Introduction
Colorectal cancer is a common malignant disease,
with an increasing incidence worldwide and remains one of the
leading causes of mortality, globally (1). In recent years, it has become the
leading cause of cancer-associated mortality in Japanese females
(2). The TNM classification and
Dukes' staging system are useful for determining the colorectal
cancer stage in patients (3).
However, these conventional staging procedures cannot precisely
predict the cancer prognosis, as many patients at the same
colorectal cancer stage experience various clinical outcomes.
Therefore, the identification of novel prognostic factors to
improve adjuvant therapeutic strategies or postoperative monitoring
is required (4).
Angiogenesis, commonly assessed by the microvessel
density (MVD), is involved in the formation of new blood vessels,
and is important for tumor growth and metastasis (5,6). The
association between angiogenesis and the clinical outcome of
colorectal cancer is currently controversial and inconclusive,
although colorectal cancer has been one of the most widely
investigated types of tumor (7–9). In
a previous study, a regimen of combined chemotherapy with a
monoclonal anti-vascular endothelial growth factor (VEGF) antibody
resulted in improved patient survival (10). Therefore, angiogenesis in
colorectal cancer is considered to be a particularly interesting
research field.
VEGF is a potent angiogenic factor, which stimulates
endothelial cell proliferation, survival and vascular maturation
(10). Various studies have
demonstrated that the VEGF expression level correlates with
angiogenesis (11,12) and tumor progression in colorectal
cancer (13,14); however, the prognostic value of
VEGF expression levels in colorectal cancer remains to be fully
elucidated.
Thymidine phosphorylase (TP), also termed platelet
derived endothelial cell growth factor, is an essential enzyme for
the activation of fluoropyrimidine, as well as an angiogenic factor
(15,16). TP exerts distinct and contradictory
biological functions (17) that
complicate the analysis of its contribution to predicting the
response to therapy or the prognosis. Despite various studies
regarding TP (18–24), the clinical applications of TP in
colorectal cancer continue to be insufficient (25).
In the present study, the expression of VEGF, TP and
cluster of differentiation (CD)34 as a marker of MVD were examined
in 84 cases of colorectal cancer. These factors were assessed to
elucidate whether they are interrelated, and to establish whether
expression of VEGF, TP and CD34 is correlated with
clinicopathologic features and clinical outcomes.
Materials and methods
Patients and materials
Surgically resected tissues of 84 patients with
colorectal cancer were analyzed at the Hirosaki University Hospital
(Hirosaki, Japan) between January 2005 and December 2006, after
obtaining informed consent from each patient to use their clinical
records and pathology specimens. The present study was approved by
the Research Ethics Committee of the Hirosaki University Graduate
School of Medicine (Hirosaki, Japan). Survival data were obtained
from hospital medical charts and by contacting patients or their
families, and the median postoperative follow-up period was 1,027
(14–1,298) days. The series consisted of 55 males and 29 females
with a median age of 66 years (range, 31–90 years). The patients
had not received chemotherapy or radiation therapy prior to
surgery. Forty-seven tumors were located in the colon and 37 tumors
were in the rectum. Of the 84 cases, 35 were well-differentiated
adenocarcinomas, 43 were moderately differentiated adenocarcinomas
and six were poorly differentiated adenocarcinomas, mucinous
adenocarcinoma, and signet-ring cell carcinoma The pathological
stage of each case at the time of surgery was defined according to
the TNM classification (3) and
Dukes' classification (26) as
follows: Dukes' stage A (n=8), invasion is not yet through the
bowel wall; Dukes' stage B (n=26), invasion through the bowel wall;
Dukes' stage C (n=27), involvement of lymph node metastasis; and
Dukes' stage D (n=23) involvement of distant organ metastasis. The
present study was retrospective and was conducted according to the
principles of the World Medical Association Declaration of Helsinki
1964 (27).
Histopathological and immunohistochemical
examinations
For histological examination, the pathology
specimens were routinely fixed with formalin, embedded in paraffin,
thin-sectioned (4 µm), and stained with hematoxylin and
eosin (all from Muto Pure Chemicals Co., Ltd., Tokyo, Japan). Each
lesion was graded histologically according to the Japanese
Classification of Colorectal Carcinoma (28). The degree of lymphatic (ly) and
venous (v) invasion were classified into the following four
categories: i) ly0/v0, no invasion; ii) ly1/v1, minimal invasion;
iii) ly2/v2, moderate invasion; and iv) ly3/v3, severe invasion.
The budding grade, defined as an isolated single cancer cell or a
cluster composed of fewer than five cancer cells, was classified as
follows: grade 0, no budding; grade 1, 1–4 foci in one histological
section; grade 2, 5–9 foci in one histological section; and grade
3, ≥10 foci in one histological section (29).
In each case, one representative histological
specimen at the deepest invaded area of the colorectal cancer
lesion was selected for immunohistochemistry. Sections (thickness,
4 µm) were mounted on silane-coated glass slides (Muto Pure
Chemicals Co., Ltd.). The primary antibodies used for
immunohistochemistry were rabbit anti-VEGF polyclonal antibody
(1:100 dilution; cat. no. sc-152; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), mouse anti-TP monoclonal antibody (clone 1C6-203;
1:500 dilution; cat. no. 1C6-203; Chugai Pharmaceutical Co., Ltd.,
Research Center, Kamakura, Japan) and mouse anti-CD34 monoclonal
antibody (1:200 dilution; cat. no. M7165; Dako, Glostrup, Denmark).
For antigen retrieval, sections were heated in a microwave oven for
20 min in 10 mM citrate buffer (Muto Pure Chemicals Co., Ltd.). The
staining was performed following a streptavidin biotin-peroxidase
procedure using a Histofine® kit (Nichirei Biosciences,
Inc., Tokyo, Japan) according to the manufacturer's instructions.
All sections were incubated overnight at 4°C. The sections were
reacted with 3,3′-diaminobenzidine tetrahydrochloride (Merck
Millipore, Darmstadt, Germany) and counterstained with
hematoxylin.
Evaluation of immunohistochemistry
Staining patterns of VEGF and TP expression were
divided into two groups: High, ≥50% of staining in the tumor cells;
and low, <50% of staining in the tumor cells.
MVD was assessed by counting the microvessels that
were immunostained for the CD34 antigen under a light microscope
(BX50 microscope; Olympus, Tokyo, Japan). The CD34-stained sections
were carefully scanned at a magnification of ×4 (low-power field)
to identify the regions with the greatest number of microvessels
(designated as 'hot spots') using a digital color camera system
(DP70; Olympus). In each case, the three most vascularized areas
within the tumors were selected and the individual vessels were
counted under a magnification of ×20 (high-power field). The mean
count from each of the three regions was recorded for analysis as
MVD and expressed as the number of vessels/high-power field
(30). In addition, the specimens
were divided into the following two groups: High, ≥81.33 (mean
value) vessels per high-power field; and low, <81.33 (mean
value) vessels per high-power field.
Statistical analysis
Statistical analyses of immunostaining were
performed using the χ2 test or Fisher's exact
probability test and Bonferroni correction. Spearman's rank
correlation test was used to assess the correlations between VEGF
and TP expression. Survival curves were calculated using the
Kaplan-Meier method and differences in survival were evaluated
using the log-rank test. Excluding the Bonferroni correction,
P<0.05 were considered to indicate a statistically significant
difference. All of the statistical evaluations were performed using
the statistical software package StatView (version 5.0; SAS
Institute, Inc., Cary, NC, USA).
Results
Immunohistochemical expression levels of
VEGF, TP, and CD34
Representative immunohistochemical expression
patterns of VEGF, TP, and CD34 are demonstrated in Fig. 1. The associations between
clinicopathologic features and VEGF/TP expression levels, and MVD
are summarized in Table I. No
significant association was identified between VEGF and TP
expression levels, and MVD for gender, tumor location, histological
type, lymph node metastasis, lymphatic invasion, venous invasion or
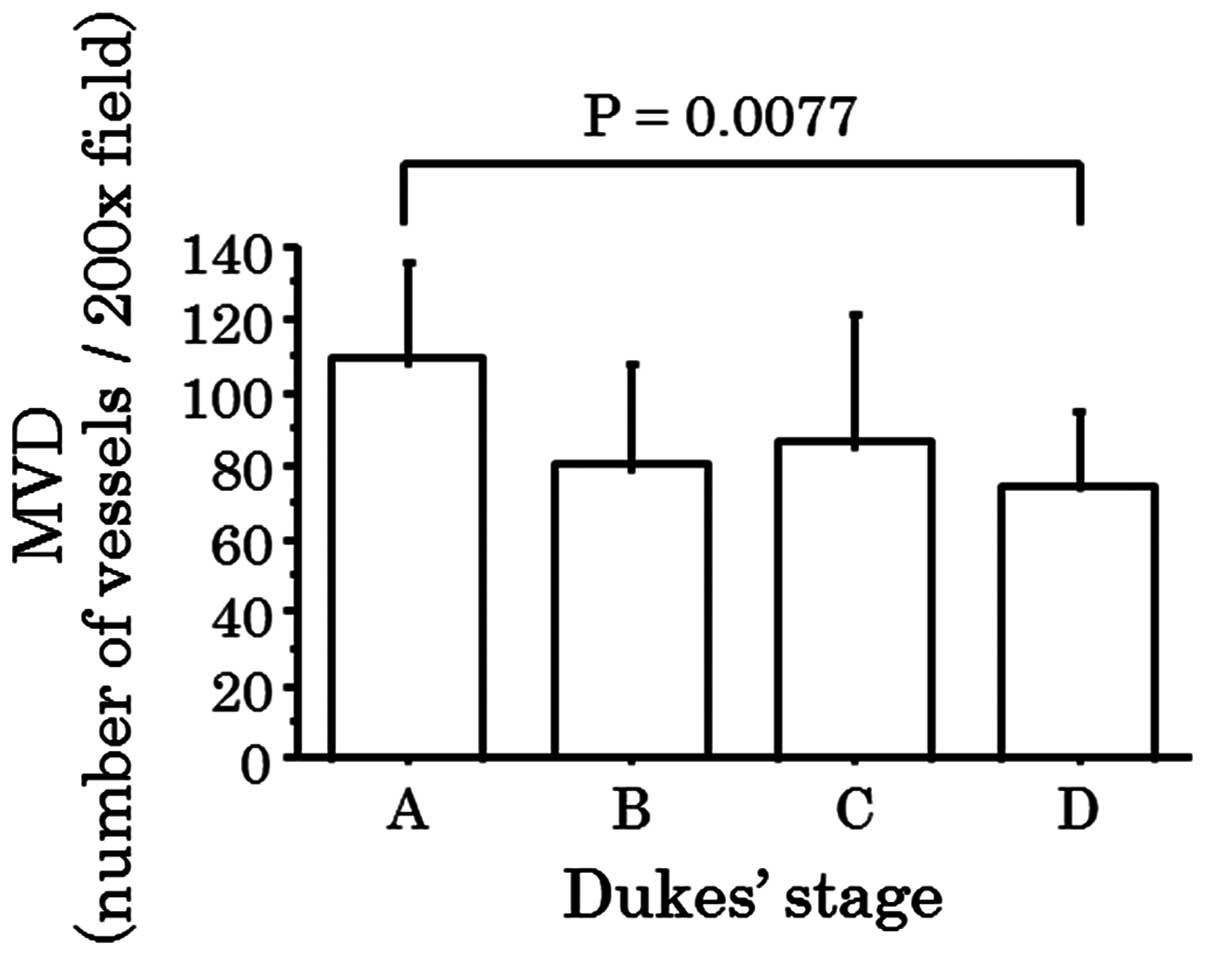
budding. MVD of Dukes' stage A cases was observed to be greater
than that in cases of Dukes' stage D, following performance of
multiple comparison with Bonferroni correction (P=0.0077; Fig. 2), while there was no significant
association identified between the Duke's classification, and VEGF
and TP expression levels (Table
I).
 | Figure 1Immunohistochemical expression
patterns of VEGF, TP and CD34 for MVD. (A) High VEGF expression,
≥50% staining in tumor cells. (B) Low VEGF expression, <50%
staining in tumor cells. (C) High TP expression, ≥50% staining in
tumor cells. (D) Low TP expression, <50% staining in tumor
cells. (E) High MVD, ≥81.33/field. (F) Low MVD, <81.33/field.
Magnification, ×20. VEGF, vascular endothelial growth factor; TP,
thymidine phosphorylase; CD34, cluster of differentiation 34; MVD,
microvessel density. |
 | Table IAssociation between clinicopathologic
features, VEGF and TP expression levels and MVD. |
Table I
Association between clinicopathologic
features, VEGF and TP expression levels and MVD.
| Clinicopathologic
featurea | Cases n=84 | VEGF expression
| TP expression
| MVD
|
|---|
High
(n=53) | Low
(n=31) | P-value | High
(n=55) | Low
(n=29) | P-value | High
(n=39) | Low
(n=45) | P-value |
|---|
| Gender | | | | 0.27 | | | 0.15 | | | 0.11 |
| Male | 55 | 37 | 18 | | 33 | 22 | | 29 | 26 | |
| Female | 29 | 16 | 13 | | 22 | 7 | | 10 | 19 | |
| Location | | | | 0.77 | | | 0.57 | | | 0.72 |
| Colon | 47 | 29 | 18 | | 32 | 15 | | 21 | 26 | |
| Rectum | 37 | 24 | 13 | | 23 | 14 | | 18 | 19 | |
| Histological
type | | | | 0.25 | | | 0.21 | | | 0.11 |
| Well | 35 | 24 | 11 | | 23 | 12 | | 21 | 14 | |
| Moderate | 43 | 27 | 16 | | 30 | 13 | | 16 | 27 | |
| Poor/Other | 6 | 2 | 4 | | 2 | 4 | | 2 | 4 | |
| Lymph node
metastasis | | | | 0.13 | | | 0.84 | | | 0.15 |
| pN0 | 36 | 26 | 10 | | 24 | 12 | | 20 | 16 | |
| pN1–4 | 48 | 27 | 21 | | 31 | 17 | | 19 | 29 | |
| Lymphatic
invasion | | | | 0.78 | | | 0.81 | | | 0.63 |
| ly0–1 | 45 | 29 | 16 | | 30 | 15 | | 22 | 23 | |
| ly2–3 | 39 | 24 | 15 | | 25 | 14 | | 17 | 22 | |
| Venous
invasion | | | | 0.57 | | | 0.76 | | | 0.37 |
| v0–1 | 62 | 38 | 24 | | 40 | 22 | | 27 | 35 | |
| v2–3 | 22 | 15 | 7 | | 15 | 7 | | 12 | 10 | |
| Budding | | | | 0.88 | | | 0.41 | | | 0.21 |
| Grade 0–1 | 47 | 30 | 17 | | 29 | 18 | | 19 | 28 | |
| Grade 2–3 | 37 | 23 | 14 | | 26 | 11 | | 20 | 17 | |
| Dukes' stage | | | | 0.49 | | | 0.58 | | | 0.047b |
| A | 8 | 6 | 2 | | 7 | 1 | | 7 | 1 | |
| B | 26 | 18 | 8 | | 16 | 10 | | 13 | 13 | |
| C | 27 | 14 | 13 | | 17 | 10 | | 12 | 15 | |
| D | 23 | 15 | 8 | | 15 | 8 | | 7 | 16 | |
Correlation between VEGF, TP and MVD
The mean MVD of the high VEGF expression group was
significantly greater than that of the low VEGF expression group
(86.5 vs. 66.9, respectively; P=0.0194; Fig. 3). In the 23 Dukes' stage D cases,
the mean MVD of the high TP expression group was significantly
greater than that of the low TP expression group (79.4 vs. 57.9,
respectively; P=0.0149; Fig. 4),
while the mean MVD of all Dukes' stage cases demonstrated no
significant difference between the high and low TP expression
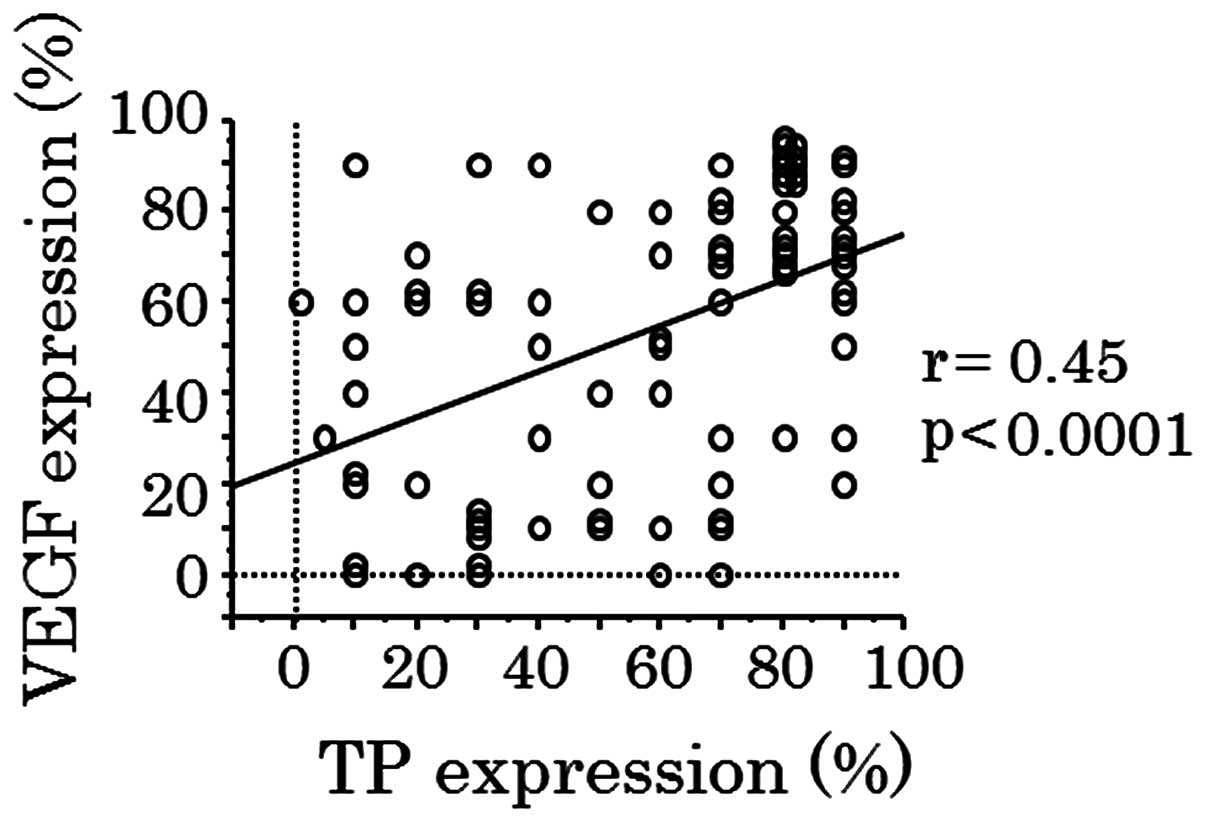
groups. A statistically significant correlation between the levels
of VEGF and TP expression was noted (r=0.45; P<0.0001; Fig. 5).
Correlation between patient prognosis and
VEGF, TP, and MVC
The patient overall survival rate in the high VEGF
expression group tended to be lower when compared with that of the
low VEGF expression group (P=0.0716; Fig. 6). In the 48 cases of lymph node
metastasis, the overall survival rate of the high VEGF expression
group was significantly lower than that of the low VEGF expression
group (P=0.0128; Fig. 7). However,
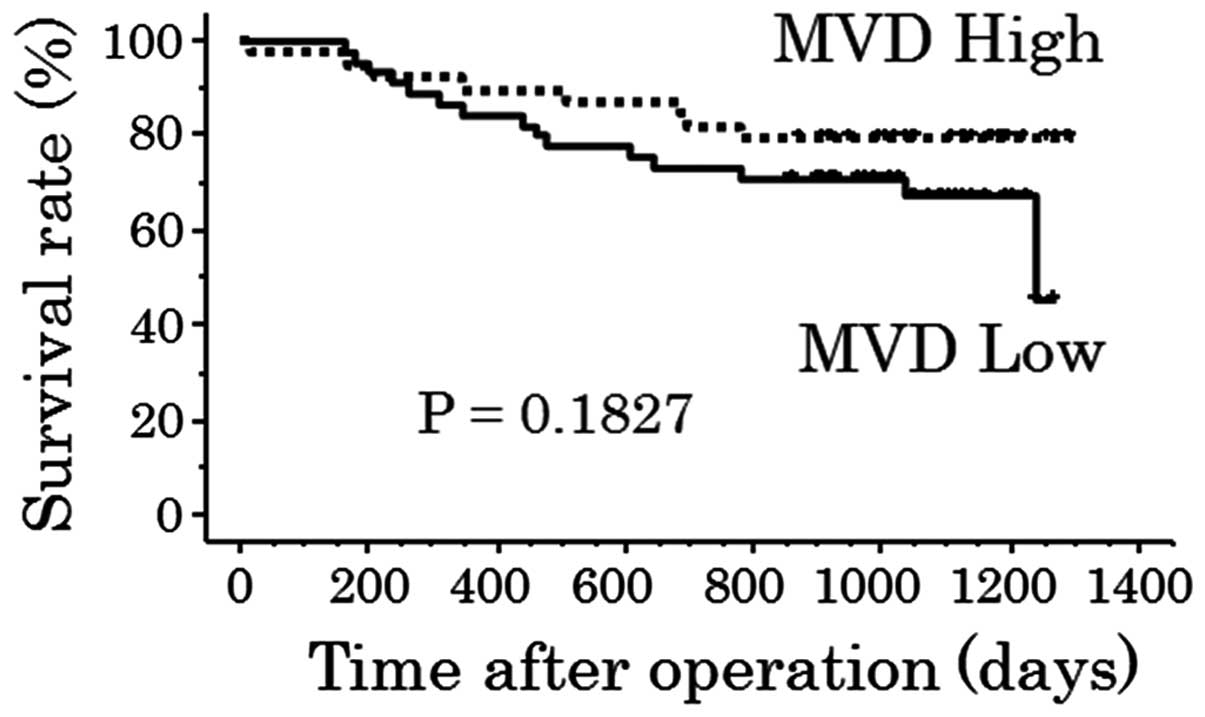
no significant differences in the overall survival rates between
the high and low TP expression groups were identified (P=0.5169;
Fig. 8), or between the high and
low MVD groups (P=0.1827; Fig.
9).
Discussion
Angiogenesis is essential for tumor growth and
metastasis (5,6). The degree of angiogenic activity in
tumors was hypothesized to define tumor aggressiveness, as a dense
vascular network ensures an adequate supply of oxygen and
nutrients, which facilitates tumor growth and invasion, and
migration to distant organs (10).
Angiogenesis-stimulating proteins are commonly known as angiogenic
factors and include VEGF and TP, amongst others (4,10).
These factors are commonly associated with increased MVD and,
therefore, to an unfavorable clinical course (10). In the present study, a positive
correlation was demonstrated between VEGF and TP expression levels,
and an association between VEGF and TP expression and MVD was also
identified. A high VEGF expression level was identified to be
correlated with a short overall survival of patients exhibiting
lymph node metastasis, and MVD of Dukes' stage A was observed to be
significantly higher than that of Dukes' stage D.
Previous studies have described an association
between VEGF expression and tumor aggressiveness in various types
of malignant tumor (31),
including colorectal cancer (13).
Furthermore, various reports regarding colorectal cancer specified
that VEGF expression was closely correlated with tumor angiogenesis
(11,12,32),
and may be considered an independent prognostic factor (1,7,33),
while a study indicated that there was no apparent correlations
(12). In addition, previous
reports analyzed the positive correlations between a high VEGF
expression level and lymph node metastasis (1,34),
while certain studies revealed negative correlations (35,36).
In the present study, MVD in the high VEGF expression group was
observed to be significantly higher than that of the low VEGF
expression group, and patients' overall survival rate in the high
VEGF expression group tended to be lower when compared with that of
the low VEGF expression group. A high VEGF expression level is
considered to be a prognostic factor of colorectal cancer,
particularly in patients exhibiting lymph node metastasis. Previous
studies have reported significant correlations between
clinicopathologic features and TP expression levels (19,35,37),
whereas other studies have reported an inverse correlation between
the TP expression level and lymph node/hematogenous metastasis
(22); thus, the association
between TP expression level and clinicopathologic features is
considered to be controversial (23,37–41).
It was hypothesized in the current study that the significance of
the TP expression levels is minimal with regard to angiogenesis
(which is assessed by MVD), when high VEGF expression levels are
sufficient to induce VEGF-associated angiogenesis.
Previous studies have demonstrated the correlations
between high MVD and lymph node or distant metastases (42–47),
while certain studies reported a significant correlation between
MVD and hematogenous metastasis, but not with lymph node metastasis
(8), and that MVD was not
associated with patient survival (9). The present study demonstrated that
MVD in Dukes' stage A specimens was higher than that of the Dukes'
stage D specimens. Therefore, it was hypothesized that MVD is an
important factor for tumor growth of primary colorectal cancer,
however, exerts a less significant affect on distant metastasis,
such as liver metastasis.
In conclusion, the results of the present study
indicate that VEGF expression levels may serve as a prognostic
factor for patients with colorectal cancer exhibiting lymph node
metastasis. Furthermore, angiogenesis, as assessed by MVD, was
shown to be an important prognostic factor for tumor growth at the
primary site.
Acknowledgments
The present study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan (Tokyo, Japan); and a Grant for Hirosaki
University Institutional Research (Hirosaki, Japan).
References
|
1
|
Zafirellis K, Agrogiannis G, Zachaki A,
Gravani K, Karameris A and Kombouras C: Prognostic significance of
VEGF expression evaluated by quantitative immunohistochemical
analysis in colorectal cancer. J Surg Res. 147:99–107. 2008.
View Article : Google Scholar
|
|
2
|
Pourhoseingholi MA: Increased burden of
colorectal cancer in Asia. World J Gastrointest Oncol. 4:68–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sobin L, Gospodarowicz MK and Wittekind C:
Colon and rectum. TNM Classification of Malignant Tumors. Sobin L,
Gospodarowicz MK and Wittekind C: 7th edition. Wiley-Blackwell; New
York: pp. 100–pp105. 2009
|
|
4
|
Papamichael D: Prognostic role of
angiogenesis in colorectal cancer. Anticancer Res. 21:4349–4353.
2001.
|
|
5
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Des Guetz G, Uzzan B, Nicolas P, Cucherat
M, Morere JF, Benamouzig R, Breau JL and Perret GY: Microvessel
density and VEGF expression are prognostic factors in colorectal
cancer. Meta-analysis of the literature. Br J Cancer. 94:1823–1832.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanigawa N, Amaya H, Matsumura M, Lu C,
Kitaoka A, Matsuyama K and Muraoka R: Tumor angiogenesis and mode
of metastasis in patients with colorectal cancer. Cancer Res.
57:1043–1046. 1997.PubMed/NCBI
|
|
9
|
Gao J, Knutsen A, Arbman G, Carstensen J,
Frånlund B and Sun XF: Clinical and biological significance of
angiogenesis and lymphan-giogenesis in colorectal cancer. Dig Liver
Dis. 41:116–122. 2009. View Article : Google Scholar
|
|
10
|
Giatromanolaki A, Sivridis E and
Koukourakis MI: Angiogenesis in colorectal cancer: Prognostic and
therapeutic implications. Am J Clin Oncol. 29:408–417. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amaya H, Tanigawa N, Lu C, Matsumura M,
Shimomatsuya T, Horiuchi T and Muraoka R: Association of vascular
endothelial growth factor expression with tumor angiogenesis,
survival and thymidine phosphorylase/platelet-derived endothelial
cell growth factor expression in human colorectal cancer. Cancer
Lett. 119:227–235. 1997. View Article : Google Scholar
|
|
12
|
Zheng S, Han MY, Xiao ZX, Peng JP and Dong
Q: Clinical significance of vascular endothelial growth factor
expression and neovascularization in colorectal carcinoma. World J
Gastroenterol. 9:1227–1230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishigami SI, Arii S, Furutani M, Niwano M,
Harada T, Mizumoto M, Mori A, Onodera H and Imamura M: Predictive
value of vascular endothelial growth factor (VEGF) in metastasis
and prognosis of human colorectal cancer. Br J Cancer.
78:1379–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cascinu S, Staccioli MP, Gasparini G,
Giordani P, Catalano V, Ghiselli R, Rossi C, Baldelli AM, Graziano
F, Saba V, et al: Expression of vascular endothelial growth factor
can predict event-free survival in stage II colon cancer. Clin
Cancer Res. 6:2803–2807. 2000.PubMed/NCBI
|
|
15
|
Furukawa T, Yoshimura A, Sumizawa T,
Haraguchi M, Akiyama S, Fukui K, Ishizawa M and Yamada Y:
Angiogenic factor. Nature. 356(668)1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishikawa F, Miyazono K, Hellman U, Drexler
H, Wernstedt C, Hagiwara K, Usuki K, Takaku F, Risau W and Heldin
CH: Identification of angiogenic activity and the cloning and
expression of platelet-derived endothelial cell growth factor.
Nature. 338:557–562. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ciccolini J, Evrard A and Cuq P: Thymidine
phosphorylase and fluoropyrimidines efficacy: A Jekyll and Hyde
story. Curr Med Chem Anticancer Agents. 4:71–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J: What is the role of thymidine
phosphorylase in tumor angiogenesis. J Natl Cancer Inst.
88:1091–1092. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takebayashi Y, Akiyama S, Akiba S, Yamada
K, Miyadera K, Sumizawa T, Yamada Y, Murata F and Aikou T:
Clinicopathologic and prognostic significance of an angiogenic
factor, thymidine phosphorylase, in human colorectal carcinoma. J
Natl Cancer Inst. 88:1110–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi Y, Bucana CD, Liu W, Yoneda J,
Kitadai Y, Cleary KR and Ellis LM: Platelet-derived endothelial
cell growth factor in human colon cancer angiogenesis: Role of
infiltrating cells. J Natl Cancer Inst. 88:1146–1151. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuura T, Kuratate I, Teramachi K, Osaki
M, Fukuda Y and Ito H: Thymidine phosphorylase expression is
associated with both increase of intratumoral microvessels and
decrease of apoptosis in human colorectal carcinomas. Cancer Res.
59:5037–5040. 1999.PubMed/NCBI
|
|
22
|
Saito S, Tsuno N, Nagawa H, Sunami E,
Zhengxi J, Osada T, Kitayama J, Shibata Y, Tsuruo T and Muto T:
Expression of platelet-derived endothelial cell growth factor
correlates with good prognosis in patients with colorectal
carcinoma. Cancer. 88:42–49. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Triest B, Pinedo HM, Blaauwgeers JL,
van Diest PJ, Schoenmakers PS, Voorn DA, Smid K, Hoekman K, Hoitsma
HF and Peters GJ: Prognostic role of thymidylate synthase,
thymidine phosphorylase/platelet-derived endothelial cell growth
factor and proliferation markers in colorectal cancer. Clin Cancer
Res. 6:1063–1072. 2000.PubMed/NCBI
|
|
24
|
van Halteren HK, Peters HM, van Krieken
JH, Coebergh JW, Roumen RM, van der Worp E, Wagener JT and
Vreugdenhil G: Tumor growth pattern and thymidine phosphorylase
expression are related with the risk of hematogenous metastasis in
patients with Astler Coller B1/B2 colorectal carcinoma. Cancer.
91:1752–1757. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr:
ASCO 2006 update of recommendations for the use of tumor markers in
gastrointestinal cancer. J Clin Oncol. 24:5313–5327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mainprize KS, Mortensen NJ and Warren BF:
Dukes' staging is poorly understood by doctors managing colorectal
cancer. Ann R Coll Surg Engl. 84:23–25. 2002.PubMed/NCBI
|
|
27
|
General Assembly of the World Medical
Association; World medical association declaration of Helsinki:
Ethical principles for medical research involving human subjects. J
Am Coll Dent. 81:14–18. 2014.
|
|
28
|
Japanese Society for Cancer of the Colon
and Rectum: Japanese classification of colorectal carcinoma. Second
English Edition. Kanehara & Co., Lit; Tokyo: 2009
|
|
29
|
Ueno H, Murphy J, Jass JR, Mochizuki H and
Talbot IC: Tumour 'budding' as an index to estimate the potential
of aggressiveness in rectal cancer. Histopathology. 40:127–132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosari S, Lee AK, DeLellis RA, Wiley BD,
Heatley GJ and Silverman ML: Microvessel quantitation and prognosis
in invasive breast carcinoma. Hum Pathol. 23:755–761. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Byrne KJ, Koukourakis MI, Giatromanolaki
A, Cox G, Turley H, Steward WP, Gatter K and Harris AL: Vascular
endothelial growth factor, platelet-derived endothelial cell growth
factor and angiogenesis in non-small-cell lung cancer. Br J Cancer.
82:1427–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi Y, Tucker SL, Kitadai Y, Koura
AN, Bucana CD, Cleary KR and Ellis LM: Vessel counts and expression
of vascular endothelial growth factor as prognostic factors in
node-negative colon cancer. Arch Surg. 132:541–546. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ogata Y, Matono K, Mizobe T, Ishibashi N,
Mori S, Akagi Y, Ikeda S, Ozasa H, Murakami H and Shirouzu K: The
expression of vascular endothelial growth factor determines the
efficacy of post-operative adjuvant chemotherapy using oral
fluoropyrimidines in stage II or III colorectal cancer. Oncol Rep.
15:1111–1116. 2006.PubMed/NCBI
|
|
34
|
George ML, Tutton MG, Janssen F, Arnaout
A, Abulafi AM, Eccles SA and Swift RI: VEGF-A, VEGF-C and VEGF-D in
colorectal cancer progression. Neoplasia. 3:420–427. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshimoto K, Kawahara H, Kobayashi S,
Kashiwagi H, Hirai K and Yanaga K: Importance of thymidine
phosphorylase expression at the invasive front of T3 rectal cancer
as a prognostic factor. Dig Surg. 23:331–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abdou AG, Aiad H, Asaad N, Abd El-Wahed M
and Serag El-Dien M: Immunohistochemical evaluation of vascular
endothelial growth factor (VEGF) in colorectal carcinoma. J Egypt
Natl Canc Inst. 18:311–322. 2006.
|
|
37
|
Aoki T, Katsumata K, Tsuchida A, Tomioka H
and Koyanagi Y: Correlation between malignancy grade and p53 gene
in relation to thymidine phosphorylase activity in colorectal
cancer patients. Oncol Rep. 9:1267–1271. 2002.PubMed/NCBI
|
|
38
|
Tsuji T, Sawai T, Yamashita H, Takeshita
H, Nakagoe T, Shindou H, Fukuoka H, Yoshinaga M, Hidaka S, Yasutake
T, et al: Platelet-derived endothelial cell growth factor
expression is an independent prognostic factor in colorectal cancer
patients after curative surgery. Eur J Surg Oncol. 30:296–302.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsumura M, Chiba Y, Lu C, Amaya H,
Shimomatsuya T, Horiuchi T, Muraoka R and Tanigawa N:
Platelet-derived endothelial cell growth factor/thymidine
phosphorylase expression correlated with tumor angiogenesis and
macrophage infiltration in colorectal cancer. Cancer Lett.
128:55–63. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Osanai T, Ichikawa W, Takagi Y, Uetake H,
Nihei Z and Sugihara K: Expression of pyrimidine nucleoside
phosphorylase (PyNPase) in colorectal cancer. Jpn J Clin Oncol.
31:500–505. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tokunaga Y, Hosogi H, Hoppou T, Nakagami
M, Tokuka A and Ohsumi K: Prognostic value of thymidine
phosphorylase/platelet-derived endothelial cell growth factor in
advanced colorectal cancer after surgery: Evaluation with a new
monoclonal antibody. Surgery. 131:541–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takebayashi Y, Aklyama S, Yamada K, Akiba
S and Aikou T: Angiogenesis as an unfavorable prognostic factor in
human colorectal carcinoma. Cancer. 78:226–231. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cianchi F, Palomba A, Messerini L, Boddi
V, Asirelli G, Perigli G, Bechi P, Taddei A, Pucciani F and
Cortesini C: Tumor angiogenesis in lymph node-negative rectal
cancer: Correlation with clinicopathological parameters and
prognosis. Ann Surg Oncol. 9:20–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakayama Y, Nagashima N, Minagawa N, Inoue
Y, Katsuki T, Onitsuka K, Sako T, Hirata K, Nagata N and Itoh H:
Relationships between tumor-associated macrophages and
clinicopathological factors in patients with colorectal cancer.
Anticancer Res. 22:4291–4296. 2002.
|
|
45
|
Rajaganeshan R, Prasad R, Guillou PJ,
Chalmers CR, Scott N, Sarkar R, Poston G and Jayne DG: The
influence of invasive growth pattern and microvessel density on
prognosis in colorectal cancer and colorectal liver metastases. Br
J Cancer. 96:1112–1117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kaneko I, Tanaka S, Oka S, Yoshida S,
Hiyama T, Arihiro K, Shimamoto F and Chayama K: Immunohistochemical
molecular markers as predictors of curability of endoscopically
resected submucosal colorectal cancer. World J Gastroenterol.
13:3829–3835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan G, Zhou XY, Cai SJ, Zhang GH, Peng JJ
and Du X: Lymphangiogenic and angiogenic microvessel density in
human primary sporadic colorectal carcinoma. World J Gastroenterol.
14:101–107. 2008. View Article : Google Scholar : PubMed/NCBI
|