subjected to PCR, and at the end of the
reaction, the results were analyzed following SDS-PAGE (1.5%;
Biowest, Barcelona, Spain) and melting curve analysis was performed
using CFX Manager (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The relative expression of target genes in the samples was
calculated from the cycle threshold (CT) values according to the
following equations: ΔCT = CTtarget gene -
CTinternal reference; and ΔΔCT = (CTtarget
gene - CTinternal reference) of the patients -
(CTtarget gene - CTinternal reference) of the
control group. The relative total amount of target genes was
2-ΔΔCT. The CT value refers to the number of cycles
required for the fluorescence signal in each reaction tube to reach
a set threshold. The CT value is negatively correlated with the
level of target gene mRNA.
Western blot analysis of the protein
expression of NF-κB p65 and inhibitor of NF-κB (IκB)
Cells were lysed using TRIzol lysis buffer on ice
and centrifuged at 12,000 g at −4°C for 5 min. The protein
concentration in the supernatants was detected using a
Bicinchoninic Acid Assay kit (cat. no. P0012; Beyotime Institute of
Biotechnology, Haimen, China). The total protein (50 µg) was
separated by 12% SDS-PAGE, electrophoretically transferred onto
polyvinylidene difluoride membranes and blocked with Tris-buffered
saline containing Tween 20 and 5% skimmed milk (Boster Systems,
Inc., Pleasanton, CA, USA) for 90 min. Membranes were incubated
with rabbit anti-human NF-κB p65 antibodies (1:400) and mouse
anti-human IκB antibodies (1:400) (both from Santa Cruz
Biotechnology, Inc. Dallas, TX, USA) or rabbit anti-mouse β-actin
antibodies (1:1,500; Beijing 4A Biotechnology) overnight at 4°C.
The membranes were then washed and incubated with horseradish
peroxidase-labeled goat anti-mouse and goat anti-rabbit
immunoglobulin G (1:1500, Zhongshan Golden Bridge Biotechnology,
Beijing, China) at room temperature for 2 h. The membranes were
washed, developed and fixed (BeyoECL Plus; cat. no. P0018; Beyotime
Institute of Biotechnology), followed by capturing and analysis of
images using an automated chemiluminescence imaging system (ChimDoc
XRS; Bio-Rad Laboratories, Inc.).
Cytokine detection by ELISA
After intervention, the levels of IL-6 and monocyte
chemoattractant protein 1 (MCP-1) in monocyte supernatants were
detected by ELISA (BioSource Pharm, Inc., New York, NY, USA). The
optical density value of each well was detected at a wavelength of
450 nm, and IL-6 and MCP-1 were expressed in pg/ml. Coefficients of
variation of the intra-batch and inter-batch analyses were <10%.
Each sample was measured in triplicate.
Statistical analysis
SPSS 15.0 (SPSS, Inc., Chicago, IL, USA) statistical
software was used for statistical analysis. Values are expressed as
the mean ± standard deviation. Paired comparisons were performed
using a paired t-test, and multiple group comparisons were
performed using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Patient characteristics
There was no difference in the age, gender, body
mass index, HBA1c levels, fasting blood glucose, diastolic blood
pressure, triglyceride levels, total cholesterol, high-density
lipoprotein, absolute leukocyte and monocyte count and GADA among
the three groups (P>0.05). However, systolic blood pressure,
kidney function and high-sensitivity C-reactive protein were
significantly different between the control, T2DM and DN groups
(P<0.01; Table I).
Effects of LPS and IL-15 on the mRNA
expression of IL-15, TLR4 and TLR9 in THP-1 monocytes in each
group
After treatment with LPS + IL-15 for 4 h, the levels
of monocyte mRNA in the T2DM and DN uremia groups were
significantly higher than those in the control group (P=0.046 and
0.008, respectively); the levels of TLR4 mRNA were also
significantly higher than those in the control group (P=0.006 and
0.002, respectively). The levels of IL-15 and TLR4 mRNA in the DN
group were significantly higher than those in the T2DM group
(P=0.015 and 0.038, respectively). However, there was no
significant difference in the levels of TLR9 mRNA among the three
groups (P>0.05) (Fig. 1 and
Table II).
 | Table IIInfluence of LPS and IL-15 on levels
of monocyte IL-15, TLR4 and TLR9 mRNA expression in each group. |
Table II
Influence of LPS and IL-15 on levels
of monocyte IL-15, TLR4 and TLR9 mRNA expression in each group.
| Group | IL-15 | TLR4 | TLR9 |
|---|
| Control group | 0.78±0.36 | 1.09±0.58 | 0.63±0.29 |
| T2DM group | 1.29±0.63a | 3.24±1.09b | 0.57±0.23 |
| DN uremia
group | 1.81±0.65b,c | 4.61±1.35b,d | 0.54±0.22 |
VD3 does not affect IL-15, TLR4 and TLR9
mRNA expression in THP-1 monocytes treated with LPS and IL-15
As shown in Table
III, pre-treatment with VD3 did not affect the mRNA levels of
IL-15, TLR4 and TLR9 following treatment with LPS and IL-15 in each
group (P>0.05).
 | Table IIIInfluence of 1,25-dihydroxyvitamin D3
pre-treatment on IL-15, TLR4 and TLR9 mRNA expression in monocytes
treated with lipopolysaccharide and IL-15 in each group. |
Table III
Influence of 1,25-dihydroxyvitamin D3
pre-treatment on IL-15, TLR4 and TLR9 mRNA expression in monocytes
treated with lipopolysaccharide and IL-15 in each group.
| Group | IL-15 | TLR4 | TLR9 |
|---|
| Control group | 0.69±0.28 | 1.00±0.34 | 0.68±0.28 |
| T2DM group | 0.70±0.24 | 1.18±0.37 | 0.72±0.25 |
| DN uremia
group | 0.60±0.28 | 0.96±0.35 | 0.65±0.29 |
Effects of VD3 on NF-κB p65 and IκB
protein expression in LPS + IL-15-treated THP-1 monocytes
After treatment with LPS and IL-15, the protein
levels of NF-κB p65 in THP-1 monocytes from the T2DM and DN uremia
groups was upregulated, compared with those in the control group
(P=0.009 and 0.002, respectively), and they were higher in the DN
uremia group than those in the T2DM group (P=0.016). However, IκB
protein expression showed an opposite trend to that of NF-κB p65
(P<0.05). Of note, pre-treatment with VD3 blocked LPS +
IL-15-induced increases in NF-κB p65 protein and decreases in IκB,
and after VD3 intervention, NF-κB p65 and IκB levels in the
control, T2DM and DN uremia groups showed no significant difference
(P>0.05) (Fig. 2 and Table IV).
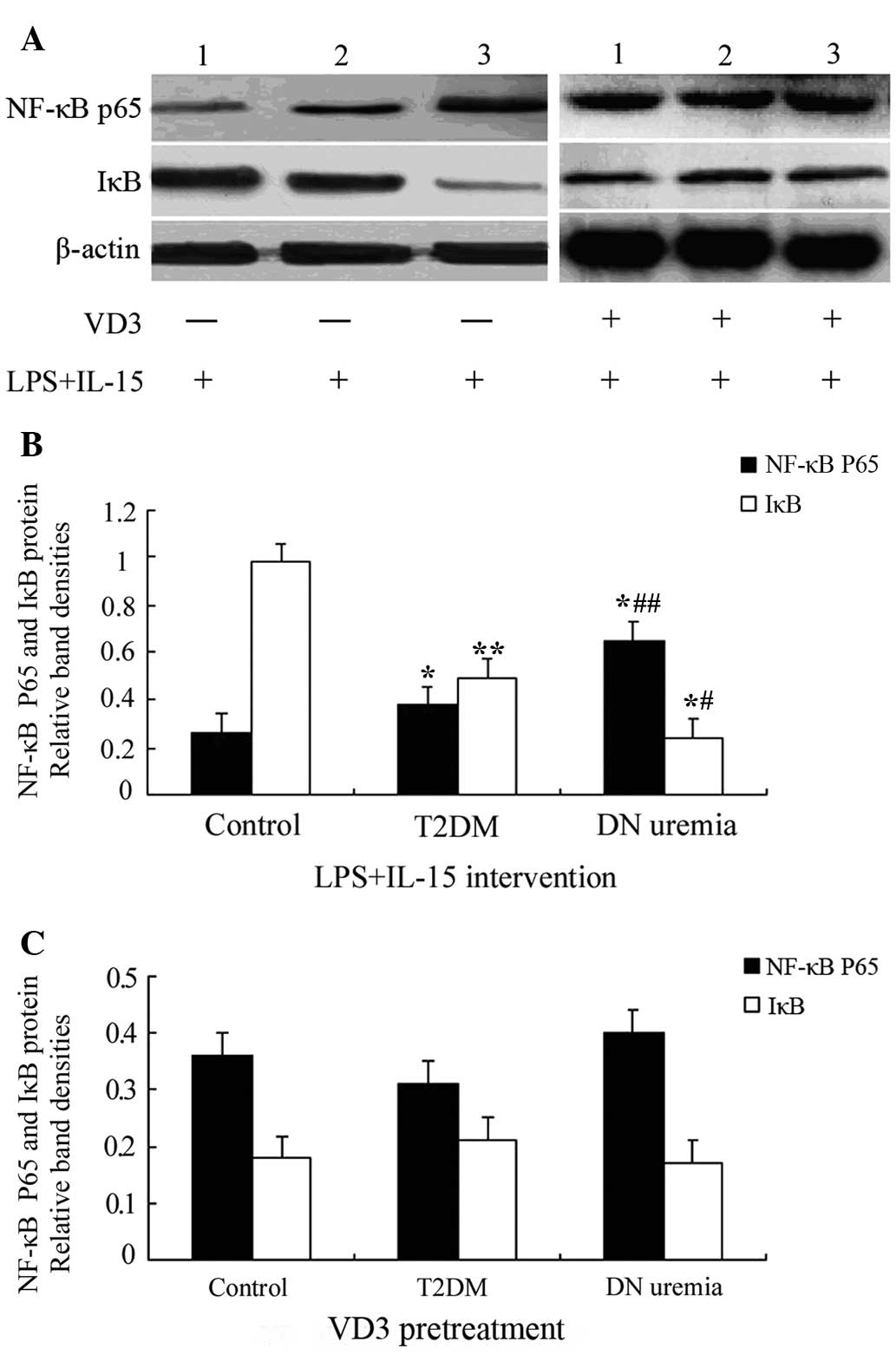 | Figure 2Western blot analysis of monocyte
NF-κB p65 and IκB protein expression. (A) Representative blots for
NF-κB p65 and IκB protein expression. Lanes: 1, Control group; 2,
T2DM group; 3, DN uremia group. (B) Bar graph showing NF-κB p65 and
IκB protein expression in THP-1 monocytes treated with LPS and
IL-15. (C) Bar graph showing NF-κB p65 and IκB protein expression
in THP-1 monocytes pre-treated with VD3; Values are expressed as
the mean ± standard deviation. *P<0.01,
**P<0.05 vs. the control group;
#P<0.01, ##P<0.05 vs. the former. VD3,
1,25-dihydroxyvitamin D3; IL, interleukin; T2DM, type 2 diabetes;
DN, diabetic nephropathy; NF-κB, nuclear factor kappa B; IκB,
inhibitor of NF-κB; LPS, lipopolysaccharide. |
 | Table IVWestern blot detection of NF-κB p65
and IκB protein expression after LPS + IL-15 intervention in the
presence or absence of VD3. |
Table IV
Western blot detection of NF-κB p65
and IκB protein expression after LPS + IL-15 intervention in the
presence or absence of VD3.
| Group | NF-κB p65
| IκB
|
|---|
| LPS + IL-15
intervention | VD3
pre-treatment | LPS + IL-15
intervention | VD3
pre-treatment |
|---|
| Control group | 0.26±0.14 | 0.36±0.12 | 0.98±0.19 | 0.18±0.09 |
| T2DM group | 0.38±0.26a | 0.31±0.11 | 0.49±0.17b | 0.21±0.13 |
| DN uremia
group | 0.65±0.13a,c | 0.40±0.14 | 0.24±0.12a,d | 0.17±0.08 |
VD3 pre-treatment on IL-6 and MCP-1
expression in monocyte supernatants after treatment with LPS +
IL-15
As shown in Fig. 3,
after LPS + IL-15 treatment, the levels of IL-6 and MCP-1 in the
supernatants of monocytes cultured in the presence of serum from
patients with DN uremia (248.87±54.69 pg/ml and 95.40±16.68 pg/ml,
respectively) or T2DM (157.55±26.38 pg/ml and 37.07±11.08 pg/ml,
respectively) were significantly higher than those in the control
group (78.33±16.95 and 26.02±8.12 pg/ml; P<0.01) (Fig. 3A and B). Furthermore, the levels of
IL-6 in the DN group were significantly higher than those in the
T2DM group (P<0.01) (Fig. 3A and
B). Of note, pre-treatment with VD3 significantly decreased
IL-6 secretion by monocytes compared with that in the monocytes
treated with LPS + IL-15 only. Following VD3 pre-treatment, no
significant difference was found in the levels of IL-6 and MCP-1
among the T2DM, DN uremia and control groups (P>0.05) (Fig. 3C and D).
Discussion
Inflammatory cytokines have been shown to
participate in each stage of DN, and T2DM monocytes have a
pro-inflammatory properties as well as a high potential for the
expression of inflammatory cytokines (1). Circulating inflammatory markers are
increased in patients with T1DM or T2DM, which may be caused by
monocyte/macrophage infiltration in the kidneys. Therefore,
according to the inflammatory immune theory, it is thought that DN
is a chronic low-grade inflammatory disease characterized by
natural immune activation (23–29).
In recent years, the anti-inflammatory and immunomodulatory effects
of VD3 have received extensive attention (30). Substantial studies have shown that
VD3 causes decreases in TLR4 and NF-κB expression (11–14)
and a reduction in inflammatory cytokines secreted by monocytes
(3). A previous study by our group
showed that VD3 alleviates inflammation, which may be associated
with STAT5-VDR crosstalk in monocytes (14). However, it has remained elusive
whether the anti-inflammatory effects of VD3 in monocytes from T2DM
and DN uremic patients are mediated via the TLR4/NF-κB signaling
pathway. Based on previous studies by our and other groups
(19,31–33),
the present study hypothesized that the anti-inflammatory effects
of VD3 on monocytes from T2DM and DN uremic patients may be
associated with the TLR4/NF-κB signaling pathway and performed
experiments to verify this hypothesis.
The present study found that, compared with those in
the control group, the mRNA expression of IL-15 and TLR4, the
protein expression of NF-κB p65 and the pro-inflammatory mediators
IL-6 and MCP-1 in the supernatant of monocytes incubated with serum
from T2DM and DN patients were all significantly upregulated
following treatment with LPS + IL-15. Furthermore, the levels of
IL-15 and TLR4 mRNA, NF-κB p65 protein, and the secretion of IL-6
and MCP-1 in the DN uremia group were all higher than those in the
T2DM group. These results, which were consistent with findings of
previous studies (9–12), indicated that the sensitivity of
monocytes treated with serum from T2DM and DN patients to LPS and
IL-15 was elevated. The mechanisms of this effect are likely to
include the TLR4/NF-κB signaling pathway, as LPS and IL-15
stimulated the expression of TLR4 and NF-κB.
As shown in Tables
III and IV, the present study
also showed that VD3 pre-treatment for 48 h significantly
attenuated the effects of LPS + IL-15 stimulation; VD3 was able to
block the pro-inflammatory effects of LPS and IL-15 by inhibiting
increases in the levels of IL-15, TLR4 mRNA and NF-κB p65 protein
expression as well as the secretion of pro-inflammatory mediators
IL-6 and MCP-1 in the supernatant. These findings are in accordance
with the findings of previous studies (9–11),
indicating that VD3 is able to alleviate inflammation. However,
pre-treatment with VD3 followed by LPS + IL-15 had no significant
effect on the mRNA expression of TLR-9 in the three groups.
Therefore, the anti-inflammatory effects of VD3 on monocytes
cultured in sera of patients with T2DM and DN uremia may be
associated with the TLR4/NF-κB signaling pathway.
The present study had certain limitations, including
a small sample size. Moreover, the present study only observed the
anti-inflammatory effects of VD3 in patients with T2DM and DN
uremia in vitro, which may not be representative of the
in vivo micro-environment. The findings of the present study
should further validated by expanding the sample size and
performing animal studies.
In conclusion, the present study preliminarily
investigated the influence of VD3 and LPS + IL-15 on TLR4 and NF-κB
expression in monocytes incubated with serum from T2DM and DN
uremic patients, and explored the mechanism of action of VD3
against the inflammatory immune response of T2DM and DN. The
results indicated that VD3 may exert its anti-inflammatory effects
via the TLR4/NF-κB signaling pathway. Supplementation of DM and DN
patients as well as other individuals who are also susceptible to
vitamin D3 deficiency, with active vitamin D3 may be suitable for
the prevention and treatment of T2DM and DN. The use of vitamin D
and its derivatives in the prevention and treatment of DM is
desirable, as it would reduce the high cost of DM treatment, due to
vitamin D being inexpensive as well as easy to administer.
Therefore, future studies by our group will perform an in-depth
analysis of the abovementioned pathway in order to provide novel
clues for the prevention and treatment for T2DM and DN.
Acknowledgments
The authors would like to thank Professor Qifu Li
and Dr Lai Han for their cooperation in patient selection. National
Natural Science Foundation of China (grant. no. 81560147), the
Science and Technology Project of Guizhou Province [grant. no. SY
(2012) No. 3116), the Science and Technology Fund Project of
Guizhou Province [grant. no. J word LKZ(20 13) No. 53], the
Doctoral Fund of the Zunyi Medical College (grant. no. F-588) and
the Outstanding Doctoral Dissertation Fund of Chongqing Medical
University, supported by the Key Project of Chongqing Municipal
Health Bureau (grant. no. 2010-1-16).
References
|
1
|
Pickup JC: Inflammation and activated
innate immunity in the pathogenesis of type 2 diabetes. Diabetes
Care. 27:813–823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baeke F, Korf H, Overbergh L, van Etten E,
Verstuyf A, Gysemans C and Mathieu C: Human T lymphocytes are
direct targets of 1,25-dihydroxy1,25(OH)2D3 in the immune system. J
Steroid Biochem Mol Biol. 121:221–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thacher TD and Clarke BL: Vitamin D
insufficiency. Mayo Clin Proc. 86:50–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giulietti A, van Etten E, Overbergh L,
Stoffels K, Bouillon R and Mathieu C: Monocytes from type 2
diabetic patients have a pro-inflammatory profile 1,
25-dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res
Clin Pract. 77:47–57. 2007. View Article : Google Scholar
|
|
5
|
Du T, Zhou ZG, You S, Huang G, Lin J, Yang
L, Li X, Zhou WD and Chao C: Modulation of monocyte
hyperresponsiveness to TLR ligands by 1,25-dihydroxy-vitamin D3
from LADA and T2DM. Diabetes Res Clin Pract. 83:208–214. 2009.
View Article : Google Scholar
|
|
6
|
Liu PT, Steffen S, Li H, Wenzel L, Tan BH,
Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al: Toll-like
receptor triggering of a vitamin D mediated human antimicrobial
response. Science. 311:1770–1773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nimitphong H, Holick MF, Fried SK and Lee
MJ: 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin
D3 promote the differentiation of human subcutaneous
preadipocytes. PLoS One. 7:e521712012. View Article : Google Scholar
|
|
8
|
Kim F, Pham M, Luttrell I, Bannerman DD,
Tupper J, Thaler J, Hawn TR, Raines EW and Schwartz MW: Toll-like
receptor 4 mediates vascular inflammation and insulin resistance in
diet-induced obesity. Circ Res. 100:1589–1596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devaraj S, Dasu MR, Rockwood J, Winter W,
Griffen SC and Jialal I: Increased Toll-like receptor (TLR) 2 and
TLR4 expression in monocytes from patients with type 1 diabetes:
further evidence of a proinflammatory state. J Clin Endocrinol
Metab. 93:578–583. 2008. View Article : Google Scholar
|
|
10
|
Dasu MR, Devaraj S, Park S and Jialal I:
Increased Toll-like receptor (TLR) activation and TLR ligands in
recently diagnosed Type 2 diabetic subjects. Diabetes Care.
33:861–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verma R, Jung JH and Kim JY:
1,25-Dihydroxyvitamin D3 upregulates TLR10 while downregulating
TLR2, 4 and 5 in human monocyte THP-1. J Steroid Biochem Mol Biol.
141:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sadeghi K, Wessner B, Laggner U, Ploder M,
Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E, et
al: Vitamin D3 downregulates monocyte TLR expression and triggers
hyporesponsiveness to pathogen-associated molecular patterns. Eur J
Immunol. 36:361–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu HR, Luan B, Qiao JY, Wang YZ and Li Q:
Effect of 1,25-(OH)2D3 on expression of HMGB1 and TLR4 in the lungs
of asthmatic mice. Zhongguo. Dang Dai Er Ke Za Zhi. 16:301–305.
2014.In Chinese.
|
|
14
|
Li F, Liu P, Zhang X, Zhang Q, Tang S, Zhu
M and Qiu M: 1,25(OH)2D3-mediated amelioration of aortic injury in
strep-tozotocin-induced diabetic rats. Inflammation. 36:1334–1343.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang M, Gan H, Shen Q, Tang W, Du X and
Chen D: Proinflammatory CD14+CD16+monocytes are associated with
microinflammation in patients with type 2 diabetes mellitus and
diabetic nephropathy uremia. Inflammation. 35:388–396. 2012.
View Article : Google Scholar
|
|
16
|
Yang M, Shen Z, Chen D, et al: Effects of
1,25-(OH)2D3 on the expressions of vitamin D receptor, STAT5 and
cytoskeletal rearrangement in human monocytes incubated with sera
from type 2 diabetes patients and diabetic nephropathy patients
with uremia. Inflamm Res. 61:511–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang M, Gan H, Shen Q, et al: Proportion
of CD14+CD16+ monocytes in peripheral blood from patients with type
2 diabetic mellitus and effect of LPS and IL-15 on expression of
STAT5 in monocytes. Chinese Pathophysiology. 1:136–141. 2012.
|
|
18
|
Alleva DG, Kaser SB, Monroy MA, Fenton MJ
and Beller DI: IL-15 functions as a potent autocrine regulator of
macrophage proinflammatory cytokine production: Evidence for
differential receptor subunit utilization associated with
stimulation or inhibition. J Immunol. 159:2941–2951.
1997.PubMed/NCBI
|
|
19
|
Yao L, Xiao Y, Liu SP, Xu AM and Zhou ZG:
Serum of obesity induce the activation of TLR4/NF-κB signaling
pathway on THP-1 cell line. Zhonghua Yi Xue Za Zhi. 90:3119–3123.
2010.In Chinese.
|
|
20
|
Ge JB and Xu YJ: Internal Medicine.
Edition 8. People Health Publishing House; Beijing: pp. 7412013
|
|
21
|
Pramanik R, Asplin JR, Lindeman C, Favus
MJ, BAi S and Coe FL: Lipopolysaccharide negatively modulates
vitamin D action by downregulating expression of vitamin D-induced
VDR in human monocytic THP-1 cells. Cell Immunol. 232:137–143.
2004. View Article : Google Scholar
|
|
22
|
Sadeghi K, Wessner B, Laggner U, Ploder M,
Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E, et
al: Vitamin D3 downregulates monocyte TLR expression and triggers
hypo-responsiveness to pathogen-associated molecular patterns. Eur
J Immunol. 36:361–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco
O, Fernandez-Vizarra P, Mallavia B, Flores C, Sanz A, Blanco J,
Mezzano S, Ortiz A, et al: Suppressors of cytokine signaling
abrogate diabetic nephropathy. J Am Soc Nephrol. 21:763–772. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Connor JC, Satpathy A, Hartman ME,
Horvath EM, Kelley KW, Dantzer R, Johnson RW and Freund GG:
IL-1beta-mediated innate immunity is amplified in the db/db mouse
model of type 2 diabetes. J Immunol. 174:4991–4997. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mora C and Navarro JF: Inflammation and
diabetic nephropathy. Curr Diab Rep. 6:463–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navarro JF and Mora C: Diabetes,
inflammation, proinflammatory cytokines and diabetic nephropathy.
Scientific World Journal. 6:908–917. 2006. View Article : Google Scholar
|
|
28
|
Tuttle KR: Linking metabolism and
immunology: Diabetic nephropathyis an inflammatory disease. J Am
Soc Nephrol. 16:1537–1538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruster C and Wolf G: The role of
chemokines and chemokine receptors in diabetic nephropathy. Front
Biosci. 13:944–955. 2008. View
Article : Google Scholar
|
|
30
|
Stubbs JR, Idiculla A, Slusser J, Menard R
and Quarles LD: Cholecalciferol supplementation alters
calcitriol-responsive monocyte proteins and decreases inflammatory
cytokines in ESRD. J Am Soc Nephrol. 21:353–361. 2010. View Article : Google Scholar :
|
|
31
|
Akira S and Takeda K: Toll-like receptor
signaling. Nat Rev Immunol. 7:499–511. 2004. View Article : Google Scholar
|
|
32
|
Cao XT: The Progress of Immunology.
People's Medical Publishing House; Beijing: pp. 1432009
|
|
33
|
Li ML, Gan H and Qiao L: The expression of
TLR4 on peripheral blood monocytes from uremic patients with
diabetic nephropathy and its relation with plasma MCP-1
concentration. Chinese Journal of Immunology. 9:848–850. 2009.
|

















