Introduction
Autophagy is an adaptive response to cellular stress
induced by conditions such as nutrient deprivation. During
autophagy, cellular components to be degraded are surrounded by
autophagosomes and catabolized after fusion with lysosomes to
provide precursors for synthetic processes and energy production,
which contributes to cellular survival (1).
Tissue kallikrein (TK), an important component of
the kallikrein-kinin system, belongs to a sub-group of serine
proteinases and processes low-molecular-weight kininogen to release
kinin peptide (2). Previous
studies by our group showed that TK exerted neuroprotective effects
in oxygen-glucose-deprived cells as well as in the ischemic brain
(3–5), in which autophagy has been reported
to have an important role in maintaining neuronal survival
(6). It has been reported that TK
is able to activate the Raf/mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase pathway (4) and adenosine
5′-monophosphate-activated protein kinase phosphorylation (7), which participate in autophagy
induction (8,9). However, to date, no direct evidence
has revealed whether autophagy is involved in TK-mediated
protective effects under nutrient deprivation-induced stress
conditions.
β-catenin is a key downstream effector in the Wnt
signaling pathway. The activation of the Wnt/β-catenin axis is
known to increase cell survival and proliferation under various
conditions (10). A recent study
demonstrated that activated β-catenin signaling was able to repress
autophagy. However, β-catenin protein contains a light chain
(LC)3-interacting motif, which is targeted for autophagic
clearance, thus facilitating autophagy induction and cell survival
under nutrient deficiency (11).
The present study used serum-starved SH-SY5Y human
neuroblastoma cells as a canonical model for investigating
autophagy. It was attempted to evaluate the role of TK in
modulating autophagy and its crosstalk with β-catenin signaling
under nutrient deprivation-induced stress conditions. It was
indicated that TK promoted the induction of protective autophagy
along with autophagic degradation of β-catenin and maintained
normal autophagic flux.
Materials and methods
Reagents
TK was purchased from Techpool Bio-Pharma Co.
(Guangzhou, China). Rabbit anti-human LC3A/B (cat no. 12741), LC3B
(cat no. 3868) and Beclin-1 (cat. no. 3495) as well as mouse
anti-human α-tubulin (cat. no. 3873) monoclonal antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Mouse anti-human sequestosome 1 (p62; cat. no. ab56416) and rabbit
anti-human β-catenin (cat. no. ab32572) monoclonal antibodies were
purchased from Abcam (Cambridge, MA, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G (cat. no.
GGHL-15P) and HRP-conjugated goat anti-mouse IgG (cat. no.
GGHL-90P) secondary antibodies were obtained from Immunology
Consultants Laboratory (Portland, OR, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT), 3-methyladenine (3-MA) and NH4Cl were obtained
from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human SH-SY5Y neuroblastoma cells were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were grown in Dulbecco's modified Eagle's medium supplemented
with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Waltham, MA, USA) supplemented with 100 U/ml penicillin and 100
µg/ml streptomycin (Invitrogen) at 37°C in a humidified
atmosphere containing 5% CO2. Cells were grown to 80%
confluency prior to the experiments.
Cell viability and apoptosis assays
SH-SY5Y cells were seeded into 96-well plates at
2×104 cells per well and allowed to adhere overnight.
Cells were treated with indicated concentrations of TK (0, 0.125,
0.25, 0.5 and 1 µM) followed by serum starvation for 12 or
24 h. Subsequently, 10 µl MTT solution (5 mg/ml) was added
to each well, followed by incubation at 37°C for 4 h in the dark.
Following removal of the MTT solution and addition of 150 µl
dimethyl sulfoxide (Sigma-Aldrich) to dissolve the formazan
crystals, the absorbance at 570 nm was measured using a plate
reader (Synergy H1, BioTek Instruments, Winooski, VT, USA). The
cell viability was determined by calculating the absorbance ratio
of treated cells vs. that of unstarved control cells (viability,
100%).
For the apoptosis assay, cells were treated as
stated above, harvested and stained using the FITC Annexin V
Apoptosis Detection Kit I (BD Pharmingen, San Jose, CA, USA)
according to the manufacturer's instructions and subsequently
analyzed using flow cytometry (BD FACSCanto; BD Pharmingen) with BD
FACSDiva software (BD Pharmingen). Quantitative analysis of cell
death by flow cytometry Annexin V/PI staining. Dead cells included
early and late apoptotic cells (lower and upper right quadrants in
dot plot) as well as necrotic cells (upper left quadrant).
Co-immunoprecipitation
Co-immunoprecipitation was performed according to
the manufacturer's instructions of the protein A/G PLUS-Agarose
Immunoprecipitation Reagent (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Briefly, SH-SY5Y cells were lysed on ice in 1 ml
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) for 30 min. Following
pre-clearing with normal IgG (Beyotime Institute of Biotechnology),
cell lysates were incubated overnight at 4°C with anti-LC3B
(diluted at 1:50), followed by precipitation with 20 µl
protein A/G Plus-Agarose (Santa Cruz Biotechnology, Inc.) overnight
at 4°C. The precipitated complexes were washed four times with
phosphate-buffered saline (PBS) and heated at 100°C for 5 min in 5X
sodium dodecyl sulfate (SDS) sample buffer (Beyotime Institute of
Biotechnology). The samples were then separated on 10%
SDS-polyacrylamide gels and immunoblotted with β-catenin monoclonal
antibody overnight at 4°C. Membranes were routinely washed four
times in Tris-buffered saline with 0.1% Tween 20 (TBST) for 5 min
and then incubated with HRP-conjugated goat anti-rabbit IgG for 1 h
at room temperature. The antigen-antibody complexes were visualized
using the Immobilon™ western chemiluminescent HRP substrate
(Millipore, Billerica, MA, USA) and images were captured using the
ChemiDoc XRS+ system (Bio-Rad Laboratories, Hercules, CA, USA).
Western blot analysis
2×107 SH-SY5Y cells were lysed on ice and
washed with ice-cold PBS and lysed on ice for 20 min with RIPA
lysis buffer supplemented with protease inhibitors (Roche
Diagnostics, Basel, Switzerland). Protein concentrations were
determined using a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology). Equal amounts of protein (20 µg) were
separated by electrophoresis on SDS-polyacrylamide gels [prepared
with 30% acrylamide-bisacrylamide, 1 M Tris-HCl (pH 6.8 and pH
8.8), ammonium persulfate and tetramethylethylenediamine; all from
Beyotime Institute of Biotechnology) and transferred to an
Immobilon-P polyvinylidene difluoride membrane (Millipore).
Immunoblotting was performed with the appropriate primary antibody
at 1:1,000 dilution overnight at 4°C, followed by HRP-conjugated
secondary antibody at 1:5,000 dilution for 1 h at room temperature.
Blotted proteins were visualized using enhanced chemiluminescence
(using the Immobilon™ western chemiluminescent HRP substrate) and
images were captured using the ChemiDoc XRS+ system (Bio-Rad
Laboratories). Densitometric analysis was performed using Image J
1.4.3.67 software (National Institutes of Health, Bethesda, MD,
USA) with α-tubulin as the loading control. Relative density values
to α-tubulin for each sample were normalized to the basal levels in
the control group to obtain fold-change values.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Following treatment as stated above, total RNA was
extracted using TRIzol reagent (Invitrogen) according to the
manufacturer's instructions. Complementary DNA was synthesized
using the PrimeScript™ RT Master Mix (no. RR036A; Takara Bio Inc.,
Otsu, Japan). qPCR was performed using a SYBR Green PCR kit (no.
DRR041A; Takara Bio Inc.) in an ABI real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific) according to the
manufacturer's instructions. The following primers (Sangon Biotech,
Shanghai, China) were used: p62 forward,
5′-AAATGGGTCCACCAGGAAACTGGA-3′ and reverse,
5′-TCAACTTCAATGCCCAGAGGGCTA-3′; GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′AAGTGGTCGTTGAGGGCAATG-3′.
The thermocycling conditions were as follows: Polymerase
activation/denaturation (95°C for 30 sec) and 45 amplification
cycles (95°C for 5 sec, 58°C for 30 sec and 72°C for 30 sec).
Relative p62 mRNA levels were normalized to GAPDH and calculated
using the 2−ΔΔCq method. ΔCq was calculated by
subtracting their Cq values from the average Cq value of GAPDH.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences were evaluated by one-way analysis of
variance followed by Bonferroni's post-hoc test with GraphPad Prism
5 software (GraphPad Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
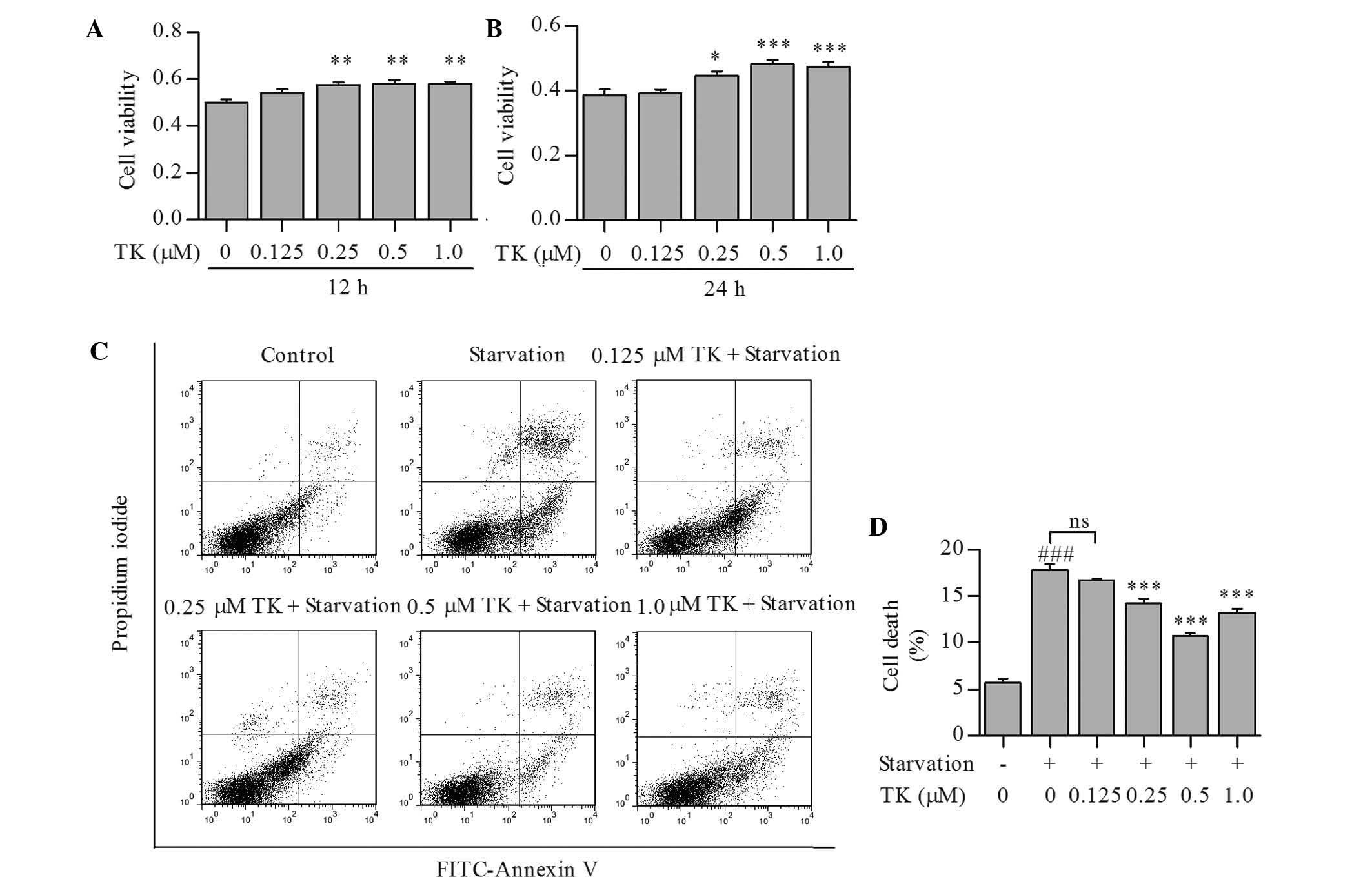
TK promotes survival of SH-SY5Y cells
under serum starvation
To determine whether TK is able to promote cell
survival under nutrient deprivation-induced stress conditions,
SH-SY5Y cells were treated with TK at 0.125–1.0 µM and then
subjected to serum starvation for 12 or 24 h. As shown in Fig. 1A and B, TK augmented the viability
of SH-SY5Y cells in a time- and concentration-dependent manner,
with 0.5 µM of TK displaying the most significant protective
effects at 24 h. Annexin V and propidium iodide staining also
showed that cell death induced by starvation for 24 h was partly
prevented by TK at 0.25–1.0 µM (Fig. 1C and D).
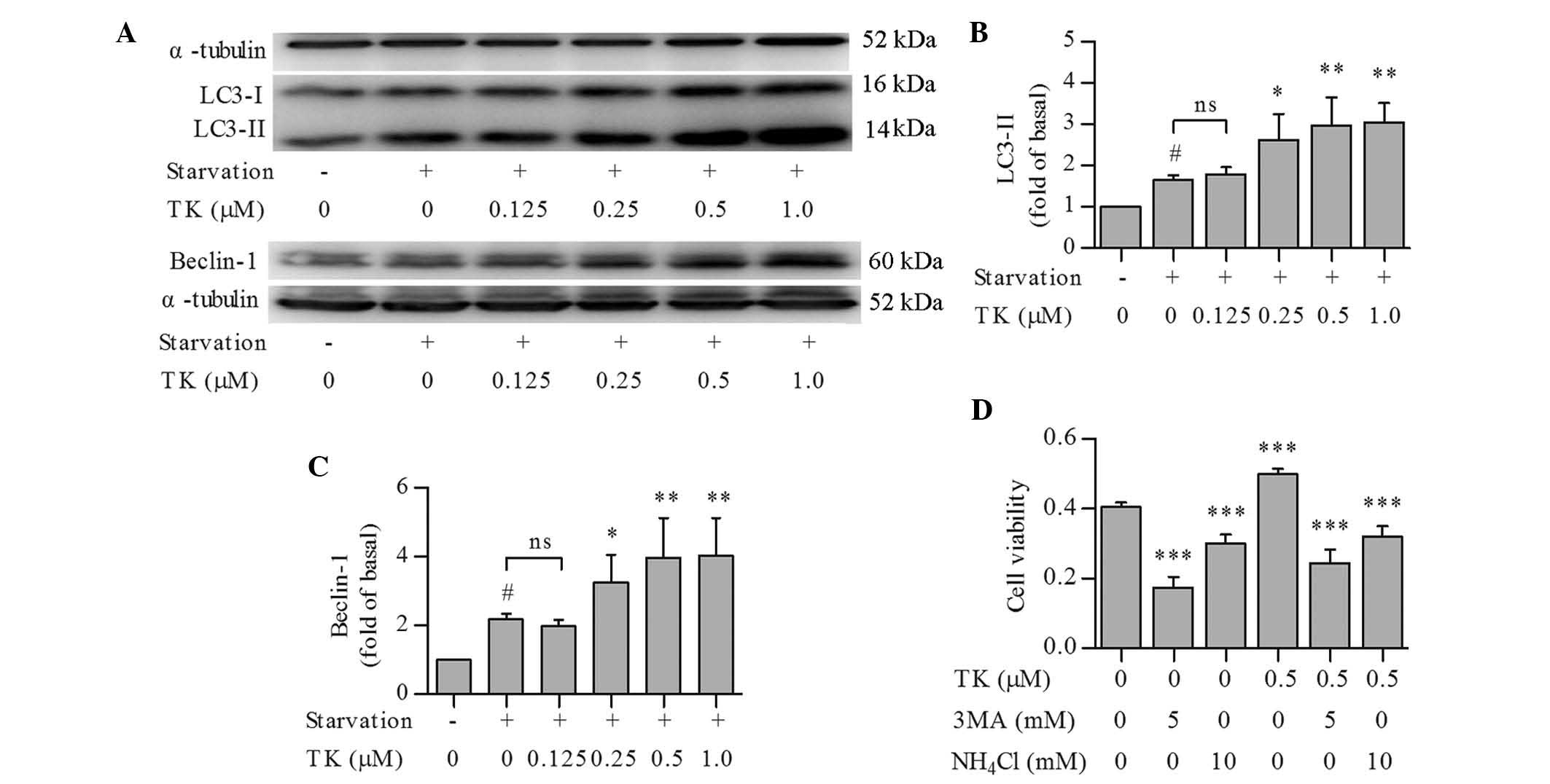
Pro-survival effects of TK are based on
the induction of autophagy
To explore whether autophagic pathways were involved
in the pro-survival effects of TK, LC3 conversion and Beclin-1
expression were assessed as autophagic markers in serum-starved
SH-SY5Y cells pre-treated with TK. Compared to serum starvation
alone, TK concentration-dependently induced a more profound
elevation of LC3-II and expression of Beclin-1 (Fig. 2A–C), with the most significant
effects at 0.5 and 1.0 µM.
In an additional experiment, SH-SY5Y cells were
pre-treated with 0.5 µM TK in combination with the autophagy
inhibitors 3-MA or NH4Cl (12), followed by serum starvation for 24
h and detection of cell viability using an MTT assay. Pre-treatment
with 3-MA or NH4Cl alone diminished the viability of
SH-SY5Y cells under serum starvation. Furthermore, the protective
effects of TK were abolished by 3-MA as well as NH4Cl
(Fig. 2D). These results suggested
that autophagy induction contributed to the pro-survival effects of
TK.
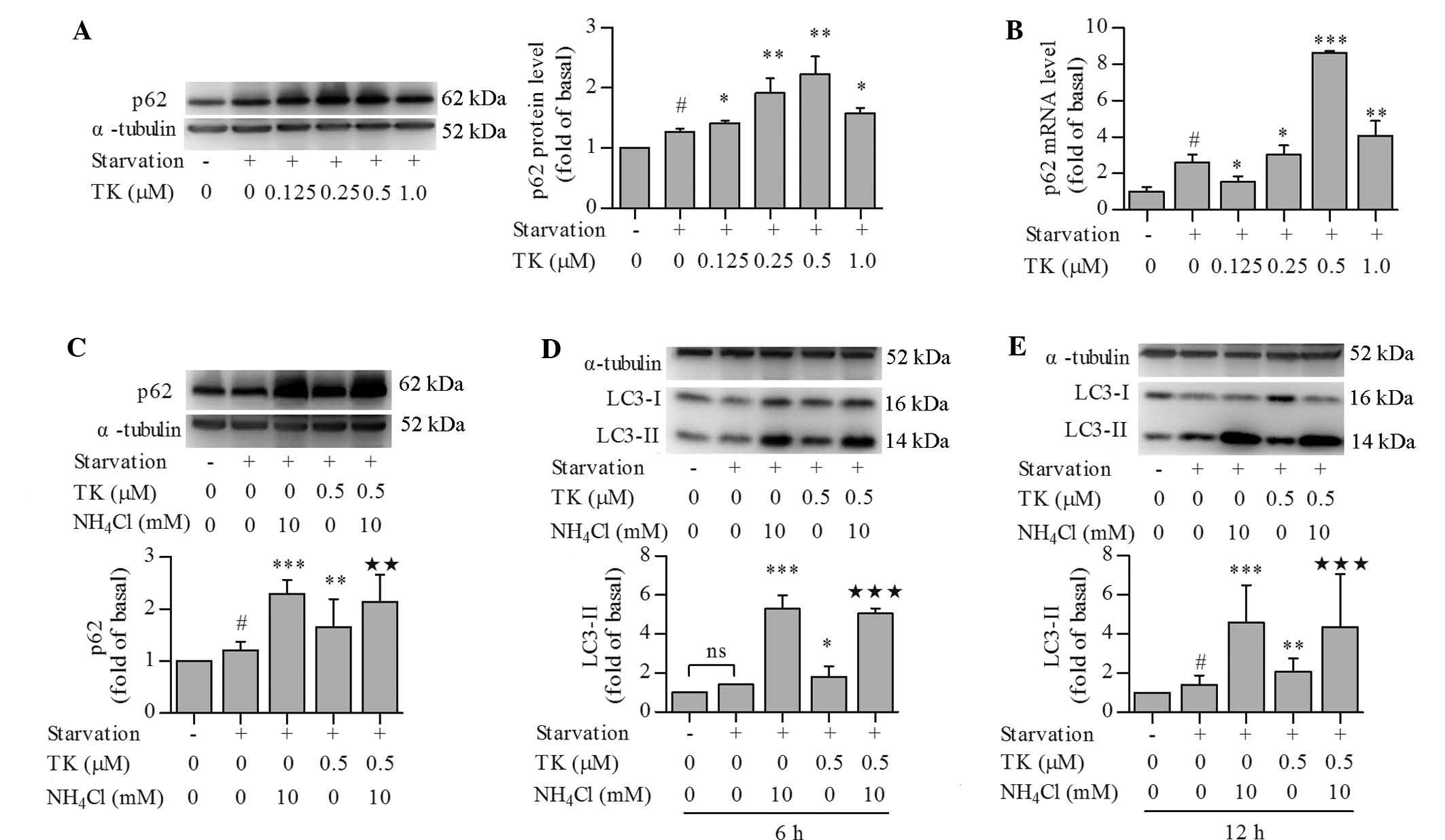
TK upregulates p62 expression while
maintaining normal autophagic flux in serum-starved SH-SY5Y
cells
p62 is known to scavenge soluble mis-folded proteins
for removal in autophagosomes (1)
and can be used as a protein marker of autophagic degradation and
autophagic flux in certain settings (12). Unexpectedly, p62 protein levels
were significantly increased by serum starvation and further
increased by TK. TK elevated the protein levels of p62 in a
concentration-dependent manner, with the most significant changes
at 0.5 µM (Fig. 3A).
Similarly to its effect on p62 protein levels, TK at 0.25–1.0
µM also promoted the transcription of p62 as compared to
that following serum starvation alone, as indicated by RT-qPCR
analysis (Fig. 3B). This result
indicated that increases in p62 protein were due to enhanced
transcription, and did not reflect autophagic flux in this
scenario. To further assess autophagic flux, cells were pre-treated
with 0.5 µM TK in combination with NH4Cl, an
inhibitor of lysosomal acidification that can inhibit autophagic
degradation (12), followed by
serum starvation. NH4Cl significantly raised the protein
levels of p62 in serum-starved SH-SY5Y cells at 12 h, irrespective
of the presence of TK (Fig. 3C),
indicating that p62 was indeed degraded in the autophagic process.
In addition, the effects of NH4Cl on LC3-II levels were
assessed at 6 h (Fig. 3D) and 12 h
(Fig. 3E), which revealed that
NH4Cl led to the accumulation of LC3-II at each
time-point with or without pre-treatment with TK. These results
confirmed that TK maintained a normal autophagic flux.
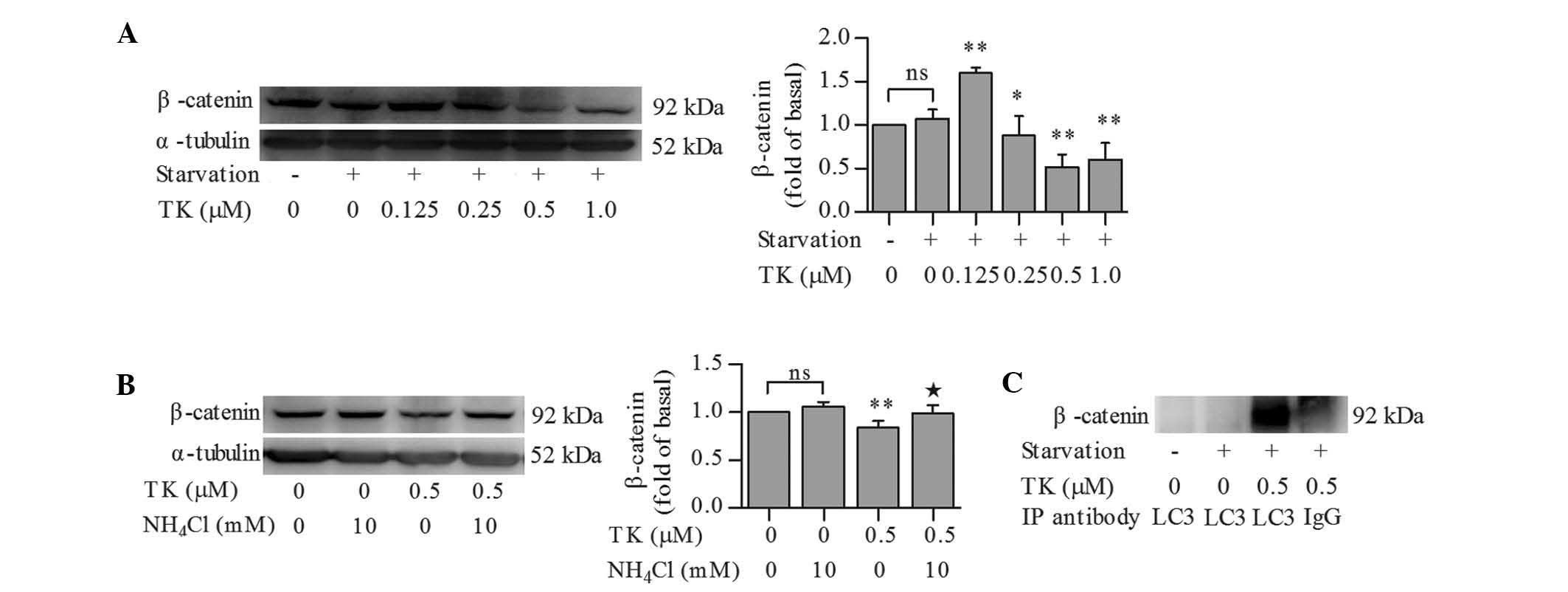
TK stimulates the autophagic degradation
of β-catenin
It was previously reported that β-catenin represses
autophagy but is also degraded during the autophagic process
(11). Therefore, β-catenin levels
in serum-starved SH-SY5Y cells treated in the presence or absence
of TK were assessed. β-catenin expression remained unchanged
following serum starvation for 12 h as compared to that in the
control cells. While 0.125 µM TK led to an upregulation of
β-catenin expression, TK at 0.25–1.0 µM significantly
decreased the levels of β-catenin, with 0.5 µM TK causing
the most signifi-cant reduction of β-catenin (Fig. 4A).
To further investigate whether downregulation of
β-catenin by TK was due to autophagic degradation, β-catenin levels
were examined in serum-starved SH-SY5Y cells pre-treated with 0.5
µM TK in combination with NH4Cl. NH4Cl
abolished the effects of 0.5 µM TK on β-catenin levels in
serum-starved SH-SY5Y cells, while treatment with NH4Cl
alone had no significant impact on β-catenin levels in
serum-starved cells (Fig. 4B).
Co-immunoprecipitation analysis then revealed that TK induced an
interaction between LC3 and β-catenin in serum-starved SH-SY5Y
cells (Fig. 4C), suggesting that
TK stimulated the autophagic degradation of β-catenin.
Discussion
TK has been reported to have important roles in
various physiological and pathological processes (13,14).
The present study revealed that TK enhanced the survival of human
SH-SY5Y cells under serum starvation in a starvation time-and TK
concentration-dependent manner. Previous studies demonstrated that
TK transactivates multiple downstream signaling pathways, including
the phosphoinositide-3 kinase (PI3K)/Akt and epidermal growth
factor receptor (EGFR) signaling (3,15,16).
PI3K/Akt activation and EGFR are able to activate Wnt/β-catenin
signaling but suppress autophagy induction (17–20).
However, the present study revealed that β-catenin expression was
significantly downregulated by TK treatment, accompanied by
increased autophagy, suggesting that the underlying molecular
mechanism is independent of the PI3K/Akt and EGFR pathways.
The findings of the present study are consistent
with a previous study, which reported that Wnt/β-catenin represses
autophagy and p62 expression, while β-catenin itself is targeted by
autophagic clearance in autolysosomes upon induction of autophagy
(11). Indeed, TK was shown to
induce β-catenin downregulation, which was abrogated by
NH4Cl; furthermore, co-immunoprecipitation analysis
revealed that TK stimulated the interaction between LC3 and
β-catenin. These findings indicated that TK promoted the autophagic
degradation of β-catenin to favor adaptation-promoting autophagy,
thereby mitigating the deleterious consequences of continued
proliferation and transcription during stress.
In the experimental model used in the present study,
serum starvation as well as TK treatment increased the protein
levels of p62, which however, is not necessarily indicative of
blocked autophagic flux in this context (8,11).
Under starvation conditions, depletion of p62 has been reported to
inhibit the recruitment of LC3 to autophagosomes and the background
level of LC3-II was shown to be elevated in cells overexpressing
p62, indicating that autophagic activity is increased with
increasing levels of p62 (21). In
the present study, RT-qPCR analysis further suggested that the
observed elevation of p62 protein was, at least partially,
attributed to increased transcription. Furthermore, pre-treatment
with the lysosome inhibitor NH4Cl in the presence as
well as absence of TK led to a recovery of the levels of p62 and
LC3-II, indicating normal autophagic flux.
In conclusion, the present study provided direct
evidence that autophagy contributed to the pro-survival effects of
TK in SH-SY5Y cells under serum starvation. TK initiated autophagy
while maintaining normal autophagic flux. The present study
enhanced the present understanding of key cellular processes
affected by TK and revealed the potential use of TK for alleviating
nutrient deprivation-induced stress conditions such as ischemic
stroke. The underlying molecular mechanisms of the modulation of
autophagy by TK require to be elucidated in future studies.
Acknowledgments
This work was supported by a grant from the National
Natural Science Foundation of China (no. 81271295).
References
|
1
|
Tanida I: Autophagosome formation and
molecular mechanism of autophagy. Antioxid Redox Signal.
14:2201–2214. 2011. View Article : Google Scholar
|
|
2
|
Bhoola KD, Figueroa CD and Worthy K:
Bioregulation of kinins: Kallikreins, kininogens and kininases.
Pharmacol Rev. 44:1–80. 1992.PubMed/NCBI
|
|
3
|
Su J, Tang Y, Zhou H, Liu L and Dong Q:
Tissue kallikrein protects neurons from
hypoxia/reoxygenation-induced cell injury through Homer1b/c. Cell
Signal. 24:2205–2215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Han X, Cui M, Fang K, Lu Z and
Dong Q: Tissue kallikrein protects rat hippocampal CA1 neurons
against cerebral ischemia/reperfusion-induced injury through the
B2R-Raf-MEK1/2-ERK1/2 pathway. J Neurosci Res. 92:651–657. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang Y, Shao Y, Su J, Zhou H, Liu L, Ren H
and Dong Q: The protein therapy of kallikrein in cerebral ischemic
reperfusion injury. Curr Med Chem. 16:4502–4510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Papadakis M, Hadley G, Xilouri M, Hoyte
LC, Nagel S, McMenamin MM, Tsaknakis G, Watt SM, Drakesmith CW,
Chen R, et al: Tsc1 (hamartin) confers neuroprotection against
ischemia by inducing autophagy. Nat Med. 19:351–357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan G, Deng J, Wang T, Zhao C, Xu X, Wang
P, Voltz JW, Edin ML, Xiao X, Chao L, et al: Tissue kallikrein
reverses insulin resistance and attenuates nephropathy in diabetic
rats by activation of phosphatidylinositol 3-kinase/protein kinaseB
and adenosine 5′-monophosphate-activated protein kinase signaling
pathways. Endocrinology. 148:2016–2026. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JH, Hong SK, Wu PK, Richards AL,
Jackson WT and Park JI: Raf/MEK/ERK can regulate cellular levels of
LC3B and SQSTM1/p62 at expression levels. Exp Cell Res.
327:340–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Whiteman MW, Lian H, Wang G, Singh
A, Huang D and Denmark T: A non-canonical MEK/ERK signaling pathway
regulates autophagy via regulating Beclin 1. J Biol Chem.
284:21412–21424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon RT: Wnt/beta-catenin pathway. Sci
STKE. 2005:2005.PubMed/NCBI
|
|
11
|
Petherick KJ, Williams AC, Lane JD,
Ordóñez-Morán P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik
K, Paraskeva C and Greenhough A: Autolysosomal β-catenin
degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J.
32:1903–1916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waeckel L, Potier L, Richer C, Roussel R,
Bouby N and Alhenc-Gelas F: Pathophysiology of genetic deficiency
in tissue kallikrein activity in mouse and man. Thromb Haemost.
110:476–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chao J, Shen B, Gao L, Xia CF, Bledsoe G
and Chao L: Tissue kallikrein in cardiovascular, cerebrovascular
and renal diseases and skin wound healing. Biol Chem. 391:345–355.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao YY, Yin H, Shen B, Smith RS Jr, Liu Y,
Gao L, Chao L and Chao J: Tissue kallikrein promotes
neovascularization and improves cardiac function by the
Akt-glycogen synthase kinase-3beta pathway. Cardiovasc Res.
80:354–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Z, Yang Q, Cui M, Liu Y, Wang T, Zhao H
and Dong Q: Tissue kallikrein induces SH-SY5Y cell proliferation
via epidermal growth factor receptor and extracellular
signal-regulated kinase1/2 pathway. Biochem Biophys Res Commun.
446:25–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chong ZZ and Maiese K: Targeting WNT,
protein kinase B and mitochondrial membrane integrity to foster
cellular survival in the nervous system. Histol Histopathol.
19:495–504. 2004.PubMed/NCBI
|
|
18
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Georgopoulos NT, Kirkwood LA and Southgate
J: A novel bidirectional positive-feedback loop between
Wnt-β-catenin and EGFR-ERK plays a role in context-specific
modulation of epithelial tissue regeneration. J Cell Sci.
127:2967–2982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Y, Zou Z, Becker N, Anderson M,
Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et
al: EGFR-mediated Beclin 1 phosphorylation in autophagy
suppression, tumor progression, and tumor chemoresistance. Cell.
154:1269–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: P62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|