Introduction
Insulin resistance and declining function of
pancreatic islet beta cells result in diabetes mellitus type 2. It
was first proposed in 2004 that endoplasmic reticulum stress (ERS)
is closely associated with insulin resistance and islet-cell
dysfunction, and ultimately diabetes (1,2).
ERS-induced apoptosis was also shown to contribute to insulin
resistance (3), and intervention
in ERS is therefore a potential strategy for diabetes treatment.
Erp29 is a protein located in the ER membrane of numerous tissue
types and is highly evolutionarily conserved in mammals, although
its exact functions have remained elusive (4) and it has yet to be clarified whether
Erp29 is involved in ERS in islet beta cells. Misfolded proteins
trigger a specific stress response, which is referred to as the
unfolded protein response (UPR) and has an important role in the
development of ERS. One of the main targets of Erp29 is
glucose-regulated protein 78 (Grp78) (5,6),
which forms a chaperone and signaling regulator complex with
binding immunoglobulin protein (BIP) that monitors ERS (7). In the present study, an ERS model of
islet beta cells was established and used to explore the role of
Erp29 in ERS in islet beta cells.
Materials and methods
Antibodies (Abs)
The following primary antibodies were used in the
present study: Anti-rat BIP/Grp78 rabbit monoclonal Ab (cat no.
ab32618; Abcam, Cambridge, MA, USA), anti-Erp29 goat polyclonal Ab
(cat no. EB08977; Everest Biotech, Heyford, UK), anti-calnexin
rabbit polyclonal Ab (ER marker; cat no. ab22595; Abcam),
anti-GAPDH mouse polyclonal Ab (cat no. AG019; Beyotime Institute
of Biotechnology, Haimen, China), anti-β-actin mouse monoclonal
antibody (cat no. AA128, Beyotime Institute of Biotechnology),
Alexa Fluor 488- and 594-conjugated secondary Ab (cat. nos. A11055
and A21442; Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA)
and the horseradish peroxidase (HRP)-conjugated secondary Abs goat
anti-rabbit (cat no. A0218), goat anti-mouse (cat no. A0216) and
donkey anti-goat (cat no. A0181) were all obtained from Beyotime
Institute of Biotechnology. All secondary antibodies used for
immunofluorescence showed minimal cross-reactivity with other
species.
Cell culture
Unless otherwise indicated, all chemicals in the
present study were purchased from Sigma-Aldrich (St. Louis, MO,
USA). INS-1 cells (provided by the Institute of Biochemistry and
Cell Biology, Academy of Sciences of China, Shanghai, China) were
routinely grown in a humidified atmosphere containing 5%
CO2 and 95% air at 37°C in RPMI-1640 medium (Thermo
Fisher Scientific) containing 11.1 mM glucose supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific), 1 mM sodium pyruvate, 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 55 µM
beta-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were passaged weekly after detachment with
trypsin-EDTA, and all experiments were performed between passages 4
and 20.
Immunofluorescence microscopy
INS-1 cells were grown on coverslips in 24-well
plates, washed twice with phosphate-buffered saline (PBS) and fixed
with 4% paraformaldehyde for 10–30 min. After two more washes with
PBS, cells were permeabilized/blocked with PBS containing 0.2%
saponin and 10% bovine serum albumin (SS-PBS) for 30 min. For
double immunostaining, coverslips were incubated with primary Abs
followed by secondary Abs in SS-PBS for 1 h and 30 min each,
followed by three washes with PBS. Coverslips were mounted onto
1-mm glass slides using fluorescent mounting medium. All steps were
performed at room temperature and samples were analyzed using a
Leica TCS SP2 confocal microscope (Leica Microsystems, Wetzlar,
Germany).
Erp29 siRNA and establishment of
INS-1-cell model of ERS
The specific siRNA for Erp29 (sense, 5′-ACG CUU AAA
CUC AUC UUG CUU-3′ and antisense, 5′-GCA AGA UGA GUU UAA GCG
UCU-3′) and non-target control siRNA (sense, 5′-UUC UCC GAA CGU GUC
ACG UTT-3′ and antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′) were
used in the current study (Shanghai GenePharma Co., Ltd., Shanghai,
China). Cells were transfected with siRNA using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific) according to the
manufacturer's instructions. To induce ER stress in vitro,
INS-1 cells in the logarithmic growth phase were cultured overnight
in the presence of 0, 2, 5, 10 and 20 µg/ml tunicamycin
(Sigma-Aldrich).
Reverse-transcription polymerase chain
reaction (RT-PCR) analysis
PCR was performed using a panel of normalized cDNAs
prepared from treated INS-1 cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific). Three different internal fragments were
amplified using an Applied Biosystems 9700 PCR machine (Thermo
Fisher Scientific) using the following primers: Erp29 sense, 5′-AAC
CCG ATT CCT GCT TTG-3′ and anti-sense, 5′-TTG CCT TGA ACA CCA
CCT-3′, (750-bp fragment); GRP78 sense, 5′-TCA GCC CAC CGT AAC
AAT-3′ and anti-sense, 5′-CAA ACT TCT CGC GTCAT-3′ (275-bp
fragment). Amplification was performed over 33–35 cycles with an
annealing temperature of 56–62°C. Normalization to GAPDH was
performed, which was amplified using the following primers: Sense,
5′-GAA GGT CGG AGT CCA CGG-3′ and anti-sense, 5′-GAA TGG TGA TGG
GATT-3′ (221-bp fragment). All PCR primers were produced by Sangon
Biotech, Co., Ltd. (Shanghai, China). A PCR mix kit (Tiangen
Biotech Co., Ltd., Beijing, China) was used in the PCR protocol.
The 25 µl total reaction volume contained 12.5 µl 2X
GC buffer, 1 µl dNTPs, 1 µl primers, 1 µl
cDNA, 0.25 µl Taq polymerase and 7.75 µl
ddH2O. The PCR cycling conditions were as follows: 94°C
for 4 min, then a total of 32 cycles of 94°C for 45 sec,
60/56/58.5°C (Grp78/Erp29/GAPDH) for 45 sec and 72°C for 60 sec, 32
cycles, then 72°C for 10 min. The PCR products were separated by
agarose gel electrophoresis, and the bands were analyzed by Gray
analysis software (Quantity One 4.5; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The DNA ladder was obtained from Beyotime
Institute of Biotechnology.
Western blot analysis
Protein was extracted from treated INS-1 cells and
the concentrations were determined using the Bio-Rad protein assay
(Bio-Rad Laboratories, Inc.). Equal amounts of protein were
subjected to 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The blots
were blocked in 5% nonfat milk for 1 h at room temperature. Then
the membranes were incubated overnight at 4°C with primary
antibodies (Erp29 and Grp78, 1:1,000; GAPDH and actin 1:2,000) and
then washed three times (10 min each) with Tris-buffered saline
(Sigma-Aldrich) containing 0.1% Tween 20 (Sigma-Aldrich).
Subsequently, membranes were incubated with HRP-conjugated
secondary antibodies (1:5,000) for 1 h at room temperature and
immunoreactive proteins were detected by enhanced chemiluminescence
reagent (Beyotime Institute of Biotechnology). The blots were then
exposed to an X-OMAT AR X-ray film (Kodak, Rochester, NY, USA) for
between 10 sex and 5 min as previously described (8).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean and statistically significant differences between two
groups were analyzed using the unpaired Student's t-test. SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
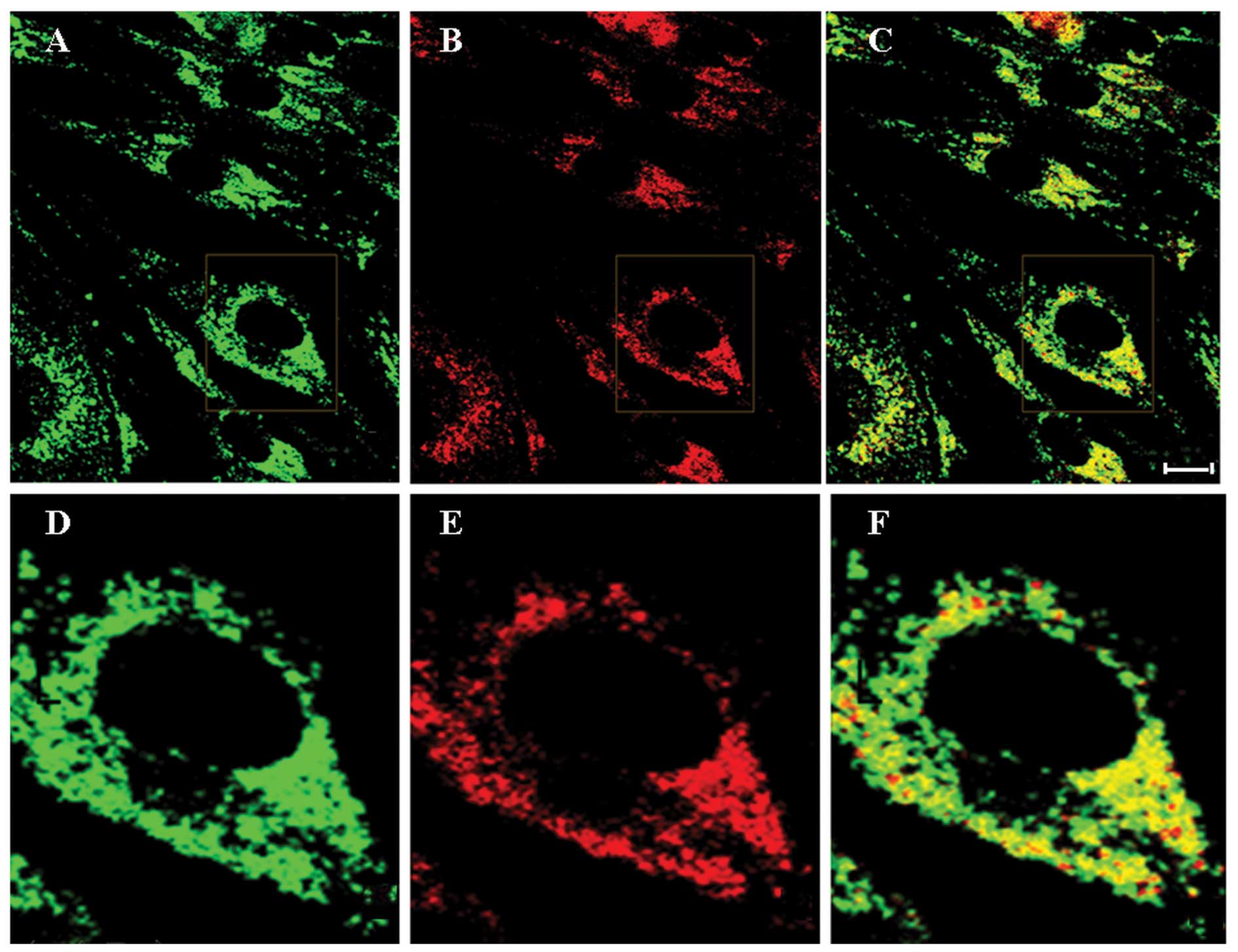
Erp29 is localized to the ER
Immunofluorescence microscopy was used to confirm
that Erp29 is localized to the ER using an ER marker
protein-specific antibody. Staining for Erp29 (green fluorescence)
was observed throughout the cytoplasm, but predominantly in the
perinuclear region, whereas no staining was present at the plasma
membrane or in the nucleus (Fig. 1A
and D). This suggested that Erp29 protein is localized to an
intracellular compartment. Co-staining for ER marker protein
calnexin (red fluorescence; Fig. 1B
and E) indicated co-localization with Erp29 (Fig. 1C and F), further indicating that
Erp29 is localized to the ER.
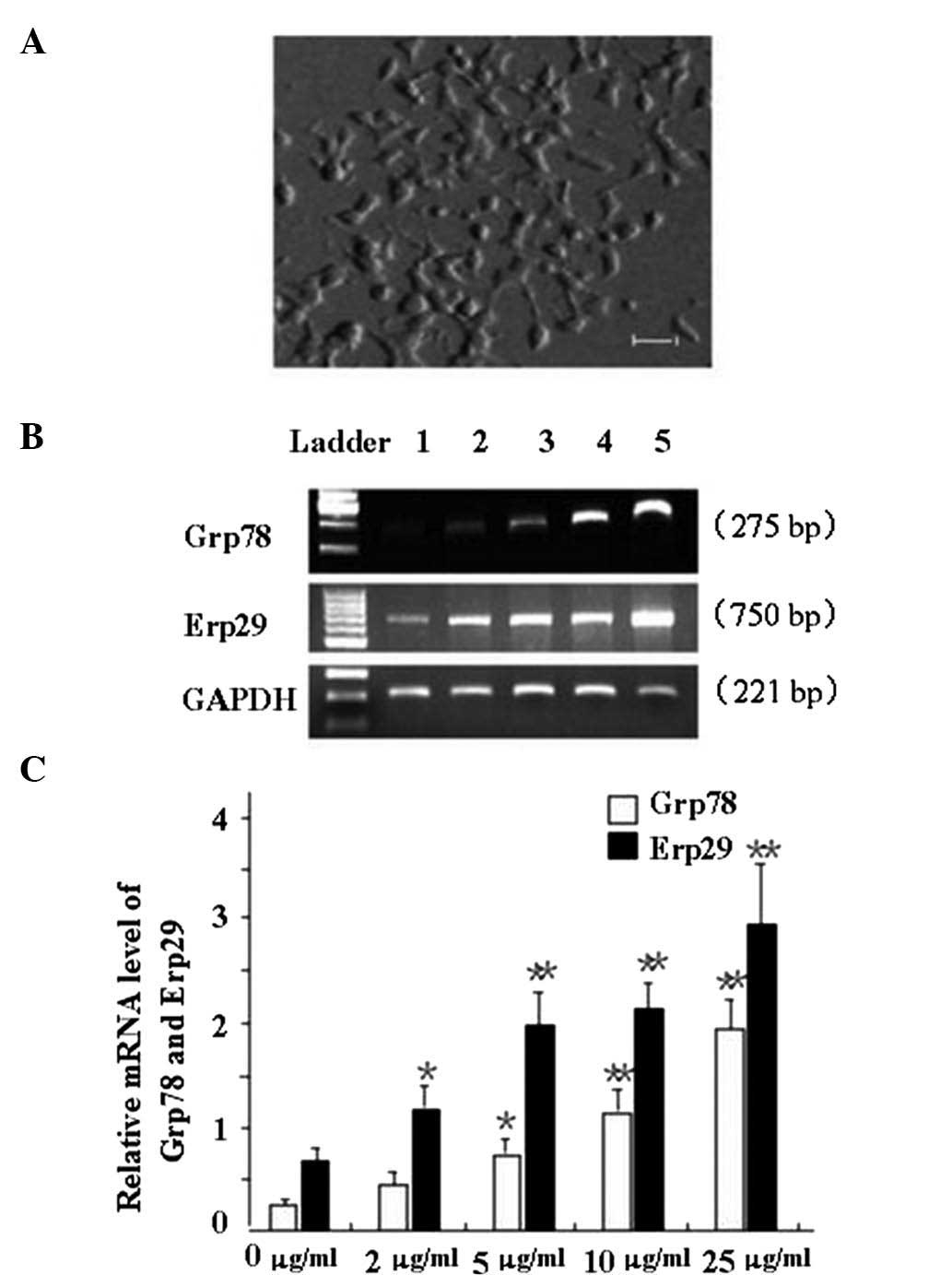
Tunicamycin-induced ERS increases the
expression of BIP/Grp78 and Erp29 in INS-1 cells
Ins-1 cells in the logarithmic growth phase
(Fig. 2A) were incubated with
various concentrations of tunicamycin to induce ERS, and Bip/GRp78
mRNA levels were assessed by RT-PCR analysis (Fig. 2). BIP/Grp78 expression was found to
be upregulated with increasing tunicamycin concentration. A
tunicamycin concentration of 5 µg/ml and above significantly
increased the mRNA expression of BIP/Grp78 (P<0.05 or P<0.01;
Fig. 2B and C). Furthermore, Erp29
mRNA expression was enhanced with increasing tunicamycin
concentration. Tunamycin at 2 µg/ml and above significantly
increased the expression of Erp29 compared with that in the control
group (P<0.05 or P<0.01; Fig. 2B
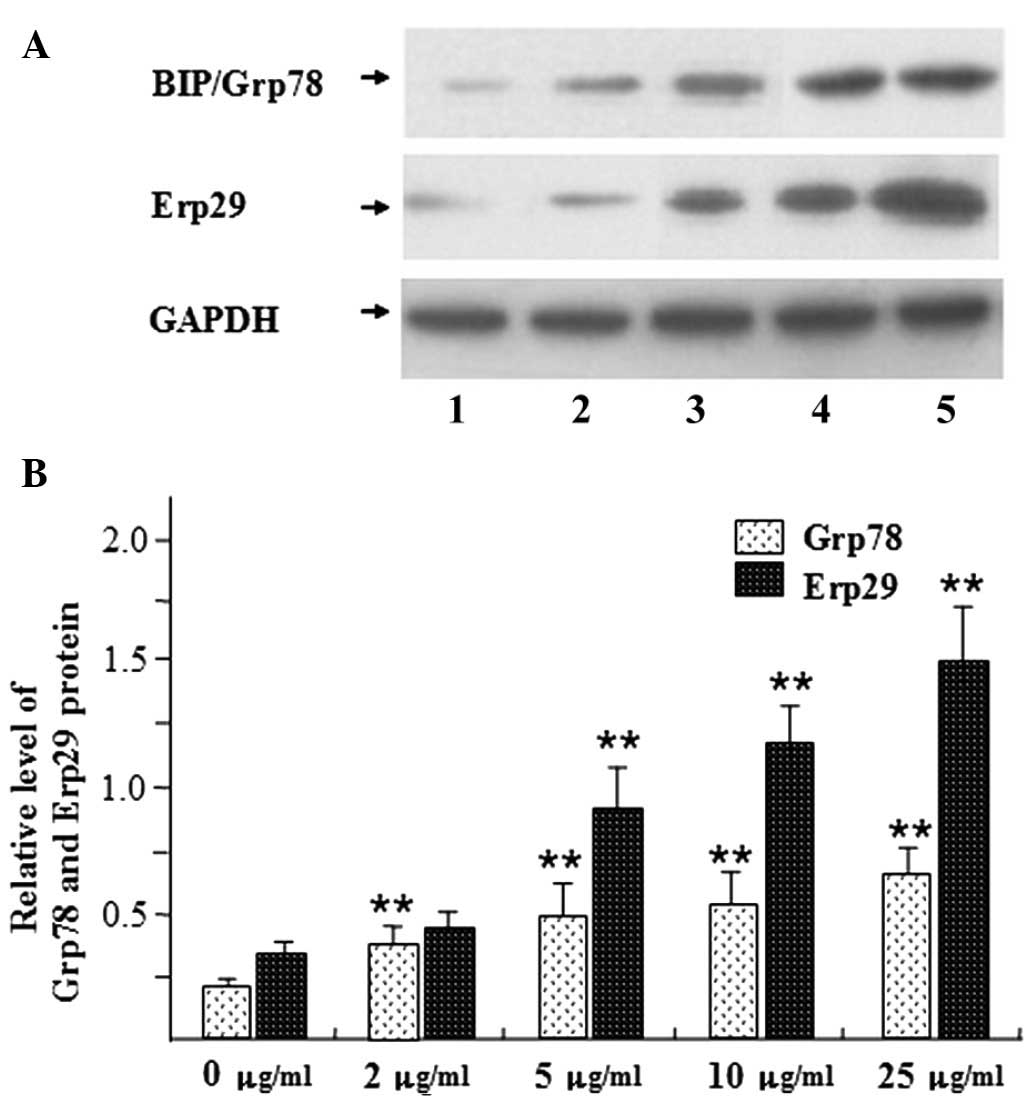
and C). Furthermore, the protein levels of BIP/Grp78 and Erp29
were assessed by western blot analysis (Fig. 3). In accordance with the changes in
mRNA expression, a tunicamycin dose of 2 mg/ml and above
significantly increased the protein expression of BIP/Grp78
compared with that in the control group (P<0.01) (Fig. 3). As BIP/Grp78 is a marker for ERS
its upregulation indicated that ERS had been induced. Furthermore,
the protein levels of Erp29 were also significantly increased
compared with those in the control group following tunicamycin
treatment at 5 µg/ml and above (P<0.01) (Fig. 3). Upregulation of Erp29 may be
associated with ERS in beta cells.
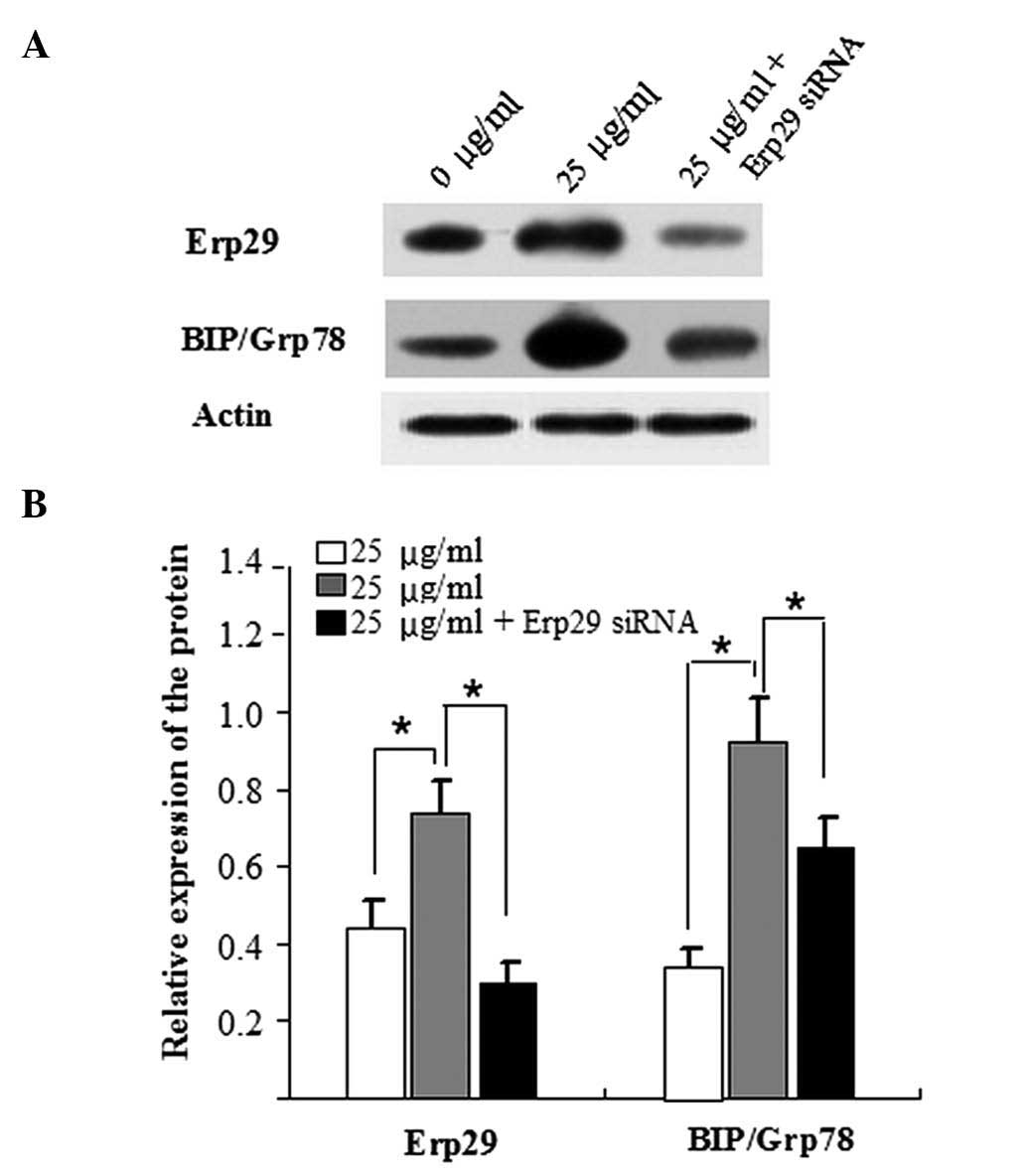
Knockdown of Erp29 decreases BIP/Grp78
expression in tunicamycin-induced ins-1 cells
In order to further investigate the association
between Erp29 and ERS, Erp29 expression was silenced using a small
interfering (si)RNA approach. After it had been confirmed that
Erp29 was successfully silenced (Fig.
4A), it was further identified that ERS responses were reduced
in tunicamycin-induced INS-1 cells transfected with Erp29 siRNA, as
indicated by a decrease in the protein levels of BIP/Grp78
(Fig. 4B).
Discussion
Erp29 is an ER protein that is widely expressed in
mammalian tissues; however, its exact functions have remained
elusive (4). The highly
evolutionarily conserved human Erp29 gene is located on chromosome
12 and the encoded protein (29 kDa) consists of 261 amino acid
residues (4). It has been
suggested that Erp29 may be involved in protein folding and
transport under ERS conditions (9). ERS-mediated apoptosis in islet cells
has an important role in the development of diabetes (10). Pancreatic β cells specifically
regulate insulin-secreting cells and the highly developed ER in
these cells is particularly sensitive to ERS. Following stimulation
of insulin secretion by elevation of blood glucose, the released
insulin activates further proinsulin biosynthesis in the ER of beta
cells. Specifically, preproinsulin is synthesized and the 16-amino
acid signaling peptide is recognized by the signal recognition
particle, which triggers synergistic translocation into the ER
lumen. Following cleavage of the signal peptide to produce
proinsulin, the peptide is folded in the ER lumen, transported to
the Golgi apparatus and packaged into secretory granules. Finally,
proinsulin is converted to the mature form and eventually released
by exocytosis. The ER of beta cells is highly sensitive to
fluctuations in blood glucose and this signaling affects the
folding of proinsulin. Upon entering the ER lumen, insulin affects
the ER protein folding capacity as well as Ca2+
homeostasis, which leads to beta cell damage, apoptosis and
ultimately ERS (11).
In the present study, double-staining
immunofluorescence microscopy was used to confirm the ER
localization of Erp29. In addition, a tunicamycin-induced in
vitro cell model of ERS was established in islet beta cells.
Erp29 as well as the ER chaperone and marker BIP/Grp78 were
increased in tunicamycin-induced beta cells, confirming that the
model of ERS had been successfully established. Erp29 expression
increased with increasing tunicamycin dose, suggesting that Erp29
is associated with ERS. While its exact role remains elusive and
requires further study, Erp29 may serve as a protective factor
against net stress in the ER and may act synergistically with
BIP/Grp78 to facilitate protein transport from the ER. This would
alleviate ERS and promote islet-cell survival, thus avoiding the
onset of apoptosis. This result is similar to the findings of a
study which assessed Erp29 expression in an IEC26-cell model of
radiation injury (12). The
present study therefore concluded that Erp29 may be involved in ERS
in islet cells and possibly other cell types and tissues. The
results indicated that a correlation between Erp29 and cell injury
may exist, which requires further study.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81200632 and
81471002), the Natural Science Foundation of Anhui, China (no.
1308085QH134) and the Introduction of Talents Foundation of
Yijishan Hospital (no. YR201104).
References
|
1
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao L, Guo H, Chen H, Petersen RB, Zheng
L, Peng A and Huang K: Effect of liraglutide on endoplasmic
reticulum stress in diabetes. Biochem Biophys Res Commun.
441:133–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ou HY, Wu HT, Hung HC, Yang YC, Wu JS and
Chang CJ: Endoplasmic reticulum stress induces the expression of
fetuin-A to develop insulin resistance. Endocrinology.
153:2974–2984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sargsyan E, Baryshev M, Backlund M,
Sharipo A and Mkrtchian S: Genomic organization and promoter
characterization of the gene encoding a putative endoplasmic
reticulum chaperone, ERp29. Gene. 285:127–139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnes JA and Smoak IW: Glucose-regulated
protein 78 (GRP78) is elevated in embryonic mouse heart and induced
following hypoglycemic stress. Anat Embryol (Berl). 202:67–74.
2000. View Article : Google Scholar
|
|
6
|
Qian Y, Falahatpisheh MH, Zheng Y, Ramos
KS and Tiffany-Castiglioni E: Induction of 78 kD glucose-regulated
protein (GRP78) expression and redox-regulated transcription factor
activity by lead and mercury in C6 rat glioma cells. Neurotox Res.
3:581–589. 2001. View Article : Google Scholar
|
|
7
|
Ma KX, Chen GW, Shi CY, Cheng FF, Dou H,
Feng CC and Liu DZ: Molecular characterization of the
glucose-regulated protein 78 (GRP78) gene in planarian Dugesia
japonica. Comp Biochem Physiol B Biochem Mol Biol. 171:12–17. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao J, Xia L, Lu M, Zhang B, Chen Y, Xu R
and Wang L: TM7SF1 (GPR137B): A novel lysosome integral membrane
protein. Mol Biol Rep. 39:8883–8889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sargsyan E, Baryshev M, Szekely L, Sharipo
A and Mkrtchian S: Identification of ERp29, an endoplasmic
reticulum lumenal protein, as a new member of the thyroglobulin
folding complex. J Biol Chem. 277:17009–17015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marchetti P, Bugliani M, Lupi R, Marselli
L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL and Cnop M:
The endoplasmic reticulum in pancreatic beta cells of type 2
diabetes patients. Diabetologia. 50:2486–2494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu M, Guo M, Fei L, Pan XQ and Liu QQ:
4-phenylbutyric acid attenuates endoplasmic reticulum
stress-mediated pancreatic beta-cell apoptosis in rats with
streptozotocin-induced diabetes. Endocrine. 47:129–137. 2014.
View Article : Google Scholar
|
|
12
|
Zhang B, Wang M, Yang Y, Wang Y, Pang X,
Su Y, Wang J, Ai G and Zou Z: ERp29 is a radiation-responsive gene
in IEC-6 cell. J Radiat Res. 49:587–596. 2008. View Article : Google Scholar : PubMed/NCBI
|