Introduction
Investigations in previous years have shown that the
predominant cause of acute renal failure is closely associated with
renal ischemia/reperfusion injury (IRI), as ischemia-reperfusion
can lead to renal vasoconstriction, tubular obstruction,
anti-leakage of glomerular filtrate and decreased glomerular
filtration rate, leading to impaired renal function, and conditions
of shock, heart failure and requirement for kidney transplant. This
is often accompanied by renal ischemia and reperfusion, which
affect treatment outcomes (1,2).
Therefore, reducing or avoiding IRI is one of the areas in renal
protection, which has received significant attention.
The generation of excessive reactive oxygen species
disrupts normal redox homeostasis of renal tissue, causing a state
of oxidative stress in renal tissues (1). Previous studies have demonstrated
that, in sustained diabetes, states of hyperglycemia and oxidative
stress in vivo, and non-enzymatic glycation (glycosylation)
reactions are evident, which are important in the pathogenesis of
diabetes and nephropathy (3–6). Jin
et al reported that C-type natriuretic peptide ameliorates
IRI-induced acute kidney injury through the inhibition of oxidative
stress (7).
The physiological concentration of nitric oxide (NO)
is involved in the functional regulation of several vital organisms
under normal circumstances, and the pathological induction of renal
IRI leads to a significant increase in NO synthesis (8). NO at high concentrations reacts
rapidly in the peroxide microenvironment at the site of injury to
produce peroxynitrite ion (ONOO-), directly or indirectly leading
to the damage of target cells and tissues (9). Inflammatory reactions can markedly
promote IRI (10). Certain reports
have suggested that IRI is a process of inflammation, although this
is debated, and reflects the importance of IRI in inflammatory
reactions (11). A cascade
network, comprising reactive oxygen species, a substantial number
of NO generated by inducible nitric oxide synthase (iNOS) and
inflammatory reactions is an important mechanism of IRI (12). A previous study demonstrated that
inhibiting the expression of lipopolysaccharide-induced iNOS
synthase and inflammation reduces the content of NO in rats with
acute myocardial ischemia (13). A
previous study demonstrated that the development of IRI is
characterized by the activation of the signal transducer and
activator of transcription (STAT) pathway, which is involved in
signaling associated with various cytokines and growth factors
(14).
Magnolia officinalis is widely used in
traditional Chinese and in Japanese Kampo medicines, which are used
clinically to treat bacterial infections, inflammation and
gastrointestinal diseases (15).
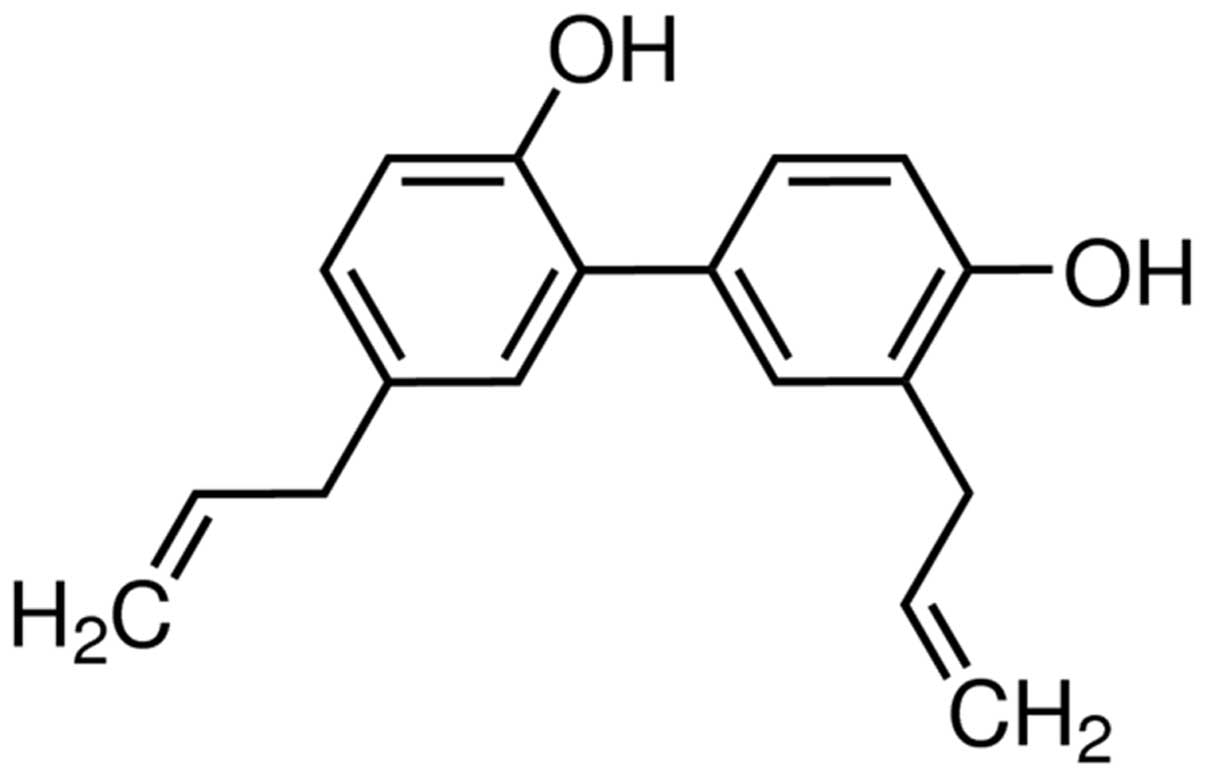
Since 1973, two of the predominant active compounds in Magnolia
officinalis have been isolated, magnolol and its isomer
honokiol (Fig. 1), and have been
investigated in several studies (16–19).
Previous studies have demonstrated that honokiol has
pharmacological functions, including central muscle relaxation,
central nervous system inhibition, anti-inflammatory,
antibacterial, anti-ulcer, anti-oxidative and anticancer
properties, and hormone regulation (20–23).
Therefore, the present study aimed to evaluate the protective
effects of honokiol against IRI and examine its possible
mechanism.
Materials and methods
Reagents and kits
Serum creatinine, blood urea nitrogen (BUN) and
nitrite commercial kits were purchased from BioAssay Systems
(Hayward, CA, USA). Malondialdehyde (MDA), superoxide dismutase
(SOD), catalase (CAT), myeloperoxidase (MPO), tumor necrosis
factor-α (TNF-α) and interleukin (IL)-6 commercial kits were
purchased from Jiancheng Bioengineering Institute (Nanjing, China).
TRIzol was purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). SYBR Green I dye was purchased from Qiagen
GmbH (Hilden, Germany). The BIOER Linegene-3320 system was
purchased from Hangzhou Bioer Technology, (Hangzhou, China). The
Bicinchoninic Acid (BCA) assay kit was purchased from Thermo Fisher
Scientific, Inc.
Animals
Male adult Wistar albino rats, weighing 250–300 g,
were provided by the Animal Experimental Center of the Navy General
Hospital of Chinese PLA and maintained at a room temperature of
23±2°C, with a 12 h light-dark cycle, and were allowed 1 week to
acclimatize to the conditions with free access to water and food.
The present study was approved by the Experimental Animal Research
Committee of the Navy General Hospital of Chinese PLA (Beijing,
China) and the ethics committee of the Navy General Hospital of
Chinese PLA (Beijing, China).
Induction of renal IRI and experimental
protocol
The Wistar rats were used to perform the renal
ischemia/reperfusion surgery, as described previously (24,25).
Briefly, the rats were anesthetized by intraperitoneal (i.p.)
injection of 10 mg/kg xylazine (Jiangsu Biological Technology, Co.,
Ltd. Jiangsu, China) and 100 mg/kg of ketamine hydrochloride
(Sigma-Aldrich, St. Louis, MO, USA). The site of surgery (abdomen)
was shaved and swabbed with betadine solution (Beyotime Institute
of Biotechnology, Jiangsu, China) and ethanol. Particular care was
taken to avoid damage to the organs throughout. A single medial
incision was made, the kidney vasculature was exposed and rats were
subjected to renal IRI injury by placing a clamp on the vessels for
45 min. In the sham operation group, surgery was performed, but the
kidneys were not clamped. After 45 min, the clamp was removed and
blood flow perfused into the kidneys. At this stage, the animals
exhibiting insufficient restoration of blood flow or with vessel
damage were excluded from the experiment.
The rats were randomly allocated into four groups:
(1) Sham group (n=10), in which
the normal rats received physiological saline (i.p.); (2) sham+honokiol group (Sham+Hon; n=10),
in which normal rats received 5 mg/kg, i.p. honokiol; (3) IRI group (IRI; n=10), in which the IRI
model rats received physiological saline (i.p.); (4) IRI+honokiol group (IRI+Hon; n=10), in
which the IRI model rats received 5 mg/kg, i.p. honokiol.
Assessment of renal function
Rats were sacrificed by decapitation and the blood
samples were collected from the rats at the room temperature. The
samples were centrifuged at 12,000 × g for 10 min, following which
the supernatants were collected. The levels of serum creatinine and
BUN in the samples were measured using commercially available kits,
according to the manufacturer's protocol (BioAssay Systems).
Nitrite levels were measured using a colorimetric assay kit,
according to the manufacturer's protocol (BioAssay Systems).
Assessment of hepatic function
Rats were anesthetized with-ketamine (75 mg/kg;
Sigma-Aldrich) and xylazine (5 mg/kg; Sigma-Aldrich) and blood
samples were collected from the eye socket at room temperature. The
samples were centrifuged at 12,000 g for 10 min, following which
the supernatants were collected. The levels of alkaline phosphatase
(ALP), aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) were measured using an autoanalyzer (Pars
Azmun, Karaj, Iran).
Assessment of oxidative stress
Rats were sacrificed by decapitation and the areas
of ischemia in the renal tissues were collected at room temperature
and homogenised using liquid nitrogen and lysed in
radioimmunoprecipitation assay bugger (Jiancheng Bioengineering
Institute). The samples were centrifuged at 12,000 × g for 10 min,
following which the supernatants were collected. The levels of MDA
and SOD and the activities of CAT and MPO in the renal ischemic
zone of the tissues were measured using colorimetric assay kits
(Jiancheng Bioengineering Institute), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of iNOS
The ischemic zone tissue samples were collected at
room temperature and homogenised using liquid nitrogen and lysed in
radioimmunoprecipitation assay bugger (Jiancheng Bioengineering
Institute). The samples were centrifuged at 12,000 g for 10 min,
following which the supernatants were collected. Total RNA (1
µg) was isolated from the supernatants using TRIzol,
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was obtained following the RT reaction
using SYBR Green I dye (Qiagen GmbH). qPCR amplifications were
performed using a BIOER Linegene-3320 system (Hangzhou Bioer
Technology). The sequences of the primers used were as follows:
iNOS, forward 5′-AGTGATGGCAAGCACGACTTC-3′ and reverse
5′-TCTGTCACTCGCTCACCACGG-3′; and β-actin, forward
5′-AAGGGACTTCCTGTAACAATGCA-3′ and reverse
5′-CTGGAACGGTGAAGGTGACA-3′. The cycling conditions were as follows:
5 min at 95°C, 40 cycles of 30 sec at 95°C, 45 sec at 60°C, and 30
sec at 72°C, followed by a cycle of 10 min at 72°C. The expression
levels was quantified by Ct value: Ct=−1/lg(1+Ex)*lgX0+lgN/lg(1+Ex)
(26,27).
Assessment of iNOS activity
The ischemic zone tissue samples were collected at
room temperature and homogenised using liquid nitrogen and lysed in
radioimmunoprecipitation assay bugger (Jiancheng Bioengineering
Institute). The samples were centrifuged at 12,000 g for 10 min and
the supernatants were collected. The renal ischemic zone tissues
were homogenized and nuclear proteins were quantified using a BCA
assay (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The supernatant was incubated with 0.6 ml
reaction buffer (containing 210 mM sucrose, 40 mM NaCl, 2 mM EGTA
and 30 mM HEPES; Beyotime Institute of Biotechnology) and 1 mmol/l
ethylene glycol tetraacetic acid (Amresco LLC, Solon, OH, USA) at
room temperature for 30 min. The activity of iNOS was then
determined at 530 nm using a TECH M200 microplate reader (Tecan
Group, Ltd., Männedorf, Switzerland).
Assessment of inflammatory cytokines
The ischemic zone tissue samples were collected at
room temperature and homogenised using liquid nitrogen and lysed in
radioimmuno-precipitation assay buffer (Jiancheng Bioengineering
Institute). The samples were centrifuged at 12,000 g for 10 min and
the supernatants were collected. The levels of TNF-α and IL-6 in
the were measured using a colorimetric assay kit, according to the
manufacturer's protocol (Jiancheng Bioengineering Institute).
Western blot analysis of the protein
expression of STAT3
The ischemic zone tissue samples were collected at
room temperature. The samples were centrifuged at 12,000 g for 10
min and the supernatants were collected. The renal ischemic zone
tissues were homogenized, and nuclear proteins were extracted using
a BCA assay (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The proteins (50 µg) were
electrophoresed on 12% SDS-polyacrylamide gels (Jiangsu Biological
Technology, Co., Ltd.) and transferred into nitrocellulose
membranes (Abcam, Cambridge, UK) at 4°C for 2 h. The membranes were
blocked with Tris-buffered saline-0.05% Tween 20 (TBST) containing
5% skim milk powder for 1 h at room temperature. Following
blocking, the membranes were incubated with rabbit polyclonal
anti-phosphorylated (p-)STAT3 (sc-135649; 1:1,500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit polyclonal anti-STAT3
(sc-7179; 1:1,000; Santa Cruz Biotechnology, Inc.) and rabbit
polyclonal anti-β-actin (sc-7210; 1:500; Santa Cruz Biotechnology,
Inc.) overnight at 4°C with agitation. Subsequently, the membranes
were washed with TBST for 1 h at room temperature and incubated
with goat anti-rabbit IgG-PerCP-Cy5.5 secondary antibody (sc-45101;
1:1,000; Santa Cruz Biotechnology, Inc.). Finally, the membranes
were visualized using an enhanced chemiluminescence system (Pierce
Biotechnology, Rockford, IL, USA). Bands were exposed in a
ChemiDoc-It TS2 imager Imager (UVP, LLC, Upland, CA, USA) and
analyzed using Image J version 2 software (National Institutes of
Health, Bethesda, MD, USA).
Assessment of caspase-3 activity
The ischemic zone tissue samples were collected at
room temperature. The samples were centrifuged at 12,000 g for 10
min and the supernatants were collected. The renal ischemic zone
tissues were homogenized and nuclear proteins were extracted using
a BCA assay (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Equal quantities of protein (30 µg)
and Ac-LEHD-pNA (Beyotime Institute of Biotechnology) were
incubated at 37°C for 2 h in the dark, following which the activity
of caspase-3 was measured using a SpectraMax M2 Microplate
Autoreader (Bio-Tek Instruments Inc., Winooski, VT, USA at an
absorbance of 405 nm.
Statistical analysis
All statistical data are presented as the mean ±
standard error of the mean, and statistical analyses were performed
using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical analysis was conducted with three or more groups using
one-way analysis of variance and Dunnett's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of honokiol on renal function
The levels of serum creatinine and BUN in the IRI
group were significantly increased, compared with those in the sham
group (Fig. 2A and B). The
elevation in the levels of serum creatinine and BUN in the IRI rats
were reduced significantly with honokiol pretreatment in the
IRI+Hon group, compared with the IRI group (Fig. 2A and B).
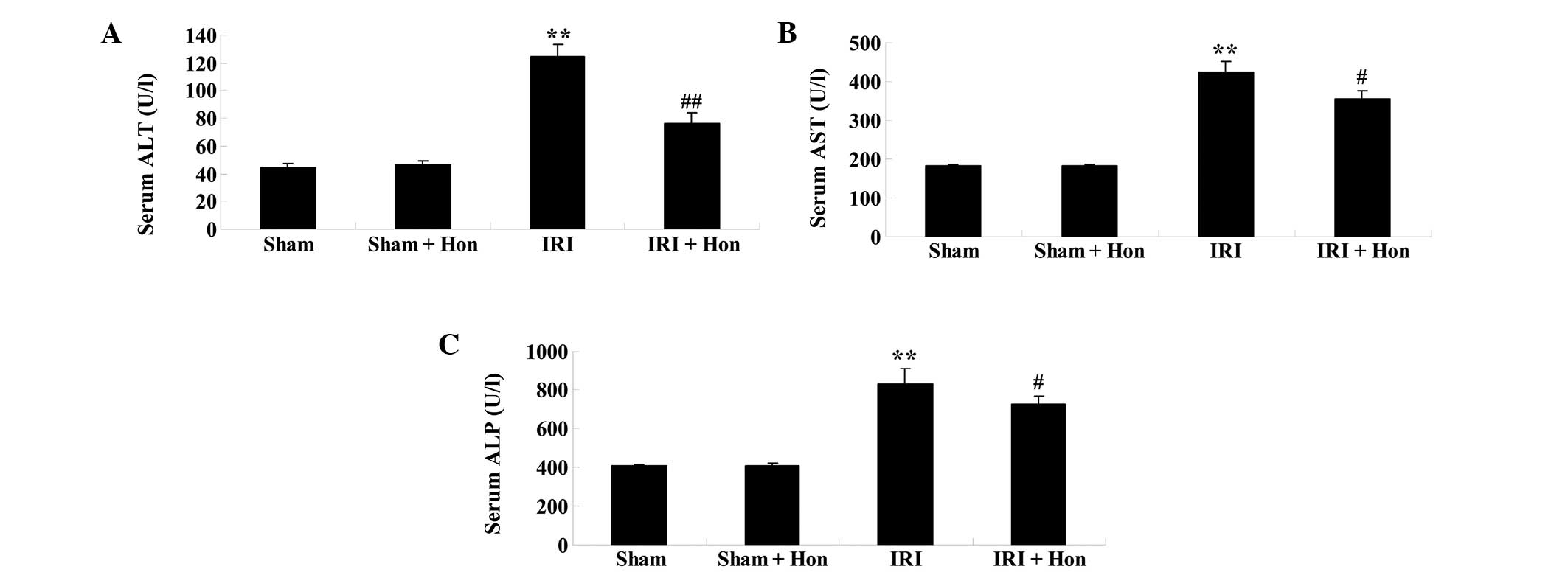
Effect of honokiol on hepatic
function
As shown in Fig.
3A–C, the serum levels of ALT, AST and ALP were elevated
following IRI, compared with the respective levels in the
sham-operated group. The administration of honokiol prior to IRI
was observed to significant reduce the serum levels of ALT, AST and
ALP, compared with the levels in the IRI group (Fig. 3A–C).
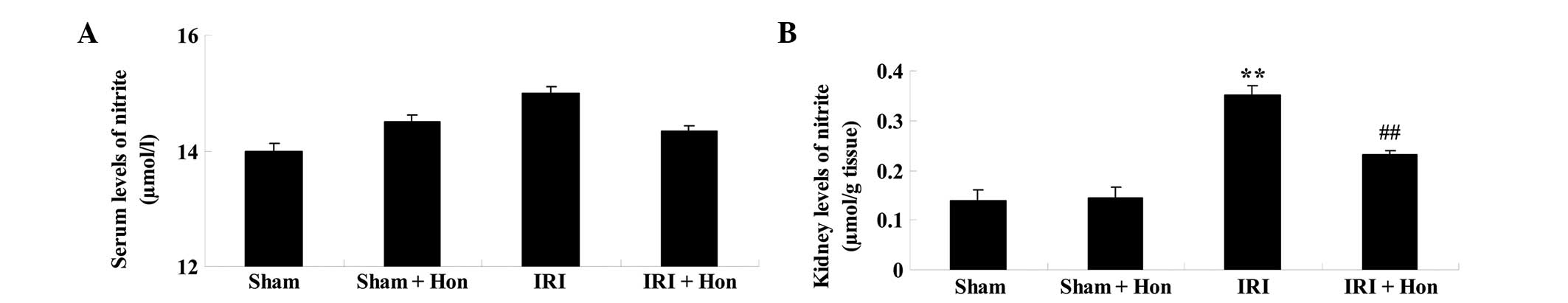
Honokiol decreases the levels of nitrite
in the kidney
As shown in Fig.
4A, the level of serum nitrite in the IRI group was higher,
compared with the levels in the Sham group, Sham+Hon group and
IRI+Hon group. However, the differences identified between the
groups were not statistically significant (Fig. 4A). The level of nitrite in the
kidney of the IRI group was increased significantly, compared with
that in the sham group (Fig. 4B).
Honokiol administration prior to IRI led to a decrease in kidney
nitrite levels, compared with the IRI group (Fig. 4B).
Honokiol reduces levels of oxidative
stress
IRI caused a significant increase in the serum level
of MDA and activity of MPO, compared with the sham group, and
honokiol treatment decreased the levels of MDA and activity of MPO,
compared with the IRI group (Fig.
5A). The activities of SOD and CAT decreased in the IRI group,
compared with the sham group (Fig. 5B
and C). However, the administration of honokiol significantly
increased the activities of SOD and CAT, compared with the IRI
group (Fig. 5B and C). Honokiol
treatment decreased the activity of MPO, compared with the IRI
group (Fig. 5D).
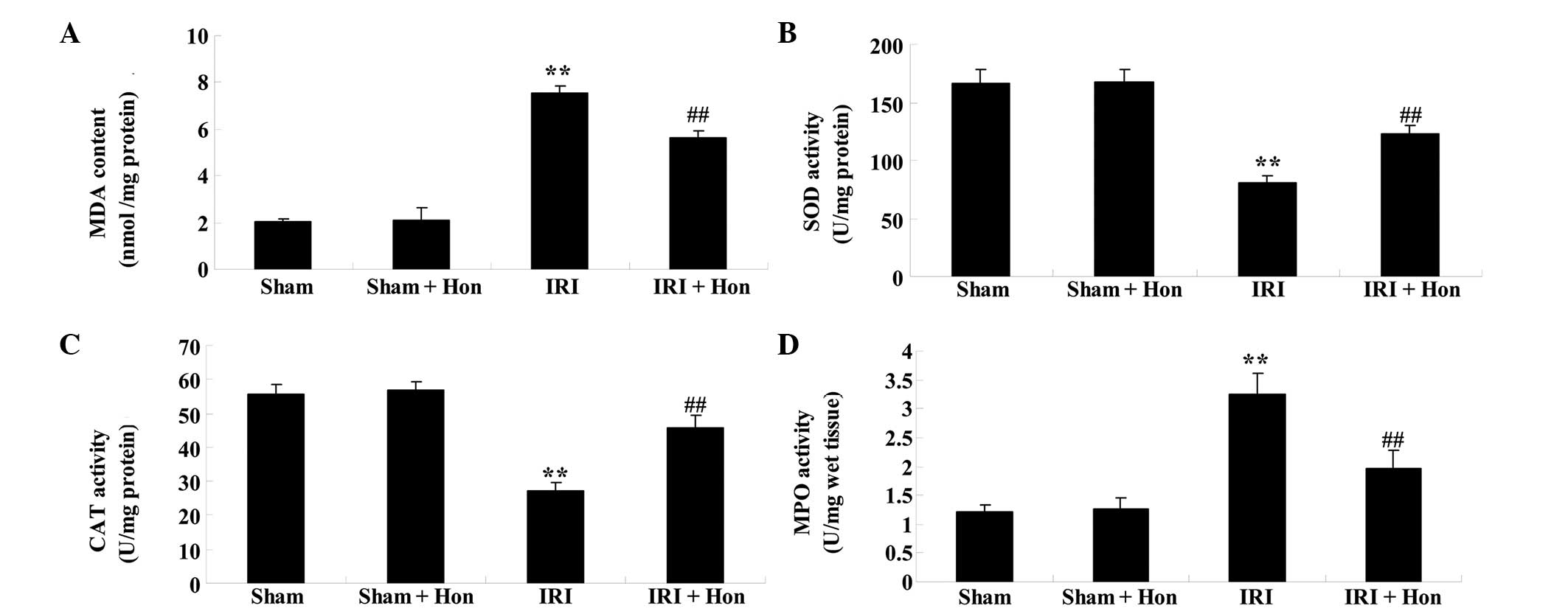 | Figure 5Effect of honokiol on oxidative
stress. Effect of honokiol on the (A) serum levels of MDA and
activities of (B) SOD, (C) CAT and (D) MPO. Data are presented as
the mean ± standard error of the mean. **P<0.01,
compared with the Sham group; ##P<0.01, compared with
the IRI group. Sham, sham surgery; Hon, honokiol treatment (5
mg/kg); IRI, ischemia/reperfusion-injury MDA, malondialdehyde; SOD,
superoxide dismutase; CAT, catalase; MPO, myeloperoxidase. |
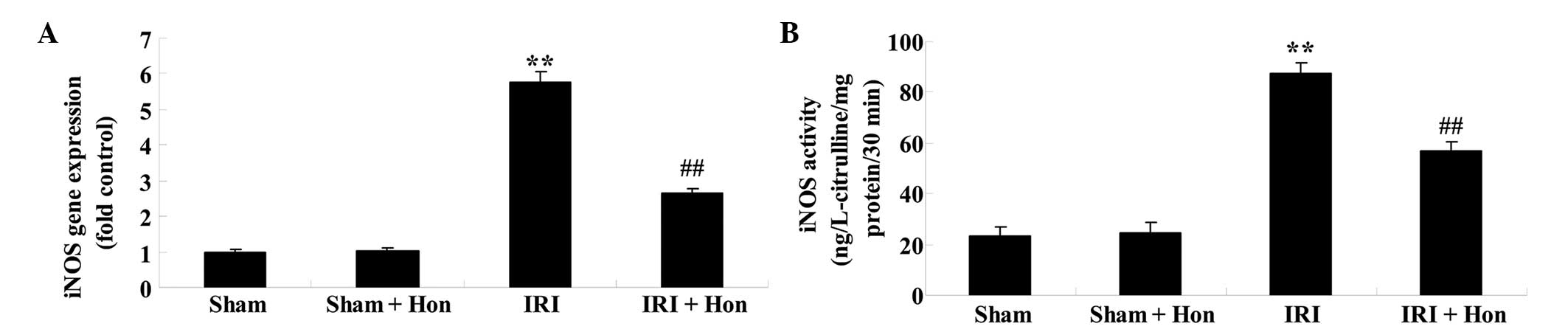
Honokiol reduces the expression and
activity of iNOS
The expression and activity levels of iNOS in the
IRI group were significantly augmented, compared with those in the
Sham group, exhibiting significant increases (Fig. 6A and B). By contrast, treatment
with honokiol prior to IRI caused a significant reduction in the
expression and activity of iNOS, compared with the IRI group
(Fig. 6A and B).
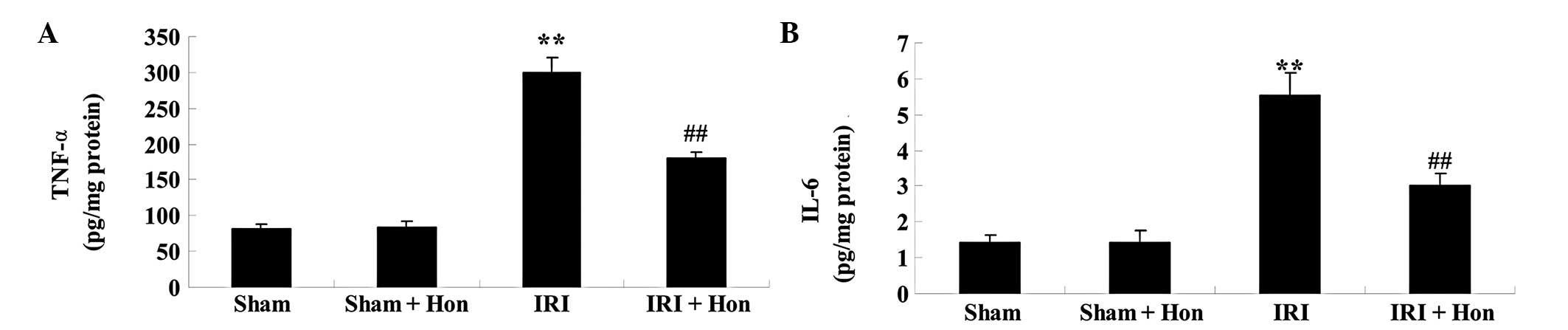
Honokiol reduces levels of inflammatory
cytokines
The levels of TNF-α and IL-6 in the IRI group
increased significantly, compared with those in the Sham group
(Fig. 7A and B). These elevated
levels of TNF-α and IL-6 in the IRI rats were reduced significantly
by honokiol pretreatment (Fig. 7A and
B).
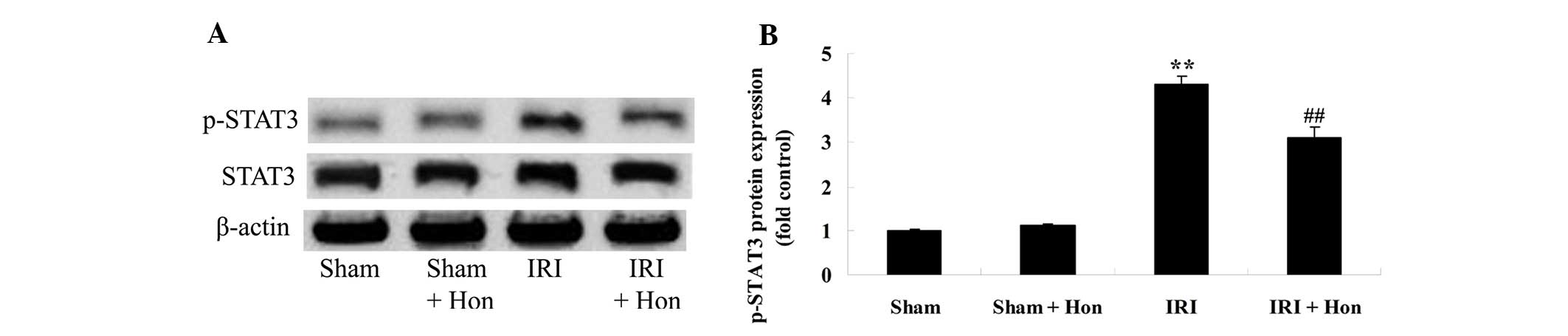
Honokiol decreases the protein expression
of STAT3
In order to elucidate the mechanistic basis of the
effects of honokiol In the IRI rats, the protein expression levels
of STAT3 were measured using western blotting. The results
suggested that the protein expression of p-STAT3 was promoted in
the IRI group, compared with the Sham group (Fig. 8A and B). The increased protein
expression of p-STAT3 by IRI was reversed in the rats pretreated
with honokiol (Fig. 8A and B).
Honokiol reduces levels of caspase-3
The results of the present study showed that
increased activity of caspase-3 was observed in the IRI group,
compared with the Sham group (Fig.
9). In addition. honokiol administration prior to IRI
significantly reduced the levels of caspase-3 levels, compared with
the levels observed in the IRI group (Fig. 9).
Discussion
The kidney is an organ with a high level of
perfusion, and is particularly sensitive to ischemia and
reperfusion. When ischemia persists for a certain duration,
following which perfusion recovers, organizational structural and
functional recovery may be impaired and kidney dysfunction and
structural damage may be aggravated, which is known as IRI
(28). Following shock, heart
failure, cardiopulmonary bypass or kidney transplantation, IRI
usually affects the treatment outcome, particularly in kidney
transplant recipients, in which IRI is one of the causes of
surgical failure. At present, the pathogenesis of IRI remains to be
fully elucidated. In the present study, honokiol pretreatment
reduced the levels of serum creatinine, BUN, ALT, AST and ALP, and
the levels of nitrite in the kidneys of IRI rats. Therefore,
honokiol may be a potential drug for treatment following IRI.
Oxidative stress is one of the important
pathogenetic mechanisms of IRI. In ischemia, a high level of ATP is
decomposed, leading to the accumulation of hypoxanthine, recovery
of blood perfusion causes the generation of a substantial number of
reactive oxygen free radicals, and peroxy radicals and their
degradation products cause tissue damage by lipid peroxidation of
mitochondria and lipid membranes (29). In the present study, honokiol
treatment depressed serum levels of MDA and MPO, and increased the
activities of SOD and CAT in the IRI rats. Hsu et al
reported that honokiol protected against heatstroke in diabetic
rats through reducing inflammation and oxidative stress (30).
NO in the body is generated by NOS, and the level of
NO is closely associated with the level and activity of NOS. In
normal kidney tissues, endothelial NOS is predominantly expressed
in the renal blood vessels and capillaries; neuronal NOS is
predominantly expressed in the juxtaglomerular macula densa; and
iNOS is expressed in the renal medullary thick ascending limb,
proximal tubule, distal convoluted tubule and interlobular
arteries, arcuate arteries and blood vessels, and other areas of
the glomerulus (31). Under a
pathophysiological state, iNOS exhibits high levels of expression
in mesangial cells, epithelial cells, smooth muscle cells and renal
tubular epithelial cells, and there are high levels of infiltrated
inflammatory cells, including in the glomeruli and renal
interstitium (32). The present
study showed that honokiol significantly decreased the gene
expression and activity of iNOS in the IRI rats. Previous studies
have suggested that honokiol prevents the inflammatory response and
the expression of iNOS in human osteoarthritis chondrocytes
(33), and the level of iNOS is
attenuated by honokiol in septic rats (34).
Inflammatory reactions, including a series of
complex pathological processes, develop and interconnect with each
other, which can be roughly divided into the following four
processes: Leukocyte activation, chemotaxis, leukocyte-endothelial
cell adhesion and migration. In IRI, polymorphonuclear cells within
the kidney or proximal tubule, activating factors, including
bradykinin, histamine, leukotrienes and platelet-activating
factors, which are generated by mesangial cells, pro-inflammatory
cytokines, including TNF-α, IL-1β, IL-6, and monocyte
chemoattractant protein-1, macrophage inflammatory protein-2,
interferon-inducible protein 10 and other chemokines, lead to
leukocyte activation and subsequent chemotaxis to the site of
injury (35). Subsequently,
following interaction with vascular endothelial cells, the
leukocytes roll and then adhere closely to the skin layer in the
endoderm of blood vessels, migrating into the skin layer and
ultimately penetrating the endoderm to reach the extravascular
tissue where it is exerts its effects (36). Inflammatory cells directly damage
cells following reperfusion by the release of oxidase and
hydrolytic enzymes, and adhered neutrophils block the capillary
bed, which further increases the circulatory disorder. The present
study showed that honokiol significantly reduced the levels of
TNF-α and IL-6 in the IRI rats. Chiang et al demonstrated
that honokiol protects against eccentric exercise-induced skeletal
muscle damage by inhibiting oxidative stress and inflammation in
rats (37), and Munroe et
al indicated that the anti-inflammatory effects of honokiol
decreased the levels of TNF-α and IL-6 in a mouse model of allergic
asthma (38).
The STAT pathway is important in cytokine signaling,
and STAT transcription factors exist in the cytoplasm during the
resting state, which can be activated by cytokines, growth factors,
and reactive oxygen species, as intracellular signal transduction
proteins and transcription factors. Once phosphorylated by Janus
kinase, STATs form homologous or heterologous dimers, which are
then translocated into the nucleus and combine with DNA, initiating
gene transcription. Ishikawa et al reported that honokiol induces
cell cycle arrest and apoptosis through inhibiting the DNA binding
of nuclear factor-ϰB and STAT3 (39). Previous studies have suggested that
the STAT signaling pathway and IRI have a specific association. IRI
can produce large quantities of reactive oxygen species, and when
it exceeds the processing ability of antioxidant enzymes, reactive
oxygen species accumulate, leading to excessive oxidative stress
and cell damage, and the direct activation of STAT3 (40). In conditions of hypoxia without
reperfusion, ATP may not be sufficient to make STAT3 fully
phosphorylated; however, following reperfusion, ATP levels rise
again, and STAT3 can be activated to a maximum degree by
phosphorylation (41). In the
present study, honokiol suppressed the protein expression of
p-STAT3 in the IRI rats. Yu et al suggested that honokiol
exerts pro-apoptotic effects on transformed Barrett's cells through
inhibition of STAT3 (42). Luan
et al reported that honokiol induces cell cycle arrest and
apoptosis through inhibiting the DNA binding of nuclear factor-κB
and STAT3 (43). In the present
study, honokiol administration significantly decreased the levels
of caspase-3 in the IRI rats. Weng et al suggested that
honokiol attenuates the severity of acute pancreatitis and lung
injury through suppression of apoptosis and caspase-3 activity
(44). Honokiol also inhibits the
activation of caspase-3 and caspase-9 in
H2O2-induced apoptosis in human lens
epithelial cells (45).
In conclusion, the present study demonstrated the
protect effect of honokiol on renal IRI through the suppression of
oxidative stress, iNOS, inflammation and STAT3 in the rats. These
results may be of potential clinical relevance, and the protective
effect of honokiol as a clinical therapeutic strategy may be of
value in the future.
References
|
1
|
Senbel AM, AbdelMoneim L and Omar AG:
Celecoxib modulates nitric oxide and reactive oxygen species in
kidney ischemia/reperfusion injury and rat aorta model of
hypoxia/reoxygenation. Vascul Pharmacol. 62:24–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai Y, Xu H, Yan J, Zhang L and Lu Y:
Molecular targets and mechanism of action of dexmedetomidine in
treatment of ischemia/reperfusion injury. Mol Med Rep. 9:1542–1550.
2014.PubMed/NCBI
|
|
3
|
Cetin N, Suleyman H, Sener E, Demirci E,
Gundogdu C and Akcay F: The prevention of ischemia/reperfusion
induced oxidative damage by venous blood in rabbit kidneys
monitored with biochemical, histopatological and
immunohistochemical analysis. J Physiol Pharmacol. 65:383–392.
2014.PubMed/NCBI
|
|
4
|
Cetin N, Suleyman H, Sener E, Demirci E,
Gundogdu C and Akcay F: The prevention of ischemia/reperfusion
induced oxidative damage by venous blood in rabbit kidneys
monitored with biochemical, histopatological and
immunohistochemical analysis. J Physiol Pharmacol. 65:383–392.
2014.PubMed/NCBI
|
|
5
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuan-Yun LI, Xue-Ping Y, Bin L, Reheman
HN, Yang G, Zhan S and Qi MA: Auricularia auricular-judae
polysaccharide attenuates lipopolysaccharide-induced acute lung
injury by inhibiting oxidative stress and inflammation. Biomed Rep.
3:478–482. 2015.PubMed/NCBI
|
|
7
|
Jin X, Zhang Y, Li X, Zhang J and Xu D:
C-type natriuretic peptide ameliorates ischemia/reperfusion-induced
acute kidney injury by inhibiting apoptosis and oxidative stress in
rats. Life Sci. 117:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tripatara P, Patel NS, Webb A, Rathod K,
Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A and
Thiemermann C: Nitrite-derived nitric oxide protects the rat kidney
against ischemia/reperfusion injury in vivo: Role for xanthine
oxidoreductase. J Am Soc Nephrol. 18:570–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reyes-Ocampo J, Ramirez-Ortega D, Vazquez
Cervantes GI, Pineda B, Montes de Oca Balderas P, González-Esquivel
D, Sánchez-Chapul L, Lugo-Huitrón R, Silva-Adaya D, Ríos C, et al:
Mitochondrial dysfunction related to cell damage induced by
3-hydroxykynurenine and 3-hydroxyanthranilic acid:
Non-dependent-effect of early reactive oxygen species production.
Neurotoxicology. 50:81–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koc M, Kumral ZN, Özkan N, Memi G, Kaçar
Ö, Bilsel S, Çetinel Ş and Yeğen BÇ: Obestatin improves
ischemia/reperfusion-induced renal injury in rats via its
antioxidant and anti-apoptotic effects: Role of the nitric oxide.
Peptides. 60:23–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan R, Li Y, Zhang L, Xia N, Liu Q, Sun H
and Guo H: Augmenter of liver regeneration attenuates inflammation
of renal ischemia/reperfusion injury through the NF-kappa B pathway
in rats. Int Urol Nephrol. 47:861–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia-Criado FJ, Rodriguez-Barca P,
Garcia-Cenador MB, Rivas-Elena JV, Grande MT, Lopez-Marcos JF,
Mourelle M and López-Novoa JM: Protective effect of new
nitrosothiols on the early inflammatory response to kidney
ischemia/reperfusion and transplantation in rats. J Interferon
Cytokine Res. 29:441–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YY, Yeh CH, So EC, Sun DP, Wang LY
and Hsing CH: Anticancer drug 2-methoxyestradiol protects against
renal ischemia/reperfusion injury by reducing inflammatory
cytokines expression. Biomed Res Int. 2014:4315242014.PubMed/NCBI
|
|
14
|
Hsu YH, Li HH, Sung JM, Chen WT, Hou YC
and Chang MS: Interleukin-19 mediates tissue damage in murine
ischemic acute kidney injury. PLoS One. 8:e560282013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seo KH, Nam YH, Lee DY, Ahn EM, Kang TH
and Baek NI: Recovery effect of phenylpropanoid glycosides from
Magnolia obovata fruit on alloxan-induced pancreatic islet damage
in zebrafish (Danio rerio). Carbohydr Res. 416:70–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar A, Kumar Singh U and Chaudhary A:
Honokiol analogs: A novel class of anticancer agents targeting cell
signaling pathways and other bioactivities. Future Med Chem.
5:809–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar A, Kumar Singh U and Chaudhary A:
Honokiol analogs: A novel class of anticancer agents targeting cell
signaling pathways and other bioactivities. Future Med Chem.
5:809–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raison-Peyron N, Cesaire A, Du-Thanh A and
Dereure O: Allergic contact dermatitis caused by Magnolia
officinalis bark extract in a facial anti-ageing cream. Contact
Dermatitis. 72:416–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghys K, De Palma A, Vandevenne A,
Werbrouck J and Goossens A: Magnolia officinalis bark extract, a
recently identified contact allergen in 'anti-ageing' cosmetics.
Contact Dermatitis. 73:130–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woodbury A, Yu SP, Wei L and Garcia P:
Neuro-modulating effects of honokiol: A review. Front Neurol.
4:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fried LE and Arbiser JL: Honokiol, a
multifunctional anti-angiogenic and antitumor agent. Antioxid Redox
Signal. 11:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian W, Xu D and Deng YC: Honokiol, a
multifunctional tumor cell death inducer. Pharmazie. 67:811–816.
2012.PubMed/NCBI
|
|
23
|
Shen JL, Man KM, Huang PH, Chen WC, Chen
DC, Cheng YW, Liu PL, Chou MC and Chen YH: Honokiol and magnolol as
multifunctional antioxidative molecules for dermatologic disorders.
Molecules. 15:6452–6465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mozaffari-Khosravi H, Ahadi Z and Fallah
Tafti M: The effect of green tea versus sour tea on insulin
resistance, lipids profiles and oxidative stress in patients with
type 2 diabetes mellitus: A randomized clinical trial. Iran J Med
Sci. 39:424–432. 2014.PubMed/NCBI
|
|
25
|
Monji A, Mitsui T, Bando YK, Aoyama M,
Shigeta T and Murohara T: Glucagon-like peptide-1 receptor
activation reverses cardiac remodeling via normalizing cardiac
steatosis and oxidative stress in type 2 diabetes. Am J Physiol
Heart Circ Physiol. 305:H295–H304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Z, Ge Y, Zhang J, et al: PUFA diets
alter the microRNA expression profiles in an inflammation rat
model. Mol Med Rep. 11:4149–4157. 2015.PubMed/NCBI
|
|
27
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sagiroglu T, Torun N, Yagci M, Yalta T,
Sagiroglu G and Oguz S: Effects of apelin and leptin on renal
functions following renal ischemia/reperfusion: An experimental
study. Exp Ther Med. 3:908–914. 2012.PubMed/NCBI
|
|
29
|
Liu ZG, Qi ZC, Liu WL and Wang WZ: Lutein
protects against ischemia/reperfusion injury in rat kidneys. Mol
Med Rep. 11:2179–2184. 2015.
|
|
30
|
Hsu CC, Chen LF, Lin MT and Tian YF:
Honokiol protected against heatstroke-induced oxidative stress and
inflammation in diabetic rats. Int J Endocrinol. 2014:1345752014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kinaci MK, Erkasap N, Kucuk A, Koken T and
Tosun M: Effects of quercetin on apoptosis, NF-κB and NOS gene
expression in renal ischemia/reperfusion injury. Exp Ther Med.
3:249–254. 2012.PubMed/NCBI
|
|
32
|
Zhang J, Li JH, Wang L, Han M, Xiao F, Lan
XQ, Li YQ, Xu G and Yao Y: Glucocorticoid receptor agonist
dexamethasone attenuates renal ischemia/reperfusion injury by
up-regulating eNOS/iNOS. J Huazhong Univ Sci Technolog Med Sci.
34:516–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YJ, Tsai KS, Chan DC, Lan KC, Chen
CF, Yang RS and Liu SH: Honokiol, a low molecular weight natural
product, prevents inflammatory response and cartilage matrix
degradation in human osteoarthritis chondrocytes. J Orthop Res.
32:573–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N, Xie H, Li L, Wang J, Fang M, Yang N
and Lin H: Effects of honokiol on sepsis-induced acute kidney
injury in an experimental model of sepsis in rats. Inflammation.
37:1191–1199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang B, Rong R, Li H, Peng X, Xiong L,
Wang Y, Yu X and Mao H: Heat shock protein 72 suppresses apoptosis
by increasing the stability of X-linked inhibitor of apoptosis
protein in renal ischemia/reperfusion injury. Mol Med Rep.
11:1793–1799. 2015.
|
|
36
|
Asaga T, Ueki M, Chujo K and Taie S:
JTE-607, an inflammatory cytokine synthesis inhibitor, attenuates
ischemia/reperfusion-induced renal injury by reducing neutrophil
activation in rats. J Biosci Bioeng. 106:22–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chiang J, Shen YC, Wang YH, Hou YC, Chen
CC, Liao JF, Yu MC, Juan CW and Liou KT: Honokiol protects rats
against eccentric exercise-induced skeletal muscle damage by
inhibiting NF-kappaB induced oxidative stress and inflammation. Eur
J Pharmacol. 610:119–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Munroe ME, Businga TR, Kline JN and Bishop
GA: Anti-inflammatory effects of the neurotransmitter agonist
Honokiol in a mouse model of allergic asthma. J Immunol.
185:5586–5597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishikawa C, Arbiser JL and Mori N:
Honokiol induces cell cycle arrest and apoptosis via inhibition of
survival signals in adult T-cell leukemia. Biochim Biophys Acta.
1820:879–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di Domenico F, Casalena G, Jia J, Sultana
R, Barone E, Cai J, Pierce WM, Cini C, Mancuso C, Perluigi M, et
al: Sex differences in brain proteomes of neuron-specific
STAT3-null mice after cerebral ischemia/reperfusion. J Neurochem.
121:680–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang
S, Wang N, Liang Z, Li Y, Chen W, et al: JAK2/STAT3 activation by
melatonin attenuates the mitochondrial oxidative damage induced by
myocardial ischemia/reperfusion injury. J Pineal Res. 55:275–286.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu C, Zhang Q, Zhang HY, Zhang X, Huo X,
Cheng E, Wang DH, Arbiser JL, Spechler SJ and Souza RF: Targeting
the intrinsic inflammatory pathway: Honokiol exerts proapoptotic
effects through STAT3 inhibition in transformed Barrett's cells. Am
J Physiol Gastrointest Liver Physiol. 303:G561–569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luan HF, Zhao ZB, Zhao QH, Zhu P, Xiu MY
and Ji Y: Hydrogen sulfide postconditioning protects isolated rat
hearts against ischemia and reperfusion injury mediated by the
JAK2/STAT3 survival pathway. Braz J Med Biol Res. 45:898–905.
2012.PubMed/NCBI
|
|
44
|
Weng TI, Wu HY, Chen BL and Liu SH:
Honokiol attenuates the severity of acute pancreatitis and
associated lung injury via acceleration of acinar cell apoptosis.
Shock. 37:478–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xia J, Wu Z, Yu C, He W, Zheng H, He Y,
Jian W, Chen L, Zhang L and Li W: miR-124 inhibits cell
proliferation in gastric cancer through down-regulation of SPHK1. J
Pathol. 227:470–480. 2012. View Article : Google Scholar : PubMed/NCBI
|