Introduction
The liver may be exposed to various harmful factors,
which result in an inflammatory response, including viruses,
certain medicines, self-immunity and alcohol consumption (1). Severe injury may result in liver
fibrosis and cirrhosis, however, the liver has a regenerative
capacity, and liver cells proliferate in response to various types
of damage (2,3). Liver precursor cells, also termed
oval cells, are key in the repair of liver tissue following injury
(4). Following severe injury,
hepatic oval cells proliferate and differentiate into several
lineages, including biliary epithelia cells, hepatocytes,
intestinal epithelial cells and, possibly, exocrine pancreas cells
(5–8). Therefore, preservation of hepatic
oval cells by natural products is a promising strategy for the
prevention and treatment of liver diseases.
Tanshinone IIA (TSA) is an extract from the sage
plant, Salvia miltiorrhiza, which was previously found to
protect rat livers from fibrosis by promoting the apoptosis of
hepatic stellate cells (9,10). TSA has also been demonstrated to
improve liver function by inhibiting the activation of hepatic
stellate cells, reducing the production of extracellular matrix and
protecting hepatocytes (11,12).
However, whether TSA improves the function of the liver, or
protects the liver from injury by modulating the biology of hepatic
oval cells, remains to be elucidated.
The molecular pharmacology of the protective effect
of TSA on the liver is also unknown. Wnt signaling is important in
the regulation of growth of various cell types, and in the
maintenance and differentiation of stem cells. Notably, Wnt
signaling is required for tissue repair and regeneration (13–15).
β-catenin is a key downstream component in the canonical Wnt
signaling pathway, and regulates liver cell proliferation (16). Therefore, TSA may protect the liver
via activation of the Wnt/β-catenin signaling pathway.
The present study aimed to investigate the effects
of TSA on the growth, proliferation and survival of hepatic oval
cells, and examine the activity of Wnt/β-catenin signaling in
hepatic oval cells following TSA treatment. The study may provide a
novel treatment option in future, for patients with liver
disease.
Materials and methods
Cell culture
The WB-F344 rat hepatic oval cell line was obtained
from the Beijing Institute of Transfusion Medicine (Beijing,
China), and is a cell line, which has been widely used in previous
studies (13,14). The cells were maintained in
Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
0.5% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere of 5% CO2. TSA
was obtained from Xi'an Haoxuan Bio-technique, Co., Ltd. (Xi'an,
China).
Cell Counting Kit-8 (CCK-8) assay
The WB-F344 cells were seeded into 96-well culture
plates (5×103 cells/well). After 6 h, increasing doses
of TSA were administered to the cells (10, 20, 40, 60 and 80
µg/ml). At 24, 48, 72 and 96 h, 10 µl CCK-8 reagent
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
to each well and incubated for 4 h. Absorbance values at a
wavelength of 450 nm were recorded using a microplate reader
(SpectraMax 250; GE Healthcare Life Sciences, Pittsburgh, PA, USA).
Viability (%) was calculated based on the optical density (OD)
values, as follows: (OD of TSA treated sample - blank) / (OD of
control sample - blank) ×100.
5-ethynyl-2′-deoxyuridine (EdU)
incorporation assay
The cells were cultured on coverslips in 24-well
plates (2×104 cells/well) and stimulated with TSA. After
24, 48, 72 and 96 h, the cells were treated with 50 µM EdU
(Guangzhou RiboBio, Co., Ltd., Guangzhou, China) for an additional
2 h at 37°C. Following treatment, the culture medium was discarded
and the cells were washed twice with Hyclone phosphate-buffered
saline (PBS; GE Healthcare Life Sciences, Logan, UT, USA). The
cells were fixed with 4% formaldehyde (Beijing Zhongyuan Huadun
Technology Trade Co., Ltd., Beijing, China) for 30 min, followed by
addition of 200 µl glycine (2 mg/ml; Amresco, LLC, Solon,
OH, USA) to each well. After 5 min, the cells were washed twice
with PBS and incubated with 0.5% Triton X-100 (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) for 10 min at
room temperature. Following washing with PBS for 5 min, 1X Apollo
reaction reagent (Guangzhou RiboBio Co., Ltd.) was added (200
µl/well) and the plates were incubated at room temperature
in the dark for 30 min, following which the cells were stained with
200 µl Hoechst 33342 (5 µg/ml; Guangzhou RiboBio Co.,
Ltd.) for an additional 30 min in the dark. Subsequent to washing
with PBS twice, the cells were analyzed using a laser-scanning
confocal microscope (TCS SP5; Leica Microsystems GmbH, Wetzlar,
Germany).
Carboxyfluorescein diacetate succinimidyl
ester (CFSE) assay
The cells, at a concentration of ~106
cells/ml, were suspended in 4°C PBS and treated with CFSE (1:1,000;
Invitrogen; Thermo Fisher Scientific, Inc.). The cells were
agitated gently for 5 min in the dark, followed by addition of FBS
to a final concentration of 10%. Following centrifugation at 80 × g
for 5 min, the supernatant was discarded. The cells were
resuspended in DMEM/F12 culture medium, with 10% FBS, and plated in
6-well plates (106 cells/well). After 6 h, the cells
were treated with various concentrations of TSA and, 24, 48, 72,
and 96 h following treatment, the cells were trypsinized (Hyclone;
GE Healthcare Life Sciences) from the plates, centrifuged at 80 × g
for 5 min, fixed with 4% formaldehyde (500 µl/well) for 30
min at room temperature, and analyzed by flow cytometry using a 488
nm argon-ion laser (BD FACSCalibur; BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
The total proteins were extracted from cells using
radioimmunoprecipitation assay lysis buffer (Merck Millipore,
Dermstadt, Germany), containing 50 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 0.5% deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mM
phenylmethylsulfonyl fluoride and 1 µg/ml protease
cocktail). The concentration of the total protein was determined
using a Micro BCA™ Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein samples (60 µg/lane) were
separated by 10% SDS-PAGE (Amresco, LLC) and transferred onto
nitrocellulose membranes (Beijing Solarbio Science & Technology
Co., Ltd.). The membranes were then blocked with 5% w/v non-fat
dried milk dissolved in Tris-buffered saline and 0.1% Tween-20, pH
8.3 (TBST; Hyclone; GE Healthcare Life Sciences) at room
temperature for 1 h. The membranes were then incubated with primary
antibodies at 4°C overnight. The primary antibodies used were as
follows: Rabbit polyclonal anti-rat β-catenin (1:400; cat. no.
BS3603; Bioworld Technology, Inc., St. Louis Park, MN, USA) and
mouse monoclonal anti-rat β-actin (1:2,000; cat. no. A5441;
Sigma-Aldrich, St. Louis, MO, USA). Antigens were retrieved for 5
min in a microwave at high power followed by 13 min at mid-low
power in 10 mM citrate buffer (pH 6.0). The membranes were
subsequently washed with TBST for 15 min and incubated with the
corresponding horseradish peroxidase-conjugated secondary
antibodies at room temperature for 1 h: Goat monoclonal anti-rabbit
Single Domain Antibody (0.05 µg/ml; cat. no. ab191866;
Abcam, Cambridge, MA, USA) and mouse monoclonal anti-actin
(1:2,000; cat. no. ab3280). The immunoreactive bands were
visualized using an ECL reagent (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), according to the manufacturer's protocol. Protein
band intensities were quantified using Quantity One®
software (version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Immunofluorescence staining
WB-F344 cells were cultured until they reached 70%
confluency in glass-bottom microwell dishes (MatTek Corporation,
Ashland, MA, USA), fixed with 4% paraformaldehyde (Nanjing Oriental
Pearl Industry & Trade Co., Ltd., Nanjing, China) and incubated
with the β-catenin antibody (1:50) following permeabilization with
0.2% Triton-X-100. All images were taken using a laser-scanning
microscope (FV1000; Olympus Corporation, Tokyo, Japan).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using RNAiso Plus
(Takara Biotechnology Co., Ltd., Dalian, China). Total RNA (1
µg) was reverse transcribed into single-stranded cDNA using
a PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.).
RT-qPCR was performed for β-catenin and β-actin using SYBR Premix
Ex Taq II (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. The primers (Beijing SBS Genetech Co.,
Ltd., Beijing, China) used were as follows: Sense
5′-GCCAGTGGATTCCGTACTGT-3′ and anti-sense
5′-GAGCTTGCTTTCCTGATTGC-3′ for β-catenin; and sense
5′-TCAGGTCATCACTATCGGCAAT-3′ and antisense
5′-AAAGAAAGGGTGTAAAACGCA-3′ for β-actin. Reactions were performed
in triplicate on an ABI PRISM® 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
following conditions: 95°C for 30 sec; followed by 40 cycles of
95°C for 15 sec; 60°C for 34 sec; a dissociation program of 95°C
for 15 sec; 60°C for 60 sec; 95°C for 30 sec; and 60°C for 15 sec.
β-actin served as an internal control, and melting curve analysis
was performed to ensure that only one PCR product was formed. The
relative quantity of RNA was calculated using the 2−ΔΔCq
method (17).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
The WB-F344 cells (cultured on cover slips in
24-well plates at a density of 2×104 cells per well)
were fixed with 4% paraformaldehyde in PBS, permeabilized by 0.1%
Triton X-100, treated with TUNEL reagent (Beyotime Institute of
Biotechnology, Haimen, China) and incubated in the dark for 30 min
at room temperature. Images were captured using an FV1000
laser-scanning microscope (Olympus Corporation, Tokyo, Japan). The
cell nuclei, which were stained with fluorescein isothiocyanate
(FITC) and DAPI, indicated apoptotic cells. The percentage of
TUNEL-positive cells in the total cell population was quantitated
to assess the apoptotic index (18).
Statistical analysis
All results are expressed as the mean ± standard
deviation and were analyzed using one-way analysis of variance.
Statistical analyses were performed using SPSS for Windows, version
17.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference. Each experiment
was repeated at least three times, and representative graphs are
presented.
Results
TSA promotes the proliferation of WB-F344
hepatic oval cells
To determine the effects of TSA on the proliferation
of WB-F344 oval cells, the cells were treated with 10, 20, 40, 60
and 80 µg/ml TSA for 24, 48, 72 and 96 h, and cell
proliferation was analyzed using CCK-8, EdU and CFSE assays. The
percentages of cell viability, indicated by CCK-8 are listed in
Table I. At 10 µg/ml, TSA
did not affect cell proliferation at any of the four time points
assayed. By contrast, 20 µg/ml TSA stimulated oval cell
proliferation within 72 h, particularly at the 48 h time point. At
40 µg/ml, TSA stimulated oval cell proliferation at each
time point, with the percentage of proliferation at 48 h higher,
compared with the percentages at 72 and 96 h. Treatment with 60 and
80 µg/ml TSA resulted in loss of cell viability at each time
point. Thus, treatment with moderate concentrations of TSA (20–40
µg/ml) for 48–72 h promoted the proliferation of WB-F344
oval cells, whereas higher concentrations of TSA (60–80
µg/ml) inhibited the proliferation of the WB-F344 oval
cells.
 | Table IConcentration- and time-dependent
effects of TSA on WB-F344 hepatic oval cell viability. |
Table I
Concentration- and time-dependent
effects of TSA on WB-F344 hepatic oval cell viability.
| Time-point (h) | Cell viability (%)
|
|---|
| 10 µg/ml
TSA | 20 µg/ml
TSA | 40 µg/ml
TSA | 60 µg/ml
TSA | 80 µg/ml
TSA |
|---|
| 24 | 97±1.82 | 122±3.41a | 140±6.42a | 98±3.80 | 80±3.10a |
| 48 | 103±1.92 | 126±1.82a | 138±1.31a | 101±0.97 | 65±0.87a |
| 72 | 105±2.01 | 118±1.66b | 116±1.62b | 90±1.10b | 70±0.86a |
| 96 | 103±1.01 | 105±1.18 | 109±0.99b | 91±0.89b | 71±0.67a |
According to the results of the CCK-8 assay, the
WB-F344 oval cells were subsequently treated with 10, 20 and 40
µg/ml TSA for 24–96 h, and EdU and CFSE assays were
performed. The EdU assay demonstrated that all three concentrations
of TSA promoted cell proliferation within 72 h, particularly in the
40 µg/ml group at 24 h, the 20 µg/ml group at 48 h
and the 10 µg/ml group at 72 h. At 96 h, the differences
between the TSA-treated groups and control group were apparent,
however they were not as marked as at 24, 48 and 72 h (Fig. 1). The proliferation index values of
the WB-F344 oval cells were obtained from CFSE assays (Fig. 2). In the 40 µg/ml group, the
proliferation index value was markedly higher than the other groups
at each time-point. However, the 10 and 20 µg/ml TSA groups
also exhibited higher proliferation indices than the untreated
control group at 24, 48 and 96 h. At 72 h, the proliferation
indices for the 10 µg/ml group was comparable with the
control group, however, this result was not consistent with the
data from the CCK-8 and EdU assays. At the 48 h time-point, 20 and
40 µg/ml TSA were found to stimulate cell proliferation,
compared with the control and 10 µg/ml TSA groups. After 72
h, the proliferation indices for all the three concentrations were
similar to those in the 24 and 48 h time-points, with no
significant differences between these three groups. After 96 h, the
proliferation index values in groups administered TSA were higher
than that of the control group, particularly in the 40 µg/ml
group. The results from the present study demonstrated that 20–40
µg/ml TSA promoted the proliferation of WB-F344 oval
cells.
TSA does not induce apoptosis of WB-F344
hepatic oval cells
It was previously reported that TSA induces the
apoptosis of rat hepatic stellate cells (9,10).
To assess whether TSA induces apoptosis of hepatic oval cells, the
WB-F344 cells were treated with 10, 20, and 40 µg/ml TSA for
24, 48, 72 and 96 h, and cellular apoptosis was determined using a
TUNEL assay (Fig. 3). No
TUNEL-positive cells, indicated by FITC and DAPI staining in the
cell nucleus, were observed in the untreated control group or
TSA-treated groups at any time point, suggesting that treatment
with TSA up to 40 µg/ml does not induce apoptosis of WB-F344
hepatic oval cells.
TSA increases the expression levels of
β-catenin in WB-F344 oval cells
β-catenin is a key component in the canonical Wnt
signaling pathway and has been demonstrated to regulate liver cell
proliferation (16). To
investigate whether TSA promotes the proliferation of WB-F344 oval
cells via activating Wnt/β-catenin signaling, the WB-F344 oval
cells were treated with 10, 20 and 40 µg/ml TSA, and the
expression levels of β-catenin were assayed using western blotting,
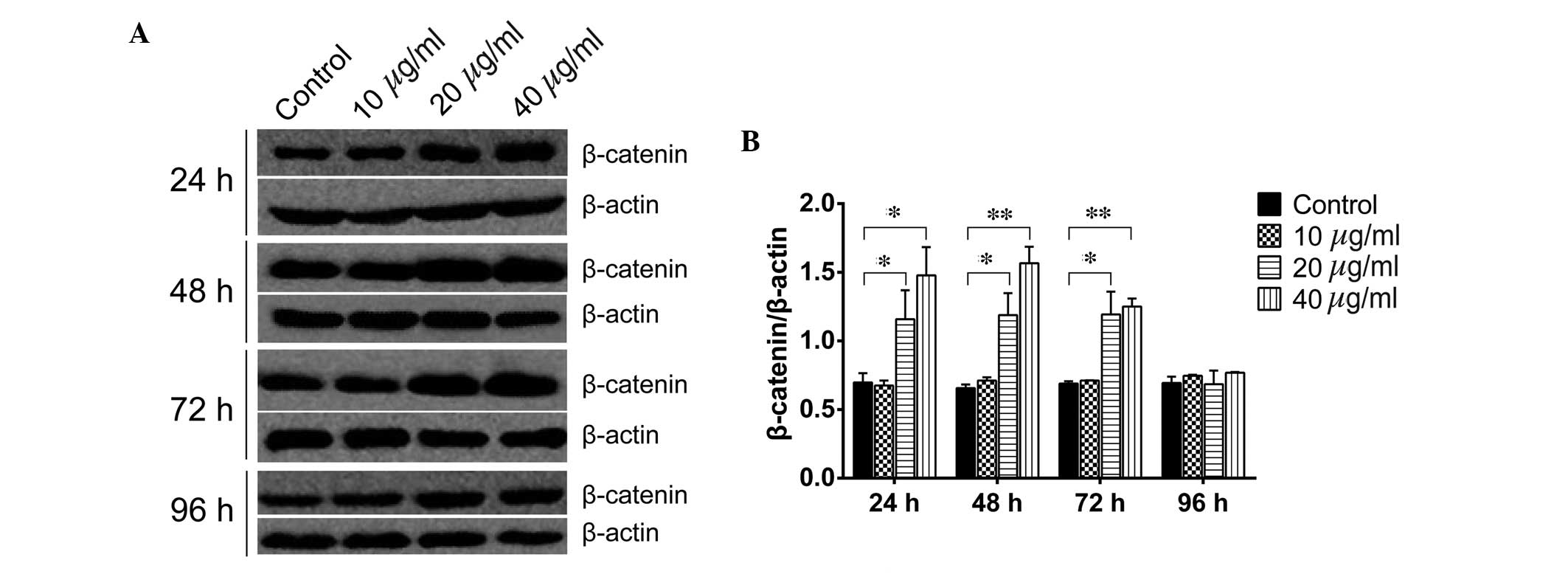
immunofluorescence and RT-qPCR analysis. As shown in Fig. 4, treatment of the WB-F344 oval
cells with 20 µg/ml TSA for 24, 48 and 72 h significantly
increased the protein levels of β-catenin (P=0.0313, P=0.0359 and
P=0.0390, respectively). Similarly, treatment of the WB-F344 oval
cells with 40 µg/ml TSA for 24, 48 and 72 h significantly
upregulated the protein expression levels of β-catenin (P=0.0202,
P=0.0063 and P=0.0071, respectively). However, no differences were
observed between the control group and the TSA groups at 96 h
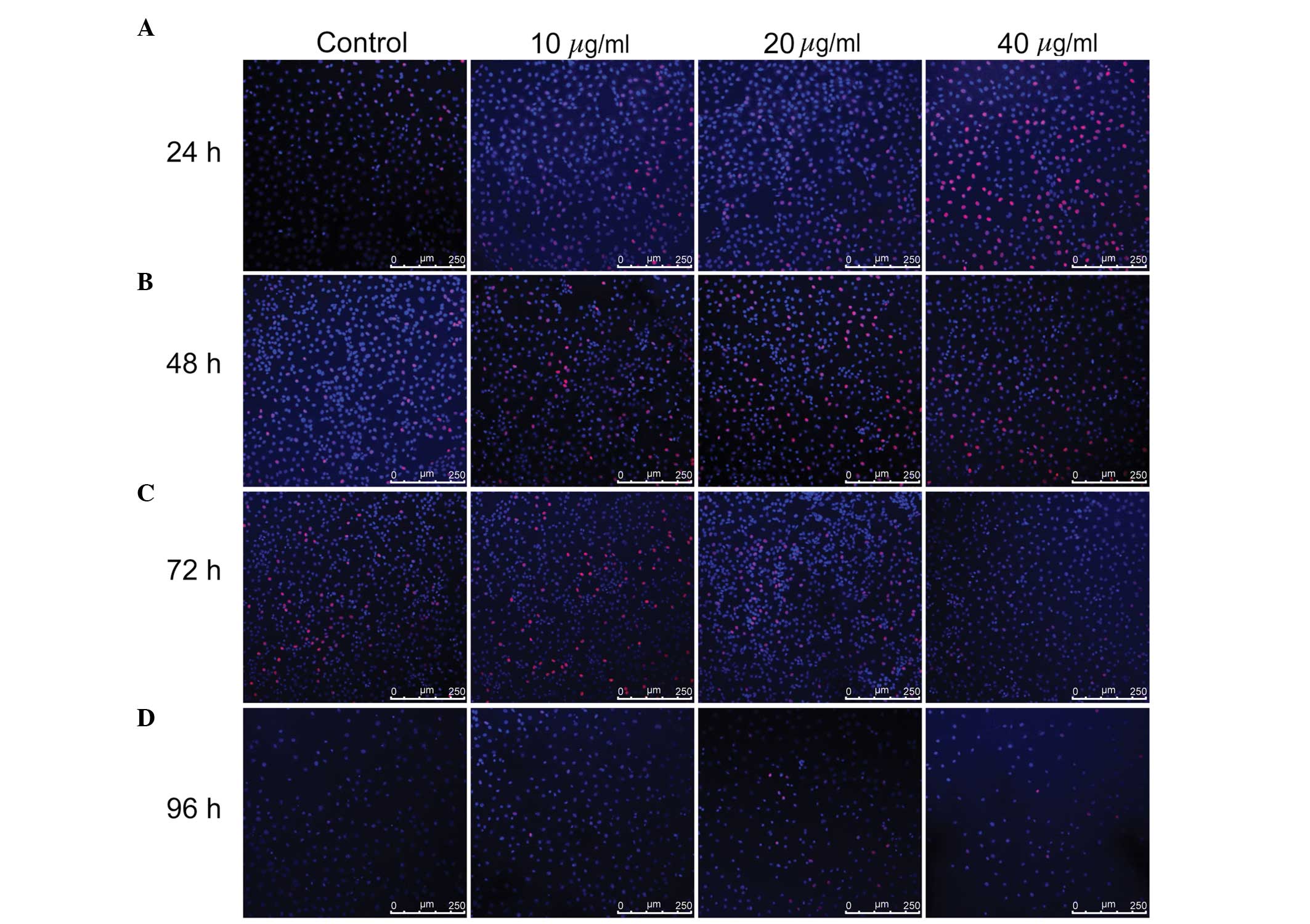
(P>0.05). Immunofluorescence staining (Fig. 5) indicated that 20 µg/ml TSA
significantly upregulated β-catenin at the 24, 48 and 72 h time
points (P=0.0006, P=0.0005 and P=0.0243, respectively), as did 40
µg/ml TSA (P=0.0004, P=0.0003 and P=0.0098, respectively).
After 96 h, neither 20 nor 40 µg/ml TSA altered the
expression levels of β-catenin (P>0.05). Consistent with the
upregulation in the protein expression levels of β-catenin, the
RT-qPCR analysis demonstrated that treatment with 20 and 40
µg/ml TSA for 24–72 h significantly increased the mRNA
expression levels of β-catenin (Fig.
6). However, 10 µg/ml TSA did not alter the mRNA
expression levels at any time point, and TSA did not affect the
mRNA level of β-catenin after 96 h at any concentration
(P>0.05). These results indicated that activation of the
Wnt/β-catenin signaling pathway is one of the underlying molecular
mechanisms by which TSA enhances the proliferation of hepatic oval
cells.
Discussion
In the present study, it was demonstrated that TSA
promoted the proliferation of WB-F344 hepatic oval cells. Notably,
TSA was observed to upregulate the expression levels of β-catenin
in the WB-F344 oval cells. These results suggested that activation
of Wnt/β-catenin signaling is one of the mechanisms by which TSA
enhances the proliferation of hepatic oval cells and protects the
liver against injury.
Hepatic progenitor cells, also termed oval cells,
proliferate and differentiate into hepatocytes and biliary
epithelial cells following stimulation, including viral infection
and cytotoxic drugs. It has been suggested that hepatic oval cells
facilitate liver regeneration following orthotopic liver
transplantation (19). Previous
studies have demonstrated that WB-F344 oval cells are important in
the repair and regeneration of injured liver (3,20).
Accordingly, the WB-F344 oval cell line is considered a hepatic
stem cell line (11,21). Furthermore, hepatic oval cells
retain progenitor cell features without spontaneous malignant
transformation following prolonged cultivation, thus, the WB-F344
oval cell line may serve as an expandable cell source for future
exploitation of stem cell technology (22).
Salvia miltiorrhiza is a plant whose roots
have been used in traditional Chinese medicine for >2,000 years
and has been shown to mediate concentration-dependent anti-fibrosis
(23). TSA has been identified as
one of the predominant extracts of Salvia miltiorrhiza, and
clinical trials have demonstrated that TSA promotes blood
circulation and improves cardiovascular disease (24,25),
improves heart function by enhancing myocardial contractility,
inhibits extracellular matrix deposition, and limits apoptosis by
cardiomyocytes and oxidative damage (26). TSA also inhibits the proliferation
of hepatic stellate cells through enhanced apoptosis, which is
induced by stimulating the extracellular signal-regulated
kinase-Bcl-2-associated X protein-caspase signaling pathways via
the RAF proto-oncogene serine/threonine-protein kinase/prohibitin
complex (9). A previous study
demonstrated that TSA interacts with a non-classical estrogen
receptor to maintain an appropriate balance between the net
deposition of collagen and elastin, while providing optimal
durability and resilience of newly deposited matrix (27). However, the effect of TSA on the
growth, proliferation and survival of hepatic progenitor cells
remains to be elucidated. In the present study, using CCK-8, EdU
and CFSE assays, TSA was demonstrated to promote the proliferation
of WB-F344 oval cells.
The results of the CCK-8 assay revealed that 10–40
µg/ml TSA significantly induced proliferation of the hepatic
oval cells within 72 h of treatment, but not at 96 h
post-treatment. However, higher concentrations of TSA (60–80
µg/ml) inhibited hepatic oval cell proliferation, which was
readily observed 72 and 96 h following treatment, indicating that
high concentrations of TSA were cytotoxic to the oval cells.
Furthermore, the EdU assay indicated that 10–40 µg/ml TSA
stimulated cell proliferation following treatment for 24 and 48 h,
and the CFSE assay demonstrated that the cell proliferative index
value of 10, 20 and 40 µg/ml TSA were higher than that of
the control group at each time point assayed. These results were
consistent with previous studies of different cell types,
indicating that TSA induces or inhibits cell proliferation
depending on the concentration of TSA administered (28–30).
In addition, the TUNEL assay performed in the present study
demonstrated that low concentrations of TSA (<40 µg/ml)
had no stimulatory effect on hepatic oval cell apoptosis.
Previous studies have indicated that the
Wnt/β-catenin and Notch signaling pathways are upregulated in
undifferentiated, proliferating and potentially migrating hepatic
progenitor cells during severe progressive canine liver disease
(31). Furthermore, the canonical
Wnt signaling pathway was found to be key in regulating the
proliferation and self-renewal of hepatic oval cells (1). In the present study, the expression
levels of β-catenin in hepatic oval cells following treatment with
various concentrations of TSA for different time periods was
investigated using western blot, immunofluorescence and RT-qPCR
analyses. β-catenin was significantly upregulated following
treatment with 20–40 µg/ml TSA for 72 h. These results
suggested that TSA may have activated the canonical Wnt signaling
pathway, which stimulated proliferation of the hepatic oval
cells.
In conclusion, the results of the present study
indicated that TSA stimulated the proliferation of WB-F344 rat
hepatic oval cells via activation of the canonical Wnt signaling
pathway. These findings suggest that TSA treatment may promote the
repair and regeneration of injured liver, or improve liver
regeneration following orthotopic liver transplantation.
Acknowledgments
The authors would like to thank Medjaden Bioscience
Limited (Hong Kong, China) for assisting in the preparation of this
manuscript.
References
|
1
|
Zhang Y, Li XM, Zhang FK and Wang BE:
Activation of canonical Wnt signaling pathway promotes
proliferation and self-renewal of rat hepatic oval cell line
WB-F344 in vitro. World J Gastroenterol. 14:6673–6680. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lowes KN, Croager EJ, Olynyk JK, Abraham
LJ and Yeoh GC: Oval cell-mediated liver regeneration: Role of
cytokines and growth factors. J Gastroenterol Hepatol. 18:4–12.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walkup MH and Gerber DA: Hepatic stem
cells: In search of. Stem Cells. 24:1833–1840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farber E: Similarities in the sequence of
early histological changes induced in the liver of the rat by
ethionine, 2-acety-lamino-fluorene, and
3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 16:142–148.
1956.PubMed/NCBI
|
|
5
|
Thorgeirsson SS: Hepatic stem cells. Am J
Pathol. 142:1331–1333. 1993.PubMed/NCBI
|
|
6
|
Fausto N: Oval cells and liver
carcinogenesis: An analysis of cell lineages in hepatic tumors
using oncogene transfection techniques. Prog Clin Biol Res.
331:325–334. 1990.PubMed/NCBI
|
|
7
|
Sell S: Is there a liver stem cell? Cancer
Res. 50:3811–3815. 1990.PubMed/NCBI
|
|
8
|
Sigal SH, Brill S, Fiorino AS and Reid LM:
The liver as a stem cell and lineage system. Am J Physiol.
263:G139–G148. 1992.PubMed/NCBI
|
|
9
|
Pan TL and Wang PW: Explore the molecular
mechanism of apoptosis induced by tanshinone IIA on activated rat
hepatic stellate cells. Evid Based Complement Alternat Med.
2012:7349872012. View Article : Google Scholar
|
|
10
|
Che XH, Park EJ, Zhao YZ, Kim WH and Sohn
DH: Tanshinone II A induces apoptosis and S phase cell cycle arrest
in activated rat hepatic stellate cells. Basic Clin Pharmacol
Toxicol. 106:30–37. 2010.
|
|
11
|
Niu XH, Hua HY, Guo WJ, Zhang Y, Liu M,
Hong Y, Wu PF, Lu P and Zhang HF: Clinical efficiency of tanshinone
IIA-sulfonate in treatment of liver fibrosis of advanced
schistosomiasis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi.
25:137–140. 2013.In Chinese. PubMed/NCBI
|
|
12
|
Sun RF, Liu LX and Zhang HY: Effect of
tanshinone II on hepatic fibrosis in mice. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 29:1012–1017. 2009.In Chinese.
|
|
13
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Willert K, Brown JD, Danenberg E, Duncan
AW, Weissman IL, Reya T, Yates JR III and Nusse R: Wnt proteins are
lipid-modified and can act as stem cell growth factors. Nature.
423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reis M and Liebner S: Wnt signaling in the
vasculature. Exp Cell Res. 319:1317–1323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Micsenyi A, Tan X, Sneddon T, Luo JH,
Michalopoulos GK and Monga SP: Beta-catenin is temporally regulated
during normal liver development. Gastroenterology. 126:1134–1146.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan L, Shi X, Liu S, Guo X, Zhao M, Cai R
and Sun G: Fluoride promotes osteoblastic differentiation through
canonical Wnt/β-catenin signaling pathway. Toxicol Lett. 225:34–42.
2014. View Article : Google Scholar
|
|
18
|
Saraste A, Arola A, Vuorinen T, Kytö V,
Kallajoki M, Pulkki K, Voipio-Pulkki LM and Hyypiä T: Cardiomyocyte
apoptosis in experimental coxsackievirus B3 myocarditis. Cardiovasc
Pathol. 12:255–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Chen J, Li L, Ran JH, Liu J, Gao TX,
Guo BY, Li XH, Liu ZH, Liu GJ, et al: In vitro proliferation and
differentiation of hepatic oval cells and their potential capacity
for intrahepatic transplantation. Braz J Med Biol Res. 46:681–688.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dezső K, Papp V, Bugyik E, Hegyesi H,
Sáfrány G, Bödör C, Nagy P and Paku S: Structural analysis of
oval-cell-mediated liver regeneration in rats. Hepatology.
56:1457–1467. 2012. View Article : Google Scholar
|
|
21
|
Li WQ, Li YM, Guo J, Liu YM, Yang XQ, Ge
HJ, Xu Y, Liu HM, He J and Yu HY: Hepatocytic precursor (stem-like)
WB-F344 cells reduce tumorigenicity of hepatoma CBRH-7919 cells via
TGF-beta/Smad pathway. Oncol Rep. 23:1601–1607. 2010.PubMed/NCBI
|
|
22
|
Wang P, Cong M, Liu TH, Yang AT, Cong R,
Wu P, Tang SZ, Xu Y, Wang H, Wang BE, et al: Primary isolated
hepatic oval cells maintain progenitor cell phenotypes after
two-year prolonged cultivation. J Hepatol. 53:863–871. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin YL, Hsu YC, Chiu YT and Huang YT:
Antifibrotic effects of a herbal combination regimen on hepatic
fibrotic rats. Phytother Res. 22:69–76. 2008. View Article : Google Scholar
|
|
24
|
Zhang H, Long M, Wu Z, Han X and Yu Y:
Sodium tanshinone IIA silate as an add-on therapy in patients with
unstable angina pectoris. J Thorac Dis. 6:1794–1799. 2014.
|
|
25
|
Mao S, Wang L, Zhao X, Shang H, Zhang M
and Hinek A: Sodium tanshinone IIA sulfonate for reduction of
periprocedural myocardial injury during percutaneous coronary
intervention (STAMP trial): Rationale and design. Int J Cardiol.
182:329–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang H, Han B, Yu T and Peng Z: The
complex regulation of tanshinone IIA in rats with
hypertension-induced left ventricular hypertrophy. PLoS One.
9:e922162014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao S, Wang Y, Zhang M and Hinek A:
Phytoestrogen, tanshinone IIA diminishes collagen deposition and
stimulates new elastogenesis in cultures of human cardiac
fibroblasts. Exp Cell Res. 323:189–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Q, Chen H, Sheng L, Liang Y and Li Q:
Sodium tanshinone IIA sulfonate prolongs the survival of skin
allografts by inhibiting inflammatory cell infiltration and T cell
proliferation. Int Immunopharmacol. 22:277–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Guo H, Dong L, Wang L, Liu C and
Wang X: Tanshinone IIA inhibits the growth, attenuates the stemness
and induces the apoptosis of human glioma stem cells. Oncol Rep.
32:1303–1311. 2014.PubMed/NCBI
|
|
30
|
Shan QQ, Guo Y and Gong YP: Effect of
tanshinone II A on leukemia cell line K562. Sichuan Da Xue Xue Bao
Yi Xue Ban. 45:410–413. 2014.PubMed/NCBI
|
|
31
|
Schotanus BA, Kruitwagen HS, van den Ingh
TS, van Wolferen ME, Rothuizen J, Penning LC and Spee B: Enhanced
Wnt/β-catenin and Notch signalling in the activated canine hepatic
progenitor cell niche. BMC Vet Res. 10:3092014. View Article : Google Scholar
|