Introduction
Pingyangmycin (PYM) was isolated from Treptomyces
verticillus var. pingyangensis n.sp in 1969 and has been
used for clinical treatment of various types of cancer since 1978
(1–5). The most successful clinical use of
PYM is in the treatment of vascular malformations and hemangioma
(6–12). Injection of PYM has been shown to
have beneficial effects in >90% of infants with low-flow orbital
or periorbital venous malformation (7) and to improve infantile hemangiomas in
oral and maxillofacial regions in 100% of cases (11). Based on these results, PYM was
recommended as a standard treatment for vascular malformations and
hemangioma (13). However, to the
best of our knowledge, the underlying molecular mechanism of action
of PYM in treating hemangioma has remained elusive. The
determination of its mechanism of action may aid in expanding the
treatment spectrum of PYM to other cancer types.
As one of most common tumor types in infants,
hemangioma have an incidence rate of 5–10% at the end of the first
year of life worldwide (14). The
etiology of hemangioma has remained elusive; however, angiogenesis
(sprouting of new vessels from existing vessels) and vasculogenesis
(de novo formation of new blood vessels from stem cells)
were proposed as mechanisms of neovascularization in hemangioma
(15). Numerous signaling pathways
are implicated in the process of angiogenesis and vasculogenesis,
including the human vascular endothelial growth factor/receptor
pathway (16), hypoxia-inducible
factor pathway (17), angiopoietin
signaling (18) and the notch
pathway (19). These pathways have
provided therapeutic targets for the development of molecular
targeted therapies.
Recent studies indicated that the phosphoinositide
3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway
was involved in the process of angiogenesis. PI3K/Akt/mTOR is one
of the major signaling pathways which is crucial for cancer-cell
survival, proliferation and angiogenesis. This pathway acts to
promote cancer-cell survival by inhibiting pro-apoptotic factors
and activating anti-apoptotic factors (20). mTOR is the key kinase downstream of
the PI3K/Akt pathway. Studies have reported that mTOR inhibitor
rapamycin has anti-angiogenic effects on endothelial cells
(21,22). Considering that mTOR is the central
downstream signaling molecule of the PI3K/Akt pathway, these
results implied that de-regulation of the PI3K/Akt/mTOR pathway may
be involved in the pathology of hemangioma. Thus, the present study
posed the hypothesis that PYM may have inhibitory effect on
hemangioma-derived endothelial cells by affecting the PI3K/Akt
pathway, which may provide a mechanistic basis for the anti-cancer
effects of PYM on hemangioma.
Originally, PYM was reported to exert its
anti-cancer effects by DNA strand breaks (23). Recent studies have reported
mechanisms including activation of the p53 pathway (24), downregulation of B-cell lymphoma 2
(Bcl-2) and upregulation of Bcl-2-associated X protein (Bax)
(25) and induction of apoptosis
by activation of caspase-3 (26)
in vitro and in vivo, which may be downstream
cellular responses to PYM treatment. The present study investigated
the underlying mechanisms of the anti-cancer activity of PYM on
hemangioma by examining its effects on cell viability, cell cycle,
invasive potential and the expression of proteins involved in the
angiogenesis-associated PI3K/Akt pathway using an MTT assay, flow
cytometry, Transwell assay and western blot analysis. The
discoveries of the present study may aid in expanding the treatment
spectrum of PYM to other cancer types.
Materials and methods
Reagents
PYM was purchased from Harbin Laiboten
Pharmaceutical (Harbin, China). It was dissolved in
dimethyl-sulfoxide (DMSO) to obtain an 8 mg/ml stock solution,
which was stored at 4°C. LY294002 was purchased from Promega Corp.
(Madison, WI, USA) and insulin-like growth factor-1 (IGF-1) was
purchased from Sino Biological (Beijing, China).
Cell culture
EOMA mouse hemangioendothelioma endothelial cells
were purchased from the American Type Tissue Collection (Manassas,
VA, USA) and were cultured in Dulbecco's modified Eagle's medium
(DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine
serum (Gen-View Scientific, Inc.,confirm El Monte, FL, USA), 100
IU/ml penicillin (Sigma-Aldrich, St. Louis, MO, USA) and 100
µg/ml streptomycin (Sigma-Aldrich) at 37°C, 5%
CO2 and a humidified atmosphere.
Cell viability assay
The effect of PYM, LY294002 and IGF-1 on the
proliferation of EOMA cells was determined using an MTT assay
(Sigma-Aldrich). EOMA cells were seeded at a density of
1×103/well in 96-well plates and incubated at 37°C for
24 h. The cells were treated with various concentrations of PYM
(0.5–100 µg/ml), LY294002 (0.5 nM), PYM (100 µg/ml)
plus IGF-1 (100 ng/ml) or an equal volume of DMSO for the control
and incubated for 24, 48 or 72 h. Subsequently, 20 µl MTT
solution (5 mg/ml) was added to each well. After 4 h of incubation
at 37°C, the supernatants were carefully removed and 150 µl
DMSO was added. The optical density (OD) value was measured at 490
nm using a Multiscan MK3 microplate reader (Thermo Fisher
Scientific, Waltham, MA, USA).
Determination of apoptotic rates
Apoptosis was determined by flow cytometry using an
Annexin V/propidium iodide (PI) apoptosis kit (Thermo Fisher
Scientific, Inc.). Briefly, after incubation with PYM (100
µg/ml), LY294002 (0.5 nM), PYM (100 µg/ml) plus IGF-1
(100 ng/ml) or an equal volume of DMSO for the control for 48 h,
the cells were collected, washed with phosphate-buffered saline
(PBS) and re-suspended in 0.5 ml 1X Annexin-binding buffer at a
density of 5×105 cells/ml. Annexin V-fluorescein
isothiocyanate and PI were added and the cells were incubated for
10 min at room temperature. Samples were immediately analyzed by
flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA, USA)
using BD Accuri C6 software version 2.8 (BD Biosciences).
Cell invasion assay
The invasive capacity of the cells was determined
using Transwell chambers (Corning-Costar, Corning, NY, USA; cat no.
3422). Cells were incubated with PYM (100 µg/ml),
LY294001221 (0.5 nM), PYM (100 µg/ml) plus IGF-1 (100 ng/ml)
or an equal volume of DMSO for the negative control in serum-free
DMEM for 12 h. Cells were then harvested and added to the upper
chamber of a Transwell insert (1×104 cells in 100
µl), which was coated with a Matrigel® mix
(Corning Incorporated, Corning, NY, USA). The lower chamber was
filled with complete medium. After 48 h of incubation at 37°C, the
cells on the upper surface of the membrane were removed. The cells
attached to the lower surface of the membrane were fixed with 4%
paraformaldehyde (Beyotime Institute of Biotechnology, Shanghai,
China) for 20 min, stained with hematoxylin (Beyotime Institute of
Biotechnology) for 5–10 min and counted under a microscope (Olympus
CX41; Olympus Corp., Tokyo, Japan).
Western blot analysis
The effects of PYM, LY294002 or PYM plus IGF-1 on
the expression of PI3K and Akt in EOMA cells was determined by
western blot analysis. After incubation with PYM (100
µg/ml), LY294001221 (0.5 nM), PYM (100 µg/ml) plus
IGF-1 (100 ng/ml) or an equal volume of DMSO for the negative
control, cells were washed with PBS (Beyotime Institute of
Biotechnology) and lysed with lysis buffer (Beyotime Institute of
Biotechnology). Following centrifugation at 14,000 × g for 10 min
at 4°C, the supernatants were collected and the protein
concentration was determined using the Pierce Bicinchoninic Acid
Protein Assay kit (Thermo Fisher Scientific). Equal amounts of
protein (20 µg) were separated by 10% SDS-PAGE (Beyotime
Institute of Biotechnology) and transferred to polyvinylidene
difluoride membranes (Millipore, Billerica, MA, USA). After washing
with Tris-buffered saline (TBS; Beyotime Institute of
Biotechnology), the membranes were blocked with TBS containing 5%
skimmed milk and incubated with primary antibodies against PI3K
(rabbit monoclonal; Abcam, Cambridge, UK; cat. no. ab40755;
1:2,000; overnight incubation at 4°C), Akt (rabbit monoclonal;
Abcam; cat. no. ab179463; 1:10,000; overnight incubation at 4°C),
phosphorylated (p)-Akt (rabbit monoclonal; Abcam; cat. no. ab81283;
1:10,000; overnight incubation at 4°C) or GAPDH (rabbit polyclonal;
Abcam; cat. no. ab181602; 1:10,000; overnight incubation at 4°C).
After washing with TBS containing Tween 20 (Beyotime Institute of
Biotechnology), the membranes were incubated with secondary
antibody (horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G; Wuhan Boster Biological Technology, Ltd., Wuhan,
China; cat. no. BA1055; 1:5,000; overnight incubation at 4°C). Then
the membranes were developed using an enhanced chemiluminescence
system (Alpha Fluochem Q; Thermo Fisher Scientific, Inc.). Finally,
the protein bands were quantified by densitometry using Image-Pro
Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) and
normalized to GAPDH.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 statistical software program (SPSS Inc., Chicago, IL, USA) or
GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Values
are expressed as the mean ± standard deviation of three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
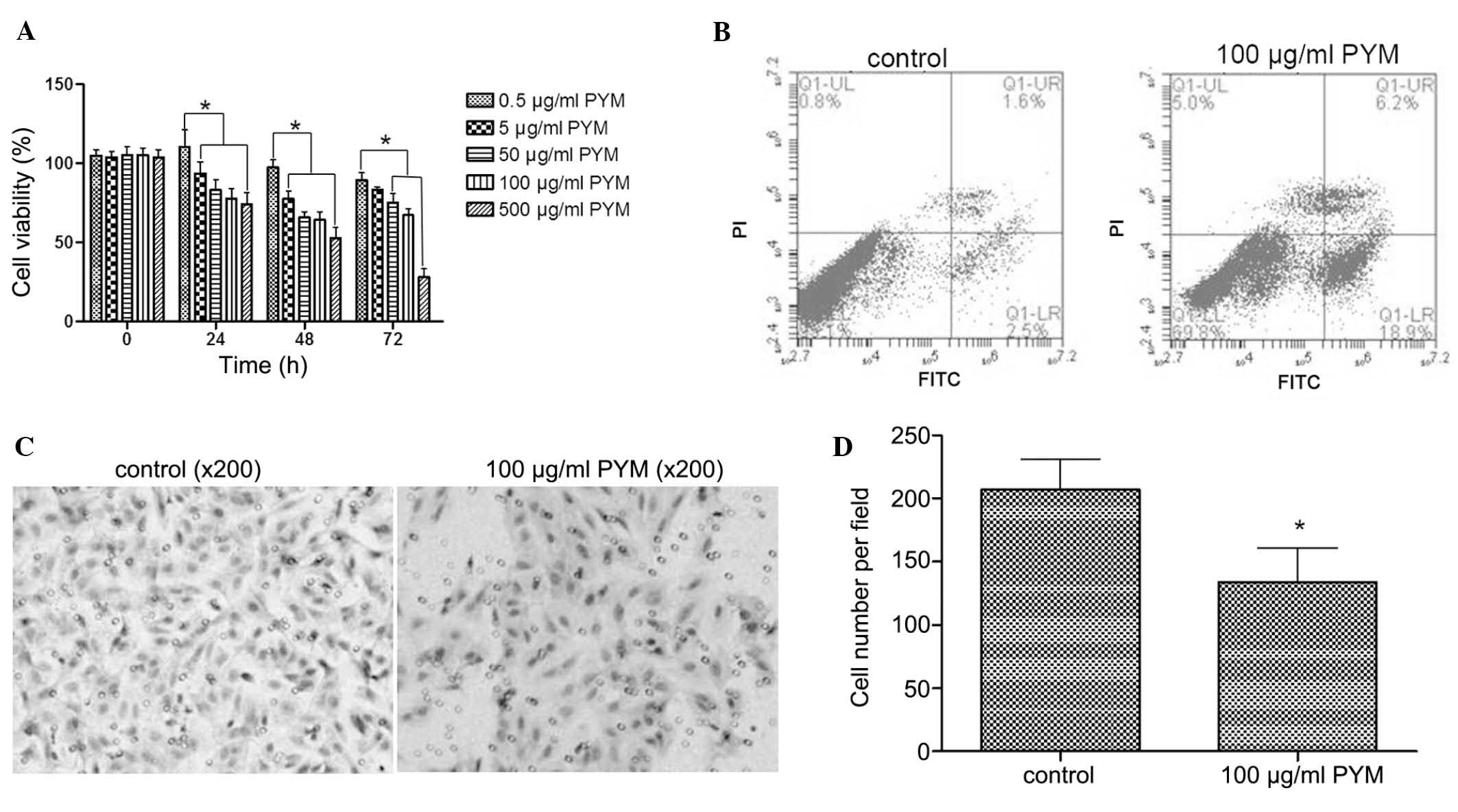
PYM inhibits the proliferation, induces
apoptosis and reduces the invasive ability of EOMA cells
The EOMA cell line is derived from
hemangioendothelioma, a type of hemangioma, of an adult mouse. In
the present study, EOMA cells were used as a pathological model of
hemangioma. First, the effects of PYM treatment on the biological
behavior of EOMA cells were tested. Treatment with PYM (0.5–500
µM) inhibited the growth of EOMA cells in a time-and
dose-dependant manner (Fig. 1A).
In particular, after treatment with PYM at 500 µM for 72 h,
cell growth was inhibited by 70%. There were fewer differences
between 48- and 72-h treatments with 100 µM PYM compared to
lower doses of PYM. Based on these results, 100 µM PYM was
selected as the concentration to be used in the subsequent
experiments.
Necrosis and apoptosis are two main modes of cell
death. To explore the mode of growth inhibition induced by PYM,
flow cytometry was used to determine the apoptotic rate after
treatment with PYM. As shown in Fig.
1B, PYM treatment at 100 µM for 48 h significantly
induced a higher apoptotic rate than that of untreated cells (18.9
vs. 2.5%). Thus, apoptosis represented a mode of cell death caused
by PYM treatment.
Tumor invasion is an important step of tumor
progression and in the development of metastasis. Therefore,
inhibition of the invasive ability of tumor cells is a desired
property of anti-tumor agents. In the present study, a Transwell
assay demonstrated that PYM treatment at 100 µM for 48 h
markedly reduced the number of EOMA cells that transgressed though
the filters of the Transwell membranes (Fig. 1C), which indicated that the
invasive ability of EOMA cells was inhibited by PYM.
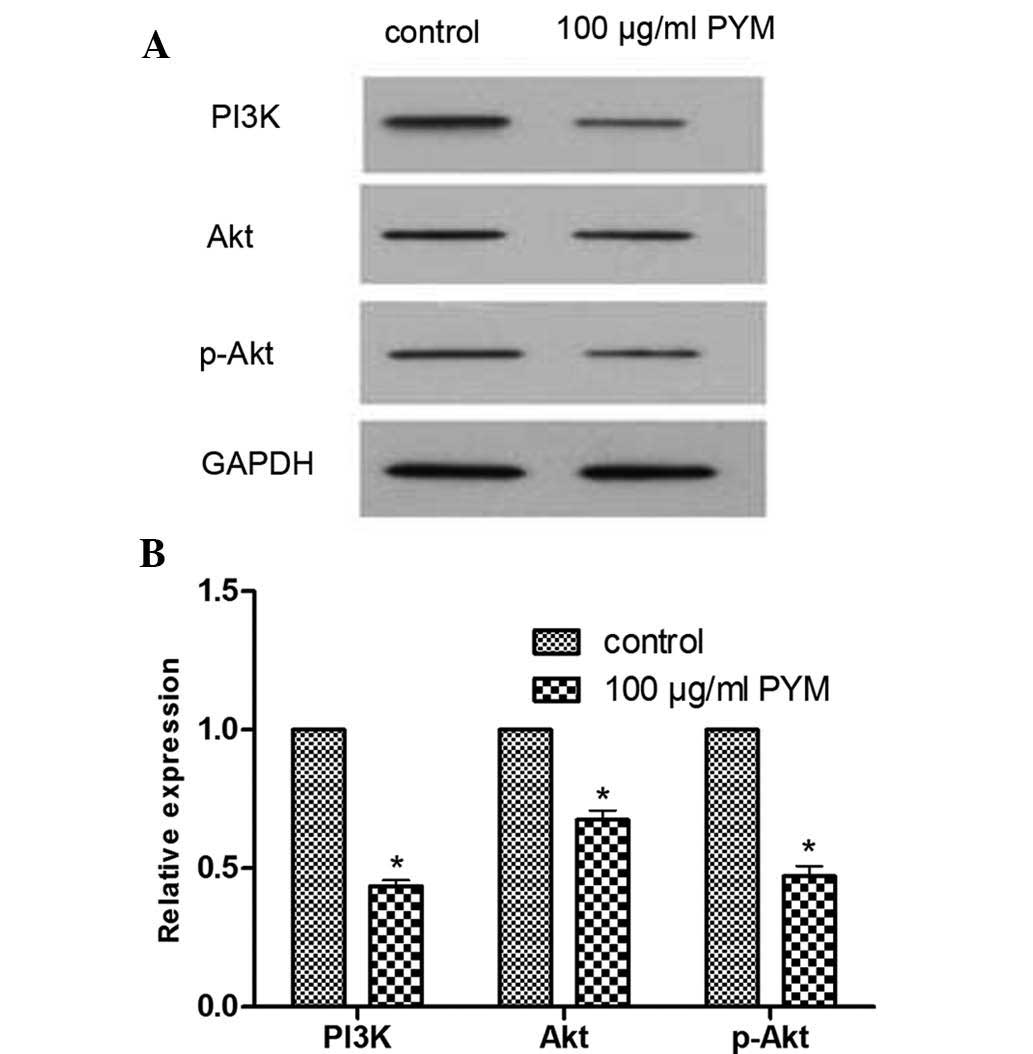
PYM reduces the expression of PI3K, Akt
and p-Akt proteins
The effects of PYM on the expression of
angiogenesis-associated PI3K/Akt signaling proteins in EOMA cells
were determined by western blot analysis. After treatment with PYM
(100 µM), the expression levels of PI3K, Akt and p-Akt were
markedly reduced (Fig. 2). These
results provided direct evidence that PYM treatment affects the
PI3K/Akt signaling pathway. However, the association between
reduction of protein expression and changes in the biological
behavior of EOMA cells following PYM treatment require further
study.
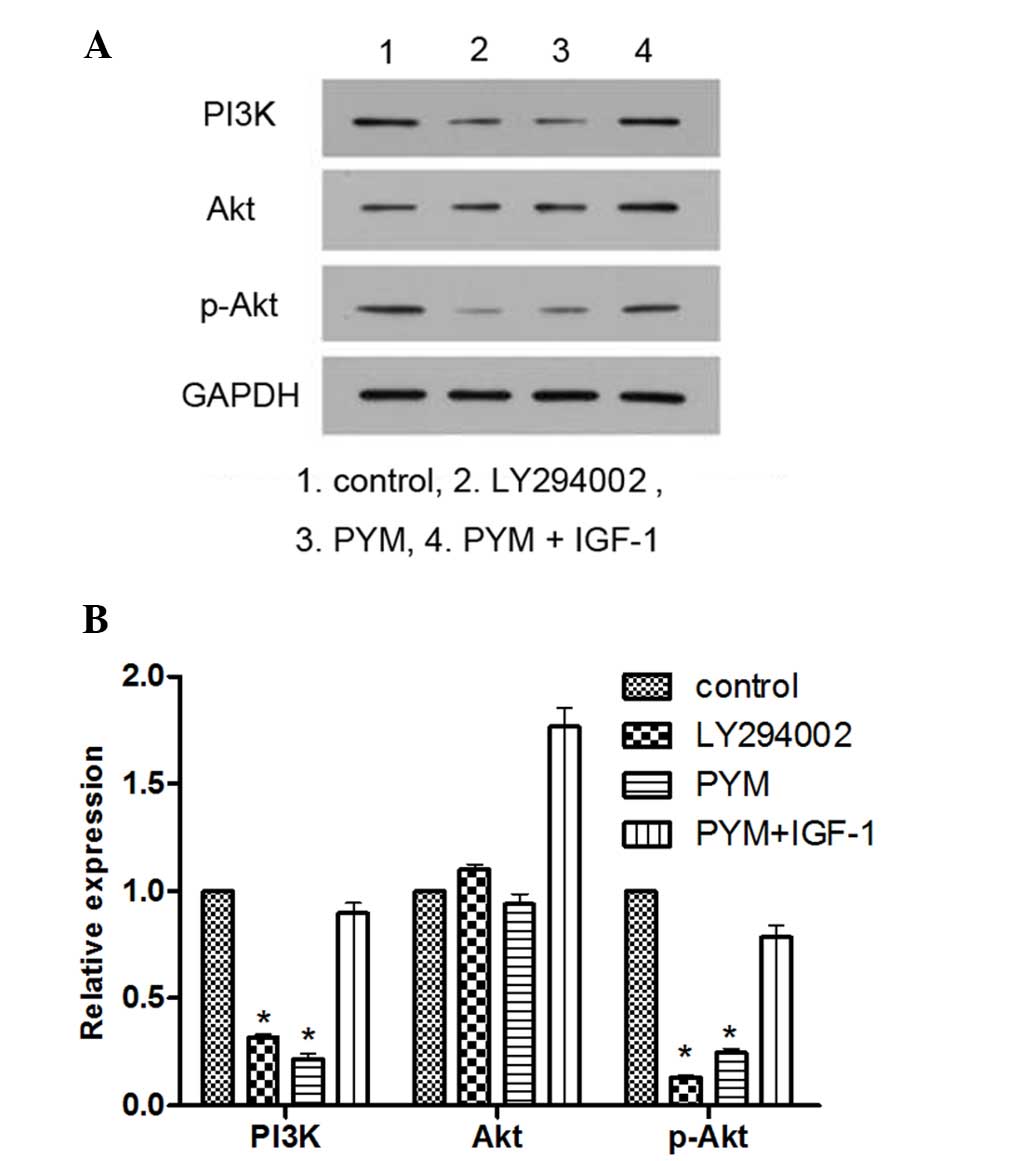
PI3K inhibitor LY294002 and PYM inhibit
the expression of PI3K and p-Akt, while the effects of PYM are
blocked by PI3K activator IGF-1
To explore the association between inhibition of the
PI3K/Akt pathway and changes in the biological behavior of EOMA
cells in response to PYM treatment, the PI3K inhibitor LY294002 and
the PI3K activator IGF-1 were employed. LY294002 was used as a
positive control for comparison with the effects of PYM, while
IGF-1 was used to explore the possible role of the PI3K/Akt pathway
in the mechanism of action of PYM.
As shown in Fig. 3,
treatment with LY294002 or PYM significantly reduced the levels of
PI3K and p-Akt expression. However, when the EOMA cells were
treated by PYM together with IGF-1, the expression of PI3K was
significantly increased and p-Akt was not inhibited. These results
provided direct evidence that PYM exerts its anti-cancer effects by
inhibiting PI3K/Akt signaling.
IGF-1 reverses the effects of PYM on the
viability, apoptosis and invasive ability of EOMA cells
The effects of PI3K activator IGF-1 on the
anti-cancer effects of PYM in EOMA cells were further investigated.
Cell viability studies showed that, compared with PYM at 100
µg/ml, LY294002 at 0.5 nM was slightly more potent (Fig. 4A). Furthermore, the
anti-proliferative effects of PYM on EOMA cells were attenuated by
co-treatment with IGF-1 (100 ng/ml). These results suggested that
inhibition of PI3K-associated signaling pathways may be involved in
the anti-proliferative effects of PYM.
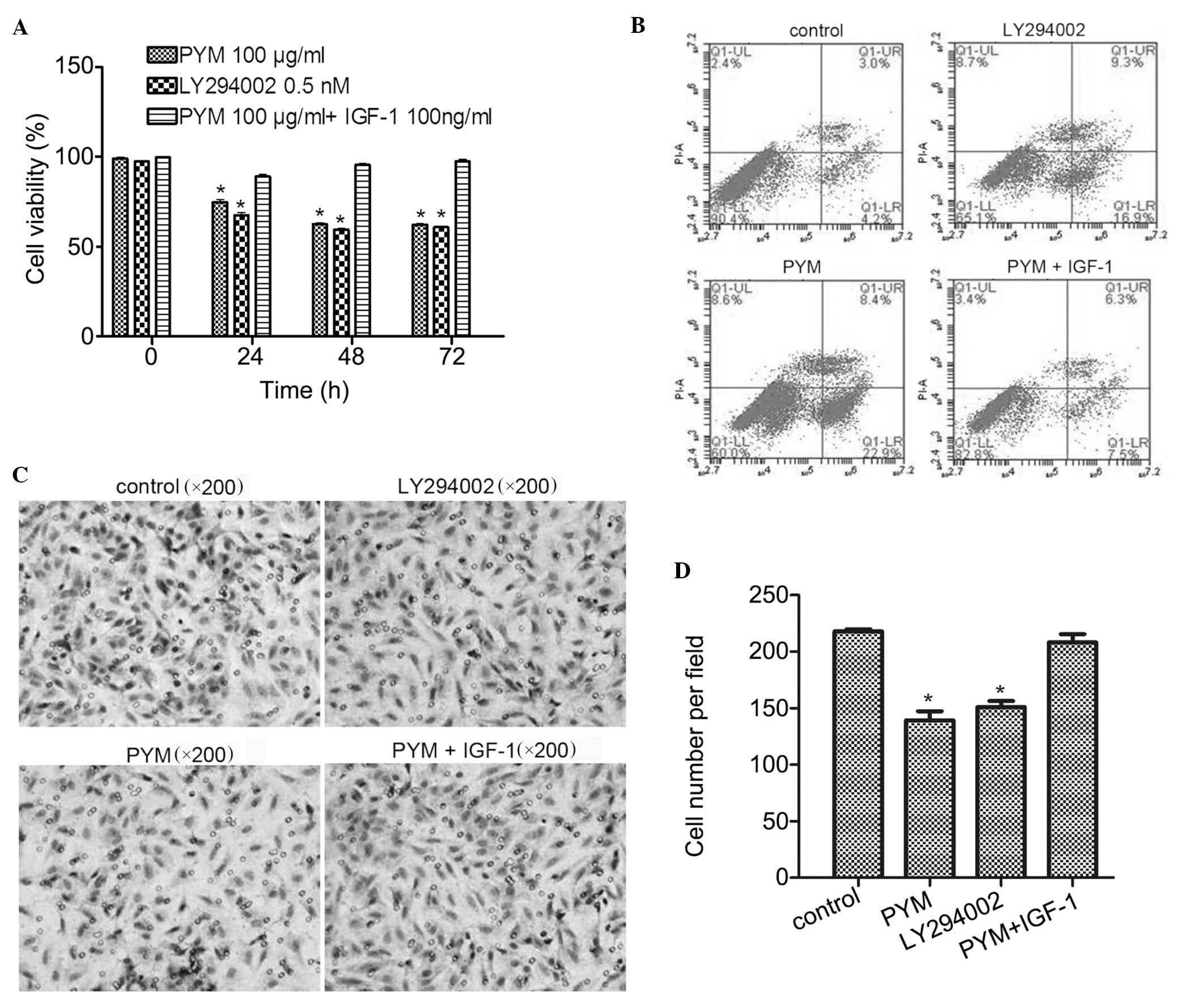 | Figure 4IGF-1 co-treatment with PYM reverses
the effects of PYM on the viability, apoptosis and invasive ability
of EOMA cells. (A) Growth-inhibitory effects of PI3K inhibitor
LY294002 and protective effects of PI3K activator IGF-1 against
PYM-induced toxicity. Cell viability was determined using an MTT
assay. (B) Induction of apoptosis by PYM, LY294002 or PYM + IGF-1.
Apoptosis was determined by flow cytometry. (C) Effects of PYM,
LY294002 and PYM + IGF-1 on the invasive ability of EOMA cells as
assessed via Transwell invasion assay. (D) Cell invasion was
quantified by counting invaded cells in six fields of view. In all
graphs, values are expressed as the mean ± standard deviation of
three independent experiments. *P<0.05 vs. control.
PYM, pingyangmycin; IGF, insulin-like growth factor; PI3K,
phosphoinositide 3-kinase; FITC, fluorescein isothiocyanate; PI,
propidium iodide; Q, quadrant; UR, upper right; LL, lower left. |
In the apoptosis assay, as shown in Fig. 4B, treatment with PYM or PI3K
inhibitor LY294002 resulted in a significant induction of apoptosis
by 22.9 and 16.9%, respectively, compared with that in the control
group (4.2%). However, combined treatment with PYM and IGF-1
resulted in a similar apoptotic rate (7.5%) to that in the control
group. These results were consistent with those of the MTT assay
and confirmed the involvement of the PI3K pathway in PYM-induced
apoptosis of EOMA cells.
Finally, in the Transwell invasion assays, treatment
with PI3K inhibitor LY294002 significantly reduced the invasion
ability of EOMA cells (P<0.01) and treatment with PYM caused a
similar extent of reduction of the invasive potential (Fig. 4C). However, co-treatment with PI3K
activator IGF-1 blocked this inhibitory effect of PYM. Again, these
results demonstrated that PYM exerts it effects via a
PI3K-associated mechanism.
In conclusion, PYM affected the biological behavior
of EOMA cells, including cell viability, cell apoptosis and
invasive ability, by inhibiting the PI3K/Akt pathway.
Discussion
PYM is a glycopeptide antibiotic which was
identified and developed into a pharmaceutical drug in China, and
which has been clinically used for the treatment of various tumor
types (1–5). The most common and effective use of
PYM is in the treatment of hemangioma, which is a benign type of
tumor derived from endothelial cells. After treatment with PYM
alone or in combination with dexamethasone, patients with
hemangioma were cured at rates of 70–100% (2,9–12).
The effects of PYM on hemangioma-derived endothelial cells have
been studied in vitro. PYM treatment (100–300 µg/ml)
resulted in a significant and dose-dependent induction of apoptosis
in human hemangioma-derived endothelial cells (HemECs) (24). These results were in line with the
results of the present study, which showed that PYM inhibited the
proliferation of EOMA cells in a dose- and time-dependent manner
and significantly induced apoptosis at 100 µg/ml. Thus,
induction of apoptosis may be an important mechanism of action of
drugs used for the clinical treatment of hemangioma.
It is thought that PYM exerts its anti-proliferative
activity by inhibiting the synthesis of DNA and DNA strands breaks
in oral cancer cells (23), and
these damages may lead to the apoptosis or necrosis of KB cells
(27). However, to date, the
underlying mechanisms of PYM-mediated DNA damage and induction of
apoptosis have not been sufficiently elucidated. Yang et al
(28) reported that PYM may induce
apoptosis by activating c-Jun N-terminal kinases and inhibiting the
activity of extracellular signal-regulated kinases 1 and -2 in KB
cells. Zhao et al (25)
reported that patients with maxillofacial squamous cell carcinomas
subjected to microwave-induced hyperthermia followed by intravenous
injection of PYM, exhibited an increased number of apoptotic cancer
cells; this effect was likely to be mediated via downregulation of
Bcl-2 and upregulation of Bax. In HemECs, the induction of
apoptosis was attributed to the activation of the p53 pathway
(24). The present study
demonstrated that the PI3K/Akt pathway has a significant role in
PYM-induced inhibition of cell viability and invasive ability as
well as induction of apoptosis in EOMA cells.
PI3K/Akt/mTOR signaling is the key regulatory
pathway for certain essential cellular processes, including cell
survival, growth and differentiation. As this pathway is
over-activated in various cancer types, it is considered as an
ideal target for anti-cancer drugs (29–31).
In the present study, PYM at 100 µg/ml acted in a similar
manner to PI3K inhibitor LY294002 at 0.5 nM; the two compounds
significantly inhibited EOMA-cell growth, induced apoptosis and
decreased the invasive ability of the cells. Furthermore, all of
these PYM-induced effects were blocked by PI3K activator IGF-1.
Finally, western blot analysis confirmed that PYM treatment
significantly decreased the expression of PI3K and p-Akt. It was
therefore concluded that PYM affects the biological behavior of
EOMA cells by inhibiting the PI3K/Akt pathway.
mTOR is one of main downstream signal regulators of
the PI3K/Akt pathway. It has a central role in positively
regulating cell growth, survival and other cell functions (32). When mTOR binds
rapamycin-insensitive companion of mTOR (rictor), the resulting
mTORC2 complex can activate the PI3K/Akt pathway by phosphorylating
Akt (33), which forms a positive
feedback loop to promote tumor-cell proliferation. Zheng et
al (34) reported that small
hairpin RNA-mediated knockdown of rictor in EOMA cells reduced the
phosphorylation of Akt, which suppressed cell proliferation and
invasion. The effects of PYM on mTOR and associated binding units
should be explored in future studies in order to further specify
the exact mechanisms of action of PYM.
Similar to the effects of other bleomycins, such as
those of a combined drug composed of 69% bleomycin A2 29.3%
bleomycin B2 and 1.7% of PYM (35), PYM has adverse effects of pulmonary
fibrosis (36). Recent studies
showed that bleomycin-induced pulmonary fibrosis may be associated
with PI3Kγ. In vivo, PI3Kγ knockout mice exhibited reduced
mortality and fibrosis compared with those of C57Bl/6j mice after
instillation of bleomycin (37).
In vitro, PI3Kγ inhibitor AS605240 protected against
bleomycin-induced pulmonary injury, angiogenesis and fibrosis
through the modulation of leukocyte, fibroblast and endothelial
cell functions (37,38). The above studies implied that
bleomycin induces fibrosis by activating the PI3Kγ pathway;
however, in the present study, PYM was demonstrated to inhibit the
PI3K/Akt pathway. A reasonable explanation for this apparent
inconsistency in results may be the possibility that PYM
selectively activates PI3Kγ, while specifically inhibiting other
PI3K sub-family members. This hypothesis should be examined in
future studies.
In conclusion, the results of the present study
showed that PYM inhibited the viability, induced apoptosis and
reduced the invasive ability of EOMA cells by inhibiting the
PI3K/Akt pathway. Further studies should focus on the effect of PYM
on downstream signaling proteins of the PI3K/Akt pathway and
sub-families of PI3K.
Acknowledgments
This study was supported by the Science and
Technology Planning Project of Guangdong Province, China (no.
2011B060300019).
References
|
1
|
Meisheng X: Histopathologic study of
esophageal squamous cell carcinoma treated preoperatively with
Pingyangmycin. Chin Med J (Engl). 92:343–348. 1979.
|
|
2
|
Zheng JW, Zhou Q, Yang XJ, He Y, Wang YA,
Ye WM, Zhu HG and Zhang ZY: Intralesional injection of
Pingyangmycin may be an effective treatment for epulis. Med
Hypotheses. 72:453–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang XH, He YW, Tang YL, Wu JL, Cao XP,
Xiao GZ and Mao ZY: Thermochemotherapy of lower lip squamous cell
carcinoma without metastases: An experience of 31 cases. J
Craniomaxillofac Surg. 38:260–265. 2010. View Article : Google Scholar
|
|
4
|
Guan JY, He XF, Chen Y, Zeng QL, Mei QL
and Li YH: Percutaneous intratumoral injection with pingyangmycin
lipiodol emulsion for the treatment of recurrent sacrococcygeal
chordomas. J Vasc Interv Radiol. 22:1216–1220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi W, Guo J, Wu S, Su B, Zhang L, Pan J
and Zhang J: Synergistic effect of nanosecond pulsed electric field
combined with low-dose of pingyangmycin on salivary adenoid cystic
carcinoma. Oncol Rep. 31:2220–2228. 2014.PubMed/NCBI
|
|
6
|
Bai N, Chen YZ, Fu YJ, Wu P and Zhang WN:
A clinical study of pingyangmycin sclerotherapy for venous
malformation: An evaluation of 281 consecutive patients. J Clin
Pharm Ther. 39:521–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia R, Xu S, Huang X, Song X, Pan H, Zhang
L, He F, Lin M, Ge S and Fan X: Pingyangmycin as first-line
treatment for low-flow orbital or periorbital venous malformations:
Evaluation of 33 consecutive patients. JAMA Ophthalmol.
132:942–948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yue H, Qian J, Elner VM, Guo J, Yuan YF,
Zhang R and Ge Q: Treatment of orbital vascular malformations with
intralesional injection of pingyangmycin. Br J Ophthalmol.
97:739–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo QF and Zhao FY: The effects of
Bleomycin A5 on infantile maxillofacial haemangioma. Head Face Med.
7:112011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Q and Zhao F: How to use bleomycin A5
for infantile maxillofacial haemangiomas: Clinical evaluation of 82
consecutive cases. J Craniomaxillofac Surg. 39:482–486. 2011.
View Article : Google Scholar
|
|
11
|
Hou J, Wang M, Tang H, Wang Y and Huang H:
Pingyangmycin sclerotherapy for infantile hemangiomas in oral and
maxillofacial regions: An evaluation of 66 consecutive patients.
Int J Oral Maxillofac Surg. 40:1246–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Sun M, Cheng X, Hu X, Zhang P, Ma
Q, Li J, Tian L and Lei D: Bleomycin A5 plus dexamethasone for
control of growth in infantile parotid hemangiomas. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 108:62–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng JW, Zhou Q, Yang XJ, Wang YA, Fan
XD, Zhou GY, Zhang ZY and Suen JY: Treatment guideline for
hemangiomas and vascular malformations of the head and neck. Head
Neck. 32:1088–1098. 2010. View Article : Google Scholar
|
|
14
|
Greenberger S and Bischoff J: Infantile
hemangioma-mechanism(s) of drug action on a vascular tumor. Cold
Spring Harb Perspect Med. 1:a0064602011. View Article : Google Scholar
|
|
15
|
Greenberger S and Bischoff J: Pathogenesis
of infantile haemangioma. Br J Dermatol. 169:12–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verheul HM and Pinedo HM: The role of
vascular endothelial growth factor (VEGF) in tumor angiogenesis and
early clinical development of VEGF-receptor kinase inhibitors. Clin
Breast Cancer. 1(Suppl 1): S80–S84. 2000. View Article : Google Scholar
|
|
17
|
Boye E and Olsen BR: Signaling mechanisms
in infantile hemangioma. Curr Opin Hematol. 16:202–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phng LK and Gerhardt H: Angiogenesis: A
team effort coordinated by notch. Dev Cell. 16:196–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauer TM, Patel MR and Infante JR:
Targeting PI3 kinase in cancer. Pharmacol Ther. 146:53–60. 2015.
View Article : Google Scholar
|
|
21
|
Phung TL, Eyiah-Mensah G, O'Donnell RK,
Bieniek R, Shechter S, Walsh K, Kuperwasser C and Benjamin LE:
Endothelial Akt signaling is rate-limiting for rapamycin inhibition
of mouse mammary tumor progression. Cancer Res. 67:5070–5075. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: Involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tai KW, Chang YC, Chou LS and Chou MY:
Cytotoxic effect of pingyangmycin on cultured KB cells. Oral Oncol.
34:219–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tu JB, Li QY, Jiang F, Hu XY, Ma RZ, Dong
Q, Zhang H, Pattar P and Li SX: Pingyangmycin stimulates apoptosis
in human hemangioma-derived endothelial cells through activation of
the p53 pathway. Mol Med Rep. 10:301–305. 2014.PubMed/NCBI
|
|
25
|
Zhao J, Wang SZ, Tang XF, Liu N, Zhao D
and Mao ZY: Analysis of thermochemotherapy-induced apoptosis and
the protein expressions of Bcl-2 and Bax in maxillofacial squamous
cell carcinomas. Med Oncol. 28(Suppl 1): S354–S359. 2011.
View Article : Google Scholar
|
|
26
|
Huang Y, Li P, Xia S, Zhuo Y and Wu L:
Proapoptotic effect and the mechanism of action of pingyangmycin on
cavernous hemangiomas. Exp Ther Med. 7:473–477. 2014.PubMed/NCBI
|
|
27
|
Tai KW, Chou MY, Hu CC, Yang JJ and Chang
YC: Induction of apoptosis in KB cells by pingyangmycin. Oral
Oncol. 36:242–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang LC, Yang SH, Tai KW, Chou MY and Yang
JJ: MEK inhibition enhances bleomycin A5-induced apoptosis in an
oral cancer cell line: Signaling mechanisms and therapeutic
opportunities. J Oral Pathol Med. 33:37–45. 2004. View Article : Google Scholar
|
|
29
|
Fruman DA and Rommel C: PI3K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hassan B, Akcakanat A, Holder AM and
Meric-Bernstam F: Targeting the PI3-kinase/Akt/mTOR signaling
pathway. Surg Oncol Clin N Am. 22:641–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan KH, Yap TA, Yan L and Cunningham D:
Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J
Cancer. 32:253–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun SY: MTOR kinase inhibitors as
potential cancer therapeutic drugs. Cancer Lett. 340:1–8. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alessi DR, Pearce LR and Garcia-Martinez
JM: New insights into mTOR signaling: MTORC2 and beyond. Sci
Signal. 2:pe272009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng NN, Ding XD and Zhang HP: Targeting
rictor inhibits mouse vascular tumor cell proliferation and
invasion in vitro and tumor growth in vivo. Neoplasma. 60:41–45.
2013. View Article : Google Scholar
|
|
35
|
Aouida M and Ramotar D: A new twist in
cellular resistance to the anticancer drug bleomycin-A5. Curr Drug
Metab. 11:595–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raisfeld IH: Pulmonary toxicity of
bleomycin analogs. Toxicol Appl Pharmacol. 56:326–336. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Russo RC, Garcia CC, Barcelos LS, Rachid
MA, Guabiraba R, Roffê E, Souza AL, Sousa LP, Mirolo M, Doni A, et
al: Phosphoinositide 3-kinase γ plays a critical role in
bleomycin-induced pulmonary inflammation and fibrosis in mice. J
Leukoc Biol. 89:269–282. 2011. View Article : Google Scholar
|
|
38
|
Wei X, Han J, Chen ZZ, Qi BW, Wang GC, Ma
YH, Zheng H, Luo YF, Wei YQ and Chen LJ: A phosphoinositide
3-kinase-gamma inhibitor, AS605240 prevents bleomycin-induced
pulmonary fibrosis in rats. Biochem Biophys Res Commun.
397:311–317. 2010. View Article : Google Scholar : PubMed/NCBI
|