Introduction
Ocular immune privilege exists in the anterior as
well as the posterior segment of the eye. Retinal pigment
epithelial (RPE) cells are important for the creation and
maintenance of ocular immune privilege in the sub-retinal space,
which is associated with intraocular suppression of immunogenic
inflammation (1,2). Antigens (Ag) presented by
non-professional antigen-presenting cells (APCs), major
histocompatibility complex (MHC) induced by RPE cells, and various
cytokines induced by local inflammation, are able to further
promote the induction of Ag-specific peripheral retinal tolerance
through interaction with autoreactive, circulating naive T cells,
which are not eliminated due to central tolerance. RPE cells with
induced expression of MHC class II hare known to be capable of
presenting Ag to Ag-experienced CD4+ T cells (3,4). In
addition, RPE cells have been shown to secrete soluble factors,
including transforming growth factor-β (TGF-β), thrombospondin-1
and prostaglandin E2, which can alter the expression of adaptive as
well as innate immune effector mechanisms in vitro (5).
CD25 is the receptor of interleukin (IL)-2. It is
widely accepted that the regulatory T cells (Tregs) are
CD4+ T cells that co-express CD25 (5–10% of peripheral
lymphocytes). Forkhead winged helix transcription factor-3 (Foxp3)
is expressed predominantly in Tregs and is necessary as well as
sufficient for their development and function; furthermore, Tregs
have immunoregulatory abilities by which they prevent
organ-specific autoimmune disease (6,7). A
number of studies have demonstrated that APCs can induce the
generation of Tregs (8–10). However, the process of Treg
generation with RPE cells acting as APCs has remained largely
elusive. The present study hypothesized that RPE cells act as APCs
and induce Treg expansion to suppress inflammation, which may
represent a potential treatment strategy for posterior uveitis. The
activity of indoleamine 2,3-dioxygenase (IDO) has a tight
correlation with the peripheral immune tolerance and immune
regulation (11). The activation
of the nuclear factor erythroid 2-related factor (Nrf2) pathway is
an anti-oxidant response, which confers cellular protection against
the detrimental effects of various insults (12). Therefore, the present study
hypothesized that TGF-β2 maintains RPE cells in an
immunosuppressive condition through IDO regulation or via an
anti-oxidant response. Furthermore, the present study investigated
the mechanism of the induction of Tregs via CD4+ T cells
cultured in vitro in the presence of RPE cells. The
immunosuppressive function of natural Tregs was compared to that of
RPE-induced Tregs.
Materials and methods
Preparation of human RPE cells and TGF-β2
stimulation
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the Institutional
Review Board of Jingling Hospital (Nanjing, China). The ARPE-19
human RPE cell line was obtained from the American Type Culture
Collection (Manassas, VA, USA). Complete Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific)
was used for the culture of ARPE-19 cells. Cultures were passaged
by dissociation in 0.25% trypsin (Thermo Fisher Scientific) in
phosphate-buffered saline (PBS; Thermo Fisher Scientific). The
cells were maintained for several weeks and the medium was replaced
every five days. The supernatants of RPE cells cultured in the
presence or absence of recombinant human TGF-β2 (5 ng/ml; R&D
Systems, Minneapolis, MN, USA) for 24–72 h were harvested for the
generation of Tregs. RPE cells were harvested for RNA isolation and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR) analysis of IDO and Nrf2.
RT-qPCR
Cellular extracts were prepared from the cultured
RPE cells. The cultured RPE cells were washed twice with PBS, and
total RNA was extracted using TRIzol reagent. (Invitrogen; Thermo
Fisher Scientific). qPCR was performed as previously described
(13). The quantity of the RNA was
assessed at an absorbance of 260/280 nm using a NanoDrop 1000
Spectrophotometer (Thermo Fisher Scientific). The RNA (500 ng) was
subjected to reverse transcription using Moloney murine leukemia
virus reverse transcriptase and oligo(dT) primer (Invitrogen). The
paired primers for IDO mRNA were 5′-CAAAGGTCATGGAGATGTCC-3′
(forward) and 5′-CCACCAATAGAGAGACCAGG-3′ (reverse). The paired
primers for Nrf2 mRNA were 5′-GAGAGCCCAGTCTTCATTGC-3′ (forward) and
5′-TGCTCAATGTCCTGTTGCAT-3′ (reverse). The thermocycling conditions
were as follows: 40 sec at 94°C, 40 sec at 56°C and 40 sec at 72°C
for 30 cycles. PCR was performed in an Opticon 2 Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc, Hercules, CA, USA)
using corresponding primers and SYBR gene PCR Master Mix
(Invitrogen). The mRNA expression levels of IDO and Nrf2 were
normalized to that of GAPDH, determined with the paired primers
5′-ACAGTCAGCCGCATCTTC-3′ (forward) and 5′-GCCCAATACGACCAAATCC-3′
(reverse). The expression levels of the mRNAs were expressed as
fold changes compared with the sham control as determined using the
2−ΔΔCq method (14).
Induction of Tregs with RPE cell culture
supernatant
The T cells were separated by magnetic cell sorting
(MACS) CD4+ T-cell isolation kits (MACS systems;
Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). To avoid
contamination with RPE cells, CD4+ T cells
(1×106/well) were exposed to RPE cell culture
supernatants in the presence of anti-human CD3 antibody (2.0
µg/ml; Clone HIT3a; BD Pharmingen, San Diego, CA, USA) and
human recombinant IL-2 (rIL-2; 200 U/ml; R&D Systems) for 72 h
in RPMI 1640 containing 10% FBS (Gibco; Thermo Fisher Scientific).
In order to investigate the most suitable immune microenvironment
for inducing Tregs, CD4+ T cells were exposed to the
supernatant of ARPE-19 cells incubated as follows: Untreated
(control) or treated with rIL-2 (200 U/ml) or TGF-β2 (5 ng/ml) or
with TGF-β2 (5 ng/ml) + rIL-2 (200 U/ml). Following 24 h of
incubation, CD4+ T cells exposed to the RPE supernatants
on 2.0 µg/ml anti-CD3-coated plates were harvested and X-ray
irradiated (2,000 rad) to inhibit cell proliferation. The number of
CD4+ CD25+ T cells was analyzed by
fluorescence-assisted cell sorting (FACS). CD4+
CD25+ T cells (2×105 cells/well) were then
added to the CD4+ CD25− T cells for the
subsequent proliferation assay.
FACS analysis
To examine the expression of CD25 on CD4+
T cells in the presence or absence of RPE cell culture
supernatants, the T cells were stained with
phycoerythrin-conjugated anti-human CD25 (BD Pharmingen) and
fluorescein isothiocyanate-conjugated anti-CD4 antibodies (BD
Pharmingen). To measure intracellular Foxp3, lymphoid cells were
stained to detect cell surface antigens and then fixed with
Cytofix/Cytoperm solution (cat. no. 2075KK; BD Pharmingen).
Isotype-matched antibodies were used as negative controls. The
cells were incubated with monoclonal antibodies (BD Pharmingen) at
4°C for 30 min in the dark and then washed twice in FACS buffer
(PBS with 1% bovine serum albumin and 0.1% sodium azide)
immediately prior to flow cytometric data acquisition. Samples were
acquired using a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and analyzed using FlowJo 7.6.1 software
(Ashland, OT, Canada). Ten thousand cells were analyzed in each
sample. The isotype controls were used to establish the gating for
positive staining of CD25. The fraction of positive cells among the
CD4+ cell population was calculated for each marker.
Cell purification
To generate RPE-induced Tregs, the CD4+ T
cells exposed to the supernatants of RPE cells cultured with TGF-β2
and/or rIL-2 and anti-CD3 as stated above were harvested.
CD4+ CD25+ T cells were purified by magnetic
bead separation using a CD4+ CD25+ T-cell
isolation kit (Miltenyi Biotec GmbH). The procedure was performed
according to the manufacturer's two-step MACS protocol.
CD4+ CD25+ T cells and the unbound fraction,
which contained CD4+ CD25− T cells, were
collected separately. The purity and phenotypes of the cells were
detected by FACS. When the T cells were stained with
anti-pancytokeratin antibody (clone PCK-26; Sigma-Aldrich, St.
Louis, MO, USA), cytokeratin-positive RPE cells were not detected
(≤0.3%) in the harvested Tregs. To generate natural Tregs,
dendritic cells (DCs) isolated from peripheral blood mononuclear
cells of five healthy volunteers were used as APCs and were
prepared by incubating CD4-depleted blood cells with CD11c
antibody-coated microbeads (BD Pharmingen) at 4°C for 15 min,
followed by positive selection. DCs were then treated with 50
µg/ml mitomycin C (Sigma-Aldrich) for 20 min at 37°C and
washed three times.
Proliferation of CD4+
CD25− T cells
To compare the immunosuppressive function of natural
Tregs and RPE-induced Tregs, the purified CD4+
CD25− T cells (2×105/well) were cultured
alone or with the CD4+ CD25+ T cells
(0.4–4×105/well; natural or RPE-induced as described
above) and in the presence of 3 µg/ml soluble anti-CD3 and
APCs (5×104/well). Cells were cultured in 96-well plates
in 200 µl RPMI supplemented with 10% fetal calf serum for
three days and pulsed with 1 µCi of [3H]thymidine
(3H-TdR; GE Healthcare, Little Chalfont, UK) for an
additional 16 h. 3H-TdR incorporation was quantified
using a Beckman scintillation counter (Beckman Coulter, Brea, CA,
USA). The results are expressed as the mean cpm of triplicate
cultures.
Statistical analysis
Values are expressed as the mean ± standard
deviation and all experiments were repeated at least twice. The
parametric data were analyzed using two-way analysis of variance
(Figs. 1 and 3) or the independent samples t-test
(Fig. 4). The non-parametric data
were analyzed using the Mann-Whitney U test (Fig. 2). P<0.05 was considered to
indicate a statistically significant difference.
Results
TGFβ2-induced mRNA upregulation of IDO
and Nrf2 in RPE cells
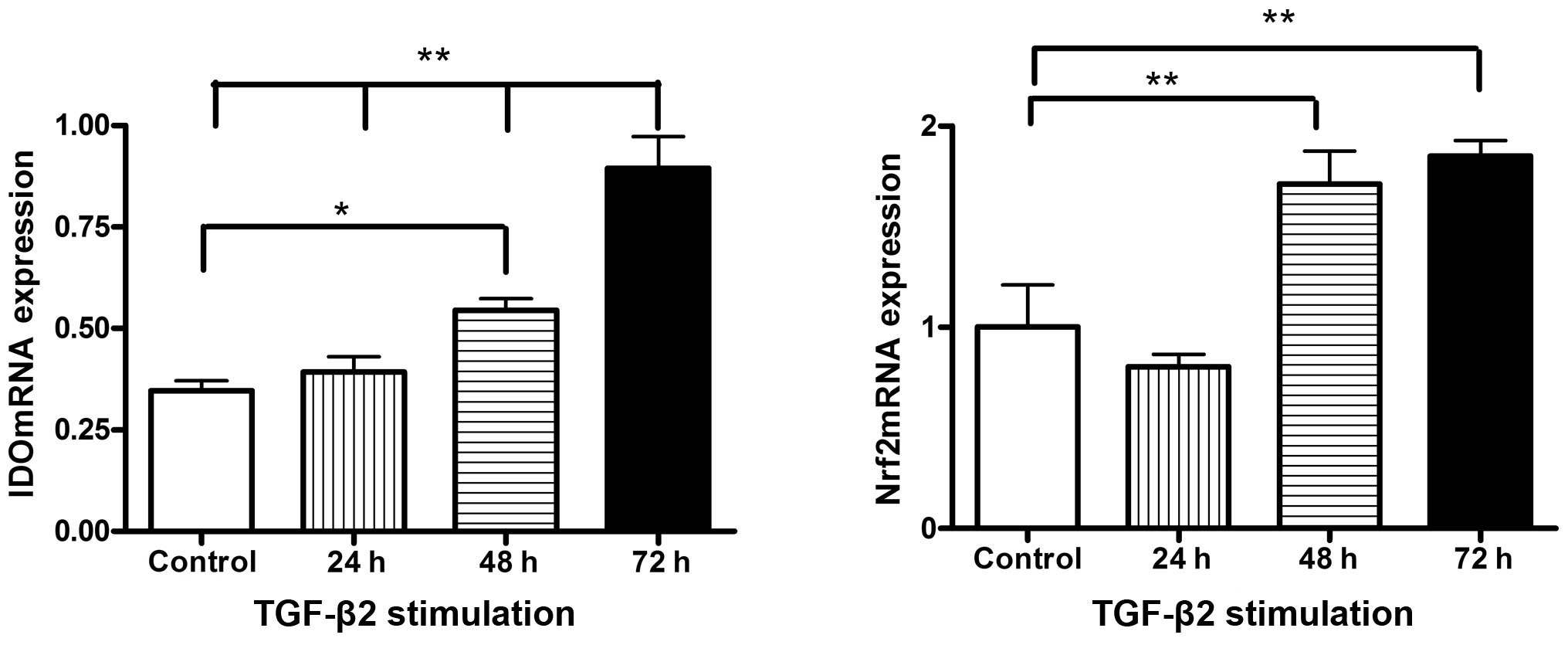
As shown in Fig. 1,
RPE cells that were not stimulated by TGF-β2 exhibited a low level
of mRNA expression of IDO, an immunoregulatory factor. Of note, IDO
was slightly upregulated after RPE cells had been treated with
recombinant TGF-β2 for 24 h (P=0.507), significantly increased
after 48 h (P=0.019) and highest after 72 h (P<0.001). However,
Nrf2 mRNA expression in RPE cells was slightly decreased after
treatment with TGF-β2 for 24 h (P=0.349) and significantly
increased after 48 h (P=0.008) and 72 h (P=0.003) of TGF-β2
stimulation (Fig. 1).
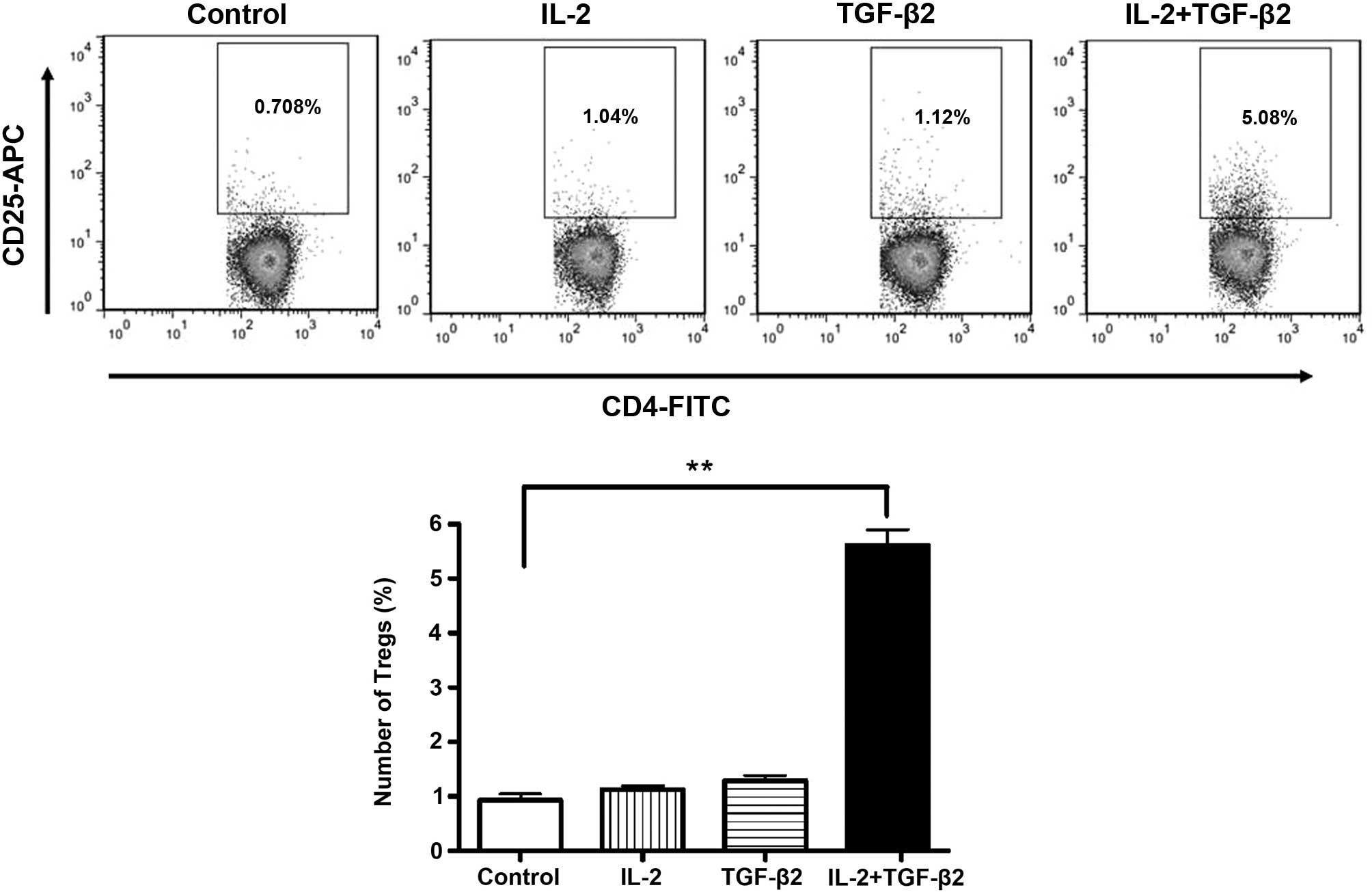
TGF-β2 enhances RPE cell-induced
differentiation of CD4+ CD25+ T cells
To test whether RPE cells are capable of inducing
differentiation of CD4+ T cells into CD4+
CD25+ Tregs, a well-established protocol for the
generation of Tregs from CD4+ T cells was applied. It
has been reported that human RPE cell lines do not convert
CD4+ T cells into Tregs (15). However, in the present study, the
supernatant of RPE cells treated with TGF-β2 induced
differentiation of CD4+ T cells into Tregs in the
presence of IL-2 and anti-CD3. The mRNA expression of IDO in the
RPE cells was increased during this process. CD4+ T
cells that were exposed to rIL-2 and the supernatants of RPE cells
treated with TGF-β2 and IL-2 combined expressed a significantly
higher level of CD25 than CD4+ T cells exposed to the
supernatant of RPE cells cultured with TGF-β2 or IL-2 alone
(P<0.001) (Fig. 2).
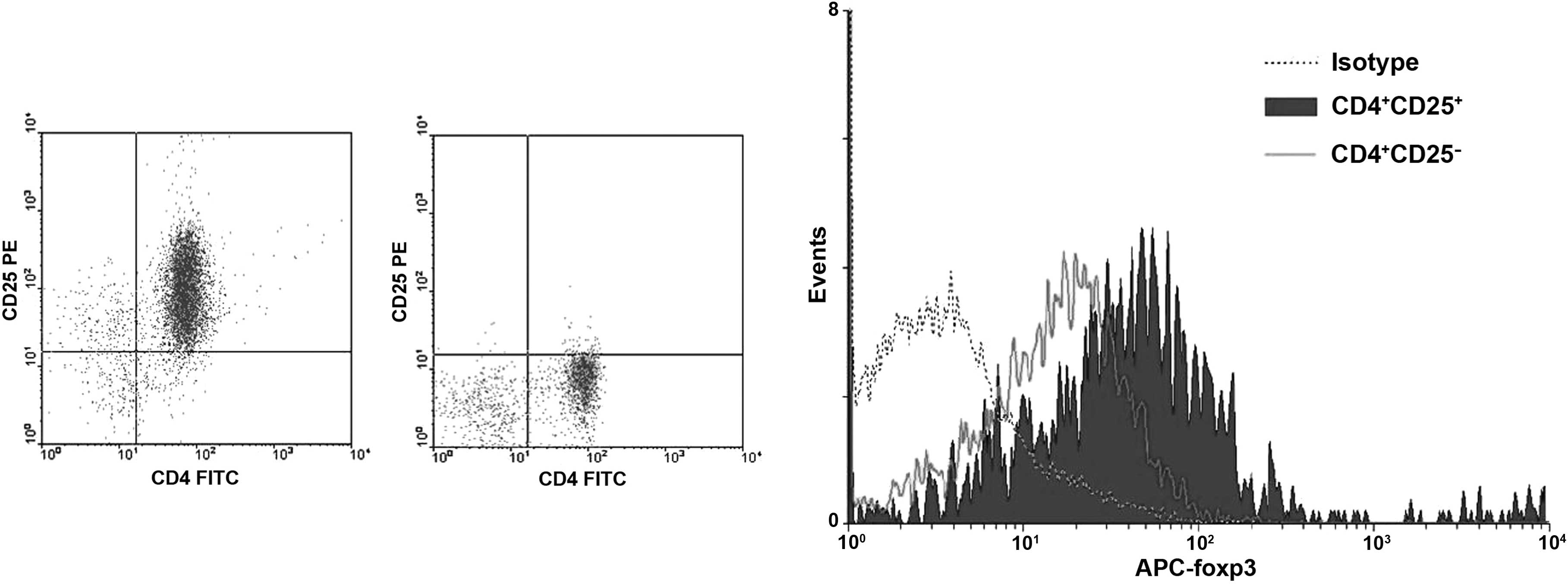
Phenotype of isolated and purified
CD4+ CD25+ Tregs
To further functionally characterize the
CD4+ CD25+ T cells induced in the present
study, the CD4+ T cells were isolated using MACS
microbeads and sorted on the basis of surface CD25 expression.
After CD4+ CD25+ T cells were isolated by
two-step MACS, 89.78% (range, 86.29–90.04%) of the cells expressed
CD4 as well as CD25. To verify the enrichment of Tregs, the
CD4+ CD25+/CD4+ CD25− T
cells were analyzed for the expression of Foxp3, a Treg-specific
transcription factor. Foxp3 was preferentially expressed by
CD4+ CD25+ T cells (64.8±1.23%), whereas the
CD4+ CD25− sub-population exhibited a low
expression level of Foxp3 (15.83±2.74%; P<0.001 for
CD4+ CD25+ vs. CD4+
CD25−) (Fig. 3).
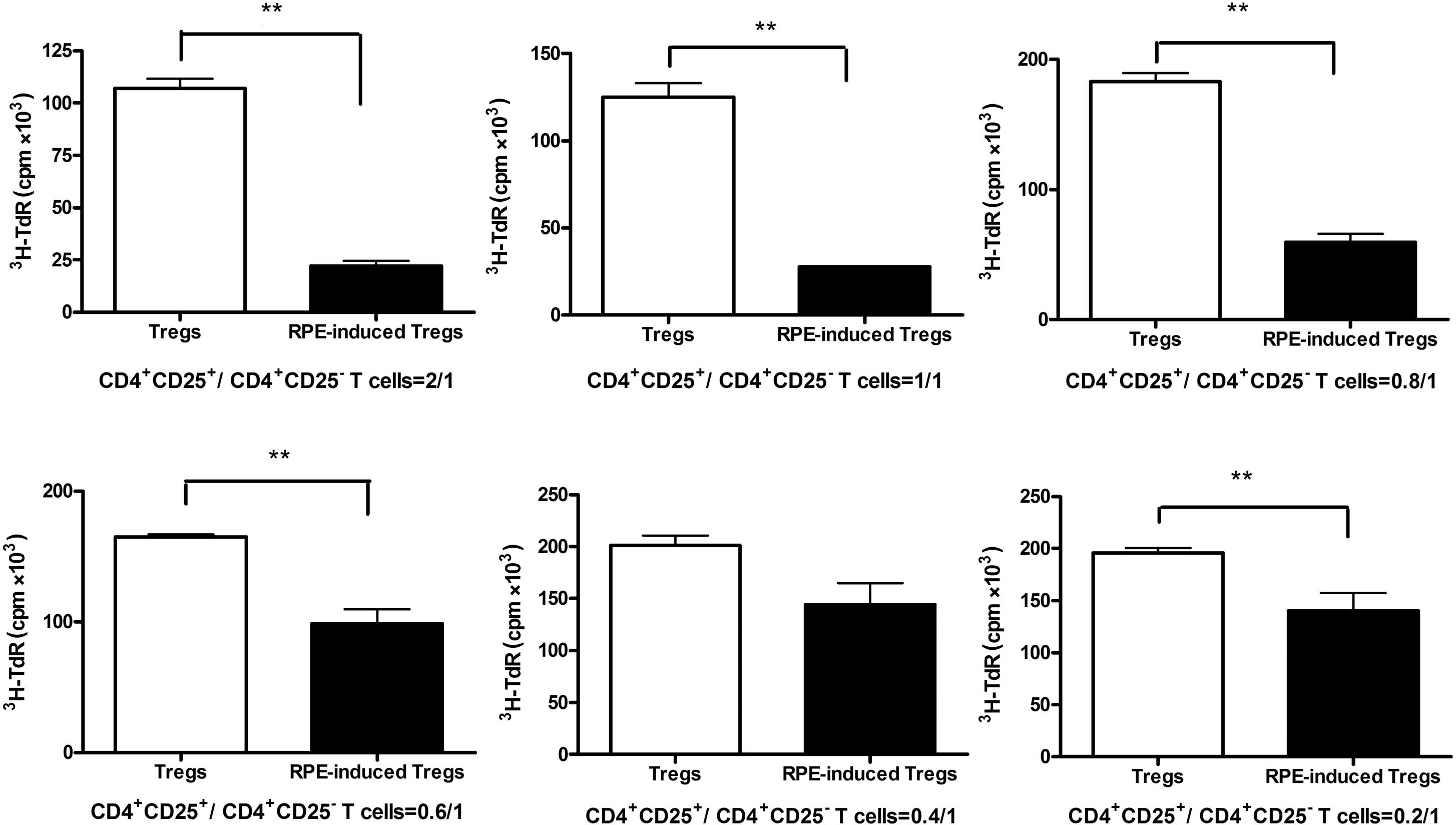
RPE-induced Tregs suppress the activation
of T cells in vitro
The ability of CD4+ CD25+ T
cells to regulate the proliferative response of CD4+
CD25− T cells was measured by mixed lymphocyte reaction
(MLR). Marked proliferative responses were observed in vitro
when CD4+ CD25− T cells were stimulated with
anti-CD3 in the presence of syngeneic APCs, whereas natural Tregs
and RPE-induced Tregs showed minimal proliferative responses (data
not shown). To compare the ability to suppress CD4+
CD25− T cell proliferation between the natural Tregs and
the RPE-induced Tregs, varying numbers of CD4+
CD25+ T cells were added to a constant number of
CD4+ CD25− T cells and activated in
vitro with anti-CD3. As shown in Fig. 4, although the natural
CD4+ CD25+ T cells inhibited the
proliferation of CD4+ CD25− T cells,
RPE-induced CD4+ CD25+ T cells were more
effective at suppressing the proliferation of CD4+
CD25− T cells (P<0.01) with ~75% suppression of
proliferation at a higher cell density (CD4+
CD25+/CD4+ CD25− T cells,
2:1).
Discussion
Immunologically, RPE cells have a pivotal role in
immune responses (1,3). RPE cells express Toll-like receptors,
complement components and Fc-γ receptors; furthermore, they respond
to treatment with certain cytokines, upregulate the expression of
both MHC class I and II molecules and participate as resident APCs
in the retina. Retinal as well as non-retinal Ag can be processed
and presented by RPE cells (3,16).
IDO is an enzyme that catabolizes the essential amino acid
tryptophan into the stable metabolite kynurenine. IDO activity has
been found to greatly impact peripheral tolerance and immune
regulation (11). It is thought
that IDO is a signaling protein in long-term tolerance by DCs
(17,18). TGF-β is known to induce IDO
expression in DCs. It is possible that a TGF-β/IDO/tryptophan
metabolite axis is involved in the maintenance of an
immunosuppressive environment (19). Park et al (20) reported that the immunosuppressive
enzyme IDO is highly expressed in RPE cells under interferon-γ
(IFN-γ) stimulation and that IDO may have a significant role in RPE
cell-mediated immune suppression and ocular immune privilege. In
normal eyes, TGF-β2 is constitutively present and actively
participates in the maintenance of ocular immune privilege. It has
been proven that the increased expression of cytotoxic T
lymphocyte-associated Ag-2 alpha in RPE cells induces Tregs through
the increased production of TGF-β (5,21).
Therefore, TGF-β2 was used as a stimulator in the present study and
led to the upregulation of IDO mRNA expression in RPE cells
following 48–72 h of incubation. However, the mRNA expression of
Nrf2 in RPE cells was slightly decreased after TGF-β2 stimulation
for 24 h, followed by marked increases following 48 and 72 h of
stimulation. The Nrf2 pathway is involved in cell survival
signaling in response to oxidants and other insults and confers
cellular protection (12).
Therefore, the results of the present study suggested that TGF-β2
maintained RPE cells in an immunosuppressive condition through IDO
regulation, which was possibly followed by an Nrf2-mediated
anti-oxidant response. The immunosuppressive enzyme IDO may have a
significant role in RPE cell-mediated immune suppression and ocular
immune privilege. Ocular immune privilege is a complex phenomenon
which has remained largely elusive; however, IDO may represent an
important modulator. The expression of IDO observed in the present
study may, at least in part, explain for the RPE-mediated
immunosuppressive effects and the role of the RPE in ocular
responses to immune, infectious and inflammatory diseases.
CD4+ CD25+ Tregs are a unique
population of Tregs that maintain peripheral immune tolerance.
Naturally occurring Tregs constitute only 1–5% of total
CD4+ T cells in human peripheral blood. A large number
of Tregs may be required for an effective Treg-based cellular
immunotherapy. Several approaches have been used for the generation
and expansion of Ag-specific human Tregs (22,23).
Hippen et al (24) reported
that CD4+ CD25+ Tregs can be expanded
3,000-fold by culture in the presence of IL-2, rapamycin and
artificial human APCs. With the goal of producing homogeneous
Tregs, Okubo et al (23)
developed a novel expansion protocol targeting tumor necrosis
factor receptors on Tregs. Golshayan et al (9) used autologous DCs as APCs to select
and expand Ag-specific CD4+ CD25+ T-cell
lines from freshly isolated polyclonal CD4+
CD25+ Tregs in vitro, and these Ag-specific
CD4+ CD25+ T cells maintained their
phenotypic characteristics and acquired potent Ag-specific
suppressive activity. It has been indicated that ex
vivo-generated inducible Tregs are significantly more potent
than natural Tregs with regard to immunosuppressive modulation
(9,25). The present study demonstrated that
the supernatant of RPE cells pre-treated with TGF-β2 and IL-2
enhanced the differentiation of CD4+ T cells into
CD4+ CD25+ Tregs. Furthermore, RPE-induced
Tregs suppressed the proliferation of CD4+
CD25− T cells more effectively than natural Tregs and
achieved a ~75% suppression of CD4+ CD25−
T-cell proliferation at a high CD4+
CD25+/CD4+ CD25− T-cell ratio.
These results indicated that TGF-β2 induced RPE cells to expand
Tregs. As it was observed that TGF-β2 induced IDO expression in RPE
cells, it may be hypothesized that IDO is a signaling protein in
RPE cells for the induction of Tregs, which should be verified in
further studies. This mechanism may have an immunosuppressive role
and the resulting RPE-induced Tregs may be applied as potential
immunotherapeutics for ocular inflammatory diseases, including
posterior uveitis or graft rejection of RPE-cell transplantation
(2). Achieving stable RPE-induced
Tregs in the eye is the key for future immunotherapy.
Intraocular migratory T cells from the choroid
vessels must pass through the RPE layer to reach the retinal space.
The present study hypothesized that intraocular effector T cells
can be converted into immunosuppressive CD4+
CD25+ Tregs in the sub-retinal space by cell-cell
contact and the cytokines produced upon their interaction with RPE
cells. Further study is required to understand the complexity of
the immune modulatory roles of RPE cells. The present study
demonstrated the role of RPE cells as a significant immune
modulatory factor contributing to ocular immune privilege. Thus, it
demonstrated that RPE-induced Tregs suppress the proliferation of
alloreactive T cells in vitro, while the in vivo
immune suppression achieved through RPE-induced Tregs need warrants
further investigation.
Acknowledgments
The present study was supported by the Postdoc
Foundation of Jiangsu Province of China (no. 0702040C). This
manuscript has been edited and proofread by Medjaden Bioscience
Ltd. (Hong Kong, China).
Abbreviations:
|
Tregs
|
regulatory T cells
|
|
RPE
|
retinal pigment epithelial
|
|
TGF-β2
|
transforming growth factor-β 2
|
|
IDO
|
indoleamine 2,3-dioxygenase
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor
|
|
MACS
|
magnetic cell sorting
|
|
rIL-2
|
recombinant interleukin-2
|
|
APCs
|
antigen-presenting cells
|
References
|
1
|
Sugita S: Role of ocular pigment
epithelial cells in immune privilege. Arch Immunol Ther Exp
(Warsz). 57:263–268. 2009. View Article : Google Scholar
|
|
2
|
Imai A, Sugita S, Kawazoe Y, Horie S,
Yamada Y, Keino H, Maruyama K and Mochizuki M: Immunosuppressive
properties of regulatory T cells generated by incubation of
peripheral blood mononuclear cells with supernatants of human RPE
cells. Invest Ophthalmol Vis Sci. 53:7299–7309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Detrick B and Hooks JJ: Immune regulation
in the retina. Immunol Res. 47:153–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gregerson DS, Heuss ND, Lew KL, McPherson
SW and Ferrington DA: Interaction of retinal pigmented epithelial
cells and CD4 T cells leads to T-cell anergy. Invest Ophthalmol Vis
Sci. 48:4654–4663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugita S, Horie S, Nakamura O, Futagami Y,
Takase H, Keino H, Aburatani H, Katunuma N, Ishidoh K, Yamamoto Y
and Mochizuki M: Retinal pigment epithelium-derived CTLA-2alpha
induces TGFbeta-producing T regulatory cells. J Immunol.
181:7525–7536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
7
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells in immunological
tolerance to self and non-self. Nat Immunol. 6:345–352. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazaki S and Morita A: Dendritic cells
in the periphery control antigen-specific natural and induced
regulatory T cells. Front Immunol. 4:1512013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Golshayan D, Jiang S, Tsang J, Garin MI,
Mottet C and Lechler RI: In vitro-expanded donor
alloantigen-specific CD4+CD25+ regulatory T cells promote
experimental transplantation tolerance. Blood. 109:827–835. 2007.
View Article : Google Scholar
|
|
10
|
Yamazaki S, Inaba K, Tarbell KV and
Steinman RM: Dendritic cells expand antigen-specific Foxp3+ CD25+
CD4+ regulatory T cells including suppressors of alloreactivity.
Immunol Rev. 212:314–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singer-Sam J, Robinson MO, Bellvé AR,
Simon MI and Riggs AD: Measurement by quantitative PCR of changes
in HPRT, PGK-1, PGK-2, APRT, MTase and Zfy gene transcripts during
mouse spermatogenesis. Nucleic Acids Res. 18:1255–1259. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horie S, Sugita S, Futagami Y, Yamada Y
and Mochizuki M: Human retinal pigment epithelium-induced CD4+CD25+
regulatory T cells suppress activation of intraocular effector T
cells. Clin Immunol. 136:83–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bodaghi B, Goureau O, Zipeto D, Laurent L,
Virelizier JL and Michelson S: Role of IFN-gamma-induced
indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in
the replication of human cytomegalovirus in retinal pigment
epithelial cells. J Immunol. 162:957–964. 1999.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Grohmann U, Fallarino F and Puccetti P:
Tolerance: DCs and tryptophan: Much ado about IDO. Trends Immunol.
24:242–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W: IDO: More than an enzyme. Nat
Immunol. 12:809–811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harden JL and Egilmez NK: Indoleamine
2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest.
41:738–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pallotta MT, Orabona C, Volpi C, Vacca C,
Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M,
Bicciato S, et al: Indoleamine 2,3-dioxygenase is a signaling
protein in long-term tolerance by dendritic cells. Nat Immunol.
12:870–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cousins SW, McCabe MM, Danielpour D and
Streilein JW: Identification of transforming growth factor-beta as
an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis
Sci. 32:2201–2211. 1991.PubMed/NCBI
|
|
20
|
Park CY, Yang SH, Chuck RS, Gehlbach PL
and Park CG: The role of indoleamine 2,3-dioxygenase in retinal
pigment epithelial cell-mediated immune modulation. Ocul Immunol
Inflamm. 18:24–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan K, Han XD and Kan YW: An important
function of Nrf2 in combating oxidative stress: Detoxification of
acetaminophen. Proc Natl Acad Sci USA. 98:4611–4616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Lu L and Jiang S: Regulatory T
cells: Customizing for the clinic. Sci Transl Med. 3:83ps192011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okubo Y, Mera T, Wang L and Faustman DL:
Homogeneous expansion of human T-regulatory cells via tumor
necrosis factor receptor 2. Sci Rep. 3:31532013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hippen KL, Merkel SC, Schirm DK, Sieben
CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL,
Riley JL, et al: Massive ex vivo expansion of human natural
regulatory T cells [T(regs)] with minimal loss of in vivo
functional activity. Sci Transl Med. 3:83ra412011. View Article : Google Scholar
|
|
25
|
Karlsson F, Martinez NE, Gray L, Zhang S,
Tsunoda I and Grisham MB: Therapeutic evaluation of ex
vivo-generated versus natural regulatory T-cells in a mouse model
of chronic gut inflammation. Inflamm Bowel Dis. 19:2282–2294. 2013.
View Article : Google Scholar : PubMed/NCBI
|