Introduction
Colon cancer (CC) is the second leading cause of
cancer-associated mortality worldwide (1). Invasion is the most difficult problem
when treating CC, however, the molecular mechanism underlying CC
invasion remains to be fully elucidated. According to previous
data, 10–25% of patients with CC have been diagnosed with liver
metastasis (2–4). The treatments for CC include surgery,
chemotherapy, endoscopic stent implant and targeted chemotherapy
(5,6). The most difficult problems for the
patients with CC are invasion and metastasis. Early detection and
targeted therapy are more practical and important for the treatment
of CC.
Focal adhesion kinase (FAK) is predominantly
distributed in the cell cytoplasm, and was initially identified in
the v-src transfected chicken embryo fibroblast. FAK causes the
accumulation and depolymerization of cytoskeleton proteins,
therefore, influencing the structures of cell adhesion sites and
membrane protrusions to regulate cell activities (7). FAK also promotes the secretion of the
matrix metalloproteinases (MMPs), which can damage the
extracellular matrix components of the intercellular matrix and
basement membrane, therefore, accelerating the growth and invasion
of CC cells (8). FAK is involved
in breast cancer, CC, prostate cancer and other malignant tumor
types (9,10), and is markedly associated with
tumor proliferation, apoptosis, adhesion and migration.
MicroRNA (miRNA), a type of highly conserved
non-coding RNA, consists of 18–25 nucleotides with endogenous
single-stranded RNA. Lee et al (11) identified the first miRNA,
let4, in Caenorhabditis elegans in 1993. miRNA
(miR)-21 and miR-264 were subsequently identified in vertebrates,
flies, worms, viruses and certain plant species. It is estimated
that there are >1,000 miRNAs in the human genome (12). miRNAs have been demonstrated to be
closely associated with tumor growth and metastasis.
Therefore, miRNAs may be targets for cancer
diagnosis and treatment (13).
miR-7 is important for the growth and metastasis of glioma, breast,
ovarian, bladder, gastric, lung, liver and colon cancer (14–20).
miR-7 inhibits the invasion of glioma cells by binding to the
3-untranslated region of FAK mRNA, which inhibits the protein
translation of FAK, leading to decreased invasion of glioma cells
(14).
The present study investigated the expression of
miR-7 in CC tissues and adjacent colon tissues. Additionally, the
possible correlations of miR-7 and the clinical pathological
factors in CC were determined, illustrating the effects of
overexpression or underexpression of miR-7 on the growth and
migration of CC in vitro. These findings may provide novel
targets for the treatment of CC.
Materials and methods
Antibodies and reagents
The miR-7 mimic (miR-7m), negative-5′-cy3, miR-7
inhibitor (miR-7i) and the negative control (NC) were purchased
from Ribobio Co., Ltd. (Guangzhou, Guangdong, China). Antibodies
were purchased as follows: Rabbit monoclonal FAK (1:1,000; Abcam,
Cambridge, MA, USA; cat. no. ab40794), rabbit polyclonal anti-MMP-2
(1:1,000; Proteintech Group, Inc., Chicago, IL, USA; cat. no.
10373-2-AP) and rabbit polyclonal anti-MMP-9 (1:1,000; Proteintech
Group, Inc.; cat. no. 10375-2-AP), and rabbit polyclonal anti-actin
(1:5,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-1616). All
antibodies were diluted with phosphate-buffered saline (PBS).
Enhanced chemiluminescence western blot detection reagents were
purchased from Thermo Fisher Scientific (Rockford, IL, USA).
Protease inhibitor cocktail, Lubrol-PX and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). An
Invitrogen cDNA synthesis kit was purchased from Thermo Fisher
Scientific (Carlsbad, CA, USA). All RNA duplexes were chemically
synthesized by GenePharma Co., Ltd. (Shanghai, China).
Cell culture and tissue specimens
Human HCT-8 and Caco-2 CC cell lines were obtained
from American Type Culture Collection (Rockville, IL, USA). The
cells were maintained in Dulbecco's modified Eagle's medium (Gibco
Life Technologies, Carlsbad, CA, USA), supplemented with 10% fetal
bovine serum (Gibco Life Technologies) and 100 µg/ml
streptomycin (NCPC Hebei Huamin Pharmaceutical Co., Ltd., Hebei,
China) in a humid incubator with 5% CO2 at 37°C.
Paired CC samples and adjacent normal colon tissues
were obtained from 60 patients who were diagnosed with CC between
2011 and 2012 in the Department of Pathology, The First Affiliated
Hospital of Nanchang University (Nanchang, China). No patient
received chemotherapy or radiotherapy prior to surgery and patients
were diagnosed by clinical pathological staging based on the 2010
American Joint Committee on Cancer criteria (21). All patients provided written
informed consent prior to surgery. The detailed clinical
pathological information of all patients and their correlation with
the expression of miR-7 are summarized in the Table I. The present study was approved by
the institutional review board of The First Affiliated Hospital of
Nanchang University medical research ethics committee.
 | Table ICorrelation between the expression of
miR-7 and clinical pathological characteristics in colon
cancer. |
Table I
Correlation between the expression of
miR-7 and clinical pathological characteristics in colon
cancer.
| Characteristic | Cases (n) | miR-7
expressionb | Spearman's (R) | P-value |
|---|
| Gender |
| Male | 29 | 0.38 (0.11–2.27) | −0.726 | 0.773 |
| Female | 31 | 0.58 (014–2.01) |
| Age (years) |
| <60 | 28 | 0.44 (0.15–2.56) | −1.089 | 0.276 |
| ≥60 | 32 | 0.49 (0.10–1.71) |
| Histopathologic
differentiation |
| WD/MD | 36 | 0.35 (0.12–1.97) | −0.800 | 0.424 |
| PD/UD | 24 | 0.49 (0.13–1.29) |
| Lymph node
metastasis |
| Yes | 27 | 0.19 (0.09–1.16) | −2.290 | 0.022a |
| No | 33 | 0.69 (0.27–2.23) |
| TNM staging |
| I+II | 30 | 0.75 (0.30–2.32) | −2.698 | 0.007 |
| III+IV | 30 | 0.17
(0.09–1.23) |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated using Invitrogen TRIzol
reagent (Thermo Fisher Scientific). The RNA was subsequently
reverse transcribed into cDNA using the stem-loop reverse
transcription primer (Ribobio Co., Ltd.) for miRNA detection. RT
was performed using Revert Aid™ reverse transcriptase (Fermentas,
Ontario, Canada) according to the manufacturer's instructions. The
cDNA results were quantified by SYBR Premix Ex Taq™ (Takara Bio.,
Inc., Otsu, Japan). The mRNA expression levels of miR-7 and FAK
were detected by RT-qPCR and analyzed on an Applied Biosystems 7500
PCR Detection system (Applied Biosystems, Inc. Foster City, CA,
USA). The mRNA expression levels of miR-7 and FAK were determined
and normalized against the expression of U6 using the comparative
cycle threshold (Ct) method (22).
Transient transfection of miR-7m or
miR-7i
The human HCT-8 and Caco-2 CC cell lines were
cultured in antibiotic-free medium for 48 h in 6-well plates. Upon
reaching a confluence of 60–70%, the miR-7m (50 nM), miR-7i (300
mM), unrelated sequence positive control (50 nM) or the NC (300 nM)
were transfected into the cells using an Invitrogen Lipofectamine
2000 reagent (Thermo Fisher Scientific), according to the
manufacturer's instructions.
Protein extraction and western
blotting
Following incubation for 72 h after transfection
with miR-7m, miR-7i or the control, the cells were washed with cold
PBS three times and were subsequently resuspended in protein lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China) at 4°C
for 30 min. The protein concentrations were measured following
centrifugation and quantified using the bicinchoninic acid assay
kit (Beyotime Institute of Biotechnology). A Bio-Rad Model 680
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to measure the results of the assay. An equal quantity of
protein was separated on 8% SDS-PAGE gels (Sigma-Aldrich) and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 50
mg/ml non-fat milk in 10 ml PBS for 2 h at room temperature and
were subsequently incubated overnight at 4°C with the appropriate
primary antibody. The membranes were washed with PBS and incubated
with horseradish peroxidase-conjugated goat anti-rat immunoglobulin
(Ig)G/IgM (cat. no. AP132P; 1:1,000; Merck Millipore, Darmstadt,
Germany) at 4°C for 4 h. The images were captured and quantified
using Gel Doc XR-Z system (controlled by Image Lab™ version 2.0;
(Bio-Rad Laboratories, Inc.).
Cell viability analysis
A cell viability assay was performed on the CC cells
using an MTT reduction assay (23). The cells were seeded into 96-well
plates for 24 h and were subsequently transfected with miR-7m/i. At
24, 48 and 72 h after transfection, 20 µl MTT reagent (5
mg/ml) was added to each well for 4 h at 4°C. The generated
formazan product was dissolved in 150 µl dimethyl sulfoxide.
The optical density at 490 nm was measured using the Bio-Rad Model
680 microplate reader. Triplicate wells were used for each cell
line and experiments were repeated in triplicate.
Wound healing assay
Cell migration was measured using a wound healing
assay. The HCT-8 and Caco-2 cells (3×105/well) were
seeded into 6-well plates. Once the cells reached a confluence
between 90 and 100% at 48–72 h after transfection, a 10-µl
pipette tip was used to scrape the cell monolayer. Fresh serum-free
medium was added and images were captured at 0, 24 and 48 h after
wounding using a phase contrast microscope (IX711; Olympus, Tokyo,
Japan). The wound-healing areas were first compared to the wound
area at 0 h, and were finally compared to the mock control using
Image J 1.48u software (National Institutes of Health, MA,
USA).
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). The data are presented as the mean
± standard deviation. The differences between the groups were
assessed by Student's t-test or one-way analysis of variance.
Pearson's correlation coefficient was used to assess the
correlation between the expression levels of FAK and miR-7 in CC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-7 is downregulated in CC and
correlates with clinical significance
A total of 60 cases of fresh specimens of CC and
adjacent normal colon tissues were used to detect the expression of
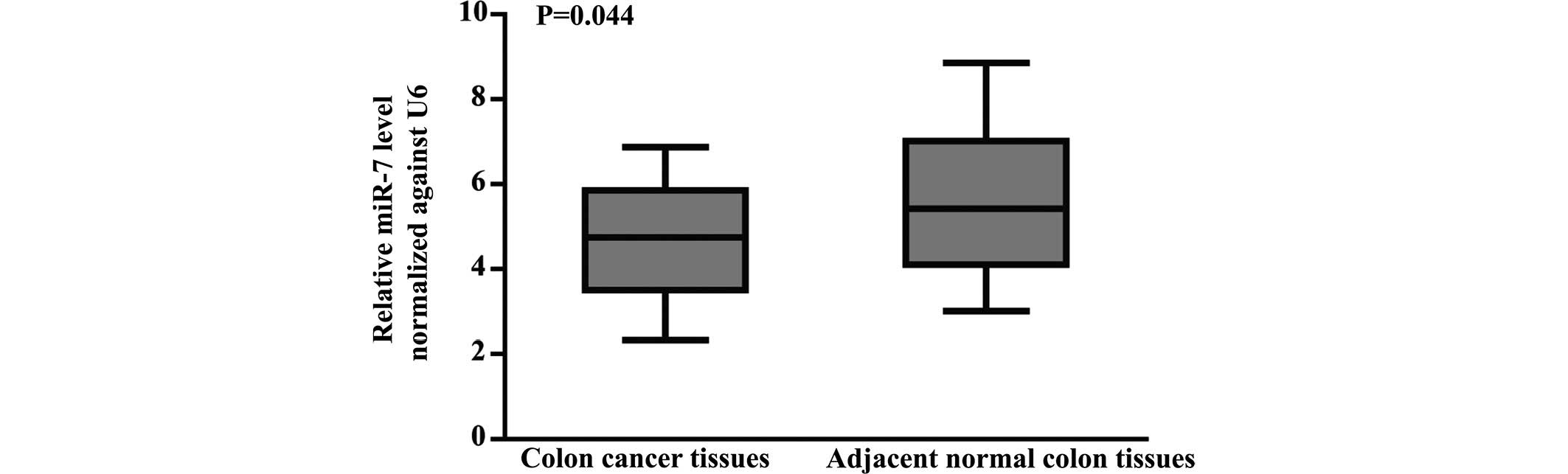
miR-7 by RT-qPCR. The expression of miR-7 was markedly
downregulated in CC compared with paired normal colon tissues
(P=0.044; Fig. 1). As shown in
Table I, the expression of miR-7
negatively correlated with lymph node metastasis (r=−2.29; P=0.022)
and tumor node metastasis (TNM) stages (r=−2.698; P=0.007),
regardless of gender, age and histopathologic differentiation of
the patients with CC. These results suggested that miR-7 may be a
potential tumor suppressor and may be important in the development
of CC.
Expression of miR-7 is associated with
the protein expression of FAK
To further determine whether FAK was a downstream
target of miR-7, miR-7m, miR-7i or NC were transfected into Caco-2
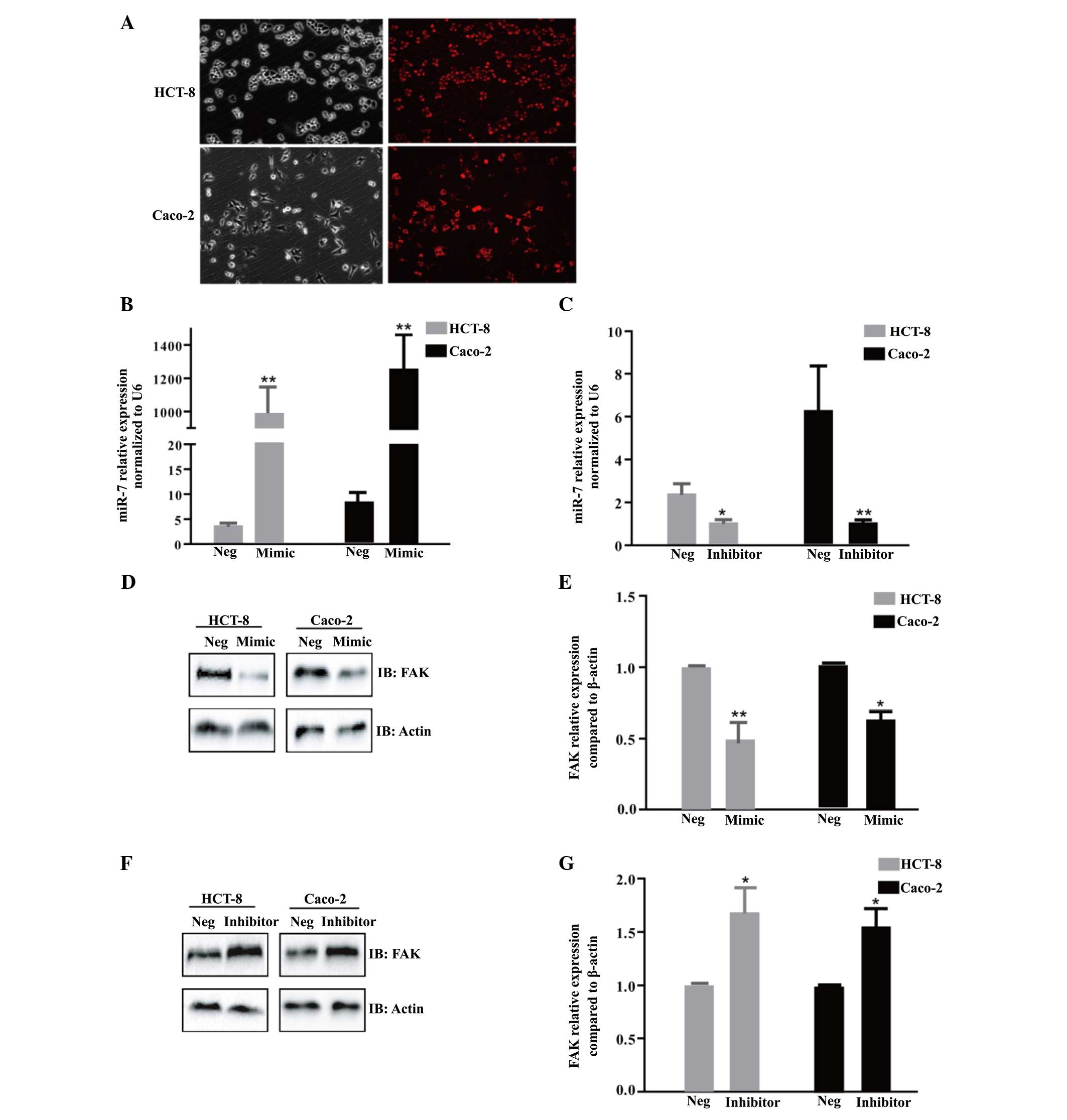
and HCT-8 cells. To confirm the transfection efficiency,
fluorescence images were caputred 6 h after transfection using
miR-7m, negative-5′-cy3 (Fig. 2A).
Compared with the NC or mock, the expression of miR-7 was
significantly increased or decreased following transfection with
miR-7m or miR-7i, respectively in the HCT-8 and Caco-2 cells
(Fig. 2B and C). The ectopic
expression of miR-7 significantly decreased the protein expression
of FAK, as determined by western blotting (P<0.05; Fig. 2D and E). However, a decrease in the
expression of miR-7 led to increased protein expression of FAK
(P<0.05; Fig. 2F and G).
miR-7 influences the proliferation and
migration of CC cells
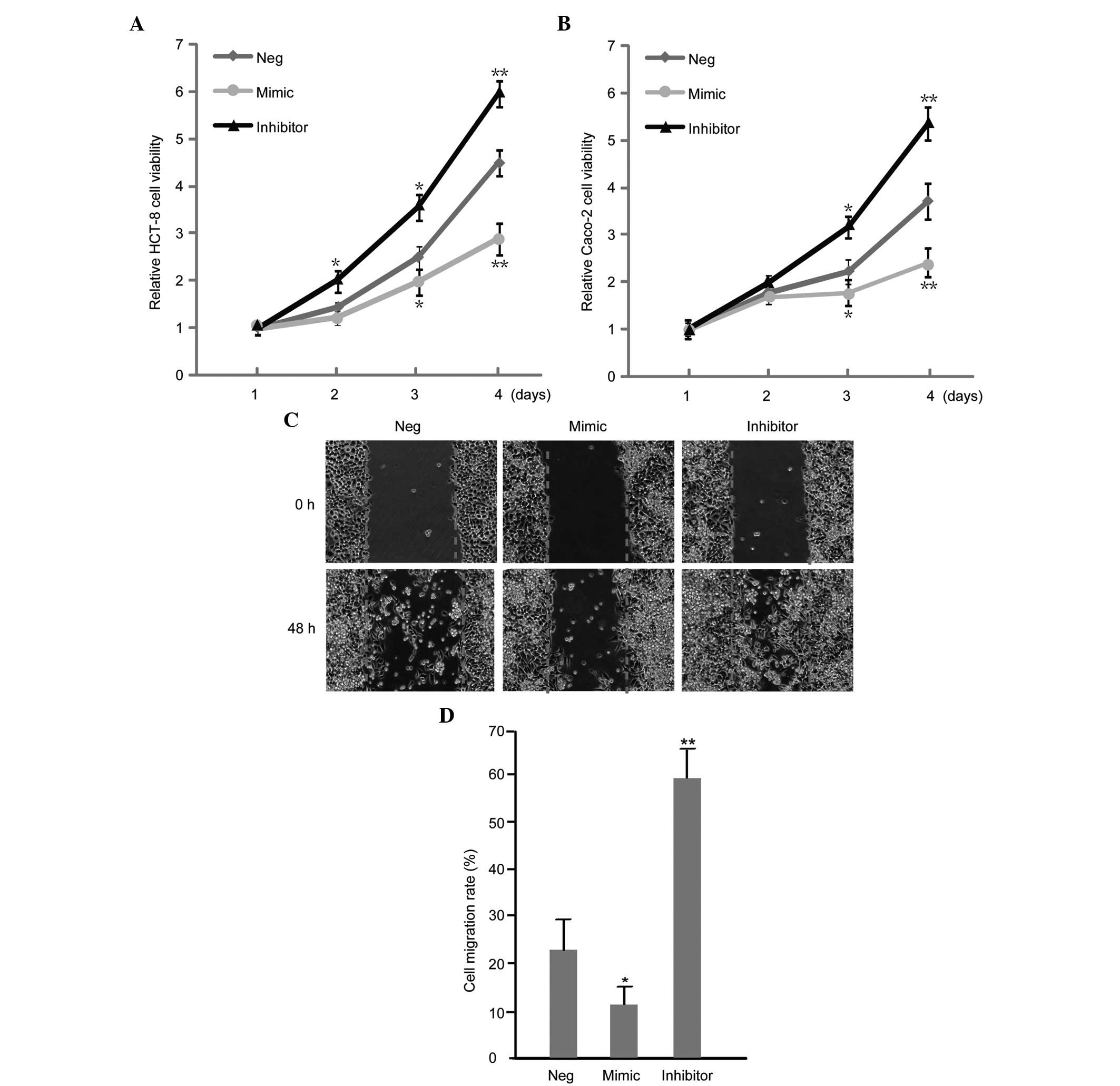
The effect of miR-7 on the proliferation and
migration of CC cells was assessed by a wound healing and MTT
assay, respectively. It was revealed that the upregulation of miR-7
inhibited the proliferation and migration of HCT-8 cells (Fig. 3A and B). Additionally,
downregulation of miR-7 enhanced the proliferation and migration of
HCT-8 and Caco-2 cell lines (Fig. 3C
and D).
miR-7 correlates with the expression
levels of MMP-2 and MMP-9
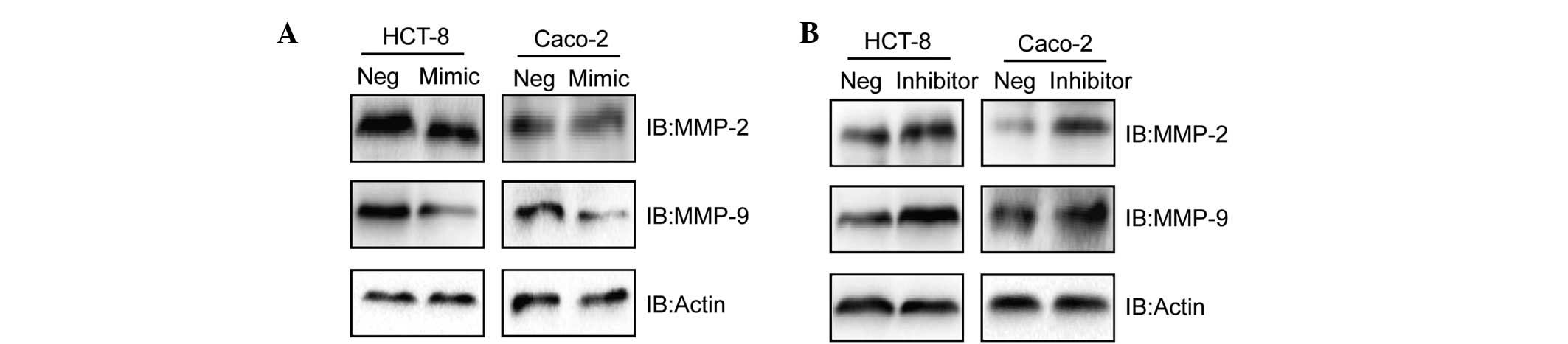
Additionally, to investigate the mechanism
underlying how miR-7 influences the biological behavior of CC cell
lines, the levels of the invasion factors, MMP-2 and MMP-9, were
assessed by western blotting following the up/down-regulation of
miR-7. Notably, it was revealed that the protein expression levels
of MMP-2 and MMP-9 were significantly decreased/increased when
HCT-8 and Caco-2 cells were transfected with miR-7m/i,
respectively, as determined by western blotting (Fig. 4A and B).
Discussion
The incidence of CC revealed an increasing trend
with recurrent cases causing liver metastasis (24). Previous studies suggested that
metastasis is the predominant reason for the high
mortality-associated with CC. In the human gene bank, >1,000
types of miRNAs are reported. Accumulating evidence suggested that
miRNAs are important in CC tumorigenesis (25). At present, the correlation of CC
with miRNAs remains to be elucidated. Balaguer et al
(26) revealed that miR-137 acted
as a tumor suppressor, however, was frequently silenced by promoter
hypermethylation in colorectal carcinoma (CRC). Kulda et al
(27) observed a higher expression
of miR-21 and lower expression of miR-143 in CRC compared with
adjacent normal colon tissue samples, which was associated with
liver metastasis of CRC. Yamakuchi et al (28) suggested that miR-107 mediates the
regulation of p53 on the hypoxic signaling and tumor angiogenesis
pathways.
miR-7 is a member of the miRNA family, and is
located on chromosome 15. The expression of miR-7 differs in
various tumor types (29). Xiong
et al (17) revealed that
increased expression of miR-7 inhibits cell growth and invasion in
the human A529 non-small cell lung cancer cell line. Notably,
similar findings were observed in malignant glioma and schwannoma
(30,31). FAK is a crucial signaling component
and functions as a biosensor or integrator to control cell
motility. Previous studies revealed that FAK, particularly
phosphorylated (p)-FAK (Y397), was highly expressed in multiple
tumor types, and was closely associated with the invasion and
metastasis of the tumor (32). How
FAK and its signaling pathway regulate the development of CC
remains to be elucidated. Currently, research on FAK inhibitors is
being performed to target its Y397 phosphorylation, for example,
p-FAKY397 antibodies or FAK-related non-kinase (33), however the treatment remains
limited. Whether there are more targets to control the invasion of
CC by inhibiting the expression of FAK remains to be
elucidated.
For the above issues, the present study aimed to
investigate the effect of miR-7 on the biological behavior of CC
and the correlation with FAK, which proved to be a promising target
for the treatment and prognosis of CC.
Firstly, the present study revealed that the
expression of miR-7 was decreased in CC tissues compared with the
paired adjacent normal tissues, by RT-qPCR, and this correlated
with lymph node metastasis and TNM stages in CC. Next, the
expression of miR-7 was investigated in vitro and revealed
that miR-7 inhibited the proliferation and invasiveness in CC cell
lines using MTT and wound healing assays, respectively. Finally, it
was revealed that the protein expression levels of FAK, MMP-2 and
MMP-9 were significantly reduced following transfection with the
miR-7m, however, increased following transfection with the miR-7i
in CC cell lines.
These results were consistent with the results of a
previous study revealing that miR-7 inhibited the growth and
metastasis of glioma by targeting a negative regulator of FAK
(14). miR-7 inhibited the
epithelial-mesenchymal transition to suppress the growth and
metastasis of breast cancer by targeting the protein expression of
FAK (34).
In conclusion, the present study revealed that miR-7
suppressed the growth and proliferation of CC cell lines by
targeting FAK. Notably, the present study provided novel insights
into the mechanisms underlying tumor growth and metastasis. miR-7
may be a potential therapeutic strategy for the treatment of CC in
the future.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of Jiangxi Province (grant no.
20142BAB205054) and the Post-graduates Innovation Foundation of
Jiangxi Province (grant no. YC2011-B007).
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cromheecke M, de Jong KP and Hoekstra HJ:
Current treatment for colorectal cancer metastatic to the liver.
Eur J Surg Oncol. 25:451–463. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warden C, Stupart D and Goldberg P:
Stenting as first-line management for all patients with
nonperforating left-sided obstructing colorectal cancer. Colorectal
Dis. 15:e389–e395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sein TT, Thant AA, Hiraiwa Y, Amin AR,
Sohara Y, Liu Y, Matsuda S, Yamamoto T and Hamaguchi M: A role for
FAK in the Concanavalin A-dependent secretion of matrix
metalloproteinase-2 and -9. Oncogene. 19:5539–5542. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cance WG, Harris JE, Iacocca MV, Roche E,
Yang X, Chang J, Simkins S and Xu L: Immunohistochemical analyses
of focal adhesion kinase expression in benign and malignant human
breast and colon tissues: Correlation with preinvasive and invasive
phenotypes. Clin Cancer Res. 6:2417–2423. 2000.PubMed/NCBI
|
|
10
|
Rovin JD, Frierson HF Jr, Ledinh W,
Parsons JT and Adams RB: Expression of focal adhesion kinase in
normal and pathologic human prostate tissues. Prostate. 53:124–132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun
LH, Wang XF, Zhang JX, Cao L, Wang XR, et al: MicroRNA-7 regulates
glioblastoma cell invasion via targeting focal adhesion kinase
expression. Chin Med J (Engl). 124:2616–2621. 2011.
|
|
15
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reddy SD, Ohshiro K, Rayala SK and Kumar
R: MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase
1 and regulates its functions. Cancer Res. 68:8195–8200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, Dou W, He L, Liang S, Tie J, Liu
C, Li T, Lu Y, Mo P, Shi Y, et al: MicroRNA-7 functions as an
anti-metastatic microRNA in gastric cancer by targeting
insulin-like growth factor-1 receptor. Oncogene. 32:1363–1372.
2013. View Article : Google Scholar
|
|
19
|
Zhang N, Li X, Wu CW, Dong Y, Cai M, Mok
MT, Wang H, Chen J, Ng SS, Chen M, et al: microRNA-7 is a novel
inhibitor of YY1 contributing to colorectal tumorigenesis.
Oncogene. 32:5078–5088. 2013. View Article : Google Scholar
|
|
20
|
Luo J, Cai Q, Wang W, Huang H, Zeng H, He
W, Deng W, Yu H, Chan E, Ng CF, et al: A microRNA-7 binding site
polymorphism in HOXB5 leads to differential gene expression in
bladder cancer. PLoS One. 7:e401272012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan KK, Dassanayake B, Deen R,
Wickramarachchi RE, Kumarage SK, Samita S and Deen KI: Young
patients with colorectal cancer have poor survival in the first
twenty months after operation and predictable survival in the
medium and long-term: Analysis of survival and prognostic markers.
World J Surg Oncol. 8:822010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bannazadeh Amirkhiz M, Rashtchizadeh N,
Nazemieh H, Abdolalizadeh J, Mohammadnejad L and Baradaran B:
Cytotoxic effects of alcoholic extract of dorema glabrum seed on
cancerous cells viability. Adv Pharm Bull. 3:403–408.
2013.PubMed/NCBI
|
|
24
|
Maruta M and Maeda K: Trends in the
treatment for liver metastasis of colorectal cancer in Japan. Rozhl
Chir. 90:669–673. 2011.
|
|
25
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balaguer F, Link A, Lozano JJ, Cuatrecasas
M, Nagasaka T, Boland CR and Goel A: Epigenetic silencing of
miR-137 is an early event in colorectal carcinogenesis. Cancer Res.
70:6609–6618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kulda V, Pesta M, Topolcan O, Liska V,
Treska V, Sutnar A, Rupert K, Ludvikova M, Babuska V, Holubec L Jr
and Cerny R: Relevance of miR-21 and miR-143 expression in tissue
samples of colorectal carcinoma and its liver metastases. Cancer
Genet Cytogenet. 200:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamakuchi M, Lotterman CD, Bao C, Hruban
RH, Karim B, Mendell JT, Huso D and Lowenstein CJ: P53-induced
microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad
Sci USA. 107:6334–6339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Foekens JA, Sieuwerts AM, Smid M, Look MP,
de Weerd V, Boersma AW, Klijn JG, Wiemer EA and Martens JW: Four
miRNAs associated with aggressiveness of lymph node-negative,
estrogen receptor-positive human breast cancer. Proc Natl Acad Sci
USA. 105:13021–13026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saydam O, Senol O, Wurdinger T, Mizrak A,
Ozdener GB, Stemmer-Rachamimov AO, Yi M, Stephens RM, Krichevsky
AM, Saydam N, et al: miRNA-7 attenuation in Schwannoma tumors
stimulates growth by upregulating three oncogenic signaling
pathways. Cancer Res. 71:852–861. 2011. View Article : Google Scholar :
|
|
31
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S and
Purow B: microRNA-7 inhibits the epidermal growth factor receptor
and the Akt pathway and is down-regulated in glioblastoma. Cancer
Res. 68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu TJ, LaFortune T, Honda T, Ohmori O,
Hatakeyama S, Meyer T, Jackson D, de Groot J and Yung WK:
Inhibition of both focal adhesion kinase and insulin-like growth
factor-I receptor kinase suppresses glioma proliferation in vitro
and in vivo. Mol Cancer Ther. 6:1357–1367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lacoste J, Aprikian AG and Chevalier S:
Focal adhesion kinase is required for bombesin-induced prostate
cancer cell motility. Mol Cell Endocrinol. 235:51–61. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kong X, Li G, Yuan Y, He Y, Wu X, Zhang W,
Wu Z, Chen T, Wu W, Lobie PE and Zhu T: MicroRNA-7 inhibits
epithelial-to-mesenchymal transition and metastasis of breast
cancer cells via targeting FAK expression. PLoS One. 7:e415232012.
View Article : Google Scholar : PubMed/NCBI
|