Introduction
Toll-like receptors (TLRs), an important family of
germ-line encoded pattern recognition receptors (PRRs), are
responsible for the recognition of pathogen-associated molecular
patterns (PAMPs) from infectious pathogens. Up to now, 10 TLRs
(TLR1-10) have been identified in humans and 12 TLRs (TLR1-9,
TLR11-13) in mice, and TLR1-9 have been found to be conserved in
the two species (1). TLR1, 2, 4, 5
and 6 are primarily expressed on the surface and predominantly
recognize bacterial and fungal cell wall components and viral
envelope proteins, as well as protozoal components (1,2). By
contrast, the nucleic acid-sensing TLRs, which include TLRs 3, 7,
8, 9 and 13, are localized within the endosomal compartments of
immune cells and recognize double-stranded RNA (dsRNA), single
stranded RNA (ssRNA) and DNA derived from viruses, bacteria, fungi
and parasites (1–3). As an important family of type I
transmembrane (TM) glycoprotein receptors, all TLRs are composed of
an ectodomain (ECD) containing multiple leucine-rich repeats (LRRs)
directly involved in the recognition of PAMPs, a TM domain required
for the sub-cellular localization of TLRs, and an intracellular
domain with a conserved cytoplasmic signaling region termed the
Toll/IL-1 receptor (TIR), which is required for the transduction of
downstream signaling (4). Upon
PAMP recognition, the cytoplasmic TIR domain of the TLRs recruits
the adaptor molecules myeloid differentiation primary response gene
88 (MyD88) and/or TIR-domain-containing adaptor-inducing
interferon-β (TRIF). This results in the activation of interferon
regulatory factor 3 (IRF3), IRF7, activator protein 1 (AP-1) and
nuclear transcription-κB (NF-κB), as well as the transcription of
inflammatory cytokines, chemokines, and type I interferons (IFNs)
that rapidly initiate innate immune responses to ensure host
protection (5).
The human TLR8 (hTLR8) gene is located on the X
chromosome, and can recognize ssRNA, short dsRNA, bacterial RNA,
oligoribonucleotides and a large number of synthetic chemical
agonists, such as imidazoquinolines (1–4).
However, although human TLR8 is able to recognize imidazoquinolines
and initiate immune responses, murine TLR8 (mTLR8) is not activated
by imidazoquinolines or ssRNA due to a five amino-acid deletion in
the ECD. As a result, mTLR8 was initially hypothesized to be
non-functional (1–4,6).
However, in 2006 it was revealed that mTLR8 could be activated by
imidazoquinoline 3M-002 combined with poly(dT)17 oligonucleotides
(ODNs), leading to NF-κB activation and TNF-α production (7), suggesting that mTLR8 is indeed
functional. In support of this, a recent study reported that
vaccinia virus (VACV) or vaccinia viral poly(A)/T-rich DNA could
activate NF-κB in an mTLR8-dependent manner. In addition, synthetic
poly(dA) and poly(dT) ODNs are capable of activating plasmacytoid
DCs (pDCs) in an mTLR8-dependent manner (8), suggesting that mTLR8 is a functional
receptor, regulating innate immunity against VACV infection.
However, research from another team has raised uncertainties about
these results, not only because they found that poly A10 and two
different polyT ODNs did not induce IFN-α or other cytokines in
sorted FL-pDCs or ex vivo-isolated pDCs, but also because of
the high levels of transcripted TLR7 and TLR9 in murine pDCs and
not TLR8 (9). Notably, mTLR8 was
found to inhibit TLR7-sensing of 3M-001 in HEK293T cells, and
mTLR8−/− DCs showed increased responses to various TLR7
ligands and NF-κB activation. These results indicate that TLR8 may
directly modulate TLR7 function (10).
In this study, to investigate the role of mTLR8 in
regulating innate immune responses, mTLR8 cDNA isolated from
peripheral blood mononuclear cells (PBMCs) was cloned and
sequenced. It was found that mTLR8 conserved the typical domains of
TLRs and had a high level of identity to other mammalian species.
Higher expression levels of mTLR8 in the heart, spleen, and lung
were also detected by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR). Furthermore, it was demonstrated that
mTLR8 can induce nuclear factor (NF)-κB activation and tumor
necrosis factor (TNF)-α production but not the activation of
interferon-sensitive response element (ISRE), AP-1 activation and
IFN-α production in HEK293T cells. Overall, these results provide a
molecular foundation for further investigation into the potential
role of mTLR8 in anti-viral therapeutics, oncotherapy, autoimmune
diseases and vaccine design.
Materials and methods
Animals and cells
C57BL/6 mice (n=30) were purchased from the
Laboratory Animal Center of Lanzhou University (Lanzhou, China).
Groups of 6–10-week-old mice were selected for this study (Animals
were treated in accordance with the Guide for the Care and Use of
Laboratory Animals and approved by the Committee of Transgenic
Bio-safety Evaluation of Agriculture Ministry, Lanzhou, China).
Animals were housed separately under 12 h light/dark cycles at a
temperature of 22°C and a humidity of 60%, with free access to food
and water. HEK293T cells were purchased from the Type Culture
Collection of the Chinese Academy of Science (Shanghai, China) and
were maintained in complete Dulbecco's modified Eagle's medium
(Gibco, Thermo Fisher Scientific Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher
Scientific Inc.), and 50 mg/ml penicillin/streptomycin (Shanghai
Sangon Biological Engineering Biotechnology Company, Shanghai,
China).
Tissue sample collection and total RNA
isolation
The mice were sacrificed by enucleation of the eye
using 2% pentobarbital sodium (Wuhan Kehaojia Biological
Technology, Wuhan, China) and blood samples were collected for
mononuclear cell isolation using lymphocyte separation medium
(Sigma-Aldrich, St. Louis, MO, USA). The heart, liver, spleen,
lung, kidney, intestine and muscle were dissected, washed three
times in phosphate-buffered saline (PBS, pH 7.2) and immediately
snap-frozen in liquid nitrogen prior to being stored at −80°C until
required. Total RNA was extracted from the collected tissue samples
and cells using Takara MiniBEST Universal RNA Extraction kit
(Takara, Dalian, China) according to the manufacturer's
instructions. The RNA quality was detected by 1% agarose gel
electrophoresis (Shanghai Sangon Biological Engineering
Biotechnology Company) which was stained with 10 µg/ml
ethidium bromide (Shanghai Sangon Biological Engineering
Biotechnology Company). The total RNA concentration was determined
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.) and the optical density (OD)260:OD280 ratio of the RNA was
between 1.8 and 2.0.
Reverse transcription
Synthesis of the first strand of cDNA was performed
with the Primescript 1st strand cDNA synthesis kit (Takara Bio
Inc., Dalian, China) according to the manufacturer's instructions.
Briefly, 800 ng RNA, 0.5 µl Oligo dT Primer (50 µM),
0.5 µl random 6 mers (50 µM), 1 µl dNTP
mixture (10 mM) and an appropriate volume of RNase-free water up to
10 µl were mixed gently. The mixture was heated to 65°C for
5 min, and then quick-chilled on ice. Then, the reverse
transcription mixture was prepared as follows: 4 µl 5X
PrimeScript Buffer, 0.5 µl RNase inhibitor (40
U/µl),1 µl PrimeScriptRTase (200 U/µl) and
4.5µl RNase free water were mixed with the above mixture.
Cycle parameters of the RT procedure were 1 cycle of 30°C for 10
min, 42°C for 60 min, and 95°C for 5 min. The cDNA were stored at
−80°C for mTLR8 cloning and relative quantification by PCR.
Cloning of murine TLR8 cDNA and
construction of pCMV-tag2B-TLR8 eukaryotic expression vector
Based on the murine genomic sequence obtained from
the BLAST search, the primers c-mTLR8-F and c-mTLR8-R (Table I) were designed, and used to
amplify the potential mTLR8cDNA by reverse transcription-PCR from
total RNA extracted from PBMCs. A pair of primers (c-mTLR8) were
designed based on the coding sequence (CDS) of Mus musculus
TLR8 (GenBank ID: NM_133212) with Primer Premier 5.0 and with
XhoI and KpnI site (underlined). The PCR was
performed in a 50 µl reaction volume containing 4 µl
of the first-strand cDNA, 3 µl each of forward and reverse
primers (10 µM), 10 µl 5X PrimeSTAR Buffer (including
Mg2+; Takara Bio Inc.), 4 µl dNTP mixture, 0.5
µl PrimeSTAR HS DNA Polymerase (Takara Bio Inc.) and 25.5
µl sterile water. PCR conditions were as follows:
Denaturation at 95°C for 4 min, 30 cycles at 98°C for 10 sec, 64°C
for 15 sec, 72°C for 3 min 40 sec, with a final extension of 72°C
for 10 min.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer | Sequence
(5′-3′) | Amplicon length
(bp) | GenBank accession
no. |
|---|
| q-mTLR8 | Forward |
ACCTGAGCCACAATGGCATTTAC | 121 | NM_133212 |
| Reverse |
TTGCCATCATTTGCATTCCAC | | |
| β-actin | Forward |
CATCCGTAAAGACCTCTATGCCAAC | 171 | NM_007393 |
| Reverse |
ATGGAGCCACCGATCCACA | | |
| c-mTLR8 | Forward | TTTCTCGAGATGGAAAACATGCCCCCTCAGTC | 3099 | NM_133212 |
| Reverse | CGGGGTACCCTAGTATTGCCTAATGGAATCAATG | | |
| mTNF-α | Forward | CAGGGTACCGTTTTCCGAGGGTTGAATGAG | 243 | GQ917239 |
| Reverse | CAGCTCGAGGTCTTTTCTGGAGGGAGATGTG | | |
| mIFN-α2 | Forward | CCGGGTACCCAGAGAGTGAAGTAAAGAAAGTG | 180 | X01969 |
| Reverse | TGTCTCGAGTGTGGGTCTTGCAGAGGTTGAT | | |
The PCR products were analyzed by electrophoresis on
1.0% agarose gel with 10 µg/ml ethidium bromide, and
purified by a gel extraction kit (Axygen Scientific Inc., Union
City, CA, USA). The purified products and the vector pCMV-Tag2B
(Stratagene, La Jolla, CA, USA) were digested by XhoI and
KpnI (Takara Bio Inc.) and the products were purified by
electrophoresis and a gel extraction kit. The digested PCR product
was inserted into the vector pCMV-Tag2B. Proper construction was
confirmed by sequencing and digesting with XhoI and
KpnI and was termed pCMV-Tag2B-mTLR8.
Sequence, structure and phylogenetic
analysis
Amino acid sequences were aligned using the Clustal
Omega online sequence alignment tool (http://www.ebi.ac.uk/Tools/msa/clustalo/) and the
Sequence Manipulation Suite (SMS; http://www.bio-soft.net/sms/), and the phylogenetics
of the molecular evolutionary analysis were conducted using the
MEGA 5.1 program (http://www.megasoftware.net/). Prediction of the mTLR8
and hTLR8 domains, motifs and features was performed on the SMART
website (http://smart.embl-heidelberg.de/). Swiss-model
(http://swissmodel.expasy.org/) was used
to simulate the crystal structure of mTLR8. The predicted structure
was analyzed and compared to that of hTLR8 (PDB ID: 3w3 g) using
Swiss-pdb Viewer 4.0.1 software (Swiss Institute of
Bioinformatics).
Construction of reporter plasmids,
transfection and reporter luciferase assays
Murine TNF-α promoter region (GenBank ID: GQ917239)
and IFN-α2 promoter region (GenBanK ID: X01969) was amplified from
murine genomic DNA, which was extracted from murine splenic
lymphocytes, and then cloned into the pGL4.10 basic vector capable
of expressing firefly luciferase. The primers are shown in Table I with KpnI and XhoI
sites (underlined). HEK293T cells were seeded at a density of
2×105 cells/well in 500 µl culture medium into
24-well plates and incubated. LyoVec (25 µl; Invivogen, San
Diego, CA, USA) was brought to room temperature and gently vortexed
to homogenize, after which it was mixed gently with 0.5
µg/well of pCMV-Tag2B-mTLR8 plasmid (pCMV-Tag2B empty
plasmid as controlled), 0.2 µg/well of the reporter plasmids
including pGL4.32 [luc2P/NF-κB-RE/Hygro] vector, pGL4.44
[luc2P-AP1-RE-Hygro] vector, pGL4.45 [luc2P-ISRE-Hygro] vector
(Promega Corporation, Madison, WI, USA), TNF-α-Luc and IFN-α2-Luc,
and 0.01 µg/well pGL4.74 [hRluc/TK] vector (Promega
Corporation), in a sterile 0.5 ml microfuge tube. When the cells
had grown to 70–90% confluency, the culture medium was gently
replaced with 475 µl opti-MEM (Gibco, Thermo Fisher
Scientific Inc.) and 25 µl LyoVec-DNA complexes were
directly added to the medium. The mixture was agitated to
distribute the complexes uniformly and incubated for 24 h for the
subsequent assay. After a 24-h transfection, the dual luciferase
reporter system was used to detect the luciferase activity (Promega
Corporation), according to the manufacturer's protocol. The
reporter assays were repeated three times in duplicate.
Tissue distribution of murine TLR8
detected by RT-qPCR
Primers for mTLR8 (q-mTLR8) were designed based on
the Mus musculus TLR8 cDNA sequence and β-actin was used as
an internal reference gene to normalize target gene transcript
levels (Table I). Quantitative PCR
was performed with the Mx3005P Real-time PCR detection system
(Agilent Technologies Deutschland GmbH, Waldbronn, Germany). PCR
was performed in 20 µl reactions with 2 µl of the
cDNA sample, 0.8 µl each of the forward and reverse primers
(10 µM), 6.4 µl DEPC-treated water, and 10 µl
2xSYBR Premix Ex Taq II (Takara Bio Inc.). The PCR parameters
include 95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C for
34 sec, and then 95°C for 15 sec, 60°C for 1 min, and 95°C for 15
sec. The primers for TLR8 and β-actin yielded a single peak in the
melting curve and a single band of the expected size on an agarose
gel. Data were analyzed according to the efficiency-corrected
comparative Cq method and normalized using β-actin expression
levels.
Statistical analysis
All results represent the mean of three separate
experiments. RT-qPCR data are expressed as the mean ± standard
deviation. The statistical significance of differences was assessed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cloning, sequence and structure analysis
of murine TLR8
Based on the murine genomic sequence obtained from
the BLAST search, the primers c-mTLR8-F and c-mTLR8-R (Table I) were designed and used to amplify
the potential mTLR8cDNA by RT-PCR from total RNA extracted from
PBMCs. The open reading frame (ORF) of mTLR8 is 3,099 bps and it
encodes 1,032 amino acid residues. It was determined that the
nucleotide sequence recorded for mTLR8 was completely identical to
that held in the NCBI (GenBank ID: NM_133212, AY035890.1 and
BC132054.1) (Fig. 1).
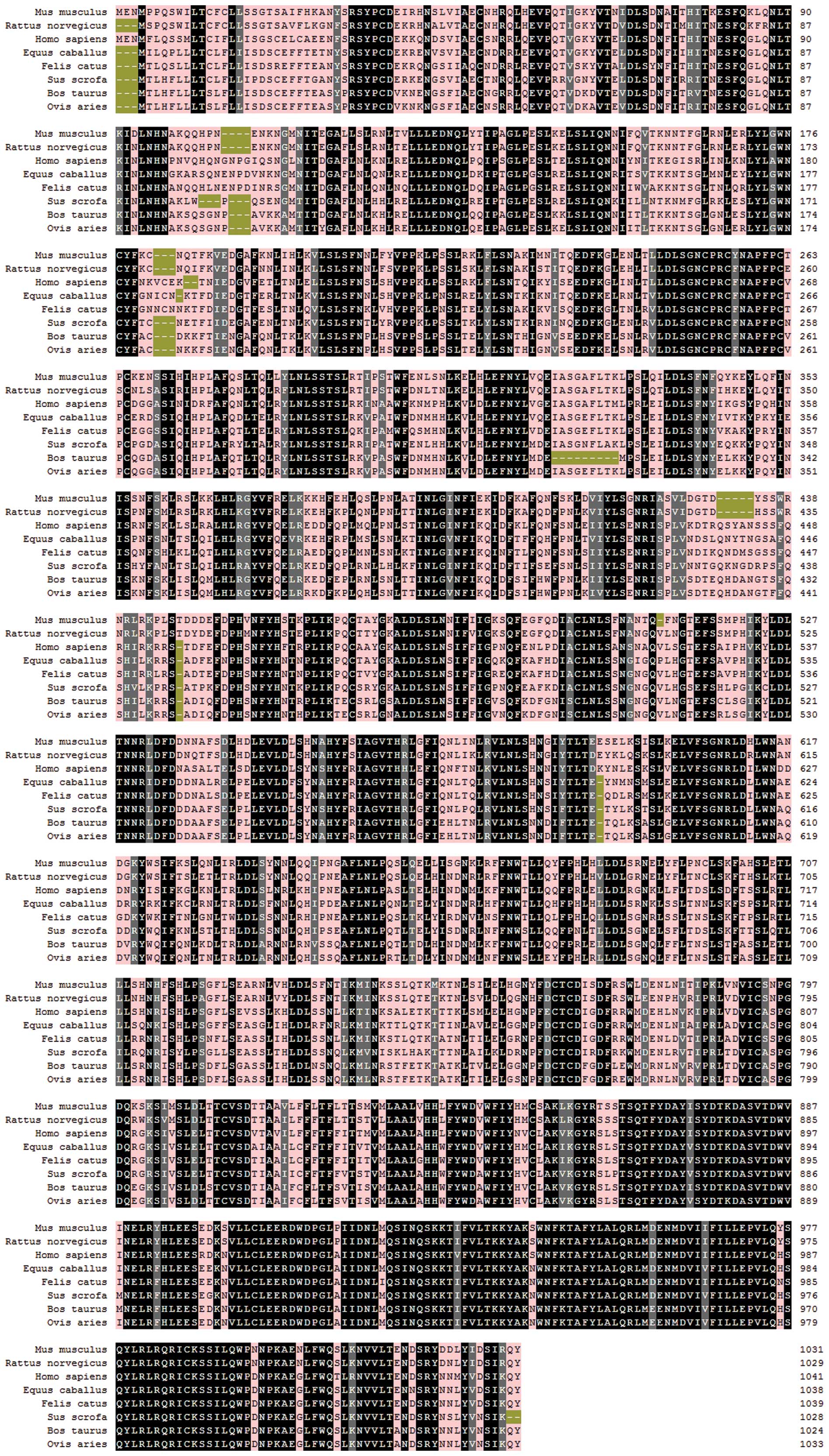
Multiple sequence alignment against other TLR8
sequences showed that the nucleotide sequence of mTLR8 was 91.1%
identical to that of rat TLR8, 76.1% identical to that of human
TLR8, 73.0% identical to that of bovine TLR8, 73.5% identical to
that of ovine TLR8, 76.7% identical to that of equine TLR8, 76.0%
identical to that of feline TLR8 and 74.0% identical to that of
porcine TLR8. At the amino acid level, the levels of identity were
88.2, 70.9, 67.3, 68.8, 72.1, 71.6 and 69.6%, respectively
(Fig. 1). In addition,
phylogenetic analysis showed that mTLR8 is closely associated with
rat, compared with other species in genetic evolution. Therefore,
mTLR8 was more diverse from TLR8 from other species (Fig. 2).
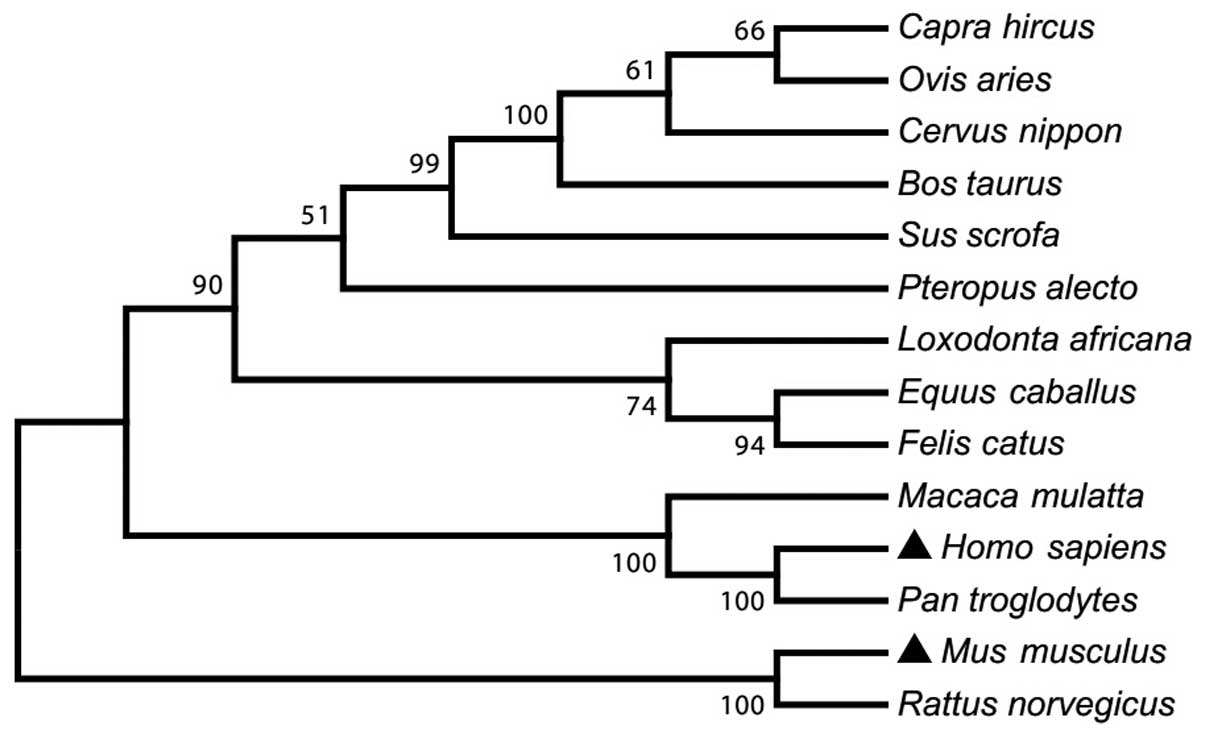 | Figure 2A phylogenetic tree of the amino acid
sequences of TLR8 in 14 mammalian species. The sequences were
obtained from GenBank with accession numbers: NP_001029109 (Bos
taurus), NP_001272686 (Capra hircus), ADZ17145
(Cervus nippon), NP_001104771 (Equus caballus),
ABS28967 (Felis catus), NP_619542 (Homo sapiens),
ABS28968 (Loxodonta africana), NP_001123899 (Macaca
mulatta), AAK62677 (Mus musculus), NP_001129401 (Ovis
aries), NP_001123944 (Pan troglodytes), ADO01610
(Pteropus alecto), ABM92444 (Rattus norvegicus) and
NP_999352 (Sus scrofa). The unrooted tree was built using
ClustalX by neighbour-joining method. Bootstrap values are
indicated on the branches. TLR8, toll-like receptor 8. |
To predict the structural domains in mTLR8, the
putative amino acid sequences were analyzed using the SMART
program. As shown in Fig. 3, mTLR8
contains features common to TLRs, namely multiple extracellular
LRR, a TM domain and an intracellular TIR domain. Additionally,
similar domains can be predicted in TLR8 from bovine, equine and
canine, indicating that the ligand-binding domain may be a common
characteristic of TLRs in ligand recognition among a number of
species (Fig. 3). Other domains
with high inter-species conservation included the N- and C-terminal
cysteine-flanked LRRs, as well as the TM and TIR domains (Fig. 3). Moreover, the LRRs of mTLR8
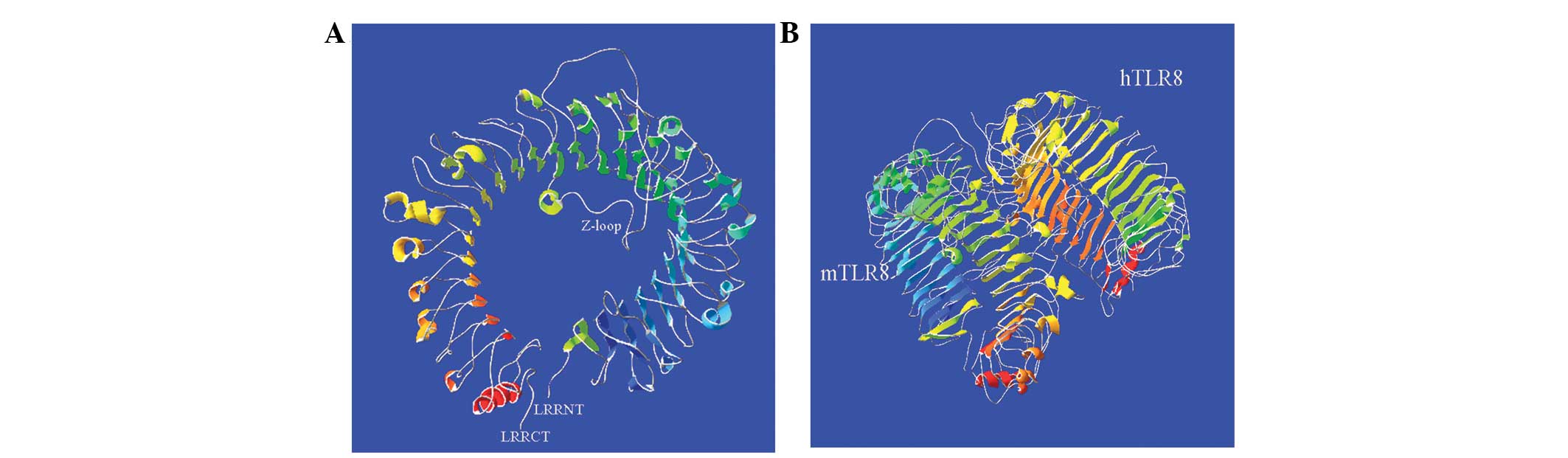
displayed a space structure like a solenoid coil and contained 38
β-ovinets located on its concave surface and 23 α-helixes on the
convex side (Fig. 4A). Compared
with the structure of hTLR8, except for extra 7 α-helixes, no
differences between mTLR8 and hTLR8 were identified, suggesting
that their crystal structures were almost identical (Fig. 4B).
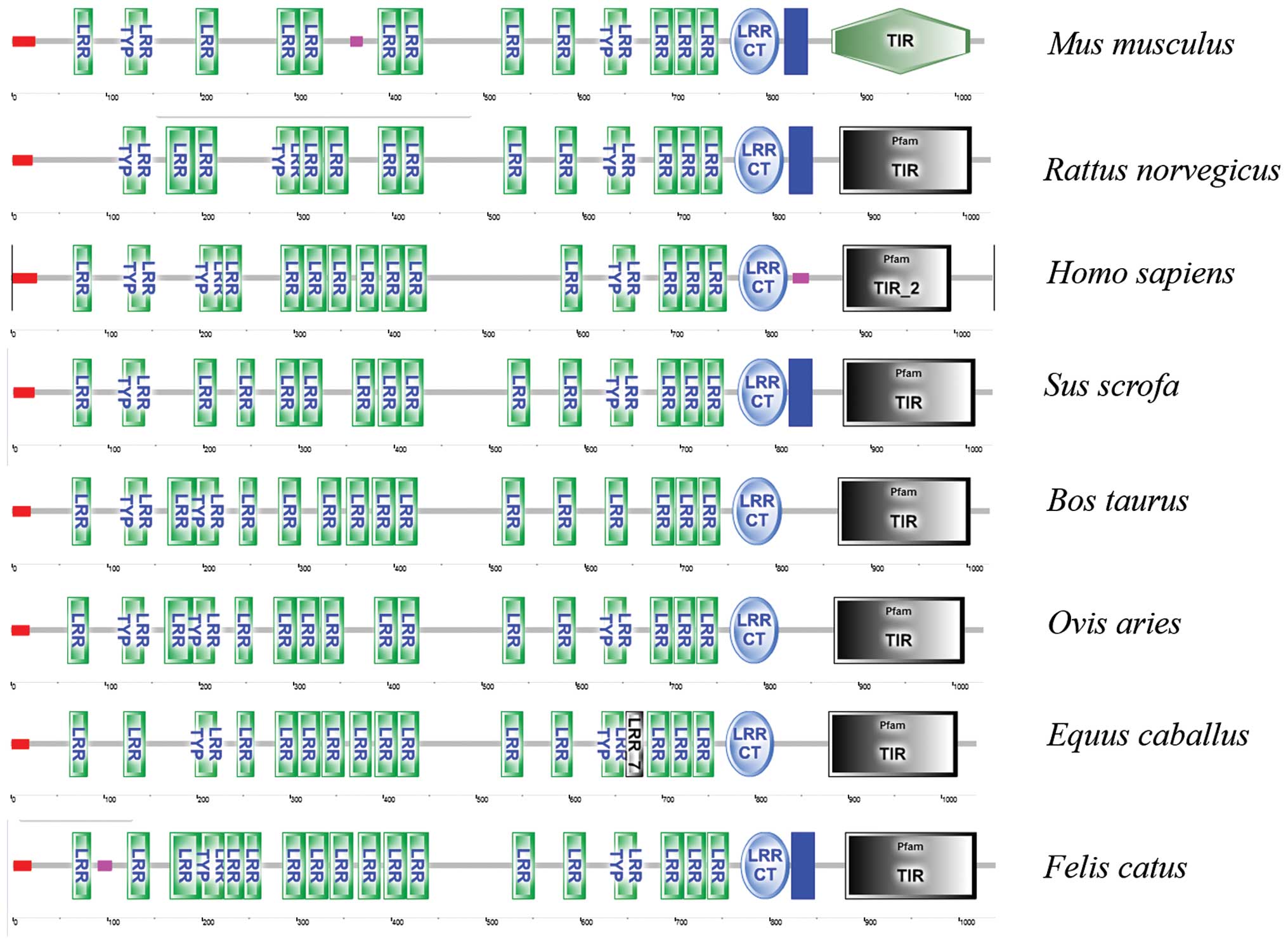 | Figure 3Predicted secondary structures of TLR8
by SMART including Mus musculus, Rattus norvegicus,
Homo sapiens, Sus scrofa, Bos taurus, Ovis
aries, Equus caballus and Felis catus. TLR8,
toll-like receptor 8; LRR, leucine-rich repeats; TYP, typical; CT,
C-terminal; TIR, Toll/IL-1 receptor. |
Expression of murine TLR8 in different
tissues
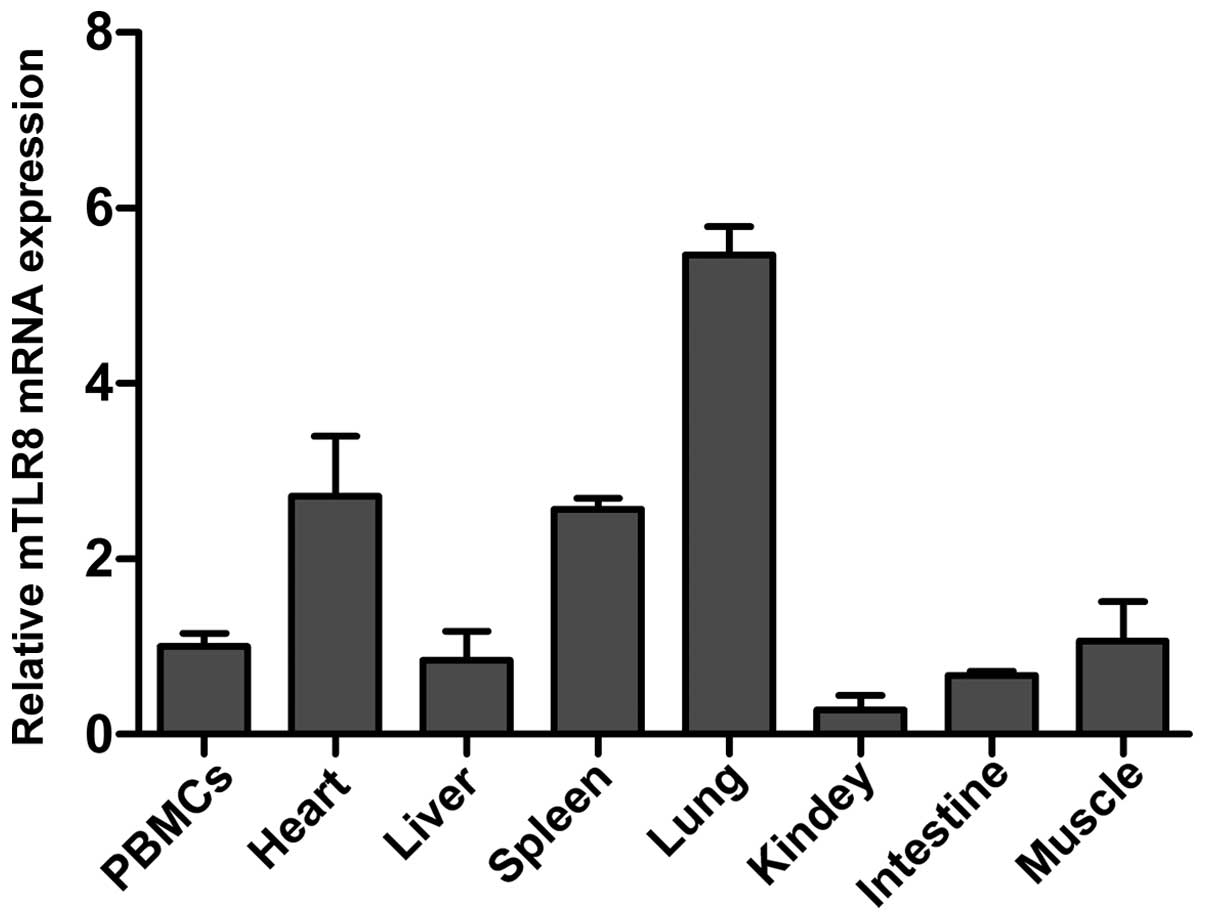
The mRNA expression profile of mTLR8 mRNA in
different tissues was analyzed by RT-qPCR using the β-actin
housekeeping gene as a control. As shown in Fig. 5, mTLR8 mRNA was expressed at a
detectable level in all the monitored tissues, including PBMCs,
heart, liver, spleen, lung, kidney, small intestine and muscle.
Notably, expression was most abundant in the lung, followed by the
heart and spleen, with somewhat less in PBMCs, liver, small
intestine, muscle and kidney. This indicated differences in the
expression levels of mTLR8 between different murine tissues. The
biological implications of the varying levels of expression in
differing tissues in mice, particularly in immune cells (including
pDCs and cDCs), is unclear and requires further investigation
(9,10). In humans, the most abundant
expression of TLR8 has been found in the lung and PBMCs, indicating
that the expression profiles of mTLR8 in tissue are similar to
hTLR8 (11,12).
Functional analysis of murine TLR8
To investigate whether mTLR8 is able to activate the
signal pathways of innate immunity, HEK293T cells were transfected
with an mTLR8 expression construct together with murine NF-κB-,
ISRE-, AP-1-, TNF-α- and IFN-α2-luciferase reporter plasmids. The
dual luciferase report system was used to record the activity of
the luciferase reporter plasmid. As shown in Fig. 6A and B, NF-κB and TNF-α promoter
activity was significantly activated in cells over-expressing mTLR8
compared with cells transfected with the empty vector (P<0.001).
A study has revealed that mTLR8 induced NF-κB activation and TNF-α
production in murine PBMCs and mTLR8-transfected HEK293 cells
(7). In order to further explore
whether mTLR8 could activate other regulatory elements or promote
type I IFNs, the luciferase activity of the ISRE, AP-1 and IFN-α2
promoters was also investigated. The results showed that
over-expression of mTLR8 in the HEK293T cells did not activate
ISRE, AP-1, or IFN-α2 (Fig. 6C–E).
Therefore, these results showed that over-expression of mTLR8 can
activate NF-κB and TNF-α but does not have the ability to induce
the activation of IRF3, AP-1 and IFN-α2 in the HEK293T cells.
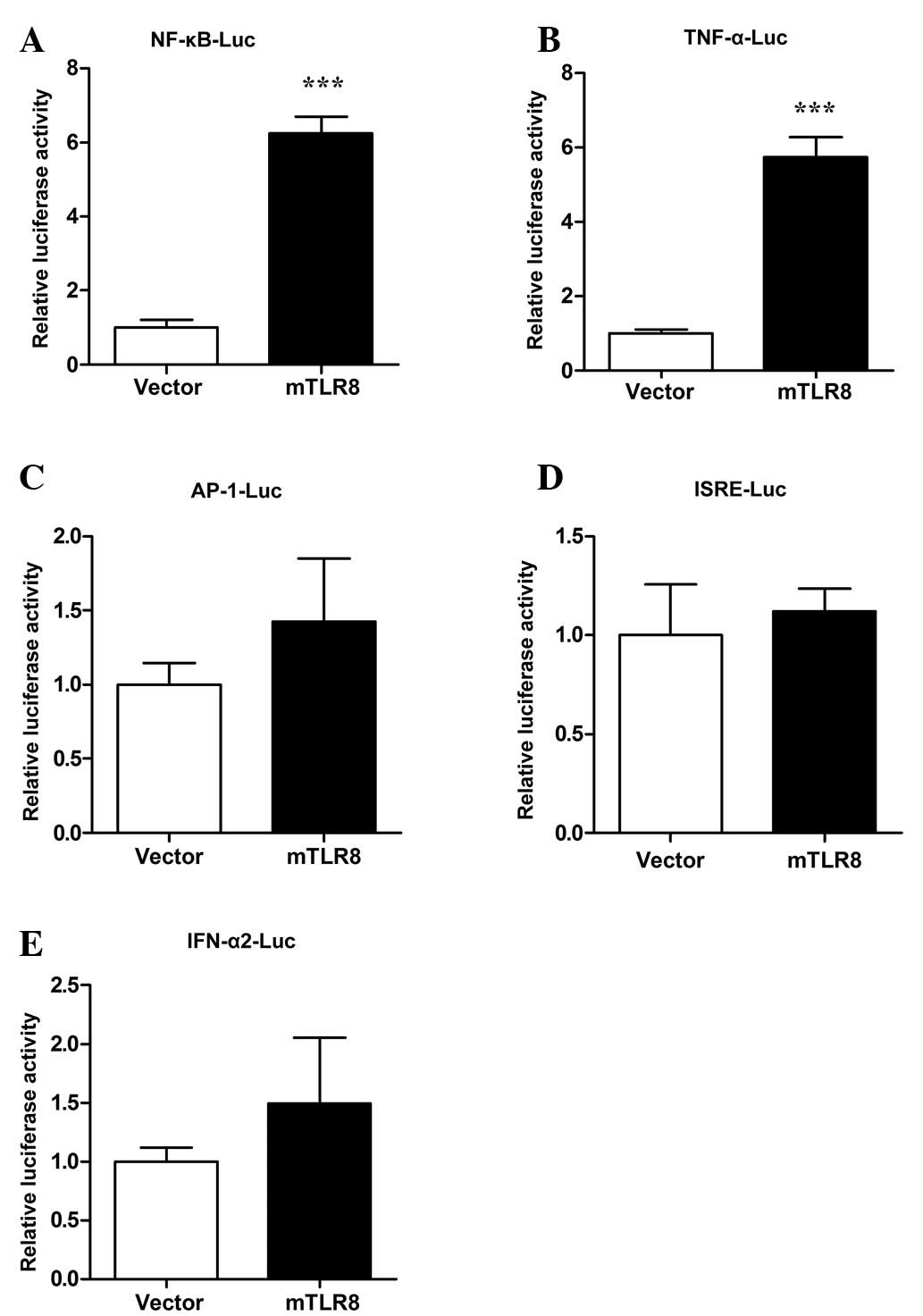 | Figure 6Murine TLR8 stimulates NF-κB to induce
TNF-α expression in HEK293T cells. HEK293T cells were
co-transfected with 500 ng/well expression plasmids (mTLR8 or empty
vector) together with 200 ng/well of a reporter plasmid: (A)
NF-κB-Luc, (B) TNF-α-Luc, (C) AP-1-Luc, (D) ISRE-Luc, (E)
IFN-α2-Luc, along with 10 ng/well phRL-TK using LyoVec. Luciferase
activity of NF-κB was quantified with the Dual-Glo Luciferase Assay
kit 24 h after transfection. The reporter assays were repeated
three times in duplicate. ***P<0.001 as compared with
the vector. TLR8, toll-like receptor 8; NF, nuclear factor; TNF,
tumor necrosis factor; AP-1, activator protein 1; ISRE,
interferon-sensitive response element. |
Discussion
As an important member of the endosomal TLRs, mTLR8
was hypothesized to be non-functional as it was initially observed
that TLR7−/− mice are unresponsive to the TLR7/8 agonist
R848, or TLR8 RNA ligands, even though human TLR8 and mTLR8 are
close relatives (13–15). However, recent studies have
revealed that mTLR8 can be activated and is important in
controlling TLR7 expression (10).
In the present study, mTLR8 was cloned and characterized. The
results clearly show that mTLR8 is an important endosomal TLR and
is critical in the innate immune signaling pathway.
The ORF of mTLR8 comprises 3,099 bps, encoding a
1,032-amino-acid polypeptide. In addition to human and mTLR8, the
TLR8 sequences from rats (ABM92444), bovine (NP_001029109), ovine
(NP_001129401), equines (NP_001104771), cats (ABS28967), porcines
(NP_999352), and other mammalian species have already been
identified. Notably, mTLR8 is more similar to rat TLR8 at the
nucleotide and amino acid level, compareed with other mammalian
species, suggesting mTLR8 is also conserved in the process of
evolution and the mTLR8 acts as a good model to research the innate
immune system of humans or other species. In addition, mTLR8 is
also composed of LRRs, a TM domain and a TIR domain. High
conservation of the three subsections of the TIR domain (boxes 1,
2, 3) known to be important in signaling (boxes 1 and 2) and
receptor localization (box 3), indicate that the ligand-binding
domain may be a common characteristic of TLRs in ligand recognition
among a range of species (4).
Furthermore, like the crystal structure of hTLR8, the extracellular
domain of mTLR8 can also form a ring-shaped structure in which each
half of the ring is produced by the N-and C-terminal fragments, and
this ring-shaped structure is different from the typical
equineshoe-shaped structure exhibited by other TLRs (4,16).
Comparative analysis has found that mTLR8 and hTLR8 have the same
numbers of β-ovinets, spanning the whole concave face of the
ring-shaped structure, as well as which, it features a Z-loop.
Although there is no difference in the crystal structures of mTLR8
and hTLR8, mTLR8 was unresponsive to hTLR8 ligand stimulation. A
recent study has shown that a five-amino-acid-residue motif (RQSYA)
that is conserved with varying sequencing in several non-rodent
species, is absent in rodent species mTLR8 and that rat(r) TLR8 was
not responsive to ligand stimulation in the absence of poly(dT) ODN
(17). As shown in Fig. 1, mTLR8 also lacks a five-amino-acid
motif (RQSYA) that is located in an undefined region following
LRR-14 of the mTLR8 ECD. The Z-loop is located between LRR14 and
LRR15 which may also be the location of the five-amino-acid motif
(RQSYA). Thus, species-specific ligand recognition is predicted on
the differences between the Z-loops of mTLR8 and hTLR8.
Expression of TLRs in different tissues is an
important determinant of their function. Previous studies have
revealed that hTLR8 is highly expressed in lung and PBMCs and
modestly expressed in the spleen, lymph nodes, bone marrow and
placenta (11,12). In the present study, mTLR8 was
expressed in all analyzed tissues, including PBMCs, heart, liver,
spleen, lung, intestine and muscle, with the highest expression
identified in the lung. However, it was modestly expressed in the
heart and spleen, and rarely in the kidney, reflecting possible
species-specific differences in the expression of TLR8 between mice
and humans. Notably, significant amounts of mTLR8 were detected in
the heart, a non-immune tissue, that usually produces little TLR8
in either humans or mice. The biological implications of these
different expression patterns of TLR8 in different tissues are
unclear and require further investigation. In addition, although
there are differences in the TLR8 mRNA expression profiles of the
different species, abundant TLR8 mRNA expression can be observed in
the lung, spleen, PBMCs, liver and lymph nodes of humans, mice and
porcines, suggesting that TLR8 is important in the mammalian immune
system (11,12,18).
Furthermore, hTLR8 is primarily expressed in monocytes/macrophages
and myeloid DCs, and predisposes the induction of inflammatory
cytokines, whereas mTLR8 is selectively expressed in DCs derived
from splenocytes, namely CD4+DCs, CD8+DCs,
CD4−CD8−DCs and pDCs (19–21).
However, similar to hTLR8, murine pDCs are also generally known to
express TLR7 and TLR9, but not TLR8, however, that the precise
expression of mTLR8 mRNA in pDCs and cDCs remains to be
elucidated.
As an important PRR that is critical in virally
induced type I IFNs and inflammatory cytokine expression,
overexpression of hTLR8 can activate IRF3 and NF-κB and suppress
viral replications. Previous studies have revealed that hTLR8 can
recognize antiviral compounds, ssRNA, oligoribonucleotide and small
interfering RNA (1–4,6). In
addition, bovine and porcine TLR8 are highly responsive to two TLR7
ligands, imiquimod and gardiquimod, in transfected HEK293T cells
and Cos-7 cells (18,22). However, a combination of poly(dT)
ODN plus the hTLR8 ligand, R848, could activate mTLR8 and induce
the transcription of NF-κB and the secretion of TNF-α in HEK293
cells and PBMCs derived from mice (7). In the present study, it was also
demonstrated that the overexpression of mTLR8 activated NF-κB and
induced TNF-α in HEK293T cells. Additionally, a previous report
demonstrated that VACV and its poly(A)/T-rich DNA motifs are potent
inducers of pDC-derived IFN-α in TLR9-independent, and exclusively
TLR8-dependent, pathways (8).
However, another previous study provided evidence that
poly(A)/T-rich DNA caused no activation of NF-κB and TNF-α in the
TLR8-dependent pathway (9). This
controversy is not only because DNA has not been previously linked
to TLR8 activation, but also because murine pDCs are generally
known to express TLR7 and TLR9, and not TLR8. Therefore, future
studies are required to elucidate the precise expression of mTLR8
mRNA in pDCs and cDCs and the functions of mTLR8 in the induction
of NF-κB activation and in TNF-α or other cytokine production with
combinations of poly(dT) ODN and R848, or poly(A)/T-rich DNA.
In conclusion, the present study cloned the ORF
sequence of mTLR8 and analyzed the sequence signature, secondary
structure and crystal structure, as well as the evolutionary
association among mammalian species. The expression profile of
mTLR8 in different tissues was also demonstrated. It was also
confirmed that mTLR8 is functional and involved in the innate
immune signaling pathways to contribute to the activation of NF-κB
and the induction of TNF-α. Clarification of the role of mTLR8 in
innate immune recognition system is important for it development as
a model for the human tumor and autoimmune diseases.
Acknowledgments
The present study was funded by the National Natural
Science Funds (nos. 31302072, 31372423 and 30871884) from the
National Natural Science Foundation of China.
References
|
1
|
Wu JR and Chen ZJ: Innate immune sensing
and signaling of cytosolic nucleic acids. Annu Rev Immunol.
32:461–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He XB, Jia HJ, Jing ZZ and Liu DX:
Recognition of pathogen-associated nucleic acids by endosomal
nucleic acid-sensing toll-like receptors. Acta Biochim Biophys Sin
(Shanghai). 45:241–258. 2013. View Article : Google Scholar
|
|
3
|
Unterholzner L: The interferon response to
intracellular DNA: Why so many receptors? Immunobiology.
218:1312–1321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Botos I, Segal DM and Davies DR: The
structural biology of toll-like receptors. Structure. 19:447–459.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim KH and Staudt LM: Toll-like receptor
signaling. Cold Spring Harb Perspect Biol. 5:a0112472013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cervantes JL, Weinerman B, Basole C and
Salazar JC: TLR8: The forgotten relative revindicated. Cell Mol
Immunol. 9:434–438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gorden KK, Qiu XX, Binsfeld CC, Vasilakos
JP and Alkan SS: Cutting edge: Activation of murine TLR8 by a
combination of imidazoquinoline immune response modifiers and polyT
oligodeoxynucleotides. J Immunol. 177:6584–6587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martinez J, Huang X and Yang Y: Toll-like
receptor 8-mediated activation of murine plasmacytoid dendritic
cells by vaccinia viral DNA. Proc Natl Acad Sci USA. 107:6442–6447.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauer S, Bathke B, Lauterbach H, Pätzold
J, Kassub R, Luber CA, Schlatter B, Hamm S, Chaplin P, Suter M and
Hochrein H: A major role for TLR8 in the recognition of vaccinia
viral DNA by murine pDC? Proc Natl Acad Sci USA. 107:E1392010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demaria O, Pagni PP, Traub S, de Gassart
A, Branzk N, Murphy AJ, Valenzuela DM, Yancopoulos GD, Flavell RA
and Alexopoulou L: TLR8 deficiency leads to autoimmunity in mice. J
Clin Invest. 120:3651–3662. 2010.PubMed/NCBI
|
|
11
|
Ma Y, Haynes RL, Sidman RL and Vartanian
T: TLR8: An innate immune receptor in brain, neurons and axons.
Cell Cycle. 6:2859–2868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuang TH and Ulevitch RJ: Cloning and
characterization of a sub-family of human toll-like receptors:
hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 11:372–378.
2000.PubMed/NCBI
|
|
13
|
Heil F, Hemmi H, Hochrein H, Ampenberger
F, Kirschning C, Akira S, Lipford G, Wagner H and Bauer S:
Species-specific recognition of single-stranded RNA via toll-like
receptor 7 and 8. Science. 303:1526–1529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jurk M, Heil F, Vollmer J, Schetter C,
Krieg AM, Wagner H, Lipford G and Bauer S: Human TLR7 or TLR8
independently confer responsiveness to the antiviral compound
R-848. Nat Immunol. 3(499)2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hemmi H, Kaisho T, Takeuchi O, Sato S,
Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K and Akira S:
Small anti-viral compounds activate immune cells via the TLR7
MyD88-dependent signaling pathway. Nat Immunol. 3:196–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohto U, Tanji H and Shimizu T: Structure
and function of toll-like receptor 8. Microbes Infect. 16:273–282.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Xu C, Hsu LC, Luo YP, Xiang R and
Chuang TH: A five-amino-acid motif in the undefined region of the
TLR8 ectodomain is required for species-specific ligand
recognition. Mol Immunol. 47:1083–1090. 2010. View Article : Google Scholar :
|
|
18
|
Zhu J, Lai K, Brownile R, Babiuk LA and
Mutwiri GK: Porcine TLR8 and TLR7 are both activated by a selective
TLR7 ligand, imiquimod. Mol Immunol. 45:3238–3243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ziegler-Heitbrock L: The CD14+ CD16+ blood
monocytes: Their role in infection and inflammation. J Leukocyte
Biol. 81:584–592. 2007. View Article : Google Scholar
|
|
20
|
Alexopoulou L, Desnues B and Demaria O:
Toll-like receptor 8: The awkward TLR. Med Sci (Paris). 28:96–102.
2012.In French. View Article : Google Scholar
|
|
21
|
Edwards AD, Diebold SS, Slack EM, Tomizawa
H, Hemmi H, Kaisho T, Akira S and Reis e Sousa C: Toll-like
receptor expression in murine DC subsets: Lack of TLR7 expression
by CD8 alpha+ DC correlates with unresponsiveness to
imidazoquinolines. Eur J Immunol. 33:827–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu JZ, Brownlie R, Liu Q, Babiuk LA,
Potter A and Mutwiri GK: Characterization of bovine Toll-like
receptor 8: Ligand specificity, signaling essential sites and
dimerization. Mol Immunol. 46:978–990. 2009. View Article : Google Scholar
|