Introduction
Retinal neovascularization is one of the leading
causes of visual impairment in numerous diseases, including
proliferative diabetic retinopathy (PDR), neovascular age-related
macular degeneration (NVAMD), central retinal vein occlusion and
retinopathy of prematurity (1).
Vascular endothelial growth factor (VEGF) is an important
stimulator of new vessel growth in the processes of these diseases,
and the development of anti-VEGF treatment has provided substantial
benefits in patients with these diseases (2). However, the side effects, including
loss of peripheral vision and reduction of night vision, greatly
limit its value and more importantly, there remains a lack of
methods for a final cure. Hypoxia is an important
pathophysiological signal and can cascade down in a series of
angiogenic processes in retinal neovascularization diseases.
Therefore, exploring the biological alterations of endothelial
cells under hypoxic conditions may be helpful to provide an
improved understanding of the mechanism of retinal
neovascularization diseases and provide potential molecular
therapies.
Hypoxia inducible factor (HIF)-1α is a key oxygen
sensor and is important in the response to retinal hypoxia, and the
regulation of angiogenesis in PDR and NVAMD (3). Through the hypoxia response element
binding site, HIF-1α regulates the production of VEGF (4–7).
Silencing HIF-1α by RNA interference during hypoxia reduces the
expression levels of VEGF and other clinically important angiogenic
factors, leading to the inhibition of angiogenesis (8). Notably, HIF-1α activates diverse
genes involved in both cell growth and cell apoptosis under hypoxic
conditions (9–11).
Prolyl hydroxylase (PHD)2 also serves as an oxygen
sensor, which regulates the stability or degradation of HIF-1α in
an oxygen-dependent manner. In normoxia or hyperoxia, PHD2
hydroxylates the proline residues of HIF-1α, which are captured by
the Von Hippel Lindau protein (pVHL) ubiquitin E3 ligase complex
and are degraded by the proteasome. By contrast, in hypoxia, PHD2
fails to initiate this reaction due to a shortage of O2
and, therefore, HIF-1α is stabilized (12,13).
Tetramethylpyrazine (TMP), one of the most important
active ingredients of Chuan Xiong and is applied widely in the
treatment of neurovascular disorders, including ischemic stroke and
pulmonary hypertension secondary to chronic obstructive pulmonary
diseases in China (14–16). Previous studies have suggested the
potential antiangiogenic properties of TMP in choroidal and retinal
neovascularization in vivo (17,18).
Our previous study demonstrated that TMP improved neurovascular
recovery by preventing neovascularization, and protecting retinal
astroglia cells and neurons from ischemia-induced cell death,
partially due to the down-regulation of the expression levels of
HIF-1α and VEGF (18). In
addition, several previous studies revealed that TMP can protect
endothelial cells from apoptosis under hyperoxia and oxidative
stress in vitro (15,19–23).
However, the molecular and cellular mechanisms of TMP in the
protection of endothelial cells remain to be elucidated. As a
result of the protection of TMP in apoptotic endothelial cells, and
the downregulation of HIF-1α and VEGF following TMP treatment, it
is reasonable to hypothesize that the antiapoptotic effect of TMP
in endothelial cells occurs via the regulation of the PHD2/HIF-1α
signaling pathway.
The present study confirmed the antiapoptotic effect
of TMP in human umbilical vein endothelial cells (HUVECs) following
pre-incubation with CoCl2 and revealed that its
anti-apoptotic effect was, at least in part, via the regulation of
the of PHD2/HIF-1α signaling pathway. These results may provide
useful insight into the pathology of endothelial cell apoptosis and
may serve as a potential novel therapeutic target in retinal
neovascularization diseases.
Materials and methods
Reagents
CoCl2 and TMP were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum,
3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyl tetrazolium bromide (MTT)
and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Rabbit monoclonal PHD2
(cat. no. 4835; 1:500), HIF-1α (cat. no. 3716; 1:500) and VEGF
(cat. no. 2839; 1:500) antibodies were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA), rabbit monoclonal
cleaved caspase-3 (AB3623; 1:500), rabbit polyclonal cleaved
caspase-8 (ASP387; 1:500) and rabbit polyclonal cleaved caspase-9
(ASP315; 1:500) were purchased from EMD Millipore (Billerica, MA,
USA), and the mouse monoclonal β-actin (sc-69879; 1:500) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) was performed using the in situ Cell Death Detection
kit (EMD Millipore).
Cell culture and drug treatment
Normal human umbilical cords were obtained from The
Third Affiliated Hospital of Sun Yat-sen University, Zhujiang
Hospital of Southern Medical University, and Foshan Nanhai
Maternity and Child Health Hospital. HUVECs were isolated from the
vein of normal human umbilical cord, as described previously
(24). The cells were cultured in
DMEM-F12, supplemented with 10% heat-inactivated foetal bovine
serum, 100 U/ml penicillin, 100 U/ml streptomycin (Thermo Fisher
Scientific, Inc.), 30 lg/ml endothelial cell growth supplement
(Gibco; Thermo Fisher Scientific, Inc.) and 5 units/ml heparin
(Thermo Fisher Scientific, Inc.) at 37°C, with 5% CO2
and 95% air. The cells used in the present study were between
passage 3 and 4. Once the cells had grown to 90% confluence, they
were pre-incubated with CoCl2 (150 µM/ml) for 4
h. Following incubation, the cells were washed twice with
phosphate-buffered saline (pH 7.4) and were subsequently exposed to
TMP at different concentrations (50, 100 and 200 µM/ml) for
8 h. TMP was dissolved in dimethyl sulfoxide (<0.1%), which
caused no deleterious effect on the viability of HUVECs in
preliminary studies.
The present study was performed in accordance with
the Declaration of Helsinki. The institutional review board of Sun
Yat-sen University approved the protocol for collection human
umbilical cords. Written informed consent was obtained from all
patients following an explanation of the purpose and procedures of
the investigation.
Cell viability assay
Cell viability was assessed using an MTT assay
(25). HUVECs (1×104
cells/well) were cultured in 96-well plates at 37°C for 24 h, and
were subsequently divided into four groups: i) control; ii)
CoCl2 (only pre-incubated with 150 µM/ml
CoCl2 for 4 h); iii) CoCl2 + TMP
(pre-incubated with 150 µm/ml CoCl2 for 4 h and
subsequently treated with 200 µM/ml TMP for 8 h); iv)
CoCl2 + TMP + siRNA (PHD2 gene silencing with siRNA,
pre-incubation with 150 µM/ml CoCl2 for 4 h and
subsequently treated with 200 µM/ml TMP for 8 h). Following
treatments, the medium was removed and 10 µl MTT solution (5
mg/ml) was added and the plates were incubated at 37°C for 4 h in a
humidified 5% CO2 atmosphere. Next, the cells were
assessed in a microtiter plate reader (LabSystems; Thermo Fisher
Scientific, Inc.) and scanned to visualize the color development.
The cell survival rates were expressed as the percentages of the
value of normal cells.
Analysis of cell apoptosis by flow
cytometry
A FACScan Flow Cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) was used for quantifying apoptotic cells by
determining the DNA content of the cells. The apoptotic rate of
HUVECs was detected using an annexin V-fluorescein isothiocyanate
(FITC) apoptosis detection kit (Santa Cruz Biotechnology, Inc.).
Following the different treatments, the cells were harvested,
washed and double-stained with annexin V-FITC and propidium iodide
in a dark at room temperature for 20 min. Following staining, the
samples were analyzed at an excitation wavelength of 488 nm and an
emission wavelength of 530 nm using the EL340 Microtiter Plate
Reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Analysis of cell apoptosis by TUNEL
The apoptosis of HUVECs was assessed using a TUNEL
assay kit, according to the manufacturer's protocol. Following the
different treatments, the cells were seeded into 24-well plates and
incubated overnight. The cells were subsequently fixed and
incubated with 100 µM enzyme solution for 30 min at 37°C.
Following incubation, the cells were washed three times with
phosphate-buffered saline and incubated with labeled solution for 1
h and 37°C. At the same time, the cell nucleus was labeled with
DAPI (1:1,000). The ratio of cell apoptosis was calculated by
comparing the number of positively labeled cells with the total
number of cells.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total cellular RNA was isolated using TRIzol
reagent, according to manufacturer's protocol (Invitrogen; Thermo
Fisher Scientific, Inc.). The purity of the RNA was confirmed by
the ratio of optical densities at 260 and 280 nm. The primers for
target genes were obtained from the NCBI GeneBank database
(http://www.ncbi.nlm.nih.gov/genbank/). The cycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. The fluorescence signal was
detected during the extension step in each cycle. The data were
normalized against GADPH and the 2−ΔΔCq method was used
to calculate target gene expression. The result was represented as
the relative value compared with the control.
The primers of the target genes were as follows:
Bcl-2 (Genbank Accession no. NM_000657), forward: 5′-AGG AAG TGA
ACA TTT CGG TGAC-3′ and reverse: 5′-GCT CAG TTC CAG GAC CAGGC-3′;
Bax (Genbank Accession no. NM_138763), forward: 5′-TGC TTC AGG GTT
TCA TCCAG-3′ and reverse: 5′-GGC GGC AAT CAT CCT CTG-3′.
Western blotting
Following the appropriate treatment, the cell
lysates were prepared in non-denaturing buffer comprising 10 mM
Tris HCl (pH 7.4), 150 mM NaCl, 1% Tr iton X-100, 5 mM
EDTANa2, 1 mM DTT, 1 µg/ml leupeptine, 1 mM
Benzamidine and 2 µg/ml aprotinin. The protein concentration
was determined using Bradford reagent, as previously described
(26). The proteins (30 µg)
were subsequently separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (EMD Millipore), and
were transferred onto polyvinylidene difluoride membrane (EMD
Millipore). The 5% non-fat milk powder dissolved in Tris-buffered
saline containing Tween-20 (TBST) buffer was used to block the
membrane for 1–2 h at 37°C to reduce non-specific binding. Specific
primary antibodies against each target protein were used and the
membrane was incubated with these antibodies at 4°C overnight. In
order to remove any unbound primary antibody, the membrane was
washed three times with TBST for 15 min, and the membranes were
subsequently incubated with secondary antibodies, which were
conjugated to horseradish peroxidase (EMD Millipore) for 4 h.
Enhanced chemiluminescence (EMD Millipore) was used for protein
visualization, according to the manufacturer's protocol. β-actin
was used as the endogenous reference protein.
Statistical analysis
All results are representative of three independent
experiments and the data are expressed as the mean ± standard
deviation. The data were analyzed statistically using one-way
analysis of variance, followed by the Newman-Keuls test. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SPSS 11.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
Effect of TMP on the expression levels of
PHD2, HIF-1α and VEGF in CoCl2-treated HUVECs
The mRNA expression levels of PHD2, HIF-1α and VEGF
in HUVECs were determined by RT-qPCR. The CoCl2 group
showed significantly decreased mRNA expression of PHD2
(*P<0.05; Fig. 1A)
and increased mRNA expression levels of HIF-1α and VEGF compared
with the control group (*P<0.05; Fig. 1B and C). Treatment with TMP (50,
100 and 200 µM/ml) for 8 h, increased the mRNA expression of
PHD2 (#P<0.05, ##P<0.01; Fig. 1A) and reduced the expression of
VEGF (#P<0.05, ##P<0.01; Fig. 1C) in a dose-dependent manner.
Notably, the mRNA expression of HIF-1α (P>0.05; Fig. 1B) remained unaffected compared with
the CoCl2 group.
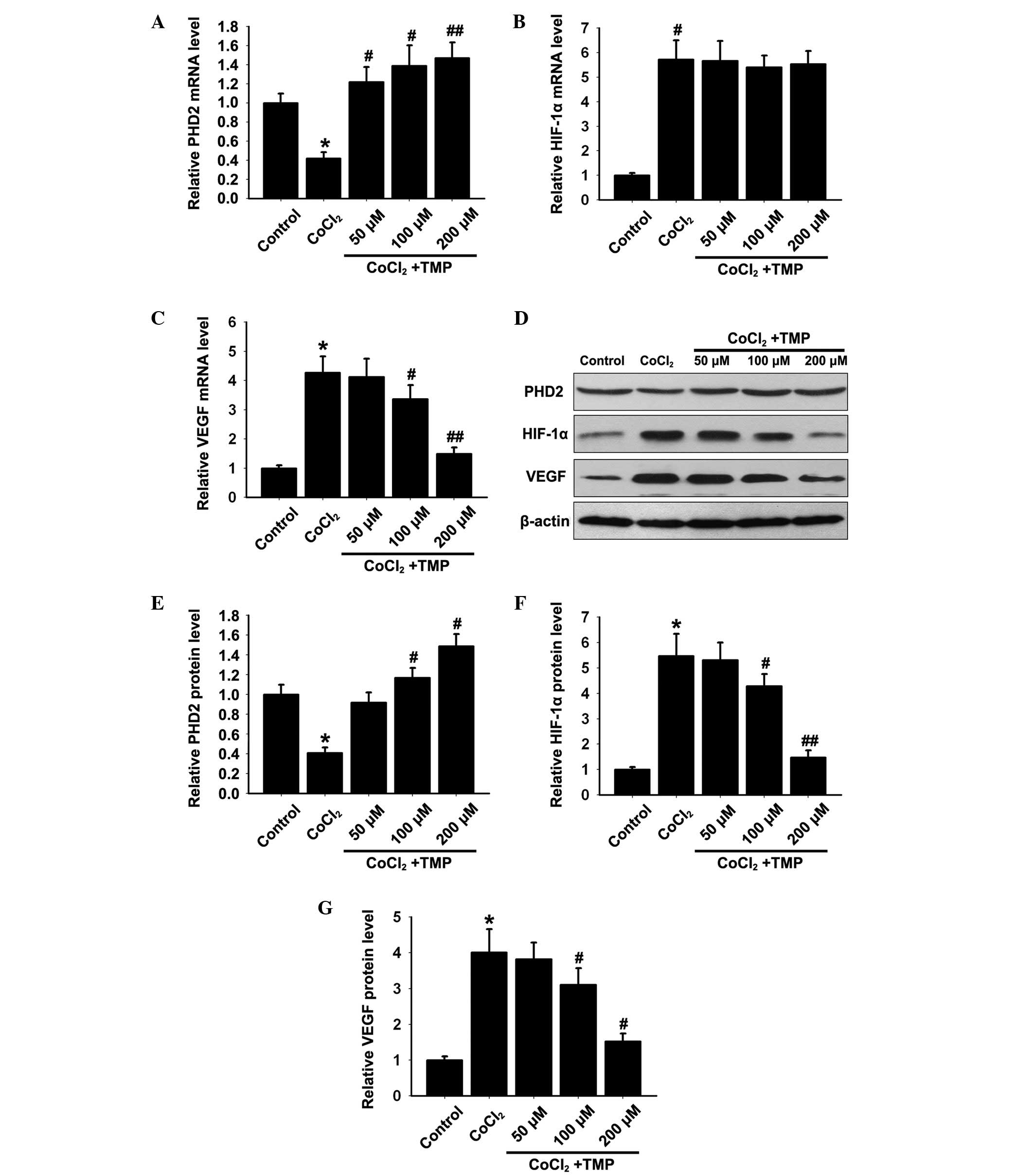 | Figure 1Effect of TMP on the expression
levels of PHD2, HIF-1α and VEGF in CoCl2-treated HUVECs.
Following pre-incubation with CoCl2 (150 µM/ml)
for 4 h (h), the HUVECs were treated with TMP at different
concentrations (50, 100 and 200 µM/ml) for 8 h. The mRNA
expression levels of (A) PHD2, (B) HIF-1α and (C) VEGF in each
group were measured by RT-qPCR. β-actin was used as internal
standard. (D) The protein expression levels of PHD2, HIF-1α and
VEGF in each group were determined by western blotting. β-actin was
used as internal standard. Quantification graphs of the expression
levels of (E) PHD2, (F) HIF-1α and (G) VEGF against β-actin in each
group. The results are representative of three independent
experiments and the data are expressed as the mean ± standard
deviation (*P<0.05, vs. control group;
#P<0.05 and ##P<0.01, vs.
CoCl2 group). TMP, tetramethylpyrazine; HUVEC, human
umbilical vein endothelial cells; PHD, prolyl hydroxylase; HIF,
hypoxia inducible factor; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; VEGF,
vascular endothelial growth factor. |
On the basis of the above mRNA expression results,
the effect of TMP on the protein expression levels of PHD2, HIF-1α
and VEGF in HUVECs was investigated by western blotting. The
CoCl2 group showed significantly reduced protein
expression of PHD2 (*P<0.05; Fig. 1D and E), and increased the protein
expression levels of HIF-1α and VEGF (*P<0.05;
Fig. 1D, F and G) compared with
the control group. Treatment with TMP (50, 100 and s200
µM/ml) significantly increased the protein expression of
PHD2 (#P<0.05; Fig. 1D
and E) and decreased the protein expression levels of HIF-1α
and VEGF (#P<0.05, ##P<0.01; Fig. 1D, F and G) in a dose-dependent
manner. These results indicated that TMP upregulated the expression
of PHD2 and initiated the degradation of HIF-1α, resulting in the
inhibition of VEGF.
PHD2-siRNA lentiviral vector inhibits the
effect of TMP on the expression levels of PHD2, HIF-1α and VEGF in
CoCl2-treated HUVECs
To further assess the role of TMP in the regulation
of the PHD2/HIF-1α signaling pathway, PHD2-siRNA lentiviral vector
or the negative control (NC) vector was transfected into the
HUVECs. Compared with the control group, no significant difference
was detected in the mRNA expression of PHD2 (P>0.05; Fig. 2A) or the protein expression
(P>0.05; Fig. 2B–C) in the NC
group. However, the mRNA (P<0.01; Fig. 2A) and protein (P<0.01; Fig. 2B and C) expression levels of PHD2
decreased significantly in the PHD2-siRNA group (P<0.01).
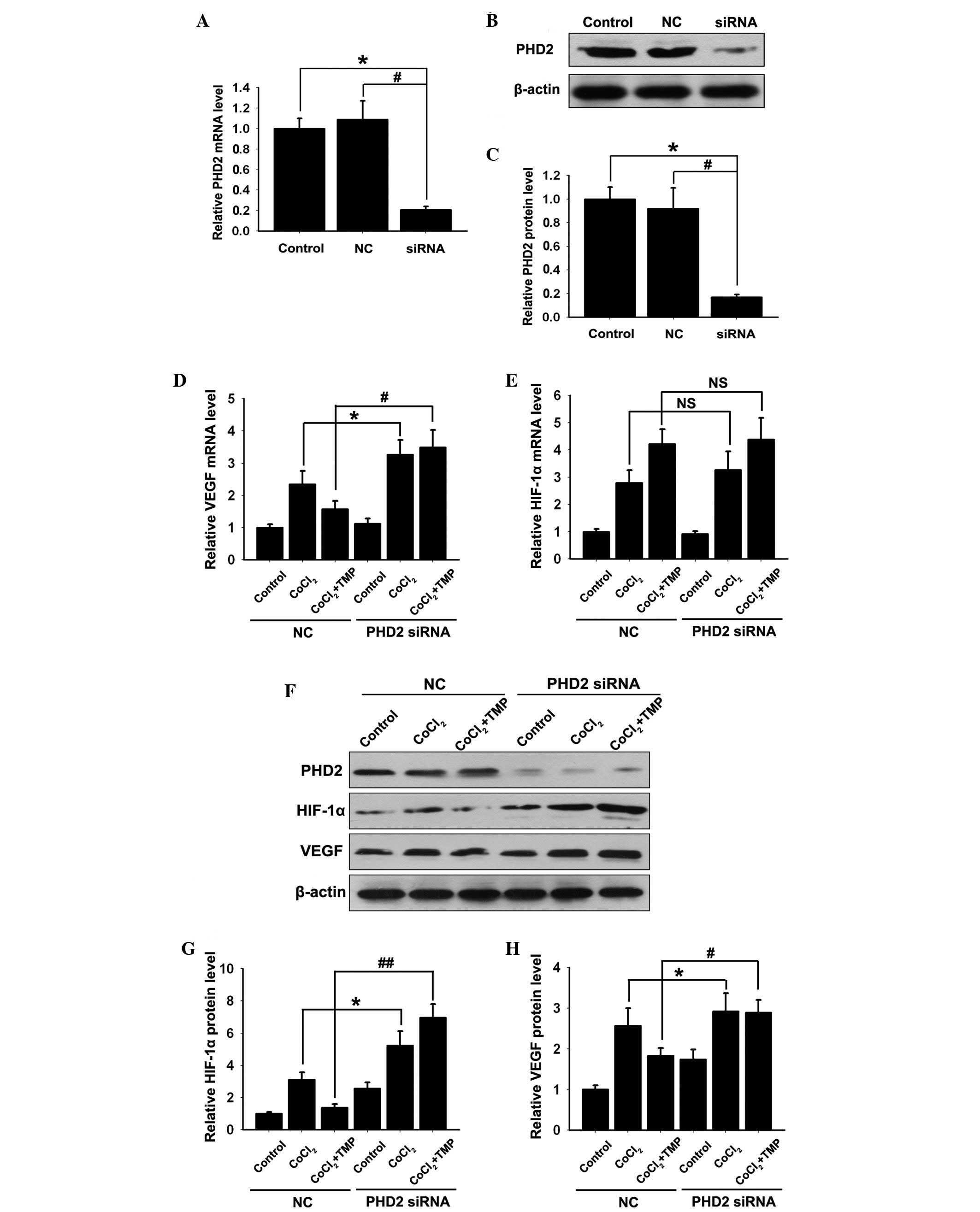 | Figure 2Effect of TMP on the expression
levels of HIF-1α and VEGF in PHD2-siRNA transfected HUVECs. The
HUVECs were transfected with PHD2-siRNA lentiviral vector and were
subsequently pre-incubated with CoCl2 (150 µM/ml)
for 4 h, followed by TMP (200 µM/ml) for 8 h. (A) The mRNA
expression of PHD2 was measured by RT-qPCR. (B) The protein
expression of PHD2 was determined by western blotting. β-actin was
used as an internal standard. (C) Quantification graph of PHD2
against β-actin. The mRNA expression levels of (D) VEGF and (E)
HIF-1α in each group were measured by RT-qPCR. (F) The protein
expression levels of PHD2, HIF-1α and VEGF in each group were
measured by western blotting. Quantification graphs of the
intensity of (G) HIF-1α and (H) VEGF bands against β-actin in each
group. The results are representative of three independent
experiments. The data are expressed as the mean ± standard
deviation (*P<0.05, #P<0.05,
##P<0.01, NSP >0.05). NS,
non-significant; NC, negative control; TMP, tetramethylpyrazine;
HUVEC, human umbilical vein endothelial cells; PHD, prolyl
hydroxylase; HIF, hypoxia inducible factor; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; VEGF,
vascular endothelial growth factor; siRNA, small interfering
RNA. |
Previous studies showed that during hypoxia, PHD2
fails to initiate the degradation of HIF-1α and, therefore, HIF-1α
is stabilized and upregulated (12–13),
resulting in the expression of VEGF. In the present study, compared
with the NC group, the upregulation of the mRNA and protein
expression levels of VEGF was observed following transfection with
the PHD2-siRNA lentiviral vector in CoCl2-treated HUVECs
(*P<0.05; Fig. 2E, F and
H). However, only the upregulation of the HIF-1α protein
(*P<0.05; Fig. 2F and
G), and not HIF-1α mRNA (Fig.
2D) was observed between the NC and PHD2 siRNA group.
Notably, compared with the NC group, the TMP induced
degradation of HIF-1α (##P<0.01; Fig. 2F and G) and the inhibition of VEGF
(#P<0.01; Fig. 2F and
H) was abolished in the PHD2-siRNA group. However, the mRNA
expression of HIF-1α in the NC and TMP groups increased
significantly following CoCl2 treatment, which remained
unaffected by the PHD2-siRNA lentiviral vector transfection
(P>0.05; Fig. 2D). These
results further indicated that TMP initiated the degradation of
HIF-1α and the inhibition of VEGF by upregulating the expression of
PHD2 in CoCl2-treated HUVECs.
TMP protects cell viability in
CoCl2-treated HUVECs
An MTT assay was used to investigate the effect of
TMP on the viability of HUVECs. Pre-incubation with
CoCl2 inhibited the cell viability of HUVECs
(#P<0.05; Fig. 3A).
Treatment with TMP (200 µM/ml) for 8 h following
pre-incubation with CoCl2 protected cell viability in
HUVECs (#P<0.05; Fig.
3A). However, following transfection with the PHD2-siRNA
lentiviral vector, the protection of TMP was partly abolished
(#P<0.05; Fig.
3A).
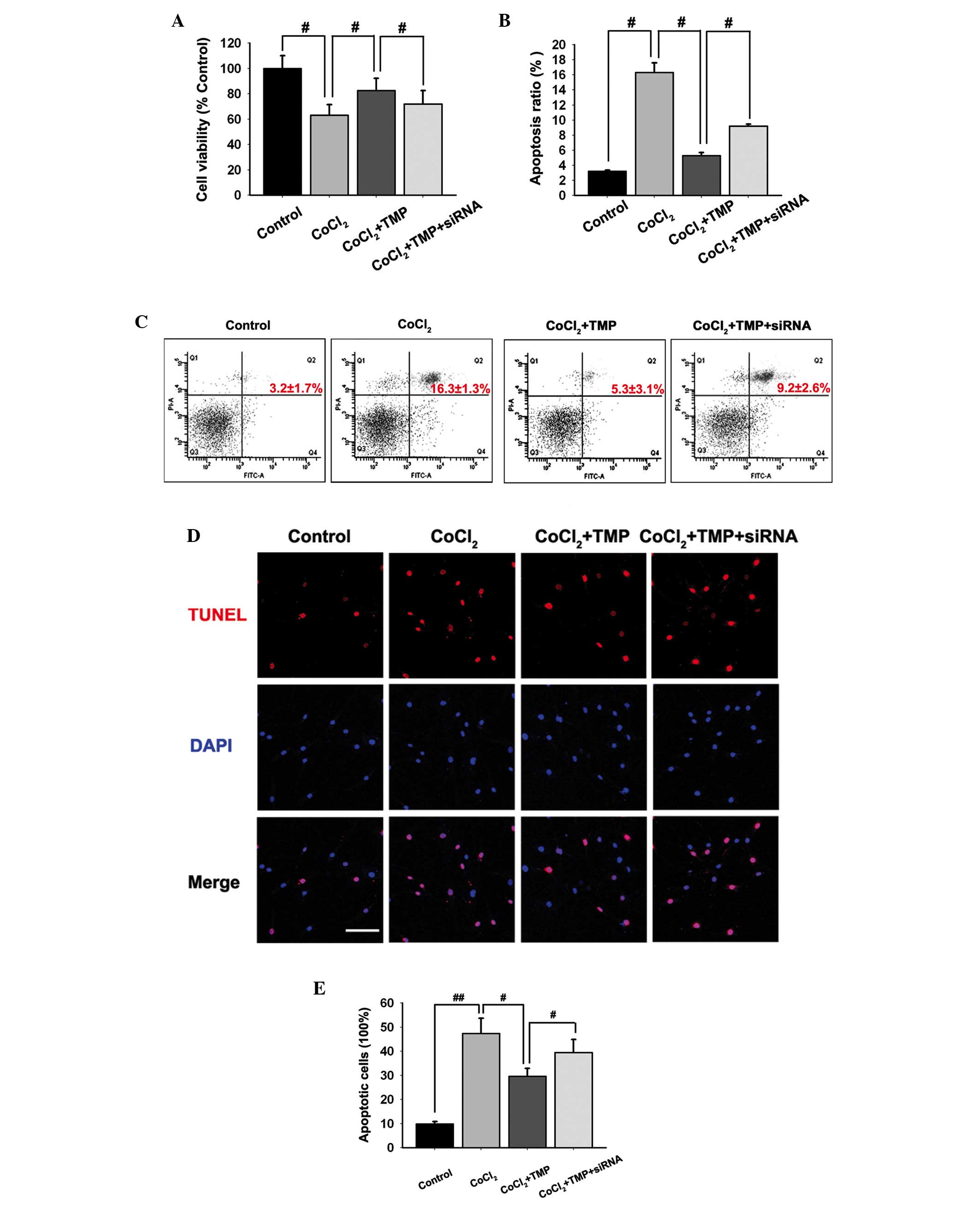 | Figure 3Effect of TMP on the apoptosis of
CoCl2-induced HUVECs. The HUVECs were transfected with
PHD2-siRNA lentiviral vector and were subsequently pre-incubated
with CoCl2 (150 µM/ml) for 4 h, followed by TMP
(200 µM/ml) for 8 h. (A) The effect of TMP on
CoCl2-induced loss of cell viability in HUVECs was
determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyl
tetrazolium bromide assay. (B and C) The apoptotic cells in
CoCl2-induced HUVECs were detected by flow cytometry
following staining with PI. (D and E) The apoptotic cells in
CoCl2-induced HUVECs were observed by TUNEL. The HUVECs
in different groups were stained with TUNEL (red) and DAPI (blue).
Images were captured (scale bar, 100 µm) and the ratio of
TUNEL-positive cells against the number of cell nuclei in each
group was calculated. The data are expressed as the mean ± standard
deviation of three independent experiments (#P<0.05
and ##P<0.01). TMP, tetramethylpyrazine; HUVEC, human
umbilical vein endothelial cells; PHD, prolyl hydroxylase; HIF,
hypoxia inducible factor; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; VEGF,
vascular endothelial growth factor; PI, propidium iodide; TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling; DAPI,
4′,6-diamidino-2-phenylindole. |
Effect of TMP on the apoptosis of HUVECs
induced by CoCl2
The present study determined whether TMP protected
from the apoptosis of HUVECs by annexin V-FITC and PI staining.
Flow cytometric analysis results indicated that CoCl2
induced apoptosis in HUVECs (Fig. 3B
and C). Following treatment with TMP (200 µM/ml), the
apoptosis in HUVECs was markedly inhibited (#P<0.05;
Fig. 3B and C). However, the
PHD2-siRNA lentiviral vector transfection partly abolished the
protection of TMP in CoCl2-induced HUVECs apoptosis
(#P<0.05; Fig. 3B and
C).
To further investigate the apoptotic ratio of
TMP-treated HUVECs, TUNEL staining was performed. It was revealed
that TMP reduced the apoptosis of HUVECs following
CoCl2-treatment (Fig.
3D). Additionally, the ratio of TUNEL-positive HUVECs reduced
by TMP was partly upregulated following transfection with the
PHD2-siRNA lentiviral vector (#P<0.05,
##P<0.05; Fig. 3E).
The results suggested that TMP protected HUVECs from apoptosis, at
least in part, via the regulation of the PHD2/HIF-1α signaling
pathway.
TMP inhibits the activation of the
caspase family in CoCl2-treated HUVECs
To further investigate downstream apoptotic
signaling in TMP-treated HUVECs, the activation of caspase-3, -8
and-9, hallmark apoptotic execution enzymes, were assessed by
western blotting. Pre-incubation with CoCl2 markedly
stimulated the activation of all caspases in HUVECs. Treatment with
TMP clearly inhibited the activation of all caspases. Following
transfection with PHD2-siRNA lentiviral vector, the inhibition of
TMP on the activation of caspase-3 and -9, however not caspase-8,
was partly abolished (#P<0.05,
##P<0.01, NSP>0.05; Fig. 4A–D). The results suggested that TMP
protected HUVECs from apoptosis, at least in part, via the
regulation of the caspase family.
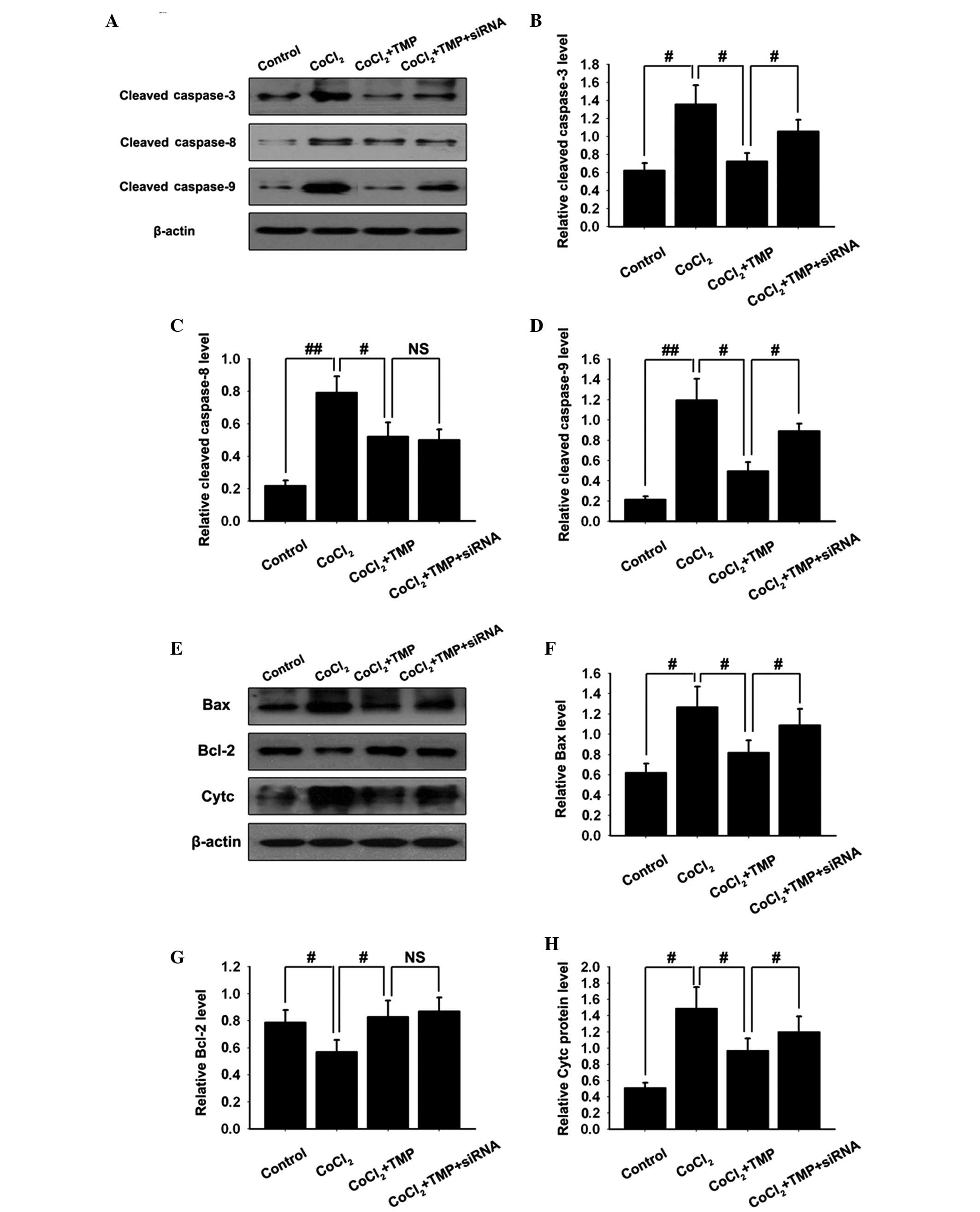 | Figure 4Effect of TMP on the expression
levels of apoptotic associated proteins in CoCl2-induced
HUVECs. The HUVECs were transfected with PHD2-siRNA lentiviral
vector and were subsequently pre-incubated with CoCl2
(150 µM/ml) for 4 h, followed by TMP (200 µM/ml) for
8 h. (A) The protein expression levels of activated caspase family
members (caspase-3, caspase-8 and caspase-9) in the
CoCl2-induced HUVECs were measured by western blotting.
β-actin was used as internal standard. Quantification graphs of the
band intensities of activated (B) caspase-3, (C) caspase-8 and (D)
caspase-9 against β-actin in each group. (E) The protein expression
levels of Bax, Bcl-2 and Cytc in CoCl2-induced HUVECs
were measured by western blotting. β-actin was used as internal
standard. Quantification graphs of the intensity of staining of (F)
Bax, (G) Bcl-2 and (H) Cytc against β-actin in each group. The
results are representative of three independent experiments. The
data are expressed as the mean ± standard deviation
(#P<0.05, ##P<0.01 and
NSP>0.05). Cytc: cytochrome c; NS,
non-significant; NC, negative control; TMP, tetramethylpyrazine;
HUVEC, human umbilical vein endothelial cells; PHD, prolyl
hydroxylase; Bcl, B-cell lymphoma. Bax, Bcl-2-associated X protein;
siRNA, small interfering RNA. |
TMP treatment modifies the expression
levels of Bcl-2 and Bax, and cytochrome c release in
CoCl2-treated HUVECs
The Bcl-2 family proteins have either proapoptotic
or antiapoptotic activities and modulate the mitochondrial
apoptosis signaling pathway. The balance between antiapoptotic and
proapoptotic proteins is critical in determining the susceptibility
of cells to death signals. Western blotting analysis demonstrated
the upregulation of the proapoptotic Bax protein and the
downregulation of antiapoptotic Bcl-2 in CoCl2-treated
HUVECs. TMP treatment resulted in the increased protein expression
of Bcl-2 and the decreased protein expression of Bax. Following
transfection with the PHD2-siRNA lentiviral vector, the inhibition
of TMP on the activation of Bax, however, not Bcl-2 was partly
abolished (#P<0.05; Fig.
4E–G). The results suggested that TMP protected HUVECs from
apoptosis by regulating the expression of the Bcl-2 family.
The disruption of the mitochondrial membrane
function is known to result in the release of the mitochondrial
enzyme, cytochrome c (27).
As detected by western blotting, stimulation of HUVECs with
CoCl2 resulted in an almost 3-fold increase in the
levels of cytochrome c compared with the control group.
Treatment with TMP inhibited this CoCl2-induced
cytochrome c release. Following transfection with the
PHD2-siRNA lentiviral vector, the inhibition of TMP on the release
of cytochrome c was partly abolished (#P<0.05;
Fig. 4E and H). These results
suggested that TMP protected HUVECs from apoptosis by inhibiting
the release of cytochrome c.
Discussion
Endothelial cells, the specialized cell type in
vessels, are involved in the physiological and pathological
processes of retinal neovascularization. It was reported that
endothelial cells are highly sensitive to the harmful effects of
hypoxia. As a traditional Chinese herb, TMP and its protective
effect against apoptosis in endothelial cells has been previously
investigated in vitro and in vivo (15,19,20,22,28,29).
For the first time, to the best of our knowledge, the present study
showed that TMP effectively ameliorated the apoptosis of
CoCl2-induced HUVECs, at least in part, via the
regulation of the PHD2/HIF-1α signaling pathway.
TMP, the active ingredient of a traditional Chinese
herb from Ligustium wallichii Franch, has long been widely
used in the treatment of patients with cerebral and cardiac
ischemic diseases in China (30).
Previous studies have demonstrated that TMP protected endothelial
cells against oxidative stress through scavenging the reactive
oxygen species, downregulating the phosphorylation of extracellular
signal-regulated kinase 1/2 and p38 mitogen-activated protein
kinase, and inhibiting nuclear factor-κB (15,23).
Although these previous studies revealed that TMP regulated certain
signaling pathways during antioxidative stress, the further
upstream signaling pathways regarding the protection effect of TMP
in endothelial cells remain to be elucidated. To further confirm
this, it is pivotal for us to understand the most effective
protective role of TMP in treating retinal neovascularization.
Considerable evidence has implicated that hypoxia,
known to be associated with angiogenesis, has a key role in the
development of retinal neovascularization. It has been well
accepted that the cellular effects of hypoxia are mostly mediated
by HIF-1α (31–33). The HIF-1α protein has been
determined to coexist with hypoxia and is associated with the
development of retinal neovascularization. PHD2, one of the PHD
family members (PHD1, 2, and 3), regulates the stability of HIF-1α
in an oxygen-dependent manner. In the present study, it was
demonstrated that TMP increased the expression of PHD2 and almost
completely abolished the accumulation of HIF-1α, resulting in
decreased expression of VEGF in CoCl2-treated HUVECs.
However, the mRNA expression of HIF-1α was unaffected following
treatment with TMP. It was hypothesized that TMP abolished the
HIF-1α accumulation associated with its role in upregulating the
expression of PHD2, which could degrade the protein expression of
HIF-1α, however, not the mRNA expression in
CoCl2-treated HUVECs. Furthermore, selective knockdown
of PHD2 with siRNAs abolished the production of HIF-1α and VEGF
reduced by TMP. These results indicated that TMP initiated the
degradation of HIF-1α and the inhibition of VEGF by upregulating
the expression of PHD2 in CoCl2-treated HUVECs.
Previous studies also showed that hypoxia not only
led to the activation of several transcription factors, but also
initiated the complex apoptotic cascade in endothelial cells
(34,35). HIF-1α is associated with severe
hypoxia-induced apoptosis of endothelial cells (9–11).
HIF-1α suppresses apoptosis by activating multiple antiapoptotic
genes, including VEGF (36) and
Bcl-xL (37), which leads to the
protection against hypoxic injury. Notably, HIF-1α has also been
shown to be a factor mediating hypoxia-induced apoptosis. Hypoxia
increases apoptosis of embryonic stem cells, however, certain
apoptotic effects disappear following the HIF-1α genes being
knocked out (38). In the present
study, following pre-incubation with CoCl2, HUVECs
treated with TMP showed significantly decreased cell viability and
apoptosis ratio. Treatment with TMP also clearly inhibited the
activation of the proapoptotic caspase family (caspase-3, -8 and-9)
and proapoptotic Bcl-2 family (Bax) and the release of the
cytochrome c. Additionally, the expression of the
antiapoptotic Bcl-2 family (Bax) was increased. However, selective
knockdown of PHD2 with siRNAs almost inverted cell viability,
apoptosis ratio and most of the hallmark apoptotic execution
enzymes, with the exception of caspase-8 and Bcl-2 following
treatment with TMP.
In conclusion, the present study demonstrated that
TMP had effective protection against CoCl2-induced
apoptosis of HUVECs, and the antiapoptotic effect of TMP on
CoCl2-induced HUVECs was, at least in part, via the
regulation of the PHD2/HIF-1α signaling pathway.
Acknowledgments
This work was supported by the Science and
Technology Planning Project of Guangdong Province (no.
2013B021800056).
References
|
1
|
Nyengaard JR, Ido Y, Kilo C and Williamson
JR: Interactions between hyperglycemia and hypoxia: Implications
for diabetic retinopathy. Diabetes. 53:2931–2938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenfeld PJ, Brown DM, Heier JS, Boyer
DS, Kaiser PK, Chung CY and Kim RY; MARINA Study Group: Ranibizumab
for neovascular age-related macular degeneration. N Engl J Med.
355:1419–1431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arjamaa O and Nikinmaa M: Oxygen-dependent
diseases in the retina: Role of hypoxia-inducible factors. Exp Eye
Res. 83:473–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forooghian F, Razavi R and Timms L:
Hypoxia-inducible factor expression in human RPE cells. Br J
Ophthalmol. 91:1406–1410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu LZ, Jing Y, Jiang LL, Jiang XE, Jiang
Y, Rojanasakul Y and Jiang BH: Acacetin inhibits VEGF expression,
tumor angiogenesis and growth through AKT/HIF-1α pathway. Biochem
Biophys Res Commun. 413:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu
M, Le YZ, Ge J, Johnson RS and Ma JX: Ischaemia-induced retinal
neovascularisation and diabetic retinopathy in mice with
conditional knockout of hypoxia-inducible factor-1 in retinal
Müller cells. Diabetologia. 54:1554–1566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forooghian F and Das B: Anti-angiogenic
effects of ribonucleic acid interference targeting vascular
endothelial growth factor and hypoxia-inducible factor-1alpha. Am J
Ophthalmol. 144:761–768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanyan C, Guoxian Q, Yang G and Leting W:
Mechanism of hypoxia-induced factor 1alpha expression in
endothelial cells of the human umbilical vein and its induction of
apoptosis. Mol Biol Rep. 35:285–290. 2008. View Article : Google Scholar
|
|
10
|
Zhu X, Zhou W, Cui Y, Zhu L, Li J, Feng X,
Shao B, Qi H, Zheng J, Wang H and Chen H: Pilocarpine protects
cobalt chloride-induced apoptosis of RGC-5 cells: Involvement of
muscarinic receptors and HIF-1 alpha pathway. Cell Mol Neurobiol.
30:427–435. 2010. View Article : Google Scholar
|
|
11
|
Jin Y, An X, Ye Z, Cully B, Wu J and Li J:
RGS5, a hypoxia-inducible apoptotic stimulator in endothelial
cells. J Biol Chem. 284:23436–23443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toffoli S and Michiels C: Intermittent
hypoxia is a key regulator of cancer cell and endothelial cell
interplay in tumours. FEBS J. 275:2991–3002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazure NM, Brahimi-Horn MC, Berta MA,
Benizri E, Bilton RL, Dayan F, Ginouvès A, Berra E and Pouysségur
J: HIF-1: Master and commander of the hypoxic world. A
pharmacological approach to its regulation by siRNAs. Biochem
Pharmacol. 68:971–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li SY, Jia YH, Sun WG, Tang Y, An GS, Ni
JH and Jia HT: Stabilization of mitochondrial function by
tetramethylpyrazine protects against kainate-induced oxidative
lesions in the rat hippocampus. Free Radic Biol Med. 48:597–608.
2010. View Article : Google Scholar
|
|
15
|
Li WM, Liu HT, Li XY, Wu JY, Xu G, Teng
YZ, Ding ST and Yu C: The effect of tetramethylpyrazine on hydrogen
peroxide-induced oxidative damage in human umbilical vein
endothelial cells. Basic Clin Pharmacol Toxicol. 106:45–52.
2010.
|
|
16
|
Liao SL, Kao TK, Chen WY, Lin YS, Chen SY,
Raung SL, Wu CW, Lu HC and Chen CJ: Tetramethylpyrazine reduces
ischemic brain injury in rats. Neurosci Lett. 372:40–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou Y, Jiang W and Chiou GC: Effect of
tetramethylpyrazine on rat experimental choroidal
neovascularization in vivo and endothelial cell cultures in vitro.
Curr Eye Res. 32:71–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang X, Zhou H, Ding Y, Li J, Yang C, Luo
Y, Li S, Sun G, Liao X and Min W: TMP prevents retinal
neovascularization and imparts neuroprotection in an oxygen-induced
retinopathy model. Invest Ophthalmol Vis Sci. 53:2157–2169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Zhao MQ, Kumar Durairajan SS, Xie
LX, Zhang HX, Kum WF, Goto S and Liao FL: Protective effect of
tetramethylpyrazine and salvianolic acid B on apoptosis of rat
cerebral microvascular endothelial cell under high shear stress.
Clin Hemorheol Microcirc. 38:177–187. 2008.PubMed/NCBI
|
|
20
|
Yang J, Yang S and Yuan YJ: Integrated
investigation of lipidome and related signaling pathways uncovers
molecular mechanisms of tetramethylpyrazine and butylidenephthalide
protecting endothelial cells under oxidative stress. Mol Biosyst.
8:1789–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ou Y, Dong X, Liu XY, Cheng XC, Cheng YN,
Yu LG and Guo XL: Mechanism of tetramethylpyrazine analogue CXC195
inhibition of hydrogen peroxide-induced apoptosis in human
endothelial cells. Biol Pharm Bull. 33:432–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang Y, Hu M, Zhu Y, Gao X and Wang MW:
Antioxidative effect of the herbal remedy Qin Huo Yi Hao and its
active component tetramethylpyrazine on high glucose-treated
endothelial cells. Life Sci. 84:428–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao
M, Zheng SZ, Wang AY, Zhang CB, Zhang WW and Lei N: Ligustrazine
inhibits B16F10 melanoma metastasis and suppresses angiogenesis
induced by vascular endothelial growth factor. Biochem Biophys Res
Commun. 386:374–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Martin R, Vanhove B, Cheng Q, Hofer E,
Csizmadia V, Winkler H and Bach FH: Cytokine-inducible expression
in endothelial cells of an I kappaB alpha-like gene is regulated by
NF kappa B. EMBO J. 12:2773–2779. 1993.PubMed/NCBI
|
|
25
|
Dai H, Yu Z, Fan X, Liu N, Yan M, Chen Z,
Lo EH, Hajjar KA and Wang X: Dysfunction of annexin A2 contributes
to hyperglycaemia-induced loss of human endothelial cell surface
fibrinolytic activity. Thromb Haemost. 109:1070–1078. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hedenbjörk-Lager A, Bjørndal L, Gustafsson
A, Sorsa T, Tjäderhane L, Åkerman S and Ericson D: Caries
correlates strongly to salivary levels of matrix
metalloproteinase-8. Caries Res. 49:1–8. 2015. View Article : Google Scholar
|
|
27
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome c release during apoptosis. Cell Death
Differ. 10:709–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Wei T, Hou J, Li G, Yu S and Xin
W: Iron-induced oxidative damage and apoptosis in cerebellar
granule cells: Attenuation by tetramethylpyrazine and ferulic acid.
Eur J Pharmacol. 467:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dang SC, Zhang JX, Qu JG, Wang XQ and Fan
X: Ligustrazine alleviates gastric mucosal injury in a rat model of
acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int.
6:213–218. 2007.PubMed/NCBI
|
|
30
|
Guo SK, Chen KJ, Qian ZH, Weng WL and Qian
MY: Tetramethylpyrazine in the treatment of cardiovascular and
cerebrovascular diseases. Planta Med. 47:891983. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brahimi-Horn MC and Pouysségur J: HIF at a
glance. J Cell Sci. 122:1055–1057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semenza GL: Hypoxia-inducible factor 1:
Oxygen homeostasis and disease pathophysiology. Trends Mol Med.
7:345–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee C, Cheng W, Chang M, Su Y, Chen C and
Hsieh F: Hypoxia-induced apoptosis in endothelial cells and
embryonic stem cells. Apoptosis. 10:887–894. 2005. View Article : Google Scholar
|
|
35
|
Matsushita H, Morishita R, Nata T, Aoki M,
Nakagami H, Taniyama Y, Yamamoto K, Higaki J, Yasufumi K and
Ogihara T: Hypoxia-induced endothelial apoptosis through nuclear
factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: In vivo
evidence of the importance of NF-kappaB in endothelial cell
regulation. Circ Res. 86:974–981. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Weng Q, Zhang L, He Q and Yang B:
VEGF and Bcl-2 interact via MAPKs signaling pathway in the response
to hypoxia in neuroblastoma. Cell Mol Neurobiol. 29:391–401. 2009.
View Article : Google Scholar
|
|
37
|
Chen N, Chen X, Huang R, Zeng H, Gong J,
Meng W, Lu Y, Zhao F, Wang L and Zhou Q: BCL-xL is a target gene
regulated by hypoxia-inducible factor-1{alpha}. J Biol Chem.
284:10004–10012. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|


















