Introduction
In spite of extensive research efforts regarding the
biomedical applications of functionalized nanoparticles (NPs), the
anti cancer effects of certain unmodified NPs have remained to be
studied in detail. At present, Au-NPs are utilized in various
biomedical applications, including intracellular gene regulation,
chemotherapy and drug delivery, as well as in optical and
electronic applications (1–3). Not
only can Au-NPs be used as scaffolds for the delivery of
anti-cancer drugs to enhance their potency, but they can also serve
as intrinsic anti-neoplastic agents (4–6). A
previous study demonstrated that unmodified Au-NPs inhibited the
proliferation of cancer cells by abrogating mitogen-activated
protein kinase signaling (7).
The benefits of nanomaterials in biomedical and
industrial applications for human health and the environment have
been demonstrated by a large number of studies (8,9).
Detailed studies on the broad applications of Au-NPs are available,
indicating their size-dependent physicochemical and biological
properties. Arvizo and Murphy (10) demonstrated that small Au-NPs
(diameter, <2 nm) were able to penetrate the cell nucleus,
rendering them highly toxic. However, Connor et al (11) found that Au-NPs with a diameter of
4, 12 and 18 nm were able to be endocytosed by cells, while not
showing any inherent toxicity to leukemia cells. As these previous
studies indicated differential effects of Au-NPs depending on their
size, further elucidation of their properties and determination of
a suitable particle size for cancer therapy are required.
The anti-metastatic properties of Au-NPs are the
focus of current research (12).
The process of cell invasion and metastasis begins with cell
proliferation, followed by dissociation of single cells from the
primary lesions and their migration via the blood or lymph system,
finally leading to adhesion to a secondary site of the body
(13). In spite of marked progress
in surgery, chemotherapy and radiotherapy, tumor recurrence is
almost inevitable once metastasis is present (14,15).
Previous studies have demonstrated that matrix metalloproteinase
(MMP)2 and MMP9, used as prognostic biomarkers for thyroid
carcinoma progression, have important roles in cancer cell
adhesion, invasion and migration (16–18).
Therefore, the present study assessed the effects of
Au-NPs on the proliferation, invasion and expression of MMPs in
human thyroid carcinoma, which is a major malignant tumor type in
China with an increasing incidence rate (19). The effects of Au-NPs of various
sizes (5, 10, 20, 40, 50 and 60 nm) on the proliferation and
invasion of the SW579 cell line were assessed in order to provide a
foundation for the application of Au-NPs in thyroid carcinoma
therapy.
Materials and methods
Synthesis of Au NPs
The classic citrate reduction method was used to
synthesize Au-NPs (20). For each
synthesis, 100 ml 0.01% HAuCl4 solution (Sinopharm
Chemical Reagent Co., Ltd, Beijing, China) was heated to boil.
Aliquots of 1% citrate solution (Shanghai XiBao Biological
Technology Co., Ltd., Shanghai, China) were added, followed by
heating to boil until the color of the solution turned to red. The
solution was allowed to cool to room temperature (RT), and the
morphology of the Au-NPs was observed by transmission electron
microscopy (TEM; JEM-2100EX, JEOL, Ltd., Tokyo, Japan). The
nanoparticles were purchased from Southeast University Biological
and Medical Nanotechnology Research Laboratory (Nanjing,
China).
Cell culture
The SW579 human thyroid carcinoma cell line
(Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences, Shanghai, China), were cultured in RPMI-1640 medium
containing 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin
and 100 μg/ml streptomycin (all from Gibco (Thermo Fisher
Scientific, Waltham, MA, USA) at 37°C in a humidified atmosphere
containing 5% CO2. Cells were harvested using
trypsin-EDTA (Gibco) at the logarithmic growth phase, followed by
centrifugation at 300 × g for 5 min and re-suspension in RPMI-1640
containing 10% FBS. In the experiments, cells were treated either
with or without 50 μg/ml Au-NP solution for 24 h prior to
subsequent analyses.
TEM studies
The uptake of Au-NPs by the cells was observed using
TEM. Prior to incubation with the Au-NPs, the SW579 cells were
seeded into 100-mm dishes (Corning Inc., Corning, NY, USA) at
1×106 cells per dish and incubated for 24 h. After
subsequent incubation with Au NPs for 24 h, the cells were fixed in
3.7% (v/v) paraformaldehyde (Beijing Dingguochangsheng Biotech Co.,
Ltd., Beijing, China) for 20 min at RT. The cells were then
prepared for TEM analysis as follows: Cells were fixed in 1% (w/v)
osmium tetroxide (Shanghai WeiHuan Biotech Co., Ltd., Shanghai,
China) for 2 h, dehydrated in a graded series of 30, 50, 70, 80 and
90% ethanol, and treated three times with 100% ethanol for 15 min
each. The samples were then embedded in a mixture of resin
(Shanghai Absin Bioscience Inc., Shanghai, China) in propylene
oxide (Shanghai WeiHuan Biotech Co., Ltd.) polymerized at 80°C.
Ultrathin sections (75 nm) were produced using a diamond knife and
the samples were analyzed by TEM (JEM-2100EX; JEOL, Ltd.).
Cell Counting Kit (CCK) 8 assay
SW579 cells, seeded in 96-well plates (2,000
cells/well), were allowed to attach overnight and then left
untreated or treated with Au-NPs (5, 10, 20, 40, 50 or 60 nm) for
24 h. Subsequently, assay reagent (cat. no. KGA317; Cell Counting
Kit-8; Kaiji, Nanjing, China) was added to each well followed by an
incubation for 1, 2, 3 and 4 h. Absorbance values at 450 nm were
recorded using a microplate reader (iMark Microplate Absorbance
Reader; Bio-Rad Laboratories, Inc., Hercules, CA, USA), the results
from each of the four time points were averaged, and the cell
viability was calculated as a percentage of the untreated control.
Each experiment was performed in triplicate wells and the
experiment was repeated three times.
Apoptosis detection by Annexin V
propidium iodide (PI) staining
SW579 cells, seeded onto a six-well culture plate at
a density of 1×105 cells per well, were and allowed to
attach overnight in a 37°C incubator. After treatment with or
without Au-NPs (5, 10, 20, 40, 50 or 60 nm) for 24 h, apoptosis and
necrosis were analyzed using the Annexin V-PI apoptosis detection
kit (cat. no. 556547; BD Biosciences, Franklin Lakes, NJ, USA)
following the manufacturer's instructions. The samples were
analyzed using a BD FACS CantoII instrument (BD Biosciences).
Cell cycle analysis by flow
cytometry
Cell cycle analysis was conducted using the Cell
Cycle Assay kit (cat. no. A411-01; Vazyme, Nanjing, China). The
cells were harvested using 0.25% trypsin containing 1 mM EDTA
(Gibco) and fixed for 12 h in 70% ethanol at 4°C. The fixed cells
were then centrifuged at 1,200 × g for 15 min to remove the
ethanol, washed twice with 3 ml phosphate-buffered saline (PBS),
re-suspended in 1 ml PI staining solution (20 μg/ml PI and
0.2 mg/ml RNase A in PBS) and incubated for 15 min at RT. The
samples were subsequently analyzed using a BD FACS CantoII
instrument (BD Biosciences). Twenty thousand events were collected
from each sample. The percentages of cells in the G0/G1, S, and
G2/M phases of the cell cycle were determined using ModFit LT v 3.3
software (BD Biosciences).
Invasion assay
Cell culture inserts (8.0-μm pore size;
Millicell Cell Culture Insert; Millipore, Billerica, MA, USA) were
pre-coated with 50 μg/ml Matrigel (BD Biosciences) on the
upper surface. Cells were treated either with or without 50
μg/ml Au-NP solution for 24 h, and the harvested cells
(2.5×105) were then seeded into the upper compartment in
200 μl RPMI-1640 containing 0.2% bovine serum albumin
(Shanghai WeiHuan Biotech Co., Ltd.). The lower compartment was
filled with 750 μl RPMI 1640 containing 5% FBS. The invasion
assay was performed for 24 h in a 37°C incubator.
The culture medium in the upper and lower
compartments of the chamber was then replaced with 4% formaldehyde
to fix the cells. After incubation for 15 min, the chambers were
washed with PBS and stained with 0.1% crystal violet (Sinopharm
Chemical Reagent Co., Ltd.) for 10 min. After washing the chambers
five times with deionized H2O, the cells at the top of
the Matrigel membrane were removed using cotton buds. Images of the
cells remaining on the lower side, which were those that had
transgressed through the membrane, were captured using a microscope
(DM2500; Leica Microsystems, Wetzlar, Germany).
In addition, cells invaded through the membrane were
quantified using the QCM™ 24-well Cell Invasion Fluorometric Assay
(cat. no. ECM554; Millipore). This assay provides an efficient
system for quantitative detection of cell invasion through a
basement membrane model. SW579 cells, either in the absence
(control) or in the presence of Au-NPs (5, 10, 20, 40, 50 or 60
nm), were cultured in complete medium for 24 h. Subsequently, cells
were harvested, re-suspended in serum-free medium and seeded
(2.5×105 cells/250 μl) into a plate chamber.
RPMI-1640 containing 10% FBS was used as chemoattractant added to
the lower chamber. After incubation for 24 h, cells remaining on
the top of the membrane were removed and the inserts were placed in
a fresh well. Cell detachment solution was added, followed by
incubation at 37°C for 30 min. After removing the inserts from the
wells, lysis buffer/dye solution was added to the detached cells
for 15 min at RT. Finally, the relative fluorescence of the stained
lysates was assessed using a fluorescence plate reader (Synergy HT,
Bio-Tek, Winooski, VT, USA) at 480/520 nm.
Reverse transcription quantitative
polymerase chain reaction (RT qPCR) analysis
Total RNA was isolated from SW579 cells in each
group using TRIzol reagent (Invitrogen; Thermo Fisher Scientific).
Reverse transcription into cDNA (cat. no. R122-01; HiScript Q RT
SuperMix for qPCR; Vazyme) was performed using 1 μg total
RNA with oligo dT primer, and PCR was performed using SYBR Green
Mix (cat. no. Q111-02/03; AceO qPCR SYBR Green Master Mix; Vazyme)
and ABI7300 Real-Time PCR System; Applied Biosystems; Thermo Fisher
Scientific). The primer sequences were as follows: GAPDH forward,
GGAGCCAAACGGGTCATCATCTC and reverse, GAGGGGCCATCCACAGTCTTCT; MMP2
forward, TGATCTTGACCAGAATACCATCGA and reverse,
GGCTTGCGAGGGAAGAAGTT; MMP9 forward, GGCTACGTGACCTATGACATCCT and
reverse, TCCTCCCTTTCCTCCAGAACA (Invitrogen; Thermo Fisher
Scientific). PCR was performed at 95°C for 5 min, followed by 95°C
for 30 sec at 60°C for 30 sec and 1 min at 70°C for 35 cycles.
Melting curve analysis was performed to determine the specificity
of the PCR products. The comparative Ct method (21) was used to evaluate the relative
abundance of mRNA and target gene expression was normalized to that
of GAPDH. Three independent experiments were performed.
Western blot analysis
SW579 cells (1×106) were placed in 75
cm2 culture flasks and treated with or without Au NPs
(5, 10, 20, 40, 50 and 60 nm). After 24 h, 5–10×106
cells were harvested and lysed with ice-cold
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich, St.
Louis, MO, USA). Protein concentration was determined using
Bicinchoninic Acid Protein Assay kit (cat. no. P0012A; Beyotime
Institute of Biotechnology, Shanghai, China). Equal amounts of
protein (50 μg) were separated by 10% SDS-PAGE and
electrophoretically transferred onto polyvinylidene fluoride
membranes (Millipore). Membranes were blocked with 5% non-fat dry
milk for 2 h and incubated overnight at 4°C with rabbit anti MMP9
antibody (1:1,000; cat. no. ab38898; Abcam, Cambridge, MA, USA),
rabbit anti-MMP2 antibody (1:1,000; cat. no. 13132; Cell Signaling
Technology, Inc., Danvers, MA, USA), or mouse anti-GAPDH antibody
(1:1,0000; cat. no. M20006; Abmart, Berkeley Heights, NJ, USA).
Blots were washed five times with Tris-buffered saline containing
0.1% Tween 20, and were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. H0912; dilution
1:1,000) or goat anti-mouse (cat. no. A8592; dilution 1:1,000)
secondary antibodies (Sigma-Aldrich) and visualized with
chemiluminescence reagents included in the ECL kit (Bio-Rad
Laboratories, Inc.). GAPDH was used as the housekeeping gene
control and the expression levels of the MMP2 and MMP9 were
normalized to GAPDH. Immunoreactive bands were detected by enhanced
chemiluminescence and quantified using a ChemiDoc XRS molecular
imager (Bio-Rad Laboratories, Inc.).
Statistical analysis
GraphPad Prism 5.0 software for Windows (GraphPad,
Inc., La Jolla, CA, USA) was used for all statistical analyses in
this study. Values are expressed as the mean ± standard deviation.
Statistical comparisons were performed using one-way analysis of
variance, followed by the Dunnett's t-test for comparison with the
control group. P<0.05 was considered to indicate a statistically
significant difference.
Results
Synthesis and characterization of Au
NPs
Au-NPs without any further modification were used in
the present study. To explore the size-dependent effects of the
nanoparticles, Au-NPs of six different sizes (5, 10, 20, 40, 50 or
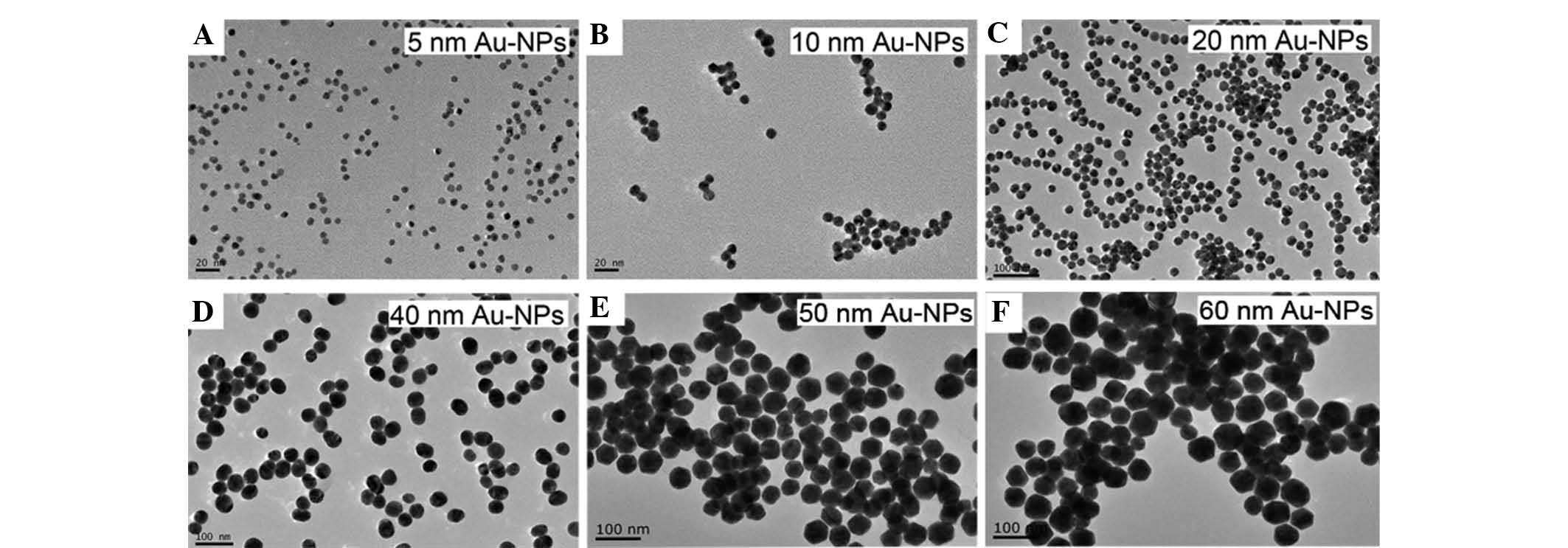
60 nm) were synthesized and characterized by TEM (Fig. 1). The particles exhibited a
spherical shape and were uniform in size within each group.
Internalization of Au NPs
To prove that Au-NPs were able to enter cells, SW579
cells were cultured in complete medium containing Au-NPs (5, 10,
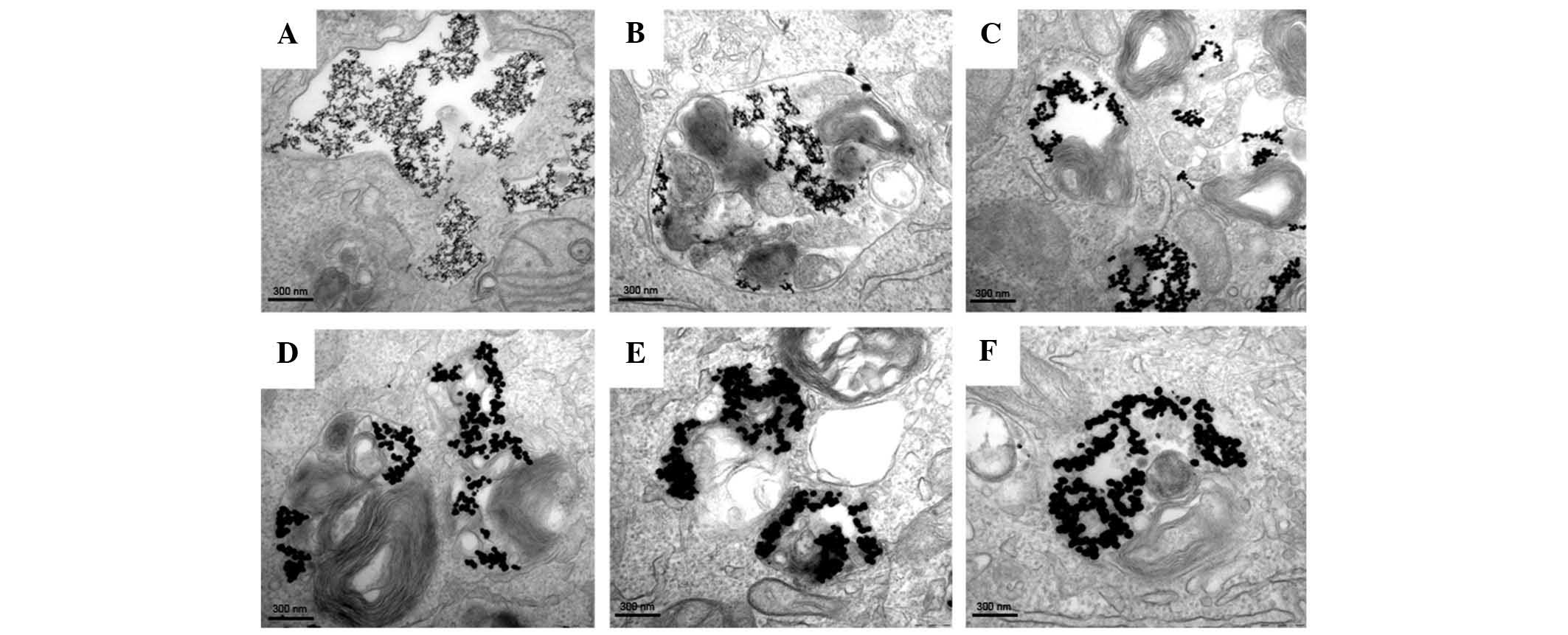
20, 40, 50 or 60 nm) for 24 h and visualized using TEM. Fig. 2 shows the internalization and
distribution of Au-NPs with various sizes in SW579 cells. Most of
the particles appeared in vesicles or the perinuclear region within
the cells.
Small sized Au NPs reduce the
proliferation of SW579 cells, and induce apoptosis and cell cycle
arrest
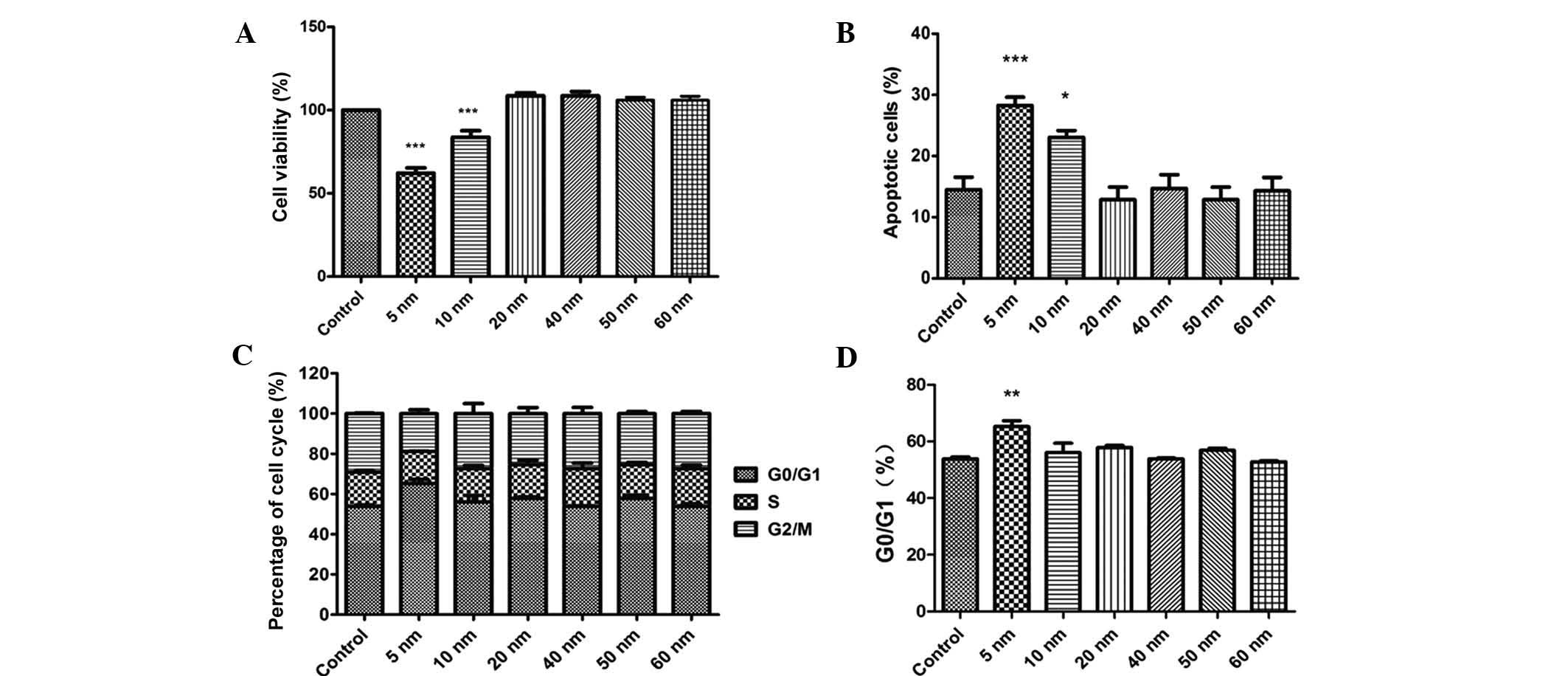
The CCK-8 assay showed that only 5- and 10-nm Au-NPs
exerted obvious inhibitory effects on the viability of SW579 cells
and promoted apoptosis (Fig. 3A and
B). In addition, only 5-nm Au NPs caused significant cell cycle
arrest in G0/G1 phase (Fig. 3C and
D). By contrast, Au-NPs sized 20–60 nm showed no significant
effects on the viability, apoptosis and cell cycle distribution of
SW579 cells.
Small sized Au NPs reduce the invasive
capacity of SW579 cells
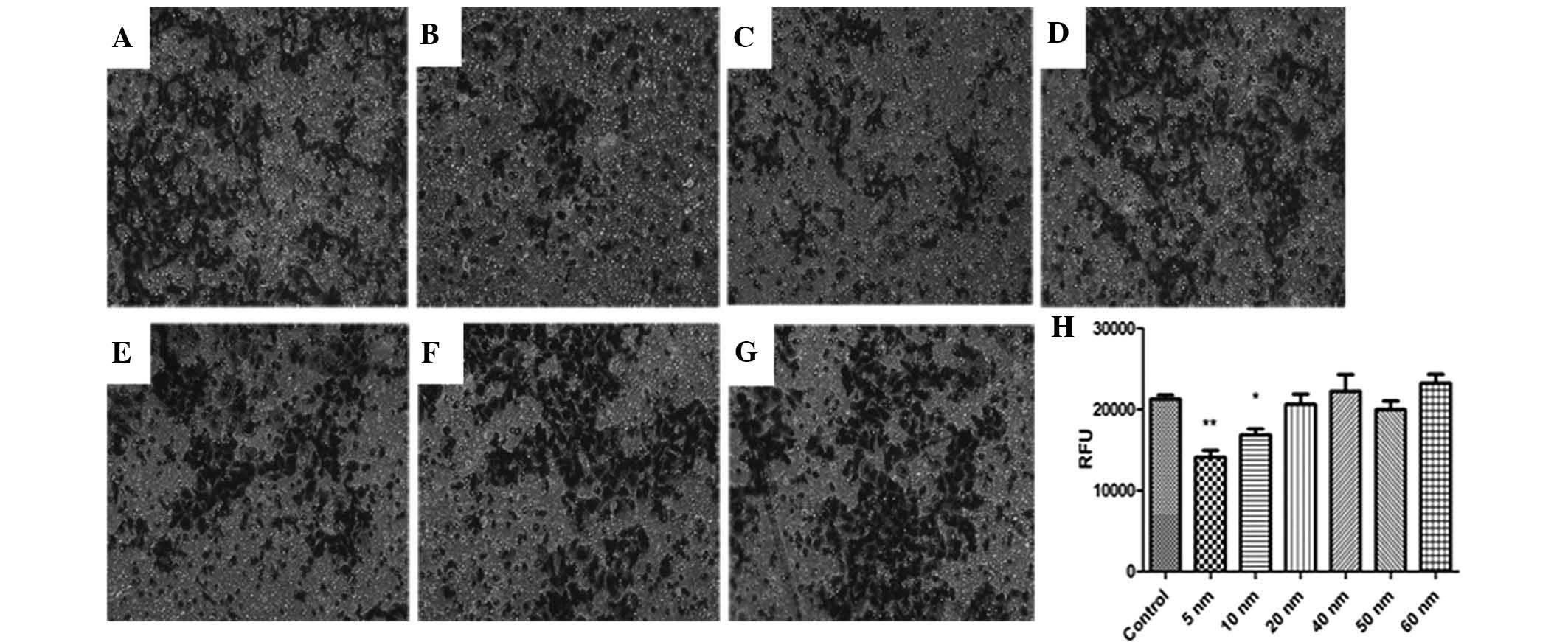
The invasive capacity of SW579 cells was determined
using a classic Transwell assay. As shown in Fig. 4, cell invasion was significantly
suppressed by 5- and 10-nm Au-NPs (P<0.05), while Au-NPs sized
20–60 nm did not significantly affect the invasiveness of SW579
cells. These findings indicated that the effects of Au NPs on cell
invasion may be size-dependent.
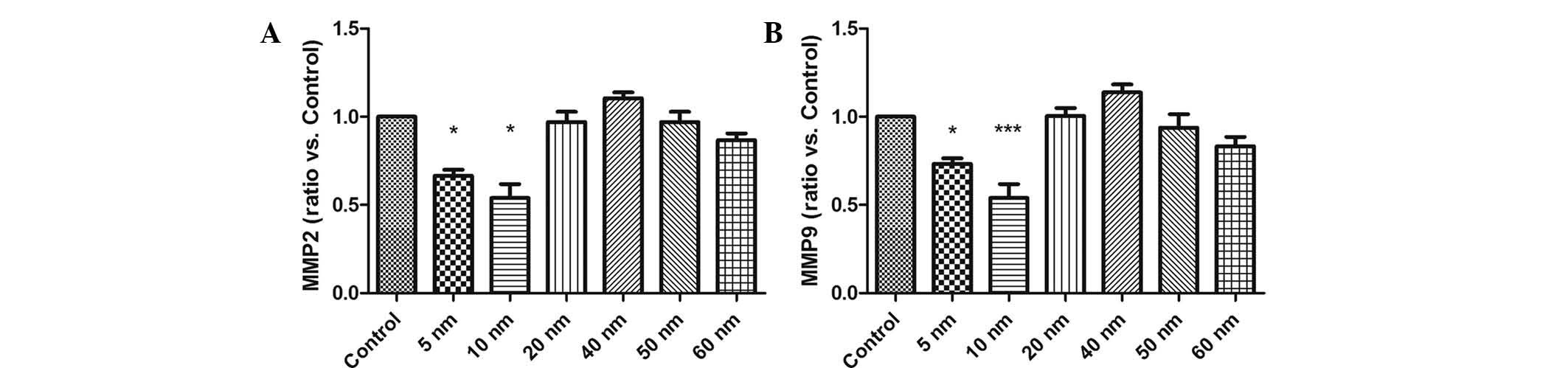
Small sized Au NPs inhibit the expression
of MMP2 and MMP9 in SW579 cells
To elucidate the underlying mechanisms of the
inhibitory effects of Au-NPS on SW579 cells, RT-qPCR analysis was
performed to evaluate the mRNA expression of MMP2 and MMP9 in the
presence of Au-NPs. The results showed that 5- and 10-nm Au-NPs
markedly reduced the mRNA expression of MMP2 and MMP9 in SW579
cells (Fig. 5), while no
significant effects were exerted by Au-NPs sized 20–60 nm.
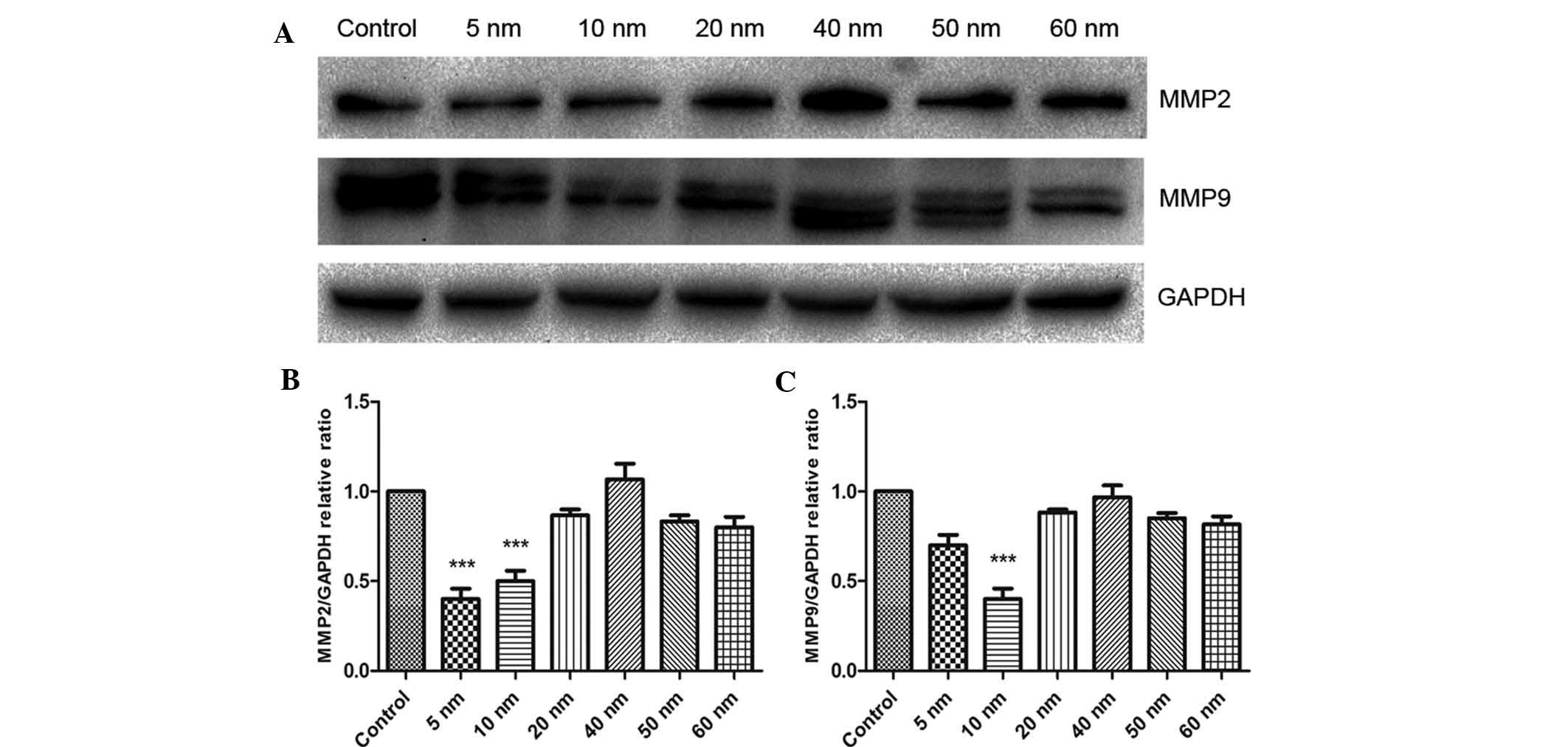
Furthermore, the present study assessed the protein
expression of MMP2 and -9 using western blot analysis. As shown in
Fig. 6, treatment with 5 nm Au NPs
significantly decreased the protein expression of MMP2 (P<0.001)
and obviously decreased the protein expression of MMP9.
Furthermore, 10 nm Au NPs significantly decreased the protein
expression of MMP2 and MMP9 in SW579 cells (P<0.001), while no
significant effects were observed for Au NPs sized 20–60 nm.
Discussion
Major research efforts in biomedical nanotechnology
have focused on drug delivery and biosensor applications. Although
physicochemical and optoelectronic properties of inorganic
nanoparticles have been studied in detail, their biological
properties remain to be fully elucidated. Among them, Au-NPs have
gained interest regarding their utilization in biomedical
applications due to their low production cost and high synthetic
accessibility (22–24). However, the basic knowledge
regarding the interactions between nanomaterials and biological
systems is required to be broadened prior to the clinical use of Au
NPs. Similar to the findings of several other studies (25,26),
the present study observed that Au-NPs were easily taken up by
SW579 cells and localized in vesicles and the perinuclear regions.
No marked differences were observed in cell uptake/localization,
which may be due to the small sample size of the TEM selection.
The present study revealed that small Au-NPs with a
diameter of 5 and 10 nm obviously inhibited the proliferation and
induced apoptosis and G0/G1 phase cell cycle arrest of SW579 cells.
By contrast, 20–60 nm-sized Au-NPs exerted no marked cytotoxic
effects on SW579 cells, which is in line with the findings of
previous studies. Arvizo et al (27) came to the conclusion that surface
size, but not surface charge, has a significant effect on the
biological effects of Au NPs. However, other studies did not
observe any cytotoxic effects of Au-NPs; for instance, Connor et
al (11) reported that Au-NPs
sized 4, 12 and 18 nm were not acutely toxic to K562 leukemia
cells, and hypothesized that the previously observed cytotoxicity
was an effect of the cetyltrimethyl ammonium bromide coating of the
Au-NPs. Cui et al (28)
even observed that Au-NPs promoted cell proliferation when
accumulated on the cell surface instead of within the cells.
Furthermore, Patra et al (29) demonstrated that Au-NPs did not
universally target all cell types, which may explain for the
controversy among the abovementioned studies.
To date, the underlying mechanisms of the
anti-proliferative effects of Au-NPs have remained elusive. Most
studies indicated that Au-NP-derived cytotoxicity is mainly based
on the generation of reactive oxygen species (30,31).
Furthermore, Au-NPs have been indicated to cause cell-morphological
changes and cytoskeletal defects, leading to cell damage and
inhibition of proliferation (29).
In addition, Au-NPs have been demonstrated to interfere with the
expression of genes associated with proliferation (32).
The present study revealed that the Au-NP-induced
reduction of the invasive ability of SW579 cells was accompanied by
a marked downregulation of MMP2 and MMP9 expression. The most
important step in tumor metastasis is the invasion of tumor cells
through the extracellular matrix (ECM). Tumor cells initiate
invasion by adhering to and migrating along the blood or lymph
vessel wall. MMPs, which are endopeptidases, are able to degrade
ECM components, allowing tumor cells to access the vasculature and
lymphatic systems (33,34). MMPs have attracted much attention
due to their ability to degrade type IV collagen, the basic
component of the basement membrane. Increased expression of MMP9 in
patients with thyroid carcinoma was shown to be correlated with a
greater risk of advanced cancer (35,36);
therefore, drugs restraining the expression of MMPs may suppress
tumor cell migration and invasion. It has been reported that MMP2
is highly expressed in human thyroid carcinoma (37,38).
Marecko et al (39)
revealed that downregulation of MMP2 mRNA or protein markedly
inhibited human thyroid carcinoma cell invasion. The present study
found that 5- and 10-nm Au-NPs effectively suppressed the
expression of MMP2 and MMP9 in SW579 cells, which partially
explained for the inhibitory effects of the nanoparticles on tumor
cell invasion. Since the downregulation of Au-NPs on MMP2 and MMP9
expression in SW579 cells indicated that small nanoparticles may
own the ability to suppress the invasion of thyroid carcinoma
cells, further in vivo studies are required to confirm the
mechanisms.
To the best of our knowledge, the present study was
the first to evidence the inhibitory effects of Au-NPs on thyroid
carcinoma cell proliferation, viability and invasion in
vitro, which contributes to the development of novel therapies
for thyroid carcinoma utilizing Au-NPs. The present study suggested
that the anti-cancer efficacy of unmodified Au NPs largely depended
on the particle size.
The present study assessed the inhibitory effects of
unmodified Au NPs of different sizes (5, 10, 20, 40, 50 and 60 nm)
on the proliferation, viability and invasion of thyroid carcinoma
cells. NP size is an essential factor determining their efficacy
with regard to the inhibition of cell proliferation and invasion.
Only 5- and 10-nm Au-NPs were able to inhibit the proliferation and
invasion of SW579 cells, which was indicated to be attributed to
the downregulation of MMP2 and MMP9 expression. The present study
provided useful information on the effects of Au-NPs on cell
proliferation and invasion, which may contribute to the utilization
of Au-NPs in thyroid carcinoma therapy.
References
|
1
|
Arvizo RR, Bhattacharyya S, Kudgus RA,
Giri K, Bhattacharya R and Mukherjee P: Intrinsic therapeutic
applications of noble metal nanoparticles: Past, present and
future. Chem Soc Rev. 41:2943–2970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dreaden EC, Alkilany AM, Huang X, Murphy
CJ and El-Sayed MA: The golden age: Gold nanoparticles for
biomedicine. Chem Soc Rev. 41:2740–2779. 2012. View Article : Google Scholar
|
|
3
|
Dykman LA and Khlebtsov NG: Gold
nanoparticles in biology and medicine: Recent advances and
prospects. Acta Naturae. 3:34–55. 2011.PubMed/NCBI
|
|
4
|
Huang X, Jain PK, El-Sayed IH and El-Sayed
MA: Gold nanoparticles: Interesting optical properties and recent
applications in cancer diagnostics and therapy. Nanomedicine
(Lond). 2:681–693. 2007. View Article : Google Scholar
|
|
5
|
Giljohann DA, Seferos DS, Daniel WL,
Massich MD, Patel PC and Mirkin CA: Gold nanoparticles for biology
and medicine. Angew Chem Int Ed Engl. 49:3280–3294. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dreaden EC, Mackey MA, Huang X, Kang B and
El-Sayed MA: Beating cancer in multiple ways using nanogold. Chem
Soc Rev. 40:3391–3404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arvizo RR, Saha S, Wang E, Robertson JD,
Bhattacharya R and Mukherjee P: Inhibition of tumor growth and
metastasis by a self-therapeutic nanoparticle. Proc Natl Acad Sci
USA. 110:6700–6705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palui G, Aldeek F, Wang W and Mattoussi H:
Strategies for interfacing inorganic nanocrystals with biological
systems based on polymer-coating. Chem Soc Rev. 44:193–227. 2015.
View Article : Google Scholar
|
|
9
|
Hauser CA, Maurer-Stroh S and Martins IC:
Amyloid-based nanosensors and nanodevices. Chem Soc Rev.
43:5326–5345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alkilany AM and Murphy CJ: Toxicity and
cellular uptake of gold nanoparticles: What we have learned so far?
J Nanopart Res. 12:2313–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Connor EE, Mwamuka J, Gole A, Murphy CJ
and Wyatt MD: Gold nanoparticles are taken up by human cells but do
not cause acute cytotoxicity. Small. 1:325–327. 2005. View Article : Google Scholar
|
|
12
|
Liu Z, Wu Y, Guo Z, Liu Y, Shen Y, Zhou P
and Lu X: Effects of internalized gold particles with respect to
cytotoxicity and invasion activity in lung cancer cells. PLoS One.
9:e991752014. View Article : Google Scholar
|
|
13
|
Ju D, Sun D, Xiu L, Meng X, Zhang C and
Wei P: Interleukin-8 is associated with adhesion, migration and
invasion in human gastric cancer SCG-7901 cells. Med Oncol.
29:91–99. 2012. View Article : Google Scholar
|
|
14
|
Machens A and Dralle H: Follicular thyroid
carcinoma: Metastasis to the sternum or adjacent tumour invasion by
continuity? Int J Clin Pract. 61:5212007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cvejic DS, Savin SB, Petrovic IM, Paunovic
IR, Tatic SB and Havelka MJ: Galectin-3 expression in papillary
thyroid carcinoma: Relation to histomorphologic growth pattern,
lymph node metastasis, extrathyroid invasion, and tumor size. Head
Neck. 27:1049–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang H, Zhong Y, Luo Z, Huang Y, Lin H,
Luo M, Zhan S, Xie K, Ma Y and Li QQ: Assessment of biomarkers for
clinical diagnosis of papillary thyroid carcinoma with distant
metastasis. Int J Biol Markers. 25:38–45. 2010.PubMed/NCBI
|
|
17
|
Liang H, Zhong Y, Luo Z, Huang Y, Lin H,
Zhan S, Xie K and Li QQ: Diagnostic value of 16 cellular tumor
markers for metastatic thyroid cancer: An immunohistochemical
study. Anticancer Res. 31:3433–3440. 2011.PubMed/NCBI
|
|
18
|
Mlika M, Makhlouf C, Boudaya MS, Haddouchi
C, Tritar F and Mezni F: Evaluation of the microvessel density and
the expression of metalloproteases 2 and 9 and ttf1 in the
different subtypes of lung adenocarcinoma in Tunisia: A
retrospective study of 46 cases. J Immunoassay Immunochem.
36:111–118. 2015. View Article : Google Scholar
|
|
19
|
Sun C, Li Q, Hu Z, He J, Li C, Li G, Tao X
and Yang A: Treatment and prognosis of anaplastic thyroid
carcinoma: Experience from a single institution in China. PLoS One.
8:e800112013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
GF: Controlled nucleation for the
regulation of the particle size in monodisperse gold suspensions.
Nature. 241:20–22. 1973.
|
|
21
|
Cikos S, Bukovská A and Koppel J: Relative
quantification of mRNA: Comparison of methods currently used for
real-time PCR data analysis. BMC Mol Biol. 8:1132007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khlebtsov N and Dykman L: Biodistribution
and toxicity of engineered gold nanoparticles: A review of in vitro
and in vivo studies. Chem Soc Rev. 40:1647–1671. 2011. View Article : Google Scholar
|
|
23
|
Mesbahi A: A review on gold nanoparticles
radiosensitization effect in radiation therapy of cancer. Rep Pract
Oncol Radiother. 15:176–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levy R, Shaheen U, Cesbron Y and Sée V:
Gold nanoparticles delivery in mammalian live cells: A critical
review. Nano Rev. 1:2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Gao H and Bao G: Physical
principles of nanoparticle cellular endocytosis. ACS Nano.
9:8655–8671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh N and Park JH: Endocytosis and
exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine.
9(Suppl 1): 51–63. 2014.PubMed/NCBI
|
|
27
|
Arvizo R, Bhattacharya R and Mukherjee P:
Gold nanoparticles: Opportunities and challenges in nanomedicine.
Expert Opin Drug Deliv. 7:753–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui W, Li J, Zhang Y, Rong H, Lu W and
Jiang L: Effects of aggregation and the surface properties of gold
nanoparticles on cytotoxicity and cell growth. Nanomedicine.
8:46–53. 2012. View Article : Google Scholar
|
|
29
|
Patra HK, Banerjee S, Chaudhuri U, Lahiri
P and Dasgupta AK: Cell selective response to gold nanoparticles.
Nanomedicine. 3:111–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thakor AS, Paulmurugan R, Kempen P,
Zavaleta C, Sinclair R, Massoud TF and Gambhir SS: Oxidative stress
mediates the effects of Raman-active gold nanoparticles in human
cells. Small. 7:126–136. 2011. View Article : Google Scholar
|
|
31
|
Pan Y, Leifert A, Ruau D, Neuss S,
Bornemann J, Schmid G, Brandau W, Simon U and Jahnen-Dechent W:
Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative
stress and mitochondrial damage. Small. 5:2067–2076. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Qu Y and Lü X: Global gene
expression analysis of the effects of gold nanoparticles on human
dermal fibroblasts. J Biomed Nanotechnol. 6:234–246. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamano Y, Zeisberg M, Sugimoto H, Lively
JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A and Kalluri R:
Physiological levels of tumstatin, a fragment of collagen IV alpha3
chain, are generated by MMP-9 proteolysis and suppress angiogenesis
via alphaV beta3 integrin. Cancer Cell. 3:589–601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Borrello MG, Alberti L, Fischer A,
Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P,
Greco A, Collini P, et al: Induction of a proinflammatory program
in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad
Sci USA. 102:14825–14830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mesa C Jr, Mirza M, Mitsutake N, Sartor M,
Medvedovic M, Tomlinson C, Knauf JA, Weber GF and Fagin JA:
Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells
is associated with gene expression profiles that predict a
preferential role of BRAF in extracellular matrix remodeling.
Cancer Res. 66:6521–6529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanii S, Saffar H, Tabriz HM, Qorbani M,
Haghpanah V and Tavangar SM: Expression of matrix
metalloproteinase-2, but not caspase-3, facilitates distinction
between benign and malignant thyroid follicular neoplasms. Asian
Pac J Cancer Prev. 13:2175–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Delektorskaia VV, Smirnova EA, Ponomareva
MV, Pavlova TV and Pavlov IA: Expression of matrix
metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in
papillary thyroid cancer: An association with the clinical,
morphological and ultrastructural characteristics of a tumor. Arkh
Patol. 72:3–6. 2010.In Russian. PubMed/NCBI
|
|
39
|
Marecko I, Cvejić D, Tatić S, Dragutinović
V, Paunović I and Savin S: Expression of matrix metalloproteinase-2
and its tissue inhibitor-2 in fetal and neoplastic thyroid tissue
and their significance as diagnostic and prognostic markers in
papillary carcinoma. Cancer Biomark. 11:49–58. 2011.PubMed/NCBI
|