Introduction
Gastric cancer has become a nationwide epidemic due
to changes in lifestyle and deterioration of the environment
threatening human health. Gastric cancer has the second highest
rate of incidence of cancer in males in China, and is one of the
top five causes of cancer-associated mortality (1,2). Due
to its critical malignant properties and poor clinical prognosis,
several investigations have been performed to identify curative
therapies, with little success. Surgical resection at the early
stage and chemotherapy at the late stages prevails as the current
treatment strategy, which limits the amelioration of long-term
efficacy (3,4). The key to developing novel
therapeutics is to clarify the molecular mechanisms underlying the
development and expansion of cancer, which allow for specific
targeted regimens, rather than less specific measures.
The sonic hedgehog (Shh) signaling pathway has
increased in interest in the scientific community, owing to its
potential in cancer (5–7). Shh is a highly-conservative pathway
in mammals. The soluble ligand, Shh, is excreted into the
extracellular matrix and makes contact with the trans-membrane
receptor, Patched, which induces an inhibitory effect on the
downstream smoothened (Smo) molecule. Subsequently, the released
Smo transfers into the primary cilium, leading to an intracellular
cascade and activation of Gli transcription factor family. Gli1 is
a positive-regulator of cell proliferation as a result of
stimulating the expression of downstream genes (8–11).
It has been demonstrated that the inhibition of any component of
the pathway results in early death and deformity, particularly for
Smo and Gli1, indicating its possible role in controlling cancer
growth (12,13).
It is well-known that embryogenesis shares similar
biological behavior with tumorigenesis in its pattern of cell
proliferation and invasion (14,15).
The interplay between these two processes suggests the potential
effect Shh may have on a neoplasm (16). Several studies have confirmed that
the Shh pathway is abnormally activated in patients with cancer,
and artificially induced inhibition of signaling leads to tumor
narrowing (17–19). Following exposure to an
Shh-antagonist, a decrease in pancreatic cancer malignancy was
observed, in the form of proliferation restriction and apoptosis
increase (20,21). In addition, malignant hepatic
carcinoma showed a similar reduction in progression as that
observed in pancreatic cancer (19,22,23).
However, the association between gastric cancer and Shh signaling
remains to be fully elucidated, warranting investigation of the
interconnection of the two. Shh-targeted inhibitors may offer
potential benefit for patients with gastric cancer, if
theoretically and clinically approved.
GDC-0449 is an Smo antagonist, and its clinical
superiority has been approved by the Food and Drug Administration
(FDA) for the treatment of basal cell skin cancer (24,25).
The efficacy of GDC-0449 has been assessed in several types of
solid tumor in vivo and in vitro (25,26),
although the effects in gastric cancer remain to be elucidated. Due
to its promising anticancer effect in various tumors, the present
study aimed to investigate whether GDC-0449 exerts similar effects
in gastric cancer and examine the possible underlying
mechanisms.
Materials and methods
Antibodies and reagents
Western blot detection reagents were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). GDC-0449 was
purchased from Selleck Chemicals (Houston, TX, USA). All other
chemicals used in the present study were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.) and other domestic qualified
providers.
Cell culture
The SGC-7901 cell line was obtained from American
Type Culture Collection (Manassas, VA, USA) and cultured in
high-glucose Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Sijiqing Bio-engineering Co., Ltd., Hangzhou, China), and 1%
penicillin and streptomycin (Thermo Fisher Scientific, Inc.) at
37°C, in a humidified atmosphere of 95% air and 5% CO2.
Passages were used when the cultured cells were at stable status of
70–80% confluence. Routine renewal of culture medium and cell
cryopreservation were managed in a standard, sterile
environment.
Cell Counting Kit-8 (CCK-8)
assessment
The SGC-7901 cell line was cultured in complete
medium in a 96-well plate (100 µl in each well) at a cell
density of 1×105/ml, and each group comprised five
replicates. Following 24 h pre-culture, dimethyl sulfoxide (DMSO)
and 5–50 µM GDC-0449 were added into the wells and
co-cultured for 24–48 h prior to assessment. Subsequently, CCK-8
reagent (Vazyme Biotech Co., Ltd., Nanjing, China) was added to the
wells, and the optical density (OD) values were determined using a
FLx800™ Fluorescence Microplate Reader (BioTek Instruments, Inc.,
Winooski, VT, USA) after 4 h. At a wavelength of 450 nm, the OD
values were counted and used to calculate the proliferation rate. A
single replication plate was used at each time point, and the whole
assessment was repeated at least three times.
Apoptosis assessment
The cells were incubated with complete medium at a
cell density of 1×105/ml. Following 24 h pre-culture,
DMSO and GDC-0449 (5, 10, 20 and 50 µM) were added to the
wells and co-cultured for 24 and 48 h prior to assessment.
Subsequently 37°C trypsin (Beyotime Institute of Biotechnology,
Haimen, China) was used for isolation, prior to three applications
of centrifugation (300 × g, 5 min, 4°C) and phosphate-buffered
saline (PBS) washing steps to purify the cells. Annexin V-propidium
iodide antibodies (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) were added and incubated for 15 min at 25°C. Flow cytometry
(FACSCanto™ II; BD Biosceiences, San Jose, CA, USA) was then
performed for the assessment of apoptosis, to calculate the
percentage of cells in Q4, which indicates early apoptosis. The
assays were performed in triplicate.
Western blot analysis
Cells grown in dishes were washed once in PBS, and
lysed in radioimmunoprecipitation assay buffer [50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and protease
inhibitors] for 10 min on ice. After being cleared by
centrifugation (13,200 × g, 20 min) at 4°C, the protein
concentrations were determined using a Bicinchoninic Acid Protein
Assay kit. (Aspen Biological, Wuhan, China). The lysates (40
µg protein) were then mixed with protein loading buffer,
separated by 8 or 10% SDS-polyacrylamide gel electrophoresis, and
transferred to a nitrocellulose membrane (EMD Millipore, Billerica,
MA, USA). A working dilution of the primary antibodies was made
using 1% non-fat milk-PBS with Tween (PBST). The membrane was
incubated with the diluted primary antibodies for 1 h at room
temperature or overnight at 4°C with gentle agitation. The membrane
was then washed in 1X PBST three times (10 min/wash) with
agitation. Subsequently, the membrane was incubated for 1–2 h at
room temperature in an appropriately diluted secondary antibody
solution, prepared in the same blocking buffer as the primary
antibody. The membrane was washed a further three times in 1X PBST
(10 min/wash) with agitation. The chemiluminescent substrate (KPL,
Gaithersburg, MD, USA) was prepared prior to use, according to the
manufacturer's protocol. The membrane was incubated in the
substrate as instructed by the manufacturer. The blots were then
scanned (LiDE 110; Canon, Inc., Tokyo, Japan), and analyzed using
ImageJ2 software (National Institutes of Health, Bethesda, MD, USA)
All results were verified in triplicate. The primary antibodies
used were as follows: Rabbit anti-human glyceraldehyde 3-phosphate
dehydrogenase (1:10,000; cat. no. ab37168; Abcam, Shanghai, China);
rabbit anti-human Gli1 (1:1,000; cat. no. sc-20687; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); and rabbit anti-human Bcl-2
(1:1,000; cat. no. 2870S; Cell Signaling Technology, Inc., Danvers,
MA, USA). The secondary antibody used was a peroxidase-conjugated
goat anti-rabbit immuno-globulin G (H+L) (1:5,000; cat. no.
074-1506) purchased from Jackson ImmunoResearch Laboratories, Inc.
(West Grove, PA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNAs were extracted from the colon cancer
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcription was performed using PrimeScript RT
Master mix (Takara Biotechnology, Co., Ltd., Dalian, China),
according to the manufacturer's protocol. The cDNAs (2 µl)
were amplified in a 25 µl reaction system using SYBR Premix
Ex TaqTM (Takara Biotechnology, Co., Ltd.). Primers (10 µM
each) specific for each of the signaling molecules were designed
using NCBI/Primer-BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and used to
generate the PCR products. For the quantification of gene
amplification, qPCR was performed using an Applied Biosystems
StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific.,
Inc.). The following gene specific primers were used: Smo forward
5′-CTG GTA CGA GGA CGT GGAGG-3′ and reverse 5′-AGG GTG AAG AGC GTG
CAGAG-3′; Gli1, forward 5′-TTC CTA CCA GAG TCC CAAGT-3′ and reverse
5′-CCC TAT GTG AAG CCC TATTT-3′; CD44, forward 5′-GTA GTA CAA CGG
AAG AAACA-3′ and reverse 5′-TGT GAG ATT GGG TTG AAGAA-3′; CD133,
forward 5′-GCA CTC TAT ACC AAA GCG TCAA-3′ and reverse 5′-CAC GAT
GCC ACT TTC TCACT-3′; Bcl-2, forward 5′-GAC TTC GCC GAG ATG
TCCAG-3′ and reverse 5′-GGT GCC GGT TCA GGT ACTCA-3′; β-actin,
forward 5′-TCA CCC ACA CTG TGC CCA TCT ACG-3′ and reverse 5′-CAG
CGG AAC CGC TCA TTG CCA ATGG-3′. Target sequences were amplified at
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. β-actin was used as an endogenous normalization control.
All assays were performed in triplicate and were calculated on the
basis of ΔΔCq. The n-fold change in mRNA expression was determined
according to the 2−ΔΔCq method (27).
Statistical analysis
The data in each group are expressed as the mean ±
standard deviation. Differences between groups were analyzed using
one- or two-way analysis of variance using Prism 5 statistical
analysis software (GraphPad Software, Inc., San Diego, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
GDC-0449 downregulates the Ssh pathway by
antagonizing the activity of Smo
As described above, GDC-0449 is a Smo antagonist,
which attenuates the progression of skin cancer and several types
of solid tumor. The results of the RT-qPCR assays performed in the
present study confirmed that the expression of Gli1 reduced
markedly in the SGC-7901 cell line when treated with GDC-0449,
which occurred in a dose- (5–50 µM) and time-dependent
manner (Fig. 1A). A higher dose
and longer exposure duration resulted in lower expression levels,
and vice versa. Up to 70% of the expression level was restricted at
the highest state of inhibition. Western blot analysis confirmed
the inhibitory effects of 10 µM, compared with the control
group (Fig. 1B). These findings
indicated that GDC-0449 was a Smo antagonist and was used in the
following experiments.
Cell reproduction rate is inhibited in
the SGC-7901 cell line on GDC-0449 exposure
Shh signaling is vital for cell proliferation,
therefore, the present study hypothesized that the Smo antagonist,
GDC-0449, can reduce the proliferation of the SGC-7901 cell line. A
notable decrease in cell proliferation rate was observed following
24 and 48 h exposure of the SGC-7901 cell line to GDC-0449
(Fig. 2). In further data
analysis, 48 h exposure restricted cell growth more markedly,
compared with that observed at 24 h (Fig. 2). The highest dose suppressed over
half of the reproduction rate observed in the blank group.
GDC-0449 increases the apoptotic rate of
the SGC-7901 cell line
The inhibitory effect on growth, described above,
led to the investigation of whether the induction of apoptosis by
GDC-0449 contributed to the apoptotic effect. Flow cytometry is the
standard measure to calculate apoptotic rate. The Q4 percentage
refers to early apoptosis and provides a good representation of
drug-induced apoptosis. As expected, an increase in the apoptotic
rate was observed in the SGC-7901 cell line, in a dose- and
time-dependent manner following GDC-0449 exposure (Fig. 3). In contrast to the results of the
CCK-8 test, a 50 µM dose led to a reduction in the early
apoptotic rate, compared with the rate at 20 µM, with a
higher ratio of necrosis, suggesting that 20 µM may
represent the peak anti-apoptotic efficacy, rather than 50
µM (Fig. 3), which exerted
a more toxic effect. This result may partially explain the
antigrowth efficacy of GDC-0449.
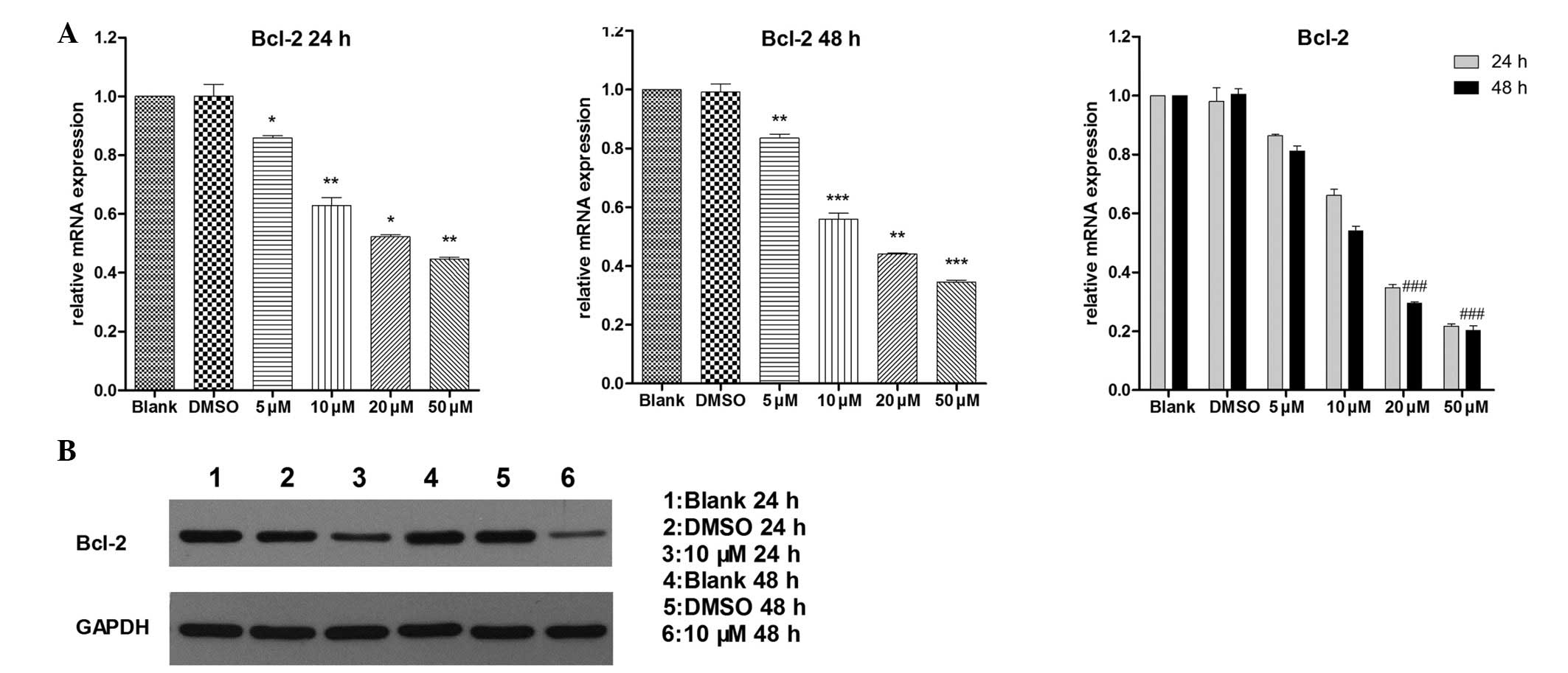
Bcl-2 is a downstream target of
GDC-0449
Bcl-2 is a widely investigated confirmed
anti-apoptoric gene, which is involved in different procedures of
cell fate determination. The pro-apoptotic activity of GDC-0449,
described above, suggested that Bcl-2 may contribute to this
effect. RT-qPCR (Fig. 4A) and
western blot (Fig. 4B) analyses
were performed to confirm this hypothesis by determining the
expression levels of RNA and protein. The expression level of Bcl-2
was reduced in a dose-dependent manner. At the peak level of
inhibition, over half of the expression was decreased, which
demonstrated the critical role Bcl-2 may have in the GDC-0449's
downstream target.
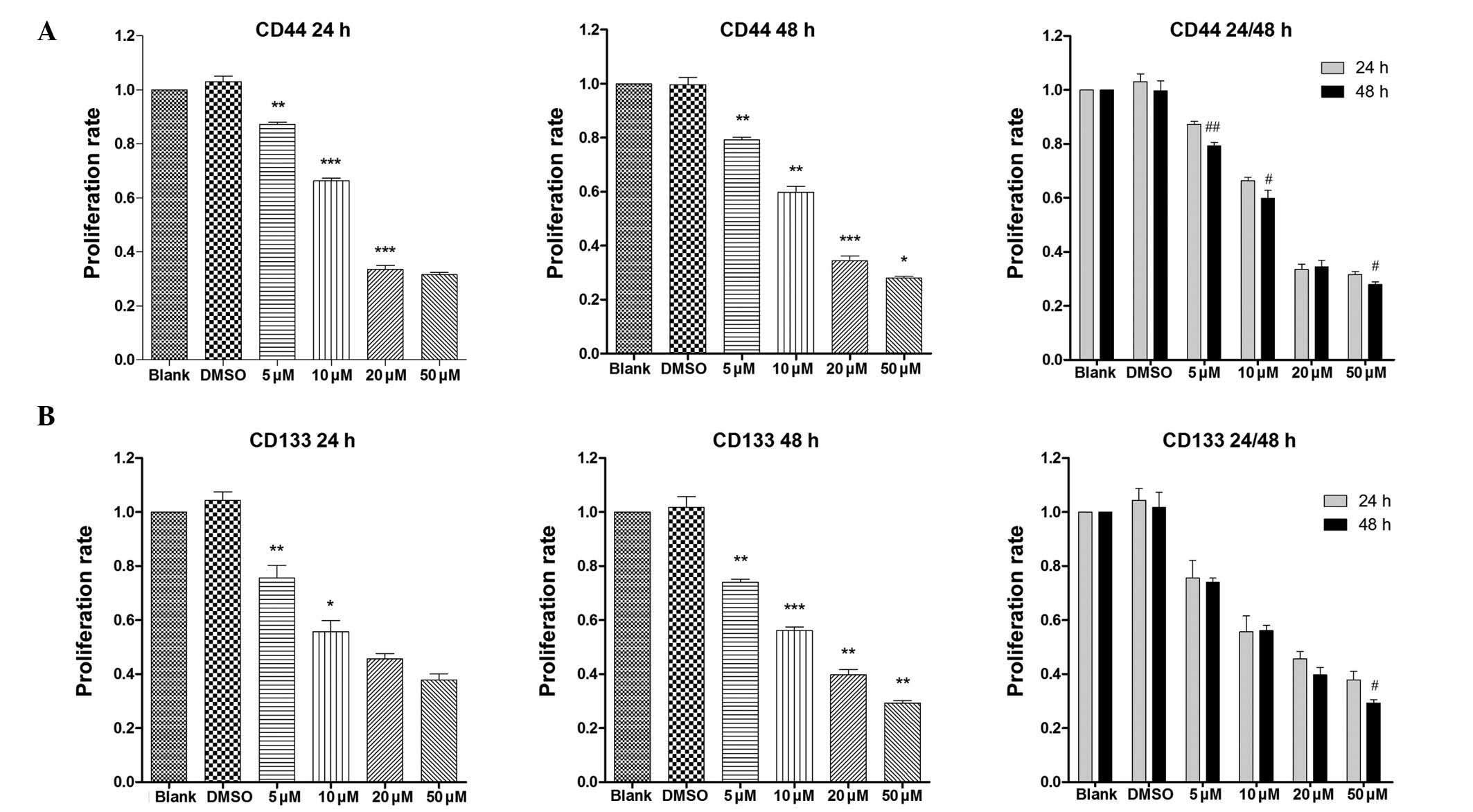
Cancer stem cell surface markers share
inhibitory effects with Smo
CD133 and CD44 have been recognized as gastric
cancer stem cell surface markers. Markers represent the status and
stem-ness of the cells. No previous studies have reported the
latent association between GDC-0449 and surface markers. In the
present study, the compelling RT-qPCR data showed a notable decline
in the expression of surface markers in a dose- and time-dependent
manner, compared with the blank group (Fig. 5). These results suggested that
GDC-0449 and the Ssh signaling may affect cancer stem cells via a
specific pathway, which remains to be elucidated.
Discussion
Gastric cancer has the highest incidence and
cancer-associated mortality rates of all types of cancer of the
digestive system (28). At
present, there are no targeted treatment regimens, limiting the
improvement of 5-year survival rates in patients with advanced
disease (29). Several clinicians
and researchers have devoted substantial efforts to improving
treatment of the malignancy.
The Ssh pathway has been actively investigated, in
terms of its regulation of embryonic growth, and is key in tissue
differentiation and in the formation of the central nervous system
in mammals (30–32). As the expansive proliferation of
cancer cells is similarity to that of embryonic development, the
critical role of Shh in cell proliferation and differentiation has
led to it being associated with tumorigenesis in cancer. Previously
published evidence has indicated an interaction between Ssh
signaling and tumor malignancies. In almost every type of solid
tumor, marked abnormal activation of the Ssh pathway exists, often
in contrast to the reduced expression levels observed among normal
differentiated tissues (33,34).
Previous studies have demonstrated that Shh-target treatment
conveys superior antitumor value in skin cancer, and several types
of solid tumors are in accordance with its theoretical mechanisms
(21,35–37).
This has led it to become a focal area of investigation for
oncologists and pharmacologists.
GDC-0449 has risen in prominence as a novel Smo
inhibitor. Owing to its clinical approval by the FDA for the
treatment of basal cell carcinoma, GDC-0449 provides superior
effects, compared with its relatives in the Shh-antagonist family
in terms of its medical efficacy (38,39).
According to in vivo and in vitro assays, pancreatic
cancer is also affected by the GDC-0449, indicating a probable
assault in digestive tumors as well (24,40,41).
The fundamental preliminary experiments in the present study also
exhibited clinical excellence in treating colon cancer cells. Taken
together, the present study on gastric cancer provided significant
and timely results, indicating the irreplaceable role of the
'Shh-Smo-Gli' pathway and the underlying antagonist in gastric
cancer. Further investigation of the downstream mechanisms
identified Bcl-2 as a target site for GDC-0449, required to exert
its functions.
However, a convincing and comprehensive set of
results includes in vivo and in vitro confirmation,
regardless of successful but restricted in vitro outcomes.
Consequently, more detailed assays are required to obtain these
data, with the aim of providing potential clinical benefit in the
future.
Cancer stem cells have attracted attention in the
scientific community. Ssh signaling is considered to be involved in
regulating the stem-ness of CSC (42). Tumor invasiveness, relapse and
metastasis are markedly associated with CSC (43). The suppression of CSC and its
natural properties may reduce malignancy and improve patient
prognosis. This interplay between Ssh signaling and cancer stem
cells requires further investigation. In the present study, CD133
and CD44, which are surface markers of gastric cancer stem cells,
showed significantly reduced expression levels in the SGC-7901 cell
line following exposure to GDC-0449, indicating that GDC-0449 and
the Ssh signaling pathway may affect the maintenance of gastric
cancer stem cells.
In the present study, GDC-0449 demonstrated similar
antitumor efficacy in the SGC-7901 cell line as skin and pancreatic
cancer. In a dose- and time-dependent manner, exposure to GDC-0449
reduced the normal replication of the cells and triggered apoptosis
in a destructive manner. The anti-apoptotic Bcl-2 gene was
identified as a downstream target of GDC-0449 in the repression of
expression. Furthermore, the effects on the CSC surface markers
indicates the requirement to investigate potential mechanisms
underlying the interactions of GDC-0449 and Shh with cancer stem
cells. The results of the present study confirm that GDC-0449 may
be a suitable candidate for use in gastric cancer therapy, and may
benefit future patients.
Acknowledgments
The authors would like to thank all team members for
methodological guidance and support. The authors would also like to
thank Dr Guangchun Jin (Department of Medicine, Columbia University
Medical Center, New York, NY, USA) for his advice and instruction.
This study was supported by the National Natural Science Foundation
of China to KaiXiong Tao (grant. no. 81172294) and the Research
Fund of Public Welfare in the Health Industry (grant no. 201202015)
and the Health and Family Plan Committee of China, 2014.
References
|
1
|
Anderson WF, Camargo MC, Fraumeni JF Jr,
Correa P, Rosenberg PS and Rabkin CS: Age-specific trends in
incidence of noncardia gastric cancer in US adults. JAMA.
303:1723–1728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang WH, Chen XZ, Liu K, Chen XL, Yang K,
Zhang B, Chen ZX, Chen JP, Zhou ZG and Hu JK: Outcomes of surgical
treatment for gastric cancer patients: 11-year experience of a
Chinese high-volume hospital. Med Oncol. 31:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon IG, Cho I, Choi YY, Hyung WJ, Kim CB
and Noh SH: Risk factors for complications during surgical
treatment of remnant gastric cancer. Gastric Cancer. 18:390–396.
2015. View Article : Google Scholar
|
|
5
|
Vorechovský I, Benediktsson KP and
Toftgård R: The patched/hedgehog/smoothened signalling pathway in
human breast cancer: No evidence for H133Y SHH, PTCH and SMO
mutations. Eur J Cancer. 35:711–713. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romer J and Curran T: Targeting
medulloblastoma: Small-molecule inhibitors of the Sonic Hedgehog
pathway as potential cancer therapeutics. Cancer Res. 65:4975–4978.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Athar M, Li C, Kim AL, Spiegelman VS and
Bickers DR: Sonic hedgehog signaling in Basal cell nevus syndrome.
Cancer Res. 74:4967–4975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Wang Y, Dong J, Wei W, Song B, Min
H, Yu Y, Lei X, Zhao M, Teng W and Chen J: Developmental
hypothyroxinemia and hypothyroidism reduce proliferation of
cerebellar granule neuron precursors in rat offspring by
downregulation of the sonic hedgehog signaling pathway. Mol
Neurobiol. 49:1143–1152. 2014. View Article : Google Scholar
|
|
9
|
Petrova E, Rios-Esteves J, Ouerfelli O,
Glickman JF and Resh MD: Inhibitors of Hedgehog acyltransferase
block Sonic Hedgehog signaling. Nat Chem Biol. 9:247–249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Álvarez-Buylla A and Ihrie RA: Sonic
hedgehog signaling in the postnatal brain. Semin Cell Dev Biol.
33:105–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen M, Qian Y, Dai J and Chu R: The sonic
hedgehog signaling pathway induces myopic development by activating
matrix metalloproteinase (MMP)-2 in Guinea pigs. PLoS One.
9:e969522014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaefer GI, Perez JR, Duvall JR, Stanton
BZ, Shamji AF and Schreiber SL: Discovery of small-molecule
modulators of the Sonic Hedgehog pathway. J Am Chem Soc.
135:9675–9680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stanton BZ and Peng LF: Small-molecule
modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst.
6:44–54. 2010. View
Article : Google Scholar
|
|
14
|
Woodland HR: Some studies on early
embryonic development relevant to the study of cancer. J Clin
Pathol Suppl (R Coll Pathol). 7:26–30. 1974. View Article : Google Scholar
|
|
15
|
Martin GR: Teratocarcinomas as a model
system for the study of embryogenesis and neoplasia. Cell.
5:229–243. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omenetti A and Diehl AM: The adventures of
sonic hedgehog in development and repair II. Sonic hedgehog and
liver development, inflammation and cancer. Am J Physiol
Gastrointest Liver Physiol. 294:G595–G598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Batsaikhan BE, Yoshikawa K, Kurita N,
Iwata T, Takasu C, Kashihara H and Shimada M: Cyclopamine decreased
the expression of sonic hedgehog and its downstream genes in colon
cancer stem cells. Anticancer Res. 34:6339–6344. 2014.PubMed/NCBI
|
|
18
|
Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W,
Lei J, Ma J, Wang X, Lv S, et al: Sonic hedgehog paracrine
signaling activates stromal cells to promote perineural invasion in
pancreatic cancer. Clin Cancer Res. 20:4326–4338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen JS, Huang XH, Wang Q, Huang JQ, Zhang
LJ, Chen XL, Lei J and Cheng ZX: Sonic hedgehog signaling pathway
induces cell migration and invasion through focal adhesion
kinase/AKT signaling-mediated activation of matrix
metalloproteinase (MMP)-2 and MMP-9 in liver cancer.
Carcinogenesis. 34:10–19. 2013. View Article : Google Scholar
|
|
20
|
Rosow DE, Liss AS, Strobel O, Fritz S,
Bausch D, Valsangkar NP, Alsina J, Kulemann B, Park JK, Yamaguchi
J, et al: Sonic Hedgehog in pancreatic cancer: From bench to
bedside, then back to the bench. Surgery. 152(3 Suppl 1): S19–S32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodova M, Fu J, Watkins DN, Srivastava RK
and Shankar S: Sonic hedgehog signaling inhibition provides
opportunities for targeted therapy by sulforaphane in regulating
pancreatic cancer stem cell self-renewal. PLoS One. 7:e460832012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau
HI, Chang FY and Tsai HH: Activation of the sonic hedgehog
signaling pathway occurs in the CD133 positive cells of mouse liver
cancer Hepa 1–6 cells. Onco Targets Ther. 6:1047–1055. 2013.
View Article : Google Scholar
|
|
23
|
Che L, Yuan YH, Jia J and Ren J:
Activation of sonic hedgehog signaling pathway is an independent
potential prognosis predictor in human hepatocellular carcinoma
patients. Chin J Cancer Res. 24:323–331. 2012. View Article : Google Scholar
|
|
24
|
Amin SH, Tibes R, Kim JE and Hybarger CP:
Hedgehog antagonist GDC-0449 is effective in the treatment of
advanced basal cell carcinoma. Laryngoscope. 120:2456–2459. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh BN, Fu J, Srivastava RK and Shankar
S: Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits
pancreatic cancer stem cell characteristics: Molecular mechanisms.
PLoS One. 6:e273062011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karlou M, Lu JF, Wu G, Maity S, Tzelepi V,
Navone NM, Hoang A, Logothetis CJ and Efstathiou E: Hedgehog
signaling inhibition by the small molecule smoothened inhibitor
GDC-0449 in the bone forming prostate cancer xenograft MDA PCa
118b. Prostate. 72:1638–1647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li QQ, Skinner J and Bennett JE:
Evaluation of reference genes for real-time quantitative PCR
studies in Candida glabrata following azole treatment. BMC Mol
Biol. 13:222012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.PubMed/NCBI
|
|
29
|
Papenfuss WA, Kukar M, Oxenberg J, Attwood
K, Nurkin S, Malhotra U and Wilkinson NW: Morbidity and mortality
associated with gastrectomy for gastric cancer. Ann Surg Oncol.
21:3008–3014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Luca A, Parmigiani E, Tosatto G,
Martire S, Hoshino M, Buffo A, Leto K and Rossi F: Exogenous Sonic
hedgehog modulates the pool of GABAergic interneurons during
cerebellar development. Cerebellum. 14:72–85. 2015. View Article : Google Scholar
|
|
31
|
Lewis S: Neural development: Double agent
sonic hedgehog. Nat Rev Neurosci. 14:666–667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Courchet J and Polleux F: Sonic hedgehog,
BOC and synaptic development: New players for an old game. Neuron.
73:1055–1058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Ding H, Rao G, Arora S, Saclarides
CP, Esparaz J, Gattuso P, Solorzano CC and Prinz RA: Activation of
the Sonic Hedgehog pathway in thyroid neoplasms and its potential
role in tumor cell proliferation. Endocr Relat Cancer. 19:167–179.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaw A, Gipp J and Bushman W: The Sonic
Hedgehog pathway stimulates prostate tumor growth by paracrine
signaling and recapitulates embryonic gene expression in tumor
myofibroblasts. Oncogene. 28:4480–4490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa Willd extract
suppresses Sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dahmane N, Lee J, Robins P, Heller P and
Ruiz i Altaba A: Activation of the transcription factor Gli1 and
the Sonic hedgehog signalling pathway in skin tumours. Nature.
389:876–881. 1997. View
Article : Google Scholar
|
|
37
|
Fan H, Oro AE, Scott MP and Khavari PA:
Induction of basal cell carcinoma features in transgenic human skin
expressing Sonic Hedgehog. Nat Med. 3:788–792. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dreno B, Basset-Seguin N, Caro I, Yue H
and Schadendorf D: Clinical benefit assessment of vismodegib
therapy in patients with advanced basal cell carcinoma. Oncologist.
19:790–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin VT, Sniegowski M and Esmaeli B:
Indications and limitations of vismodegib for basal cell carcinoma.
JAMA Ophthalmol. 132:905–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim EJ, Sahai V, Abel EV, Griffith KA,
Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al:
Pilot clinical trial of hedgehog pathway inhibitor GDC-0449
(Vismodegib) in combination with gemcitabine in patients with
metastatic pancreatic adenocarcinoma. Clin Cancer Res.
20:5937–5945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lorusso PM, Jimeno A, Dy G, Adjei A,
Berlin J, Leichman L, Low JA, Colburn D, Chang I, Cheeti S, et al:
Pharmacokinetic dose-scheduling study of hedgehog pathway inhibitor
vismodegib (GDC-0449) in patients with locally advanced or
metastatic solid tumors. Clin Cancer Res. 17:5774–5782. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang SN, Fu J, Nall D, Rodova M, Shankar S
and Srivastava RK: Inhibition of sonic hedgehog pathway and
pluripotency maintaining factors regulate human pancreatic cancer
stem cell characteristics. Int J Cancer. 131:30–40. 2012.
View Article : Google Scholar :
|
|
43
|
Heiden KB, Williamson AJ, Doscas ME, Ye J,
Wang Y, Liu D, Xing M, Prinz RA and Xu X: The sonic hedgehog
signaling pathway maintains the cancer stem cell self-renewal of
anaplastic thyroid cancer by inducing snail expression. J Clin
Endocrinol Metab. 99:E2178–E2187. 2014. View Article : Google Scholar : PubMed/NCBI
|