Introduction
Epithelial-mesenchymal transition (EMT), the
programmed conversion of epithelial into mesenchymal cells, is
defined by a loss of epithelial-cell characteristics, including
cell-cell adhesion, polarity and organized cytoskeleton (1). The EMT may be involved in several
physiological functions and pathological conditions, including
normal development, fibrosis and tumor metastasis, through
signaling pathways activated by various stimuli (2–4). A
previous study on the EMT in biliary epithelial cells (BECs) in
advanced liver fibrosis reported cytoskeletal rearrangements during
chronic liver injury, resulting in a mesenchymal phenotype
(5). BECs, also known as
cholangiocytes, are essential to the formation of bile components
in the liver and effective transport of bile into the duodenum,
although they comprise only 3–5% of total cells in the normal liver
(6,7). BECs line the intra-hepatic biliary
ducts, which are sites of damage in several diseases, including
primary biliary cirrhosis and primary sclerosing cholangitis, and
BECs may have active roles in the innate as well as the adaptive
immune response by producing interleukin (IL)-6 and IL-8 following
injury (8–10).
IL-6 was identified as an important pleiotropic
cytokine participating in acute inflammation, and previous
molecular and pathological findings showed that intrahepatic BECs
are able to synthesize IL-6, which is a critical mediator of the
hepatic response to systemic inflammation (11,12).
It has been suggested that stimulation of human intrahepatic (HI)
BECs with lipopolysaccharide resulted in increased secretion of
IL-6 (8). In addition, previous
studies suggested that IL-6 is a potent mitogen to BECs, and the
growth of a cholangiocarcinoma cell line was attenuated by
inhibition of IL-6-induced mitogen-mediated protein kinase
activation, indicating a growth-regulatory function of IL-6 in
HIBECs (13,14). The EMT is associated with elevated
E-cadherin and vimentin expression, as well as IL-6 production. Of
note, IL-6 has been reported to be a potent inducer of the EMT in a
number of tumor cell types (15–18).
The present study aimed to identify factors regulating EMT in
HIBECs, while a possible IL-6-mediated EMT in HIBECs has not yet
been reported, to the best of our knowledge. Therefore, the effects
of IL-6 stimulation on the EMT of HIBECs were assessed in
vitro, and the underlying mechanisms were investigated.
Materials and methods
Cell culture
HIBEC lines (P5100) were purchased from ScienCell
Research Laboratories (San Diego, CA, USA). All HIBEC lines were
cultured in a humidified incubator at 37°C and 5% CO2
and maintained in a mixture of Dulbecco's modified Eagle's medium
(DMEM)/Ham's F12 (1:1) medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 5% fetal bovine serum
(FBS), 5 ng/ml epidermal growth factor, 0.4 µg/ml succinyl
hydrocortisone, 2 nmol/l triiodothyronine, 5 µg/ml insulin
and 10 µg/ml recombinant human hepatocyte growth factor
(Gibco; Thermo Fisher Scientific, Inc.). Cells were passaged after
a confluent monolayer was obtained; subsequently, cells at passage
were detached with EDTA/trypsin (1:1; 0.2% each) for 5–8 min
(Gibco; Thermo Fisher Scientific, Inc.,) and re-seeded in fresh
culture medium. Cells were stained with hematoxylin-eosin (HE;
Sigma-Aldrich, St. Louis, MO, USA) using a standard protocol
(hematoxylin for 4 min, rinsing with water, eosin for 30 sec) and
observed under an Olympus IX83 microscope (Olympus Corporation,
Tokyo, Japan).
Treatment groups
Based on the protocol of a previous study (19), HIBECs (1×106) were
seeded in 10 cm-diameter cell culture plates at a confluency of
80%. Cells were then treated with various concentrations of IL-6
(0, 10, 20, 50 and 100 µg/l; R&D Systems Inc.,
Minneapolis, MN, USA). Cell-morphological changes were observed by
inverse phase-contrast microscopy (Olympus CKX41; Olympus
Corporation) after 24 h of stimulation.
Wound healing assay
Cell migration was assessed using a wound healing
assay, which was performed based on a procedure described by Chun
et al (19). HIBECs
(1.5×104) were seeded in 24-well plates and cultured in
DMEM, with three parallel wells for each experimental condition.
Upon reaching confluence, the cell layers were vertically scratched
using a Ziptip µ-C18 (10-µl) pipette tip (EMD
Millipore, Billerica, MA, USA) and detached cells were removed by
rinsing with phosphate-buffered saline (PBS). After 24 h of
incubation with IL-6 (0, 10, 20 50 and 100 µg/l) in a volume
of 1 ml, images were captured using an Olympus IX83 inverted
microscope (Olympus Corporation). The number of cells migrated into
the scratched region was counted and the assay was repeated three
times, followed by semi-automated enumeration of the migrated cells
(DotCount V1.2; Boston, MA, USA).
Transwell experiment
A Transwell invasion assay was performed in 24-well
Transwell plates (Corning-Costar, Corning, NY, USA). The chambers
were incubated with 3 µg/ml fibronectin at room temperature
for 2 h. After removing excess fibronectin, the chambers were
washed with PBS twice and kept overnight at 4°C. The cell
suspension (100 µl) was added to the chambers until the cell
density was adjusted to 2×104/ml, and cultured in
serum-free DMEM/F12 medium. The chambers were placed in a 24-well
plate, whereas 500 µl IL-6 (0, 10, 20 50 and 100
µg/l) was added to the lower chamber, and cultured in 0.6 mL
DMEM (high glucose) containing 10% FBS. After 24 h of incubation,
the cells that did not transgress through the membrane were removed
using a cotton swab. The cells transgressed to the lower side of
the membrane were fixed in 4% paraformaldehyde, stained with
4′6-diamidino-2-phenylindole dichlorhydrate (DAPI; Merck Millipore,
Darmstadt, Germany) and counted in four randomly selected visual
fields per well (magnification, ×200). Three independent
experiments were performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression of E-cadherin and vimentin was
determined by RT-qPCR according to the protocol of a previous study
(15). Total RNA was extracted
using a PCR kit (Takara Bio, Inc., Otsu, Japan) following the
manufacturer's instructions. RNA enzyme-free ultrapure water
(dilution, 1:10) and the absorbency at wavelengths of 260 and 280
nm were recorded using an ultraviolet spectrophotometer (UV-1100;
Shanghai Mapada Instruments Co., Ltd., Shanghai, China) to
determine purity and concentration. The RNA samples were extracted
until an optical density (OD) 260/OD280 ratio of 1.7–2.1 was
achieved. Complementary DNA synthesis was performed using the
PrimeScript RT-PCR kit (Takara Bio, Inc., Otsu, Japan) based on the
manufacturer's recommendations. RT 2 Real-Time SYBR Green (Takara
Bio, Inc.) was used for real-time qPCR. GAPDH was used as an
internal control. PCR primers were synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China), under the following reaction
condition: 95°C pre-degeneration for 15 sec, 95°C for 10 sec, 58°C
for 30 sec and 72°C for 1 min (35 cycles in total). Primers used
are listed in Table I. Results are
expressed as the averaged of three independent experiments after
normalization to GAPDH expression. The relative mRNA expression of
E-cadherin and vimentin was calculated using the 2−ΔΔCt
method (20).
 | Table IPrimers designed for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers designed for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence |
|---|
| E-cadherin | F:
5′-GCCCCGCCTTATGATTCTCTGC-3′
R: 5′-CTCGCCGCCTCCGTACATGTC-3′ |
| Vimentin | F:
5′-CAGCAGTATGAAAGCGTGG-3′
R: 5′-GGAAGAAAAGGTTGGCAGAG-3′ |
| GAPDH | F:
5′-ACCCATCACCATCTTCCAGGAG-3′
R: 5′-GAAGGGGCGGAGATGATGAC-3′ |
Western blot analysis
After washing with PBS, cells were lysed in the dish
with radioimmunoprecipitation assay buffer (EMD Millipore) for
total protein extraction. Western blot analysis was performed with
whole-cell lysates. The protein (40 µg per lane) was
electrophoresed using Nu-Page gels (Invitrogen; Thermo Fisher
Scientific, Inc.), electrotransferred onto polyvinylidene
difluoride membranes (Invitrogen; Thermo Fisher Scientific, Inc.)
over 90 min and blocked with PBS containing 5% dried skimmed milk
for 1 h. Rabbit anti-E-cadherin monoclonal antibody (1:500
dilution; cat. no. sc-7870; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and anti-vimentin monoclonal antibody (1:1,000
dilution; cat. no. vim 3B4; Dako, Hamburg, Germany) were added as
primary antibodies and incubated overnight at 4°C. Subsequently,
membranes were washed three times with Tris-buffered saline
(Sigma-Aldrich), and the appropriate secondary antibodies labeled
with horseradish peroxidase (Sigma-Aldrich) were added, followed by
incubation at room temperature for 1 h. Finally, the membranes were
rinsed with PBS and Amersham ECL™ Advance Western Blotting
Detection kit (GE Healthcare Life Sciences, Uppsala, Sweden) was
added for visualization. Images of the blots were captured using a
Gel Documentation 2000 system (Bio-Rad Laboratories, Inc. Hercules,
CA, USA)and images were processed for densitometric quantification
of the protein bands using ImageJ software (version 1.41; National
Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij). Each experimental condition
was performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). All data were tested
for normal distribution using the Shapiro-Wilk test. Values are
expressed as the mean ± standard deviation. Analysis of variance
was applied for comparisons between different groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
IL-6 induces morphological changes in
HIBECs
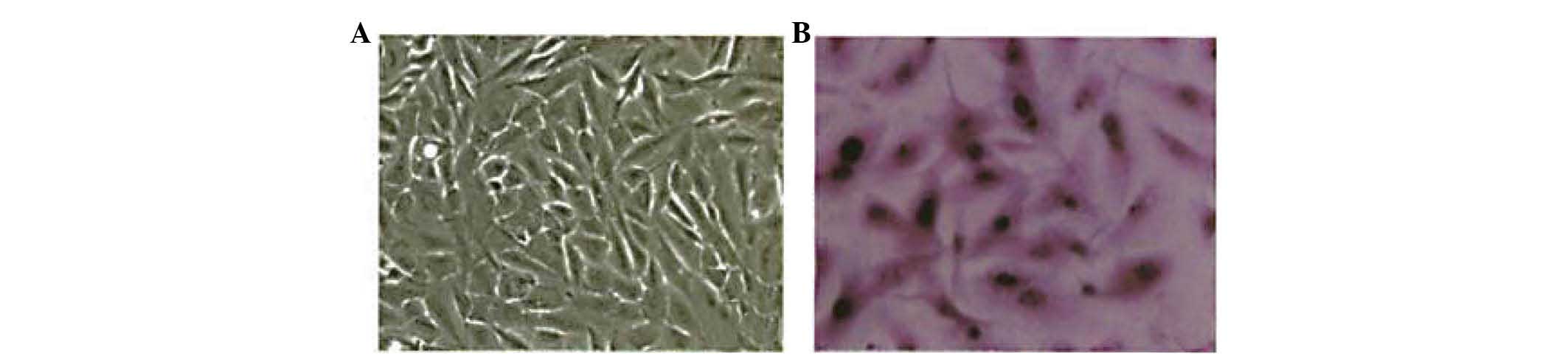
The morphology of native HIBECs was short and
fusiform or polygonal-shaped with clear, elliptical nuclei. Cells
were closely packed with strong cell-cell adhesion and cell-matrix
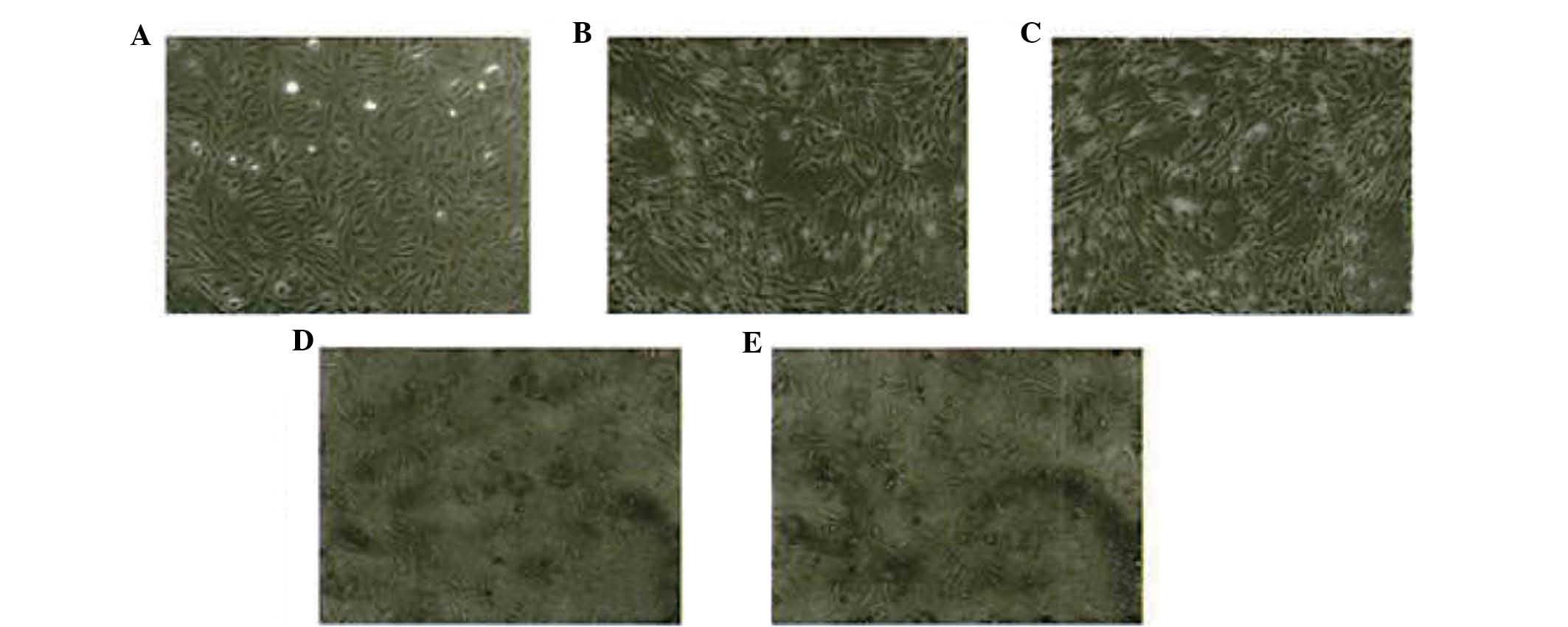
interactions (Figs. 1 and 2A). After 24 h of stimulation with IL-6
(0, 10, 20, 50 or 100 µg/l), HIBECs were observed to display
reduced cell-cell adhesion and increased intercellular gaps
(Fig. 2B–E, respectively). Obvious
morphological changes were observed in HIBECs incubated with 50 and
100 µg/l IL-6 (Fig. 2D and
E), which became long strips.
IL-6 enhances the cell-migratory and
invasive capacity of HIBECs
A wound healing assay demonstrated that the number
of cells migrated in the wounded area in the 0 µg/l IL-6
group (109±18) was lower than that in the 10 µg/l (417±24),
20 µg/l (434±22), 50 µg/l (508±31) and 100
µg/l (517±28) IL-6 groups (all P<0.05). The number of
migrated cells in the 50 and 100 µg/l groups was obviously
increased compared with that in the 10 and 20 µg/l groups.
However, no statistically significant differences in the number of
migrated cells were observed between the 10 and 20 µg/l
group or the 50 and 100 µg/l group (all P>0.05) (Figs. 3 and 4).
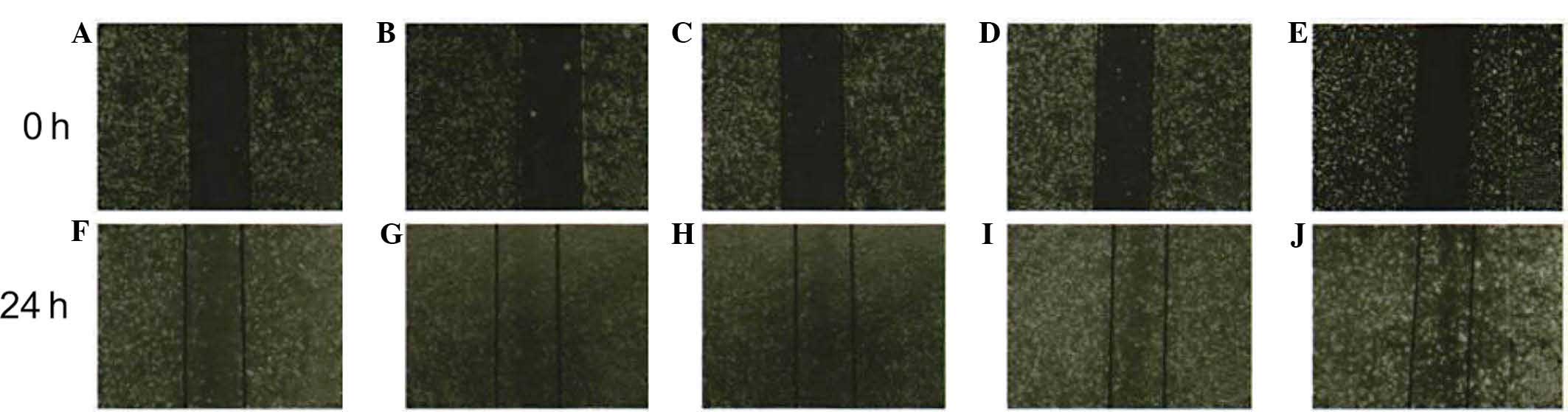 | Figure 3Migratory potential of human
intrahepatic biliary epithelial cells after stimulation with IL-6.
Inverted microscopy images of scratched cell monolayers subjected
to the wound assay (magnification, ×40). (A–E) cells at 0 h in the
presence of 0, 10, 20, 50 and 100 µg/l IL-6, respectively;
(F–J) cells following incubation with 0, 10, 20, 50 and 100
µg/l IL-6, respectively, for 24 h. IL, interleukin-6. |
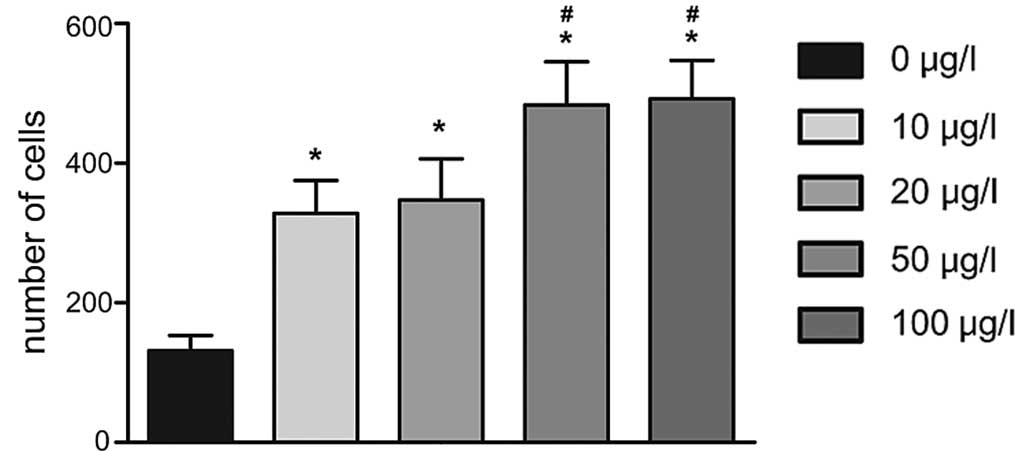
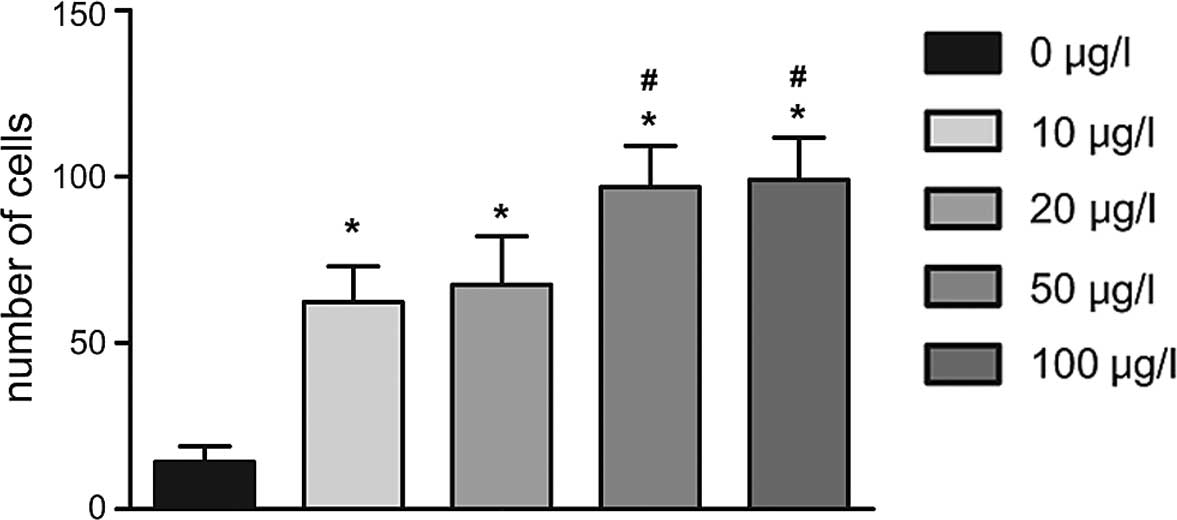
Furthermore, a Transwell invasion assay demonstrated
that cell number of cells transgressed through the membrane in the
0 µg/l IL-6 group (15±7) was lower than that in the 10
µg/l (85±1l), 20 µg/l (90±14), 50 µg/l
(121±13) and 100 µg/l (140±13) IL-6 groups following
incubation for 24 h (all P<0.05); furthermore, HIBECs stimulated
with IL-6 at concentrations of 50 and 100 µg/l showed a
significantly higher invasive capacity than those in the other
groups (P<0.05) (Figs. 5 and
6).
IL-6 alters the expression of
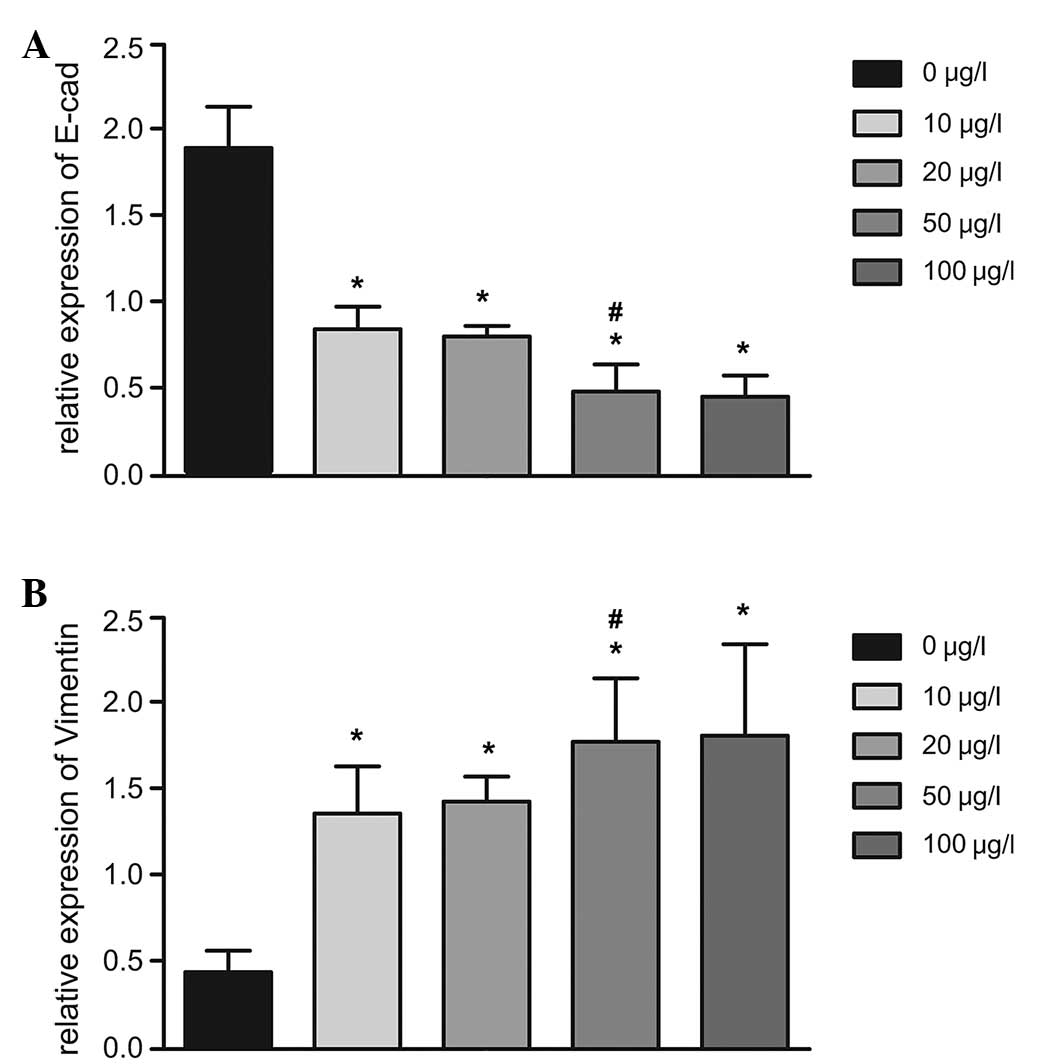
EMT-specific markers in HIBECs
The effects of IL-6 on the mRNA expression of
E-cadherin, an epithelial cell marker, and vimentin, a mesenchymal
cell marker, were assessed as indicators of the EMT of HIBECs using
RT-qPCR (Table II; Fig. 7). Following treatment with IL-6
(10, 20, 50 or 100 µg/l), the mRNA expression of E-cadherin
was significantly decreased compared with that in the untreated
group (all P<0.05). By contrast, the mRNA expression of vimentin
was significantly increased following treatment with IL-6 (10, 20,
50 or 100 µg/l) compared with that in untreated cells (all
P<0.05). In addition, the mRNA expression of E-cadherin the 50
µg/l IL-6 group was significantly lower than that in the 20
µg/l IL-6 group (0.40±0.12, vs. 0.80±0.09; P=0.010), and the
relative mRNA expression of vimentin in the 50 µg/l IL-6
group was significantly higher than that in the 20 µg/l
group (1.78±0.17, vs. 1.43±0.15; P=0.019) (Fig. 7).
 | Table IIRelative mRNA and protein expression
levels of E-cadherin and vimentin, respectively, in HIBEC lines
stimulated with interleukin-6 (0, 10, 20, 50 or 100 µg/l)
for 24 h. |
Table II
Relative mRNA and protein expression
levels of E-cadherin and vimentin, respectively, in HIBEC lines
stimulated with interleukin-6 (0, 10, 20, 50 or 100 µg/l)
for 24 h.
| Gene/Protein | 0 µg/l
group | 10 µg/l
group | 20 µg/l
group | 50 µg/l
group | 100 µg/l
group |
|---|
| Gene | | | | | |
| E-cadherin | 1.82±0.16 | 0.88±0.10a | 0.80±0.09a | 0.40±0.12a,b | 0.34±0.10a,b |
| Vimentin | 1.01±0.12 | 1.36±0.18a | 1.43±0.15a | 1.78±0.17a,b | 1.82±0.13a,b |
| Protein | | | | | |
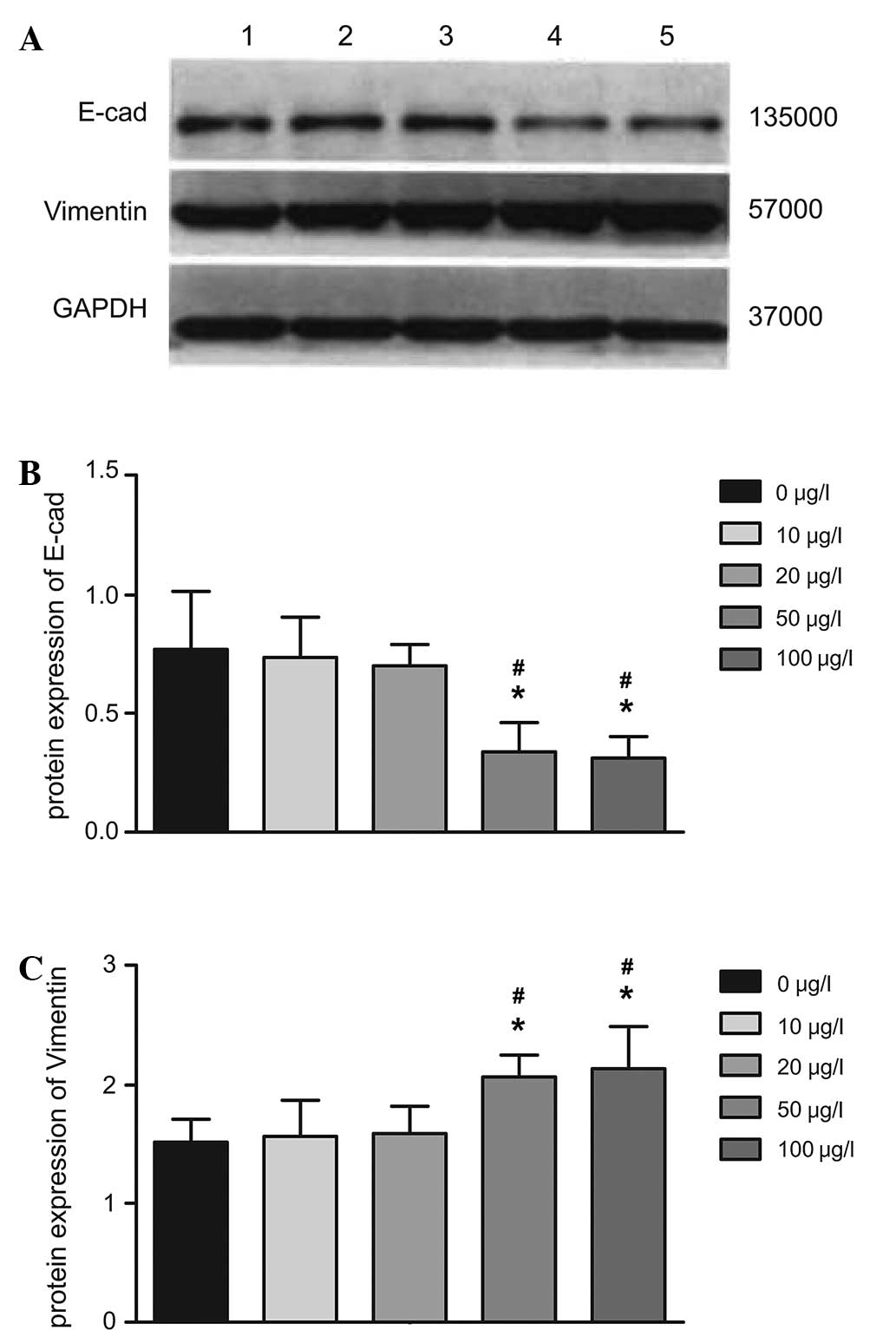
| E-cadherin | 0.76±0.11 | 0.74±0.09 | 0.71±0.09 | 0.33±0.10a,b | 0.31±0.07a,b |
| Vimentin | 1.78±0.21 | 1.86±0.23 | 1.89±0.22 | 2.36±0.18a,b | 2.40±0.20a,b |
In analogy with the RT-qPCR results, western blot
analysis revealed that the protein expression of E-cadherin in
HIBECs was significantly reduced following treatment with IL-6
(P<0.05 for 50 and 100 vs. 0, 10 and 20 µg/l). In
addition, the protein expression of vimentin was significantly
increased following treatment with IL-6 (P<0.05 for 50 and 100
vs. 0, 10 and 20 µg/l). However, E-cadherin and vimentin
expression between the 50 and the 100 µg/l groups as well as
between the 0, 10 and 20 µg/l groups was not significantly
different (all P>0.05) (Fig.
8).
Discussion
Pro-inflammatory cytokine IL-6, produced at low
levels by BECs under normal conditions, appears to contribute to
biliary tree integrity and maintenance of the hepatocyte mass
during chronic injury. However, IL-6 levels are elevated in the
cytoplasm of pathologic IBECs and IL-6 is also overexpressed at the
mRNA level (11,21–23).
Exogenous IL-6 showed mitogenic activity in BECs; furthermore, IL-6
may regulate trefoil factor family 3 (TFF3) at the mRNA and protein
level, contributing to BEC proliferation and migration in mice
(14,24,25).
The present study revealed an increased migratory and invasive
capacity of HIBECs following incubation with IL-6 at various
concentrations. Although the exact underlying mechanisms remain to
be elucidated, it is likely that IL-6 activates signaling pathways
involved in the regulation of the proliferation and biological
behavior of HIBECs (26,27). Consistent with the findings of the
present study, a previous study reported that incubation of HIBECs
with recombinant IL-6 enhanced their migratory potential, which was
indicated to be mediated via activation of the IL-6/signal
transducer and activator of transcription (STAT)3/TFF3 signaling
pathway (28).
Recent studies have suggested the implication of the
EMT in cellular process associated with migration and invasion, and
the involvement of certain EMT-associated proteins, including
E-cadherin, β-catenin, adenomatous polyposis coli, S100
calcium-binding protein A4 and vimentin, has been identified
(29,30). E-cadherin is a cell-cell adhesion
protein for homotypic cell contacts and is a cell-surface marker of
epithelial cells. Decreased E-cadherin expression disrupts
epithelial integrity and promotes EMT (31,32).
Furthermore, increased expression of vimentin is one of the
hallmarks of EMT; cellular changes accompanying the EMT include
increased vimentin expression and associated changes in cell shape,
motility and adhesion (33–35).
E-cadherin repression followed by an increase in vimentin
expression was observed in human breast cancer cells after IL-6
stimulation, indicating a potential role of IL-6 in the induction
of EMT resulting in a mesenchymal phenotype in breast cancer cells
(15,16). Of note, the present study showed
that incubation of HIBECs with IL-6 at 50 µg/l and above
resulted in a significant decrease in mRNA and protein expression
of aberrant E-cadherin and a significant increase in vimentin,
indicating a significant role of IL-6 in the EMT in the HIBECs.
Although the exact underlying mechanisms of the EMT of HIBECs
remain to be elucidated, a previous study suggested that IL-6
stimulation led to EMT-associated changes in E-cadherin and
vimentin expression via the Janus kinase/STAT3/SNAIL signaling
pathway (17). Consistent with
this, morphological changes were observed in HIBECs incubated with
IL-6, along with changes in EMT markers and increased invasive
behavior.
In conclusion, the present study demonstrated that
IL-6 has a significant role in the EMT of HIBECs, as indicated by
an increased migratory and invasive capacity of HIBECs,
characteristic changes in the aberrant mRNA and protein expression
of the EMT markers E-cadherin and vimentin, as well as typical
morphological changes indicating a mesenchymal cell type. The
present study provided novel insight into pathways which may serve
as therapeutic molecular targets for preventing HIBECs from
undergoing EMT-associated pathological processes.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China Youth Science Fund
(NSFC; grant no. 81200319) and the NSFC (grant no. 81170426).
References
|
1
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cannito S, Novo E, Di Bonzo LV, Busletta
C, Colombatto S and Parola M: Epithelial-mesenchymal transition:
From molecular mechanisms, redox regulation to implications in
human health and disease. Antioxid Redox Signal. 12:1383–1430.
2010. View Article : Google Scholar
|
|
3
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schulze F, Schardt K, Wedemeyer I, Konze
E, Wendland K, Dirsch O, Töx U, Dienes HP and Odenthal M:
Epithelial-mesenchymal transition of biliary epithelial cells in
advanced liver fibrosis. Verh Dtsch Ges Pathol. 91:250–256. 2007.In
German.
|
|
6
|
Mizuguchi YSS and Isse K: Molecular
pathology of liver diseases. Hepatology. 59:1130–1143. 2014.
View Article : Google Scholar
|
|
7
|
Rodés J, Benhamou JP, Blei A, Reichen J
and Rizzetto M: From Basic Science to Clinical Practice. Lacy A and
Francis NK: 3rd. Wiley-Blackwell; Hoboken, NJ: pp. 52–57. 2007
|
|
8
|
Yokoyama T, Komori A, Nakamura M, Takii Y,
Kamihira T, Shimoda S, Mori T, Fujiwara S, Koyabu M, Taniguchi K,
et al: Human intrahepatic biliary epithelial cells function in
innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB
and -MAPK signaling pathways. Liver Int. 26:467–476. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karrar A, Broomé U, Södergren T, Jaksch M,
Bergquist A, Björnstedt M and Sumitran-Holgersson S: Biliary
epithelial cell antibodies link adaptive and innate immune
responses in primary sclerosing cholangitis. Gastroenterology.
132:1504–1514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawata K, Kobayashi Y, Gershwin ME and
Bowlus CL: The immunophysiology and apoptosis of biliary epithelial
cells: Primary biliary cirrhosis and primary sclerosing
cholangitis. Clin Rev Allergy Immunol. 43:230–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasoshima M, Kono N, Sugawara H,
Katayanagi K, Harada K and Nakanuma Y: Increased expression of
interleukin-6 and tumor necrosis factor-alpha in pathologic biliary
epithelial cells: In situ and culture study. Lab Invest. 78:89–100.
1998.PubMed/NCBI
|
|
12
|
Kishimoto T: Interleukin-6: Discovery of a
pleiotropic cytokine. Arthritis Res Ther. 8(Suppl 2): S22006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park J, Tadlock L, Gores GJ and Patel T:
Inhibition of interleukin 6-mediated mitogen-activated protein
kinase activation attenuates growth of a cholangiocarcinoma cell
line. Hepatology. 30:1128–1133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokomuro S, Tsuji H, Lunz JG III, Sakamoto
T, Ezure T, Murase N and Demetris AJ: Growth control of human
biliary epithelial cells by interleukin 6, hepatocyte growth
factor, transforming growth factor beta1 and activin A: Comparison
of a cholangiocarcinoma cell line with primary cultures of
non-neoplastic biliary epithelial cells. Hepatology. 32:26–35.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie G, Yao Q, Liu Y, Du S, Liu A, Guo Z,
Sun A, Ruan J, Chen L, Ye C and Yuan Y: IL-6-induced
epithelial-mesenchymal transition promotes the generation of breast
cancer stem-like cells analogous to mammosphere cultures. Int J
Oncol. 40:1171–1179. 2012.
|
|
17
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colomiere M, Ward AC, Riley C, Trenerry
MK, Cameron-Smith D, Findlay J, Ackland L and Ahmed N: Cross talk
of signals between EGFR and IL-6R through JAK2/STAT3 mediate
epithelial-mesenchymal transition in ovarian carcinomas. Br J
Cancer. 100:134–144. 2009. View Article : Google Scholar :
|
|
19
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Causier B and Davies B: Analysing
protein-protein interactions with the yeast two-hybrid system.
Plant Mol Biol. 50:855–870. 2002. View Article : Google Scholar
|
|
21
|
Lamireau T, Zoltowska M, Levy E, Yousef I,
Rosenbaum J, Tuchweber B and Desmoulière A: Effects of bile acids
on biliary epithelial cells: Proliferation, cytotoxicity, and
cytokine secretion. Life Sci. 72:1401–1411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ezure T, Sakamoto T, Tsuji H, Lunz JG III,
Murase N, Fung JJ and Demetris AJ: The development and compensation
of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol.
156:1627–1639. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Sakamoto T, Ezure T, Yokomuro S,
Murase N, Michalopoulos G and Demetris AJ: Interleukin-6,
hepatocyte growth factor, and their receptors in biliary epithelial
cells during a type I ductular reaction in mice: Interactions
between the periductal inflammatory and stromal cells and the
biliary epithelium. Hepatology. 28:1260–1268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yokomuro S, Lunz JG III, Sakamoto T, Ezure
T, Murase N and Demetris AJ: The effect of interleukin-6
(IL-6)/gp130 signalling on biliary epithelial cell growth, in
vitro. Cytokine. 12:727–730. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nozaki I, Lunz JG III, Specht S, Park JI,
Giraud AS, Murase N and Demetris AJ: Regulation and function of
trefoil factor family 3 expression in the biliary tree. Am J
Pathol. 165:1907–1920. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen LP, Cai M, Zhang QH, Li ZL, Qian YY,
Bai HW, Wei X, Shi BY and Dong JH: Activation of
interleukin-6/STAT3 in rat cholangiocyte proliferation induced by
lipopolysaccharide. Dig Dis Sci. 54:547–554. 2009. View Article : Google Scholar
|
|
27
|
Chen LP, Qian YY, Li ZL, Bai HW, Cai M and
Shi BY: Role of IL-6/STAT3 in rat cholangiocyte proliferation
induced by lipopolysaccharide. Zhonghua Gan Zang Bing Za Zhi.
17:374–377. 2009.In Chinese. PubMed/NCBI
|
|
28
|
Jiang GX, Zhong XY, Cui YF, Liu W, Tai S,
Wang ZD, Shi YG, Zhao SY and Li CL: IL-6/STAT3/TFF3 signaling
regulates human biliary epithelial cell migration and wound healing
in vitro. Mol Biol Rep. 37:3813–3818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer-observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar
|
|
34
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|