Introduction
Gastric cancer is a common malignancy, and ranks
second in overall cancer-associated mortalities worldwide (1). Chemotherapy is an important
therapeutic strategy for advanced gastric cancer, with the majority
of chemotherapeutic agents functioning by inducing apoptosis of
tumor cells. However, with the prolongation of chemotherapeutic
treatment, certain cells become resistant to apoptosis, leading to
drug resistant tumor cells. Thus, novel anticancer therapeutic
agents are required and, in recent years, induction of mitotic
catastrophe has become a molecular target for developing cancer
treatments, which are effective against tumors that are resistant
to traditional chemotherapeutic agents (2).
Chelidonium majus, the greater celandine
(family, Papaveraceae), is well established in traditional Chinese
medicine. It was originally included in 'Herbal for Relief of
Famines' (3) and is currently
included in the 2015 edition of 'Chinese Pharmacopoeia' (volume 1)
(4). It typically affects the lung
and stomach. Furthermore, C. majus relieves spasms, pain,
coughs and asthma and is often administered for gastric spasm pain,
pain associated with cancer, coughing, wheezing and pertussis
(5). The essential secondary
metabolites of C. majus are isoquinoline alkaloids, such as
chelidonine, chelerythrine, sanguinarine, allocryptopine, berberine
and coptisine (6). Chelidonine,
the tertiary hexahydro-benzophenanthridine alkaloid, is a major
component of C. majus. It had been reported to exhibit a
broad spectrum of pharmacological activities, including anticancer
(7–9), analgesic (5), anti-inflammatory (10), spasmolytic (11), hepatoprotective (12,13),
antioxidative (against cadmium chloride-induced oxidative stress),
and nephroprotective effects (14). The present study focuses primarily
on the antitumor effect. Chelidonine, as the major component of the
therapeutic agent, Ukrain, has been applied in the clinical
treatment of lung, breast, prostate and pancreatic cancer (15–18).
It has been reported that chelidonine may overcome multiple types
of drug resistance and enhance cytotoxicity of chemotherapeutic
agents, particularly against leukemia cells (19), indicating that it may have
potential as an anticancer agent. The antitumor mechanisms of
chelidonine differ among cell lines. It was reported that
chelidonine reduced telomerase activity via downregulation of
telomerase reverse transcriptase expression in HepG2 cells
(6), induced apoptosis via p38-p53
and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT
signaling pathways in Hela cells (7) and induced apoptosis via the
mitochondrial signaling pathway in malignant melanoma cells,
regardless of their p53 status (20). However, chelidonine did not affect
mitochondria intactness in human CEM T-leukemia cells (21). In a previous study, chelidonine
exhibited strong antiproliferative activity in SGC-7901 human
gastric cancer cells, which indicated the morphological
characteristics of mitotic catastrophe. Thus, it was hypothesized
that chelidonine may exert its antineoplastic effect via mitotic
catastrophe. The aim of the present study was to investigate the
effects and underlying mechanisms of chelidonine on mitotic
catastrophe and apoptotic-like death in SGC-7901 human gastric
cancer cells.
Materials and methods
Chemicals, therapeutic agents and assay
kits
The following reagents were used: Chelidonine
(purity, ≥98%; Shenzhen Medherb Biotechnology, Co., Ltd., Shenzhen,
China); vincristine (VCR) sulfate for injection (Zhejiang Hisun
Chemical Co., Ltd., Taizhou, China); RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA); pancreatin
(Gibco; Thermo Fisher Scientific, Inc.); Hyclone fetal bovine serum
(FBS; GE Healthcare Life Sciences, Logan, UT, USA); dimethyl
sulfoxide (DMSO; Tianjin Bodi Chemical Co., Ltd., Tianjin, China);
methyl thiazolyl tetrazolium (MTT; Sigma-Aldrich, St. Louis, MO,
USA); propidium iodide (PI; Sigma-Aldrich); Triton X-100 (Shanghai
Huashun Bioengineering, Co., Ltd, Shanghai, China); and bovine
serum albumin (BSA; Sigma-Aldrich).
Equipment
The following equipment was used: Super-clean bench
(DL-CJ-1N; Beijing Donglian Har Instrument Manufacture Co., Ltd.,
Beijing, China), a CO2 incubator (CO-150; New Brunswick
Scientific; Eppendorf, Inc., Hamburg, Germany); a microplate reader
(Model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA); an
inverted microscope (CKX-41; Olympus Corporation, Tokyo, Japan);
flow cytometer (COULTER® EPICS®-XL, Beckman
Coulter, Inc., Brea, CA, USA); a transmission electron microscope
(TEM; Hitachi-7650; Hitachi, Ltd., Tokyo, Japan);
Mini-PROTEAN® 3 gel electrophoresis system (Bio-Rad
Laboratories, Inc.); and a laser scanning confocal microscope (SP2;
Leica Microsystems, Ltd., Wetzlar, Germany).
Cell lines and cell culture
SCG-7901 human gastric cancer cells, MCF-7 human
breast adenocarcinoma cells and HepG2 human hepatoma cells were
obtained from Obio Technology, (Shanghai) Co., Ltd. (Shanghai,
China). The cells were cultured in RPMI-1640 medium containing 10%
FBS, penicillin (100 U/ml)-streptomycin (100 µg/ml) obtained
from Beyotime Institute of Biotechnology (Haimen, China). Cells
were incubated in a humidified atmosphere with 5% CO2 at
37°C. Culture transfer was performed once every 2–3 days.
MTT assay
SGC-7901 cells were digested with 0.25% pancreatin
and seeded in a 96-well plate at a density of 1×104
cells/well. Following incubation for 24 h, the therapeutic agents,
chelidonine (100 µl) or VCR (100 µl), were added to
each well at various concentrations. The final concentrations were:
0, 5, 10, 20, 40, 80 and 160 µmol/l chelidonine; and 0, 0.1,
1, 10 and 100 µmol/l VCR. Following 48 h of incubation at
37°C, the culture medium and therapeutic agents were discarded, and
100 µl MTT (0.5 mg/ml) solution was added to each well.
After 4 h incubation at 37°C, the supernatant was removed and 100
µl DMSO was added. The absorbance of the solution was
measured at a wavelength of 570 nm using the microplate reader. The
optical density was used to calculate the inhibition rate and half
maximal inhibitory concentration (IC50).
Observation of the ultrastructure changes
of SGC-7901 cells using a TEM
Following treatment with 10 µmol/l
chelidonine at different time-points, SGC-7901, MCF-7 and HepG2
cells were fixed with 2% glutaraldehyde (Dow Chemical Co., Midland,
MI, USA) overnight, and post-fixed using 1% osmic acid (Ted Pella,
Inc., Redding, CA, USA). The samples were dehydrated in
analytically pure graded alcohol (Tianjin Dongliqu Tianda Chemical
Reagent Factory, Tianjin, China), embedded in Epon 812 resin (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) and sectioned by an
ultramicrotome (RM2126; Leica Microsystems, Ltd.). Ultra-thin
sections (70 nm) were counterstained with uranyl acetate (Hubei
Chushengwei Chemical Co., Ltd., Wuhan, China) and lead citrate
(Yingkou Tanyun Chemical Research Institute Corporation, Yingkou,
China) and observed using the TEM.
Detection of G2/M phase arrest
and apoptosis using flow cytometry (FCM)
SGC-7901 cells were treated with chelidonine (10
µmol/l) and VCR (3 µmol/l) for 24, 48 and 72 h,
collected and fixed with 70% ethanol (Shandong Lerkan Medical
Technology Co., Ltd., Dezhou, China) at 4°C overnight. Cells were
collected, washed three times with phosphate-buffered saline (PBS;
Beyotime Institute of Biotechnology) and stained with 800 µl
PI for 30 min at room temperature in the dark. The samples were
analyzed using FCM at an excitation wavelength of 488 nm.
Detection of histone H3
(Ser10) phosphorylation using LSCM
Cover slips were placed in a 6-well plate, and
SGC-7901 cells were seeded in each well (~3×104
cells/well) and allowed to attach overnight. The cells were treated
with 2.5, 5 or 10 µmol/l chelidonine for 24 h, fixed with 4%
paraformaldehyde for 30 min and were processed by 0.5% Triton X-100
for 15 min. Following washing three times with PBS, the cells were
maintained in BSA for 1 h and incubated overnight with mouse
anti-human pH3 (Ser10) polyclonal antibody (1:500; cat.
no. AH453; Beyotime Institute of Biotechnology) at 4°C.
Subsequently, the samples were incubated with 1:200 fluorescein
isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G
(heavy and light chains) [IgG(H+L); cat. no. A0568; Beyotime
Institute of Biotechnology] for 1 h at 37°C in the dark. The
samples were washed twice with PBS, mounted and observed using
LSCM.
Detection of microtubule morphology
following immunofluorescent labeling by LSCM
Cover slips were placed in a 6-well plate and
SGC-7901 cells were seeded in each well (~3×104
cells/well) and allowed to attach overnight at 37°C. Following
treatment with 2.5, 5 or 10 µmol/l chelidonine or 3
µmol/l VCR for 24 h, the cover slips with cells were washed
twice with PBS, fixed with 4% paraformaldehyde for 30 min and
treated with 0.5% Triton X-100 for 15 min. The cover slips were
blocked with BSA for 1 h, washed twice with PBS and covered on a
clean glass slide. The mouse anti-human tubulin monoclonal
antibodies (1:500; cat. no. AT819; Beyotime Institute of
Biotechnology) were added to the samples and incubated overnight at
4°C. The samples were incubated with FITC-conjugated goat
anti-mouse IgG(H+L), diluted 1:200, for 1 h at 37°C in the dark.
The samples were washed twice with PBS, mounted, and observed using
LSCM.
Detection of BUB1 mitotic checkpoint
serine/threonine kinase B (BubR1), cyclin-dependent kinase 1
(Cdk1), cyclin B1 and caspase-3 protein expression levels using
western blotting
Following treatment with 10 µmol/l
chelidonine for 0, 24, 48 and 72 h, the SGC-7901 cells were
collected and lysed with lysis buffer (Beyotime Institute of
Biotechnology) in an ice bath for 1.5 h. Subsequent to centrifuging
the lysates at 13,000 × g for 15 min at 4°C, the protein content of
the supernatant was quantified by Coomassie Brilliant Blue assay
(Beyotime Institute of Biotechnology). The cell lysates were
separated by 12% SDS-PAGE (Tianjin Kemiou Chemical Reagent Co.,
Ltd., Tianjin, China) for ~2 h at 80 V and blotted onto a
nitrocellulose membrane (Beyotime Institute of Biotechnology).
Membranes were incubated in a blocking buffer [5% non-fat dry milk
in a mixture of Tris-buffered saline (prepared from 2.5 g NaCl
obtained from Tianjin Dongliqu Tianda Chemical Reagent Factory and
2.5 ml Tris-HCl at 1 mol/l and pH 7.5 obtained from Beyotime
Institute of Biotechnology, dissolved in double-distilled water to
250 ml) and Tween-20 (Beyotime Institute of Biotechnology; TBST)]
for 2 h at room temperature, and blots were incubated with
polyclonal antibodies, as follows: Rabbit anti-human BubR1
polyclonal antibody (1:250; cat. no. bs-5726R; Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), rabbit anti-human cyclin
B1 polyclonal antibody (1:200; cat. no. bs-5072R; Beijing
Biosynthesis Biotechnology Co., Ltd.), rabbit anti-human Cdk1
polyclonal antibody (1:200; cat. no. bs-0081R; Beijing Biosynthesis
Biotechnology Co., Ltd.)or rabbit anti-human caspase-3 polyclonal
antibody (1:200; cat. no. bs-0081R, Beijing Biosynthesis
Biotechnology Co., Ltd, Beijing, China) at 4°C overnight. The
membrane was rinsed with TBST and incubated with goat anti-rabbit
IgG(H+L) labeled with alkaline phosphatase (1:5,000; cat. no.
bs-0295G; Beijing Biosynthesis Biotechnology Co., Ltd.) at room
temperature for 2 h. The membrane was rinsed three times with TBST,
and incubated with 3,3′-diaminobenzidine tetrahydrochloride (DAB;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) for ~10 min in the dark at room temperature. Finally, the
membranes were observed using a gel imaging system (GIS-219; Tanon
Science and Technology Co., Ltd., Shanghai, China).
Statistical analysis
The data obtained from the different groups were
expressed as the mean ± standard deviation, and statistical
analysis was performed using one-way analysis of variance with SPSS
for Windows, version 15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
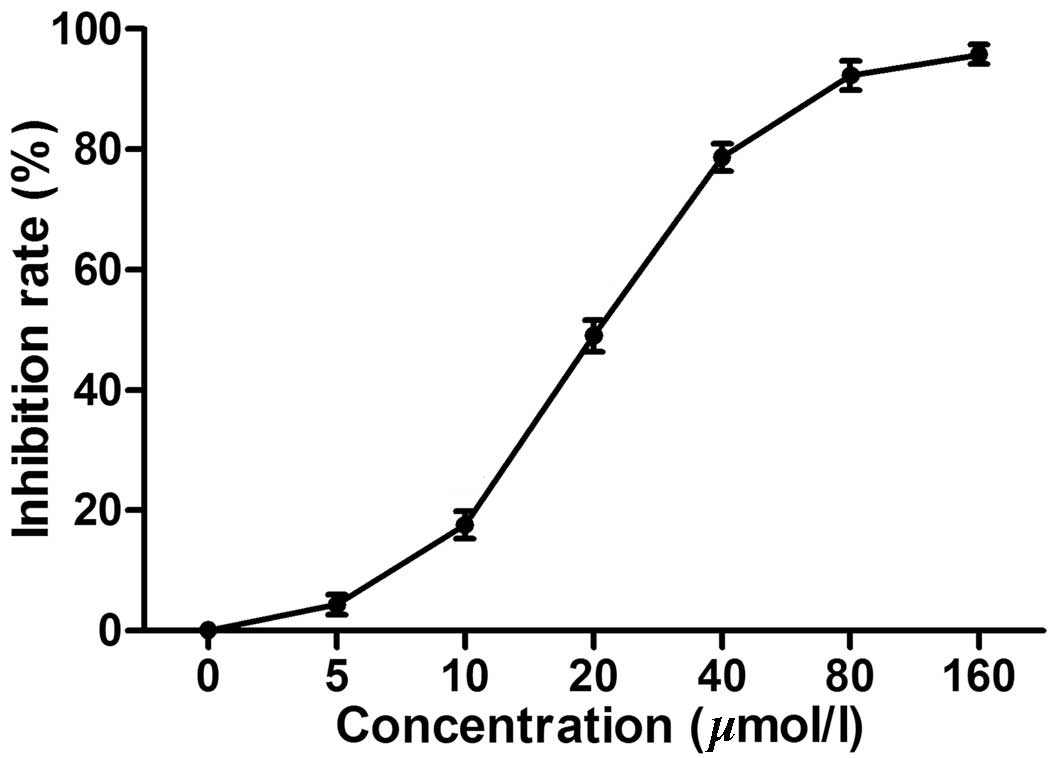
Chelidonine inhibits the proliferation of
SGC-7901 cell lines
The MTT assay demonstrated that chelidonine markedly
inhibited the proliferation of SGC-7901 cells in a dose-dependent
manner. The IC50 was 23.13 µmol/l (Fig. 1) while the IC50 of the
positive control, VCR was 5.28 µmol/l (data not shown).
Chelidonine treatment results in
multinucleated and apoptotic morphology of SGC-7901 cells
To determine the occurrence of mitotic catastrophe,
a TEM was used to observe the multinucleated cells induced by
chelidonine (Fig. 2). In the
control group, normal morphological characteristics of tumor cells
were observed, such as clear cellularity, integrated structure of
the organelles, and uniform distribution of chromatin (Fig. 2A–C). Following treatment with 10
µmol/l chelidonine for 24 h, nuclear envelopes formed around
chromatin at random, resulting in the presence of multinucleated
cells with multiple micronuclei of various sizes (Fig. 2D–F). Following treatment with
chelidonine for 48 h, the number of multinucleated cells increased
gradually. The multinuclear cells exhibited the apoptotic
morphology of reduced microvilli (Fig.
2G). Following treatment with chelidonine for 72 h, the
multinuclear cells exhibited chromatin condensation, nuclear
fragmentation and formation of the apoptotic body (Fig. 2H).
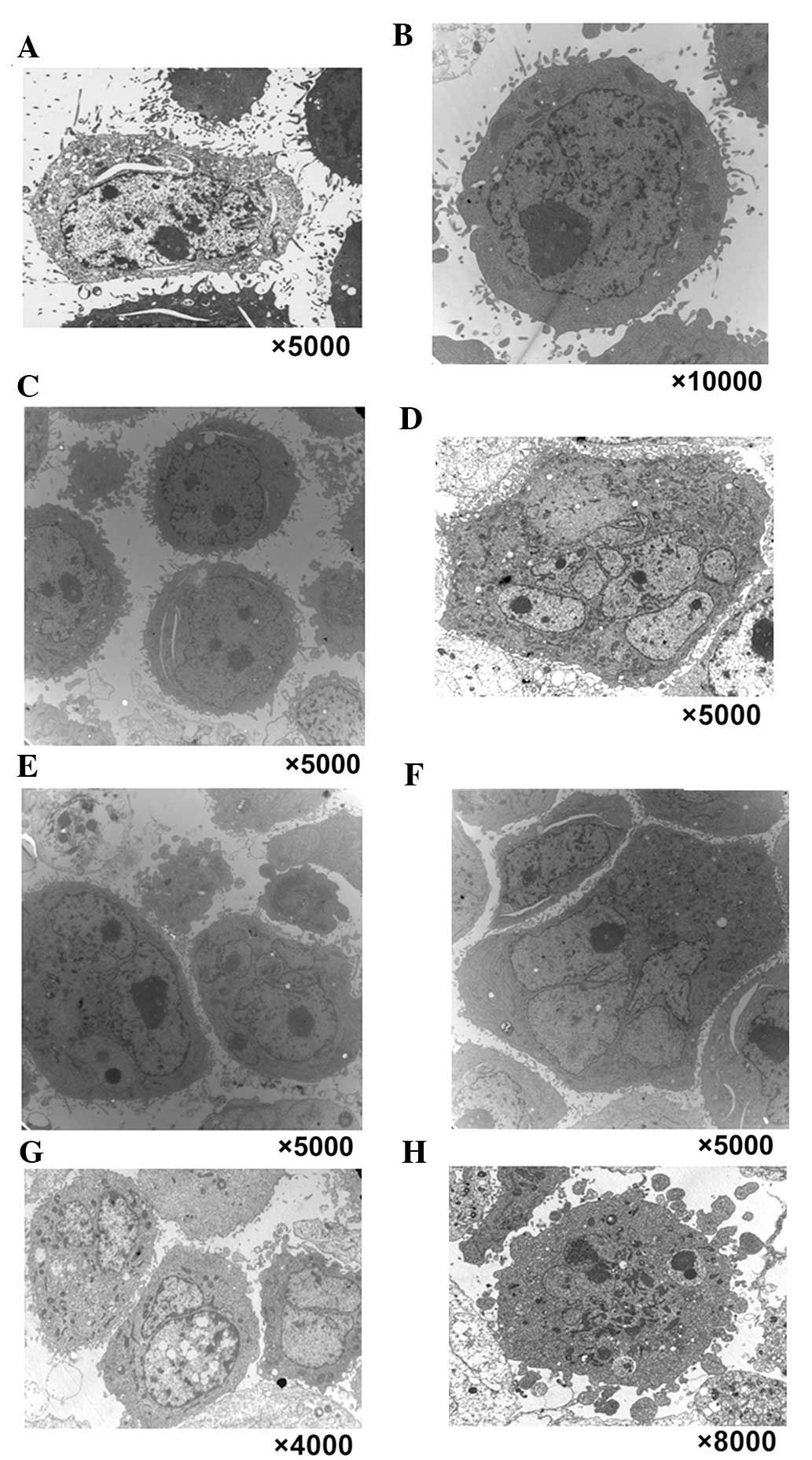 | Figure 2Typical characteristics of mitotic
catastrophe and apoptotic morphological changes were observed in
three types of tumor cell by transmission electron microscopy. In
the control group, normal morphological characteristics of
SGC-7901, MCF-7 and HepG2 tumor cells were observed, such as clear
cellularity, integrated structure of the organelles and uniform
distribution of chromatin, as demonstrated in (A) SGC-7901, (B)
MCF-7 and (C) HepG2 cells. (D–F) Following treatment with 10
µmol/l chelidonine for 24 h, nuclear envelopes randomly
formed around chromatin, resulting in multinucleated SGC-7901,
MCF-7 and HepG2 cells with multiple micronuclei of varying size.
Following treatment with chelidonine for 48 h, the number of
multinucleated cells increased gradually. (G) Following treatment
with 10 µmol/l chelidonine for 48 h, the number of
multinucleated SGC-7901 cells increased gradually. The multinuclear
cells demonstrated the apoptotic morphology of reduced microvilli.
(H) Following treatment with 10 µmol/l chelidonine for 72 h,
the multinuclear SGC-7901 cells exhibited chromatin condensation,
nuclear fragmentation and the formation of apoptotic bodies. |
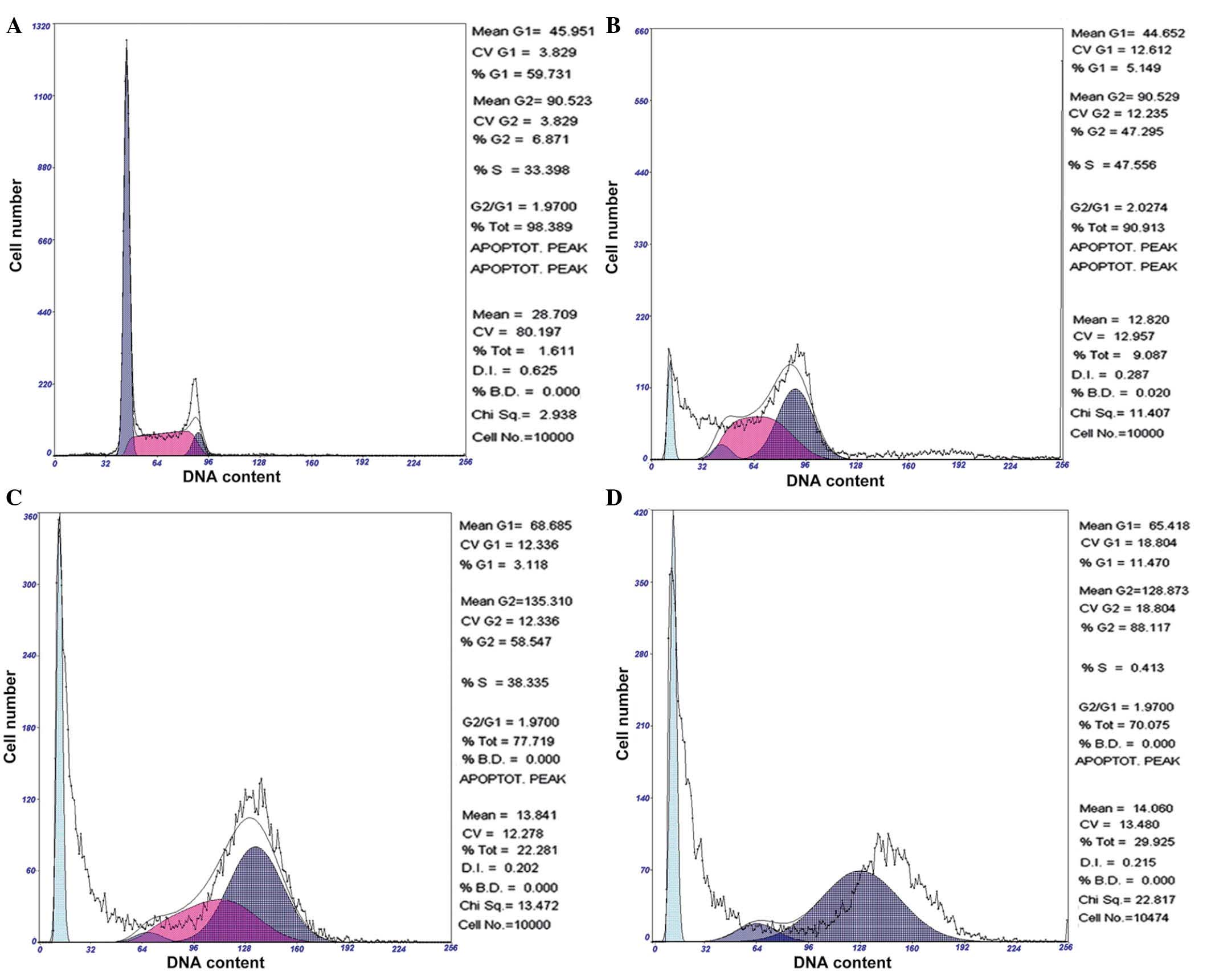
Chelidonine induces G2/M phase
arrest of SGC-7901 cells
Following 24, 48 and 72 h of exposure to 10
µmol/l chelidonine, SGC-7901 cells demonstrated significant
accumulation in the G2/M phase, which was accompanied by
a decrease of cells in the G0/G1 and S
phases. Following treatment with 10 µmol/l chelidonine for
24 h, the G2/M phase cell ratio was 47.30%, which
reached 58.55 and 88.12% at 48 and 72 h, respectively. The
apoptosis rates were 9.09, 22.28 and 29.93% following treatment
with chelidonine for 24, 48 and 72 h, respectively (Fig. 3). The result demonstrated that
chelidonine induces G2/M phase arrest and apoptosis of
SGC-7901 cells.
Chelidonine increases histone H3
(Ser10) phosphorylation in SGC-7901 cells
With the increase in chelidonine concentration, the
phosphorylation of histone H3 (Ser10) was significantly
increased following 24 h treatment (Table I). Similar to the positive control,
VCR, chelidonine induced the arrest of SGC-7901 cells in the M
phase.
 | Table IEffects of chelidonine on the
phosphorylated-histone H3 (Ser10) expression in SGC-7901
cells (n=30) following a 24-h treatment. |
Table I
Effects of chelidonine on the
phosphorylated-histone H3 (Ser10) expression in SGC-7901
cells (n=30) following a 24-h treatment.
| Group | Fluorescence
intensity |
|---|
| Control | 21.79±1.75 |
| Vincristine
(µmol/l) |
| 3 | 54.01±2.83a |
| Chelidonine
(µmol/l) |
| 2.5 | 32.15±2.00b |
| 5 | 45.72±1.52a |
| 10 | 59.66±3.04a |
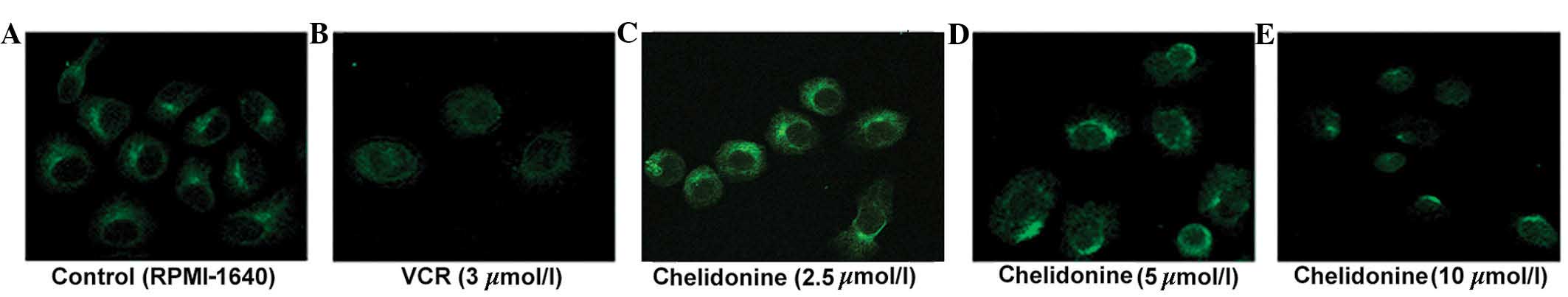
Chelidonine inhibits microtubule
polymerization in SGC-7901 cells
The control microtubules (labeled with green
fluorescence) were distributed evenly in the cytoplasm surrounding
the nucleus, and the single microtubules demonstrated a clear
outline and complete structure. The microtubules of the SGC-7901
cells treated with 5 µmol/l chelidonine for 24 h were
discontinuous and their number was reduced. The microtubules of
SGC-7901 cells treated with 10 µmol/l chelidonine were not
bundled, however, marginal fluorescence was observed with diffuse,
punctate and irregular distribution in the perinuclear region. In
the positive control group, the cells were round and the
fluorescence intensity was weak. The microtubule-targeting agent,
VCR inhibited microtubule polymerization (Fig. 4).
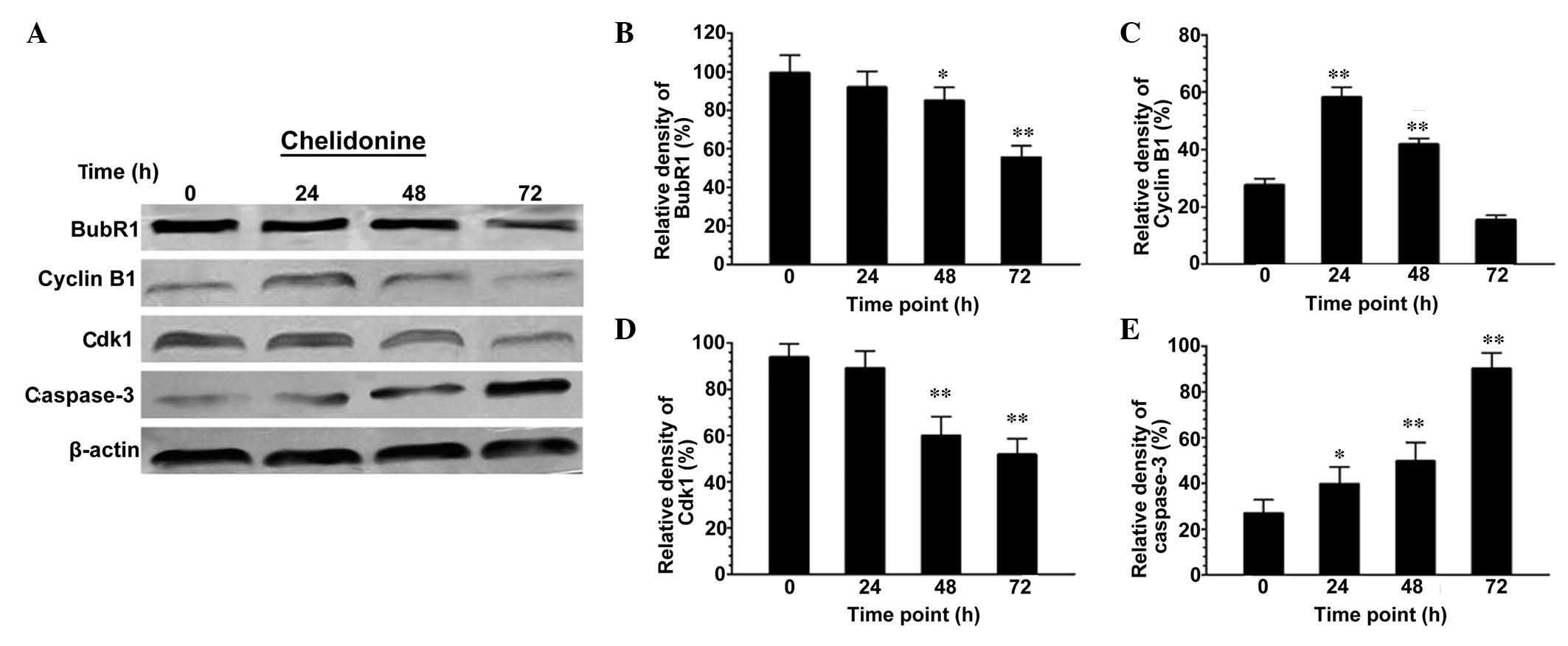
Chelidonine regulates the protein
expression levels of BubR1, Cdk1, cyclin B1 and caspase-3 in
SGC-7901 cells
Following treatment with 10 µmol/l
chelidonine for 48 and 72 h, the expression levels of BubR1 in
SGC-7901 cells decreased significantly (Fig. 5A and B). The expression level of
cyclin B1 increased significantly at 0–24 h (P<0.01), reached a
peak at 24 h, and then decreased in a time-dependent manner
(Fig. 5A and C). The expression
levels of Cdk1 in SGC-7901 cells were maintained at 0–24 h, but
then decreased significantly at 24–72 h (Fig. 5A and D; P<0.01). The expression
levels of caspase-3 increased in a time-dependent manner, and were
significantly higher than those of the control group (Fig. 5A and E; P<0.05 or
P<0.01).
Discussion
Mitotic catastrophe is a type of cell death
resulting from abnormal mitosis with the formation of large cells
that contain multiple nuclei in the mitotic phase (22). Mitotic catastrophe has been the
subject of increasing research over recent years (23). Previous studies have demonstrated
that ionizing radiation (24) and
certain antitumor therapeutic agents, such as doxorubicin (25), paclitaxel (26) and VCR (27), may result in mitotic catastrophe in
various solid tumors, which commonly contain nonfunctional p53
(28). Multiple factors determine
whether cells undergo mitotic catastrophe, including the genetic
background of the cell, the type of DNA damage, and the dosage and
treatment duration of certain therapeutic agents. Although
signaling pathways specific to mitotic catastrophe remain unclear,
it is becoming increasingly important to continue investigating the
mechanisms that result in the success of anticancer therapeutic
strategies.
It has been observed that chelidonine induced cell
apoptosis in Hela human cervical carcinoma, A375 malignant
melanoma, CEM human T-leukemia and OCM-1 human uveal melanoma cells
(7,20,21,29).
In addition, chelidonine induced G2/M phase arrest in
MT-4 human acute T-lymphoblastic leukemia cells (30) and WHCO5 human esophageal carcinoma
cells (31). As chelidonine
possesses mitotic toxicity, it remains to be elucidated whether
chelidonine induces mitotic catastrophe of tumor cells. In the
present study, SGC-7901 human gastric carcinoma cells were selected
to investigate the mitotic catastrophe-inducing effects and
mechanisms of chelidonine.
Results from the current study demonstrated that
chelidonine effectively inhibited the proliferation of SGC-7901
cells, and its IC50 was 23.13 µmol/l. The
predominant morphological characteristic of mitotic catastrophe is
the formation of large cells that contain multiple nuclei, which is
different from apoptosis (23,32,33).
The morphological changes were observed in SGC-7901 cells.
Following treatment with 10 µmol/l chelidonine for 24 h, a
number of giant cells with two or more nuclei were observed under a
TEM. The number of giant and multinucleated cells markedly
increased in a time- and dose-dependent manner, as observed under a
TEM and optical microscope. In addition, according to the clinical
application of C. majus, MCF-7 cells and HepG2 cells were
selected to investigate the mitotic catastrophe-inducing effects of
chelidonine. Following treatment with 10 µmol/l for 24 h,
the morphological characteristics of mitotic catastrophe were
observed under a TEM. FCM indicated that administration of 10
µmol/l chelidonine induced G2/M phase arrest of
SGC-7901 cells. However, the cells in the G2 and M phase
could not be effectively distinguished by FCM as the FCM histogram
of DNA content analysis was based on the DNA ploidy level in the
cell nucleus. Thus, in the present study it could not be determined
whether cheli-donine induced G2 or M phase arrest in
SGC-7901 cells. The expression of p-histone H3 (Ser10),
which is a characteristic of M phase, reached a peak at mitotic
metaphase and disappeared following the completion of mitosis.
Furthermore, histone H3 phosphorylation is important in the DNA of
the heteromorphic nuclear cells in the mitotic phase, which is an
indicator of mitotic catastrophe (34,35).
Indirect immunofluorescence assay and an LSCM were used to detect
the phosphorylation level of histone H3 (Ser10) in
SGC-7901 cells. Following treatment with chelidonine for 24 h, the
fluorescence intensity was enhanced with increasing chelidonine
concentration. The result demonstrated that chelidonine treatment
increased the phosphorylation of histone H3 (Ser10),
indicating that chelidonine arrested the SGC-7901 cells in the M
phase rather than in G2 phase. It was further
investigated whether chelidonine also inhibited microtubule
polymerization. An indirect immunofluorescence assay and LSCM were
used to observe the effect of chelidonine on microtubule morphology
in SGC-7901 cells. The results demonstrated that chelidonine
inhibits tubulin polymerization, destroys microtubule structures
and alters the cytoskeleton. As microtubules are important
components of the cell spindle, chelidonine may affect the
formation and function of the spindle; thus, inducing cell cycle
arrest at M phase and resulting in abnormal mitosis, which finally
leads to the formation of multipolar spindles and multinucleated
cells.
Cell cycle checkpoint mechanisms are strict quality
control systems that ensure the normal operation of the cell cycle.
The spindle assembly checkpoint (SAC) is key in monitoring the
proper alignment and separation of chromosomes, and ensures genome
integrity of daughter cells. For cells entering into mitosis,
whether the cells are able to exit the M phase, is controlled by
the SAC (36). Under normal
conditions, the anaphase-promoting complex (APC) is rapidly
activated in mitotic cells in metaphase, destroying the adhesion of
sister chromatids and inactivating the relevant Cdk complexes, thus
the cells complete mitosis and undergo cytokinesis (37). The inhibiting mechanism for APC
activity is the core function of the SAC. Any factors interfering
with the correct combination of sister chromatids and spindles may
directly or indirectly activate SAC signaling, and inhibit the
activation of APC resulting in cell cycle arrest in the junction
between the middle and late phase of mitosis (38). SAC is a signaling system composed
of mutually coordinated proteins, in which BubR1 is an important
receptor and implementer (39).
BubR1 ensures proper separation of chromosomes continuing the
process of cell mitosis via monitoring the status of the
microtubule gap junction and tension in the centromeres (40). BubR1 is a direct inhibitor of APC
(41). There is a dose-dependent
effect between the expression level of BubR1 and the function of
the SAC (42). The results in the
present study demonstrate that following treatment with chelidonine
for 72 h, the protein expression level of BubR1 was significantly
reduced (P<0.01). The low expression levels of BubR1 decreased
the inhibition of APC, which resulted in compromised effects at the
SAC (43,44). In the absence of a thorough repair
of damaged spindles, cells crossed the checkpoint and entered the
G1 phase of the next cell cycle, resulting in the
formation of polyploid pseudo-G1 cells. These cells with
a plurality of micronuclei remained in the G1 phase and
were unable to survive (45).
It has been demonstrated that there is a correlation
between cyclin B1-Cdk1 complexes [mitosis promoting factor (MPF)]
activity and cell mitotic slippage (46–48).
Therefore, the expression levels of cyclin B1 and Cdk1 proteins at
different time-points were detected by western blotting. The
results from the present study demonstrate that the protein
expression levels of cyclin B1 increased significantly at 0–24 h,
but then decreased in a time-dependent manner. The protein
expression levels of Cdk1 remained stable at 0–24 h, but decreased
significantly at 24–72 h. The progress of a cell from G2
to M phase is driven by the activation of the MPF. In early and
mid-mitosis, the continuous activation of MPF is required. When the
cells enter into anaphase, APC induces degradation of cyclin B1,
and MPF activity is markedly reduced to ensure the normal
completion of mitosis (49).
Following treatment with chelidonine, microtubule polymerization
was inhibited and the spindle function was affected in the SGC-7901
cells. The SAC was activated and SGC-7901 cells were arrested in
the M phase. With the prolongation of mitotic arrest, the protein
expression levels of BubR1 decreased, thus reducing the inhibition
of APC and increasing degradation of cyclin B1 by APC (50). SGC-7901 cells underwent mitotic
slippage and exited the mitotic phase, entering into a
pseudo-G1 phase and, finally, the multinucleated cells
underwent mitotic catastrophe.
Multinucleated cells cannot survive and, therefore,
undergo cell death. Cell death by mitotic catastrophe is a
complicated process and the cells may die due to apoptosis,
senescence, necrosis or another death signaling pathway (22). It is hypothesized that apoptosis is
one of the consequences of mitotic catastrophe (51). The results of the present study
indicate that following treatment with chelidonine, the number of
multinucleated cells increased gradually and demonstrated apoptotic
morphology, including chromatin condensation, nuclear fragmentation
and the formation of an apoptotic body. FCM, following staining of
cells with PI, demonstrated that the apoptotic rate of SGC-7901
cells increased gradually and in a time-dependent manner with
chelidonine treatment reaching 29.93% at 72 h. To elucidate the
underlying mechanisms of apoptosis upon treatment with chelidonine,
the protein expression level of caspase-3 was detected. The results
demonstrate that the protein expression of caspase-3 also increased
gradually and in a time-dependent manner with chelidonine
treatment. The results indicate that apoptosis, induced by
chelidonine in SGC-7901 cells undergoing mitotic catastrophe, may
involve a caspase-3-dependent signaling pathway.
In conclusion, chelidonine induced M phase arrest
and slippage of SGC-7901 human gastric carcinoma cells, which then
underwent mitotic catastrophe. A number of the multinucleated cells
underwent apoptosis-like cell death. Resistance of tumor cells to
apoptosis is one of the leading causes of cancer treatment failure,
chelidonine was demonstrated to induce mitotic catastrophe in tumor
cells and, thus, avoid the resistance to apoptosis. Due to its
unique mechanism and novel target, chelidonine is hypothesized to
be a promising therapeutic agent in the treatment of various
cancers, including gastric cancer, hepatocellular carcinoma and
breast cancer. The further elucidation of the underlying
anti-cancer molecular mechanism of chelidonine provides novel
scientific evidence for the research and application of
chelidonine-associated anticancer therapeutic agents.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81102858); the China
Postdoctoral Science Foundation (grant no. 2013M531060); the
Research Fund for the Doctoral Program of Higher Education (grant
no. 20102332120003); the Key Project of Chinese Ministry of
Education (grant no. 210059); the Natural Science Foundation of
Heilongjiang Province (grant no. D200817); the Heilongjiang
Postdoctoral Foundation (grant no. LBH-Z10103); and the
Heilongjiang Provincial Key Teachers Project (grant no.
1154G35).
References
|
1
|
De Vita F, Di Martino N, Fabozzi A,
Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V,
Sforza V, Marano L, et al: Clinical management of advanced gastric
cancer: The role of new molecular drugs. World J Gastroenterol.
20:14537–14558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cives M, Ciavarella S, Rizzo FM, De Matteo
M, Dammacco F and Silvestris F: Bendamustine overcomes resistance
to melphalan in myeloma cell lines by inducing cell death through
mitotic catastrophe. Cell Signal. 25:1108–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao GP, Dai S, Chen RS, Wen HM, Yin L,
Shi LW, Shi XD, Liu WL, Liu XH, Li Y, et al: Dictionary Chinese
materia medica. Dictionary Chinese Materia Medica. Yu HA and Liu
SF: 1. 2nd edition. Shanghai Science and Technology Press;
Shanghai: pp. p10002006
|
|
4
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. Pharmacopoeia of
the People's Republic of China. Zhao YY: 1. 10th edition. Medicine
Science and Technology Press of China; Beijing: pp. p1092015
|
|
5
|
He ZM, Tong JM and Gong FC: Study on the
analgesic effect of Chelidonium majus L. Chin Tradit Herb Drugs.
34:837–838. 2003.
|
|
6
|
Colombo ML and Bosisio E: Pharmacological
activities of Chelidonium majus L. (Papaveraceae). Pharmacol Res.
33:127–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noureini SK and Wink M: Transcriptional
down regulation of hTERT and senescence induction in HepG2 cells by
chelidonine. World J Gastroenterol. 15:3603–3610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paul A, Bishayee K, Ghosh S, Mukherjee A,
Sikdar S, Chakraborty D, Boujedaini N and Khuda-Bukhsh AR:
Chelidonine isolated from ethanolic extract of Chelidonium majus
promotes apoptosis in HeLa cells through p38-p53 and PI3K/AKT
signalling pathways. J Chin Integ Med. 10:1025–1038. 2012.
View Article : Google Scholar
|
|
9
|
Paul A, Das S, Das J, Samadder A and
Khuda-Bukhsh AR: Cytotoxicity and apoptotic signalling cascade
induced by chelidonine-loaded PLGA nanoparticles in HepG2 cells in
vitro and bioavailability of nano-chelidonine in mice in vivo.
Toxicol Lett. 222:10–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JE, Cuong TD, Hung TM, Lee I, Na M,
Kim JC, Ryoo S, Lee JH, Choi JS, Woo MH and Min BS: Alkaloids from
Chelidonium majus and their inhibitory effects on LPS-induced NO
production in RAW264.7 cells. Bioorg Med Chem Lett. 21:6960–6963.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heinle H, Hagelauer D, Pascht U, Kelber O
and Weiser D: Intestinal spasmolytic effects of STW 5 (Iberogast)
and its components. Phytomedicine. 13(Suppl 5): 75–79. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iagodina OV, Niko'/skaia EB and Faddeeva
MD: Inhibition of liver mitochondrial monoamine oxidase activity by
alkaloids isolated from Chelidonium and Macleaya and by their
derivative drugs. Tsitologiia. 45:1032–1037. 2003.In Russian.
|
|
13
|
Paul A, Das J, Das S, Samadder A and
Khuda-Bukhsh AR: Poly (lactide-co-glycolide) nano-encapsulation of
chelidonine, an active bioingredient of greater celandine
(Chelidonium majus), enhances its ameliorative potential against
cadmium induced oxidative stress and hepatic injury in mice.
Environ Toxicol Pharmacol. 36:937–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koriem KM, Arbid MS and Asaad GF:
Chelidonium majus leaves methanol extract and its chelidonine
alkaloid ingredient reduce cadmium-induced nephrotoxicity in rats.
J Nat Med. 67:159–167. 2013. View Article : Google Scholar
|
|
15
|
Staniszewski A, Slesak B, Kołodziej J,
Harłozińska-Szmyrka A and Nowicky JW: Lymphocyte subsets in
patients with lung cancer treated with thiophosphoric acid alkaloid
derivatives from Chelidonium majus L. (Ukrain). Drugs Exp Clin Res.
18(Suppl): 63–67. 1992.PubMed/NCBI
|
|
16
|
Kadan P, Korsh OB and Melnyk A: Ukrain
therapy of recurrent breast cancer with lung metastases (case
report). Drugs Exp Clin Res. 22:243–245. 1996.PubMed/NCBI
|
|
17
|
Uglyanitsa KN, Nechiporenko NA, Nefyodov
LI, Doroshenko YM, Brzosko W and Nowicky W: Results of Ukrain
monotherapy of prostate cancer. Drugs Exp Clin Res. 26:191–193.
2000.
|
|
18
|
Zemskov V, Prokopchuk O, Susak Y, Zemskov
S, Tkachenko O, Hodysh Y and Nowicky W: Efficacy of ukrain in the
treatment of pancreatic cancer. Langenbecks Arch Surg. 387:84–89.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Readi MZ, Eid S, Ashour ML, Tahrani A
and Wink M: Modulation of multidrug resistance in cancer cells by
cheli-donine and Chelidonium majus alkaloids. Phytomedicine.
20:282–294. 2013. View Article : Google Scholar
|
|
20
|
Hammerová J, Uldrijan S, Táborská E and
Slaninová I: Benzo[c] phenanthridine alkaloids exhibit strong
anti-proliferative activity in malignant melanoma cells regardless
of their p53 status. J Dermatol Sci. 62:22–35. 2011.
|
|
21
|
Kaminskyy V, Kulachkovskyy O and Stoika R:
A decisive role of mitochondria in defining rate and intensity of
apoptosis induction by different alkaloids. Toxicol Lett.
177:168–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vitale I, Galluzzi L, Castedo M and
Kroemer G: Mitotic catastrophe: A mechanism for avoiding genomic
instability. Nat Rev Mol Cell Biol. 12:385–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caruso R, Fedele F, Lucianò R, Branca G,
Parisi C, Paparo D and Parisi A: Mitotic catastrophe in malignant
epithelial tumors: The pathologis'/s viewpoint. Ultrastruct Pathol.
35:66–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindgren T, Stigbrand T, Johansson L,
Riklund K and Eriksson D: Alterations in gene expression during
radiation-induced mitotic catastrophe in HeLa Hep2 cells.
Anticancer Res. 34:3875–3880. 2014.PubMed/NCBI
|
|
25
|
Grzanka D, Marszałek A, Izdebska M,
Gackowska L, Andrzej Szczepanski M and Grzanka A: Actin
cytoskeleton reorganization correlates with cofilin nuclear
expression and ultrastructural changes in cho aa8 cell line after
apoptosis and mitotic catastrophe induction by doxorubicin.
Ultrastruct Pathol. 35:130–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Wu E, Wu J, Wang TL, Hsieh HP and
Liu X: An antimitotic and antivascular agent BPR0L075 overcomes
multidrug resistance and induces mitotic catastrophe in
paclitaxel-resistant ovarian cancer cells. PLoS One. 8:e656862013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magalska A, Sliwinska M, Szczepanowska J,
Salvioli S, Franceschi C and Sikora E: Resistance to apoptosis of
HCW-2 cells can be overcome by curcumin- or vincristine-induced
mitotic catastrophe. Int J Cancer. 119:1811–1818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mansilla S, Bataller M and Portugal J:
Mitotic catastrophe as a consequence of chemotherapy. Anticancer
Agents Med Chem. 6:589–602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kemény-Beke A, Aradi J, Damjanovich J,
Beck Z, Facskó A, Berta A and Bodnár A: Apoptotic response of uveal
melanoma cells upon treatment with chelidonine, sanguinarine and
chelerythrine. Cancer Lett. 237:67–75. 2006. View Article : Google Scholar
|
|
30
|
Philchenkov A, Kaminskyy V, Zavelevich M
and Stoika R: Apoptogenic activity of two benzophenanthridine
alkaloids from Chelidonium majus L. does not correlate with their
DNA damaging effects. Toxicol In Vitro. 22:287–295. 2008.
View Article : Google Scholar
|
|
31
|
Panzer A, Joubert AM, Bianchi PC, Hamel E
and Seegers JC: The effects of chelidonine on tubulin
polymerisation, cell cycle progression and selected signal
transmission pathways. Eur J Cell Biol. 80:111–118. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji YB, Qu ZY and Zou X: Juglone-induced
apoptosis in human gastric cancer SGC-7901 cells via the
mitochondrial pathway. Exp Toxicol Pathol. 63:69–78. 2011.
View Article : Google Scholar
|
|
33
|
Kundu S, Kim TH, Yoon JH, Shin HS, Lee J,
Jung JH and Kim HS: Viriditoxin regulates apoptosis and autophagy
via mitotic catastrophe and microtubule formation in human prostate
cancer cells. Int J Oncol. 45:2331–2340. 2014.PubMed/NCBI
|
|
34
|
Roy RV, Suman S, Das TP, Luevano JE and
Damodaran C: Withaferin A, a steroidal lactone from Withania
somnifera, induces mitotic catastrophe and growth arrest in
prostate cancer cells. J Nat Prod. 76:1909–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de-Sá-Júnior PL, Pasqualoto KF, Ferreira
AK, Tavares MT, Damião MC, de Azevedo RA, Câmara DA, Pereira A, de
Souza DM and Parise Filho R: RPF101, a new capsaicin-like analogue,
disrupts the microtubule network accompanied by arrest in the G2/M
phase, inducing apoptosis and mitotic catastrophe in the MCF-7
breast cancer cells. Toxicol Appl Pharmacol. 266:385–398. 2013.
View Article : Google Scholar
|
|
36
|
Suematsu T, Li Y, Kojima H, Nakajima K,
Oshimura M and Inoue T: Deacetylation of the mitotic checkpoint
protein BubR1 at lysine 250 by SIRT2 and subsequent effects on
BubR1 degradation during the prometaphase/anaphase transition.
Biochem Biophys Res Commun. 453:588–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu H: Regulation of APC-Cdc20 by the
spindle checkpoint. Curr Opin Cell Biol. 14:706–714. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lane SI and Jones KT: Non-canonical
function of spindle assembly checkpoint proteins after APC
activation reduces aneuploidy in mouse oocytes. Nat Commun.
5:34442014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kapanidou M, Lee S and Bolanos-Garcia VM:
BubR1 kinase: Protection against aneuploidy and premature aging.
Trends Mol Med. 21:364–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bolanos-Garcia VM, Nilsson J and Blundell
TL: The architecture of the BubR1 tetratricopeptide tandem repeat
defines a protein motif underlying mitotic checkpoint-kinetochore
communication. Bioarchitecture. 2:23–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ibrahim B: Systems biology modeling of
five pathways for regulation and potent inhibition of the
anaphase-promoting complex (APC/C): Pivotal roles for MCC and
BubR1. OMICS. 19:294–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lentini L, Piscitello D, Veneziano L and
Di Leonardo A: Simultaneous reduction of MAD2 and BUBR1 expression
induces mitotic spindle alterations associated with p53 dependent
cell cycle arrest and death. Cell Biol Int. 38:933–941. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Patel D and McCance DJ: Compromised
spindle assembly checkpoint due to altered expression of Ubch10 and
Cdc20 in human papillomavirus type 16 E6- and E7-expressing
keratinocytes. J Virol. 84:10956–10964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lara-Gonzalez P, Scott MI, Diez M, Sen O
and Taylor SS: BubR1 blocks substrate recruitment to the APC/C in a
KEN-box-dependent manner. J Cell Sci. 124:4332–4345. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brito DA and Rieder CL: Mitotic checkpoint
slippage in humans occurs via cyclin B destruction in the presence
of an active checkpoint. Curr Biol. 16:1194–1200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Galán-Malo P, Vela L, Gonzalo O,
Calvo-Sanjuán R, Gracia-Fleta L, Naval J and Marzo I: Cell fate
after mitotic arrest in different tumor cells is determined by the
balance between slippage and apoptotic threshold. Toxicol Appl
Pharmacol. 258:384–393. 2012. View Article : Google Scholar
|
|
47
|
Qi M, Yao G, Fan S, Cheng W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces mitotic
catastrophe followed by apoptotic cell death in murine fibrosarcoma
L929 cells. Eur J Pharmacol. 683:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu WT, Chen C, Lu IC, Kuo SC, Lee KH,
Chen TL, Song TS, Lu YL, Gean PW and Hour MJ: MJ-66 induces
malignant glioma cells G2/M phase arrest and mitotic catastrophe
through regulation of cyclin B1/Cdk1 complex. Neuropharmacology.
86:219–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jin SQ and Zhan QM: Cell cycle checkpoint
and tumour. Molecular Oncology. Liu S: People's Medical Publishing
House; Beijing: pp. p3642005
|
|
50
|
Giovinazzi S, Bellapu D, Morozov VM and
Ishov AM: Targeting mitotic exit with hyperthermia or APC/C
inhibition to increase paclitaxel efficacy. Cell Cycle.
12:2598–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Castedo M, Perfettini JL, Roumier T,
Valent A, Raslova H, Yakushijin K, Horne D, Feunteun J, Lenoir G,
Medema R, et al: Mitotic catastrophe constitutes a special case of
apoptosis whose suppression entails aneuploidy. Oncogene.
23:4362–4370. 2004. View Article : Google Scholar : PubMed/NCBI
|