Introduction
Medullary thyroid carcinoma (MTC), a neuroendocrine
tumor originating from thyroid parafollicular cells, accounts for
~4% of thyroid cancer cases (1).
The majority are sporadic cases, however, 20-25% occur as a
hereditary syndrome termed multiple endocrine neoplasia type 2 (MEN
2A and MEN 2B) and as familial MTC, both of which are associated
with germline mutations in the RET oncogene (2).
Mutations in the RET oncogene have previously
been identified in the tumor tissue of up to 64% of sporadic MTC
cases (3). In addition, RAS
gene mutations are observed in 10% of RET-negative cases and
are associated with a subset of tumors with less aggressive
behavior (4). While certain
studies identified that ~90% of sporadic MTCs exhibited mutually
exclusive mutations in RET, HRAS and KRAS
(4–8), Moura et al (3) reported the presence of the RAS
mutation in one case with RET-positive sporadic MTC and Rapa
et al (9) identified no
RAS mutations in 49 examined cases. Nevertheless, the
clinical phenotype of sporadic and inherited MTCs is heterogeneous
even in the presence of the same mutation; however the molecular
mechanisms underlying the pathology remain to be fully
elucidated.
In addition, it remains unclear whether there is a
modulatory role in MTC tumor progression for additional genes such
as BRAF, CDKN2A and PI3KCA. These genes
participate in the tumorigenesis of several types of human
malignancies such as tumors derived from neural crest cells,
including melanoma, pheochromocytoma and paraganglioma (10–12).
BRAF, like RET and RAS, is
involved in the mitogen-activated protein kinase pathway and has a
well-established role in the pathogenesis of malignancies such as
melanoma and papillary thyroid cancer (13). Nevertheless, the contribution in
the tumorigenesis of MTC remains controversial. A previous study
reported a high prevalence of the p.Val600Glu BRAF mutation
in sporadic MTC cases (14);
however, subsequent studies did not confirm this observation
(3,9,15,16).
An additional tumor suppressor gene,
CDKN2A/p16INK4A, is involved in the
G1/S transition in the cell cycle. Mutations and
deletions have been identified in melanoma, and polymorphisms in
its 3′ untranslated region (UTR) have been associated with earlier
progression from primary to metastatic disease (17). By contrast, polymorphisms in
another tumor suppressor gene, CDKN1B, which is in the same
CDKN family, are associated with improved outcomes (18).
Additionally, PI3KCA is a gene that serves an
important role in signaling pathways and cell growth, and
contributes to tumorigenesis in several types of human malignancy
(19,20). However, the role of this gene in
the tumorigenesis of MTC remains to be fully understood.
Therefore, the current study aimed to verify the
prevalence of somatic mutations in BRAF, CDKN2A and
PI3KCA, which have already been described in other neural
crest-derived tumors, and to determine the possible supporting role
of these genes in the tumorigenesis of MTC.
Patients and methods
Patients and tissue samples
From 128 patients with MTC assessed at the Multiple
Endocrine Neoplasia outpatient clinic at the Universidade Federal
de Sao Paulo (Sao Paulo, Brazil) between February 2007 and June
2013, formalin-fixed paraffin-embedded (FFPE) tumor tissues were
selected from 31 patients on the basis of the availability of tumor
tissues, with no other selection criteria. DNA extraction was
subsequently performed, using an in-house method as previously
described (21). Subsequent to DNA
extraction, 20 samples (from 13 males and 7 females; mean age,
40.55±16.74 years) provided the appropriate quantity and quality of
DNA. The study was approved by the Ethics and Research Committee of
the Universidade Federal de Sao Paulo (protocol number 1945/10),
and all patients provided informed consent. Additionally, 1,092
genotypes of variant frequencies (single nucleotide polymorphisms;
SNPs) were obtained from the 1000 Genomes database (http://www.1000genomes.org/) as a population genetics
control.
DNA extraction and genotyping
DNA from peripheral blood and somatic DNA from
10-µm sections of FFPE MTC tissues was extracted using an
in-house method as previously described (21). Polymerase chain reaction (PCR) was
performed to amplify DNA corresponding to hotspot exons 2, 3 and 4
of HRAS; 2, 3 and 4 of KRAS; 2 and 3 of NRAS;
15 of BRAF; 9 and 20 of PI3KCA; and exons 2, 3 and
the 3′UTR of the CDKN2A gene. The sequences of the primers
are listed in Table I. The
reactions were performed using 10 pM of each specific primer, 2.5
µl PCR buffer, 200 µM dNTP, 1.5 µM
MgCl2 and 0.2 units Taq DNA polymerase
(Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) in a
25-µl total reaction volume. The cycling conditions were as
follows: 5 min at 95°C, 38 cycles of 45 sec at 95°C, 45 sec for
annealing and 1 min at 72°C, and a final elongation for 10 min at
72°C. The PCR products were purified using the Illustra GFX PCR DNA
and Gel Purification kit (GE Healthcare Life Sciences, Chalfont,
UK) and were subject to sequencing using the Sanger method, with
the Big Dye™ Terminator Cycle Sequencing Ready Reaction kit and the
ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems; Thermo
Fisher Scientific). Gel electrophoresis of the PCR products was
performed to analyze product quality and yield using a 1.8% agarose
gel and a DNA ladder.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Gene | Forward primer | Reverse primer |
|---|
| BRAF exon 15 |
5′-AACTCAGCAGCATCTCAGGG-3′ |
5′-CTTCATAATGCTTGCTCTGATAG-3′ |
| CDKN2A exon 1 |
5′-ACCCTGGCTCTGACCATTC-3′ |
5′-CAGGTCACGGGCAGAC-3′ |
| CDKN2A exon 2 |
5′-GACCTCAGGTTTCTAACGCC-3′ |
5′-CATATATCTACGTTAAAAGGCAGGAC-3′ |
| PI3KCA exon 9 |
5′-TGGCAGTCAAACCTTCTCTC-3′ |
5′-GAGAAAGTATCTACCTAAATCCACAGA-3′ |
| PI3KCA exon 20 |
5′-AAATGTTTTGGTGTTCTTAATTTATTC-3′ |
5′-GCAGCCAGAACTCTTTATTTTG-3′ |
| C-kit exon 9 |
5′-GCCAGGGCTTTTGTTTTCTT-3′ |
5′-AGCCTAAACATCCCCTTAAATTG-3′ |
| C-kit exon 11 |
5′-AACCATTTATTTGTTCTCTCTCCA-3′ |
5′-CCACTGGAGTTCCTTAAAGTCA-3′ |
| C-kit exon 17 |
5′-TGGTTTTCTTTTCTCCTCCAAC-3′ |
5′-GGACTGTCAAGCAGAGAATGG-3′ |
| HRAS exon 2 |
5′-GGCAGGAGACCCTGTAGGAG-3′ |
5′-AGCTGCTGGCACCTGGAC-3′ |
| HRAS exon 3 |
5′-GTCCCTGAGCCCTGTCCTC-3′ |
5′-CAGCCTCACGGGGTTCAC-3′ |
| HRAS exon 4 |
5′-CTCTCGCTTTCCACCTCTCA-3′ |
5′-GGGTGGAGAGCTGCCTCA-3′ |
| KRAS exon 2 |
5′-TTAACCTTATGTGTGACATGTTCTAA-3′ |
5′-GGTCCTGCACCAGTAATATGC-3′ |
| KRAS exon 3 |
5′-AGACTGTGTTTCTCCCTTCTCA-3′ |
5′-TGGCATTAGCAAAGACTCAAA-3′ |
| KRAS exon 4 |
5′-GATATTTGTGTTACTAATGACTGTGCT-3′ |
5′-TTATGATTTTGCAGAAAACAGATC-3′ |
| NRAS exon 2 |
5′-TCGCCAATTAACCCTGATTAC-3′ |
5′-TCCGACAAGTGAGAGACAGG-3′ |
| NRAS exon 3 |
5′-TGGGCTTGAATAGTTAGATGC-3′ |
5′-AGTGTGGTAACCTCATTTCCC-3′ |
In silico analysis of HRAS mutations and
CDKN2A polymorphisms
Mutational analysis of HRAS was performed by
the use of Project HOPE to obtain structural information from the
analysis of PDB-file 1CTQ (22).
The in silico analysis for the CDKN2A polymorphisms
was performed using the Functional Single Nucleotide Polymorphism
database (http://compbio.cs.queensu.ca/F-SNP/) as previously
described (23). This database
provides information regarding potential deleterious effects of
SNPs with respect to splicing, transcription, translation and
post-translation based on SNP functional significance (FS). The FS
score for neutral SNPs is 0.1764, whereas the FS score for
disease-associated SNPs is in the range of 0.5-1.
Statistical analysis
The allele and genotype frequencies were compared
between patients with MTC and the 1000 Genomes database controls
using a χ2 test. The clinicopathological features of
patients carrying each of the polymorphisms rs11515 and rs3088440
were compared with those of patients without such polymorphisms
using the χ2 test or the Student's unpaired t-test as
appropriate. P<0.05 was considered to indicate a statistically
significant difference, and the Hardy-Weinberg equilibrium was
evaluated. Statistical analyses were performed using SPSS, version
22.0 (IBM SPSS, Armonk, NY, USA) and GraphPad Prism, version 3.0
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
Screening of the RET, HRAS, KRAS and NRAS genes
Mutational screening of the RET gene was
performed on all 20 patients. A total of 10 cases were identified
to be familial tumors as confirmed by the presence of a germline
mutation. In total, 30% of the sporadic cases (3/10) presented with
a RET somatic mutation. The clinicopathological features and
molecular analysis, including tumor staging based on the American
Joint Committee in Cancer staging system (24), are summarized in Table II.
 | Table IISummary of patient
clinicopathological features and molecular analysis. |
Table II
Summary of patient
clinicopathological features and molecular analysis.
| Patient | Gender | Age at diagnosis
(y) | pTNMa | Germline RET
allele | Somatic RET
allele | Somatic H-,
K-, NRAS allele | Somatic
CDKN2A |
|---|
| 1 | M | 28 | T2N1bMx | WT | WT |
HRAS_p.Asp33Asn | rs11515 |
| 2 | F | 25 | T3N1bMx | WT | p.Met918Thr | - | WT |
| 3 | M | 38 | T1N1aMx | WT | WT | NA |
rs11515/rs3088440 |
| 4 | M | 56 | T3N1bMx | WT | WT | WT | WT |
| 5 | F | 49 | T2NxMx | WT | p.Gln681Stop | - | WT |
| 6 | M | 69 | T2N0Mx | WT | WT |
HRAS_p.Gln61Arg | rs3088440 |
| 7 | M | 27 | T4N1Mx | WT | WT | WT | WT |
| 8 | M | 51 | T3N1bMx | WT | p.Met918Thr | - | rs11515 |
| 9 | F | 56 | T1N1bMx | WT | WT |
HRAS_p.Asp33Asn | WT |
| 10 | M | 41 | T4N1bMx | WT | WT |
HRAS_p.His94Tyr | WT |
| 11 | M | 27 | T1N1aMx | p.Cys634Arg | - | - |
rs11515/rs3088440 |
| 12 | F | 21 | T1N1aMx | p.Gly533Cys | - | - | rs11515 |
| 13 | M | 61 | T1N1aMx | p.Gly533Cys | - | - | WT |
| 14 | F | 22 | T2N0Mx | p.Cys634Arg | - | - |
rs11515/rs3088440 |
| 15 | M | 43 | T2N0Mx | p.Cys634Arg | - | - | rs11515 |
| 16 | M | 72 | T1N0Mx | p.Cys634Arg | - | - | WT |
| 17 | M | 45 | T1N1aMx | p.Cys634Arg | - | - | rs3088440 |
| 18 | F | 31 | T1NxMx | p.Cys634Arg | - | - | rs3088440 |
| 19 | F | 15 | T1N1aMx | p.Cys634Arg | - | - | rs3088440 |
| 20 | M | 40 | T1N0Mx | p.Gly533Cys | - | - | WT |
To investigate exclusive causative mutations in
cases of sporadic MTC other than RET mutations, HRAS,
KRAS and NRAS were screened for somatic mutations in
the hotspots. The majority of these patients had been previously
analyzed for RET germline mutations as part of our routine
evaluation, and for RET somatic mutations in a previous study
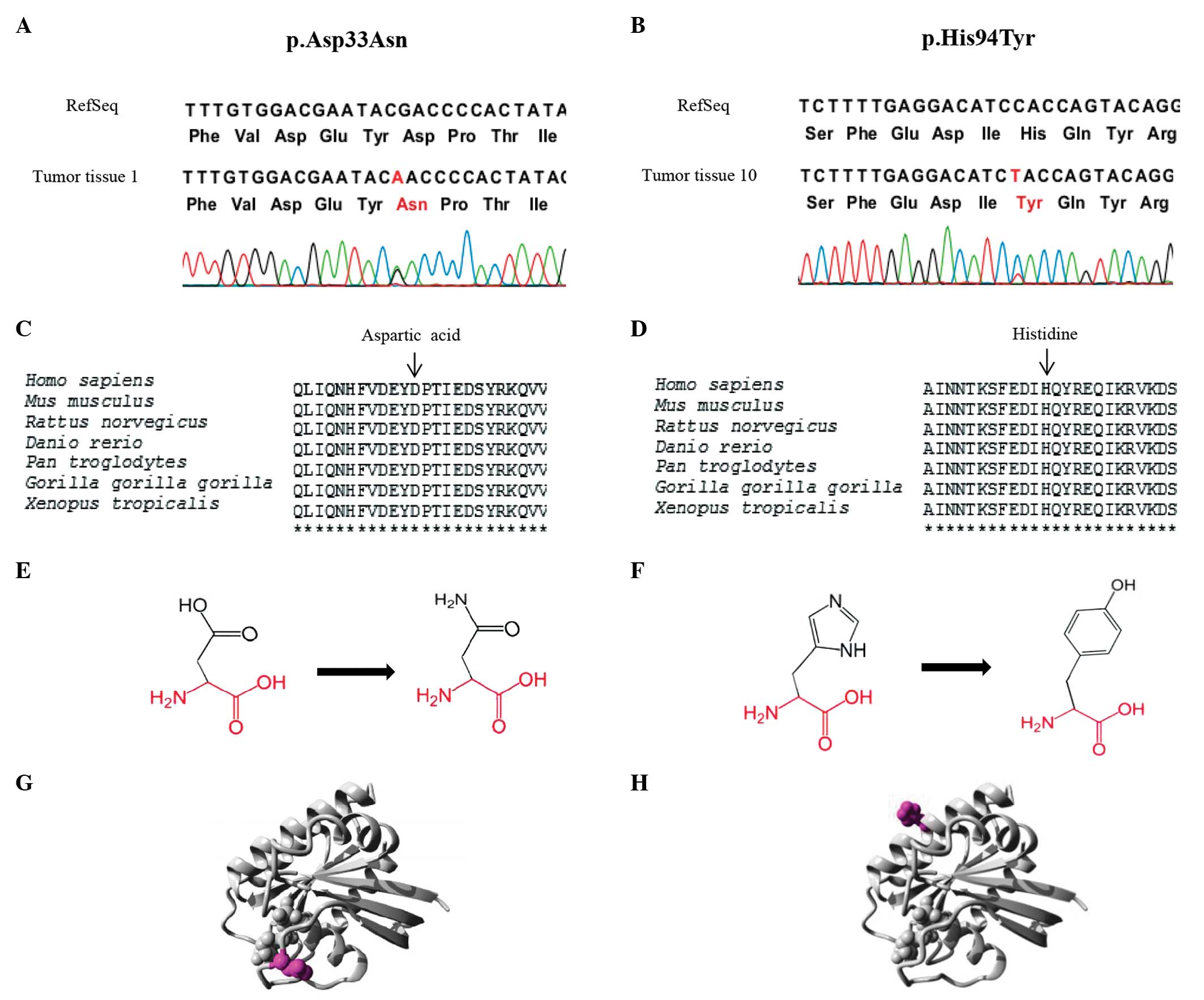
(25) Two novel HRAS
mutations, p.Asp33Asn and p.His94Tyr, were detected in
RET-negative MTC tumors. Mutational analysis using Project
HOPE suggests that the p.His94Tyr mutation is deleterious, and that
the p.Asp33Asn mutation is likely to be damaging (Fig. 1). No differences in the clinical
presentation or histological observations were noted between
patients with MTC that had a mutation in the RAS gene
(Table II).
No somatic mutations were identified in exon 15 of
BRAF or in exons 9 and 20 of PI3KCA. Patient 9 was
not analyzed for somatic mutations in PI3KCA due to an
insufficient number of tumor samples.
Despite not having identified somatic mutations in
CDKN2A hotspots, two polymorphisms in the 3′UTR regulatory
region, 500 C→G (rs11515) and 540 C→T (rs3088440), were identified
in the patients observed. The heterozygotic pattern of the two SNPs
was observed in the same proportion, 7/20 MTC (35%). The genotype
distribution was identified to be in the Hardy-Weinberg equilibrium
and was not identified to exhibit linkage disequilibrium. To
investigate whether the observed polymorphisms were limited to a
somatic event, they were further analyzed in the peripheral blood,
which confirmed germline inheritance. The in silico analysis
demonstrated that the CDKN2A polymorphisms rs11515 and
rs3088440 are located in the transcriptional regulatory region and
that the nucleotide alterations may affect the binding of
transcription factors.
In seven cases, it was possible to detect the
presence of these polymorphisms in the secondary tumors in the
lymph nodes (tumor metastases), however no differences between the
genotypes of the primary and secondary tumors were observed,
indicating that there was no additional somatic event in
CDKN2A involved in the metastatic process. This analysis was
additionally performed for BRAF and PI3KCA in
metastatic tissues.
No associations between the polymorphisms and the
clinicopathological features observed were identified (Table III). In addition, the frequency
of the SNPs was compared with a population genetics control, and
there was no significant difference between the two populations
(Table IV).
 | Table IIICorrelation between CDKN2A
SNPs and clinicopathological features in the patient cohort. |
Table III
Correlation between CDKN2A
SNPs and clinicopathological features in the patient cohort.
| Clinicopathological
feature | rs11515 (n=20)
| rs3088440 (n=20)
|
|---|
| CC (n=13) | CG (n=7) | P-value | CC (n=13) | CT (n=7) | P-value |
|---|
| Gender | | | 0.526 | | | 0.474 |
| Male (n=13) | 8/13 (61.5%) | 5/7 (71.4%) | | 9/13 (69.2%) | 4/7 (57.1%) | |
| Female (n=7) | 5/13 (38.5%) | 2/7 (28.6%) | | 4/13 (30.7%) | 3/7 (42.9%) | |
| Age at
diagnosis | | | 0.088a | | | 0.272a |
| Mean ± SD (y) | 45.41±17.49 | 31.53±11.36 | | 44.062±17.49 | 31.53±11.36 | |
| Tumor type | | | 0.500 | | | 0.175 |
| Sporadic
(n=10) | 7/13 (53.8%) | 3/7 (42.9%) | | 8/13 (61.5%) | 2/7 (28.5%) | |
| Familial
(n=10) | 6/13 (46.1%) | 4/7 (57.1%) | | 5/13 (38.5%) | 5/7 (62.5%) | |
| T category | | | 0.464 | | | 0.291 |
| T1 | 7/13 (53.8%) | 2/7(28.5%) | | 5/13 (38.5%) | 4/7 (57.1%) | |
| T2 | 2/13 (15.3%) | 3/7 (42.9%) | | 3/13 (23.1%) | 2/7 (28.5%) | |
| T3 | 2/13 (15.3%) | 2/7 (28.5%) | | 3/13 (23.1%) | 1/7 (14.4%) | |
| T4 | 2/13 (15.3%) | 0/7 (0%) | | 2/13 (15.3%) | 0/7 (0%) | |
| Tumor size | | | 0.421a | | | 0.689a |
| Mean ± SD (cm) | 1.954±1.11 | 2.34±1.03 | | 2.315±1.22 | 1.671±0.59 | |
| <2 | 8/13 (61.5%) | 2/7(28.6%) | 0.378 | 7/13 (53.8%) | 4/7 (57.1%) | 0.339 |
| ≥2 | 5/13 (38.5%) | 5/7 (71.4%) | | 6/13 (46.1%) | 3/7 (42.9%) | |
| Lymph node
metastases | | | 0.742 | | | 0.742 |
| N0 | 4/13 (30.8%) | 5/7 (71.4%) | | 3/13 (23.07%) | 3/7 (42.9%) | |
| N1 | 9/13 (69.2%) | 2/7 (28.5%) | | 10/13 (76.9%) | 4/7 (57.1%) | |
| AJCC stage | | | 0.742 | | | 0.742 |
| I and II | 4/13 (30.7%) | 2/7 (28.5%) | | 4/13 (30.7%) | 2/7 (28.5%) | |
| III and IV | 9/13 (69.2%) | 5/7 (71.4%) | | 9/13 (69.2%) | 5/7 (71.4%) | |
 | Table IVComparative analysis of the frequency
of the non-coding CDKN2A germ line single nucleotide
polymorphisms in patients with MTC and the control. |
Table IV
Comparative analysis of the frequency
of the non-coding CDKN2A germ line single nucleotide
polymorphisms in patients with MTC and the control.
A, rs11515
|
|---|
| Genotype frequency
| Allele frequency
| |
|---|
| Population | CC | CG | GG | C (32) | G (8) | P-value |
|---|
| MTC | 0.60 | 0.40 | - | 0.80 | 0.20 | 0.25 |
| 1,000
genomesa | 0.79 | 0.19 | 0.02 | 0.88 | 0.12 | |
B, rs3088440
|
| Genotype frequency
| Allele frequency
| |
| Population | CC | CT | TT | C (31) | T (9) | P-value |
|
| MTC | 0.55 | 0.45 | - | 0.78 | 0.22 | 0.65 |
| 1,000
genomesa | 0.73 | 0.24 | 0.03 | 0.85 | 0.15 | |
Discussion
The adjuvant role of additional genes in the
tumorigenesis of MTC was investigated in the current study through
analysis of tumor tissues from 20 patients. Screening in hotspot
regions of BRAF, CDKN2A and PI3KCA did not
identify any somatic mutations in the coding region. In addition,
the results of the current study were not in agreement with the
BRAF mutation frequency of 68.2% observed by Goutas et
al (14). This suggests that
BRAF does not serve an important role in the tumorigenesis
of MTC. The observations of the current study concerning MTC are
consistent with a previous study that demonstrated that somatic
mutations in genes other than RET and RAS are very
rare or even absent (5). Notably,
the present study identified two novel HRAS mutations.
Additionally, two common polymorphisms in the 3′-UTR
non-coding region of the gene CDKN2A were identified,
rs11515 and rs3088440 (26). It is
known that protein synthesis can be modulated by regulatory
elements located in the 5′-UTR and 3′-UTR regions. The 3′-UTR, the
site of the polymorphisms identified in the current study, serves
an important role in translation and mRNA stability. Alterations in
this region may be associated with the onset or progression of
disease (27).
These polymorphisms have been investigated in
various tumor types including urinary bladder neoplasm (28), esophageal adenocarcinoma (29) and cervical cancer (30) as presented in Table V. The two identified polymorphisms
have been previously associated with an earlier progression from
primary to metastatic disease in the case of melanoma (17), and rs3088440 was associated with
the mechanism of tumor invasion in bladder cancer (28). Controversially, this polymorphism
has been previously associated with a sub-group with reduced
vertical growth of melanoma and a favorable outcome (31). However, additional studies have not
identified a clinical correlation with tumor behavior (30,32,33).
 | Table VSummary of the studies on
CDKN2A polymorphisms in different tumor types. |
Table V
Summary of the studies on
CDKN2A polymorphisms in different tumor types.
| Source, year
(ref) | rs11515 (%) | rs3088440 (%) | Tumor type | n | Sample | Method used |
|---|
| Sauroja et
al, 2000 (17) | 16.67 | 16.67 | Melanoma | 48 | Frozen/FFPE
tissue |
PCR-SSCP/sequencing |
| Kumar et al,
2001 (26) | 25 | 27.27 | Melanoma | 229 | FFPE tissue | PCR-SSCP |
| Sakano et
al, 2003 (28) | 18.1 | 12.9 | Bladder | 309 | Blood | PCR-SSCP |
| Geddert et
al, 2005 (29) | 13.3 | - | ADC | 315 | FFPE tissue | PCR-RFLP |
| Chansaenroj, et
al 2013 (30) | 7.1 | 17.9 | Cervical | 56 | Cervical swab | Sequencing |
| Straume et
al, 2002 (31) | 25 | 23 | Melanoma | 185 | FFPE tissue |
PCR-SSCP/sequencing |
| Boonstra et
al, 2011 (32) | 22.07 | - | EAC | 214 | FFPE tissue | Sequencing |
| Pinheiro et
al, 2014 (33) | 21.05 | - | ESCC | 97 | FFPE tissue | Sequencing |
| 15.63 | - | HNSCC | 96 | FFPE tissue | PCR-RFLP |
| Jin et al,
2012 (34) | - | 16.7 | SGC | 156 | Blood | PCR-RFLP |
| Polakova et
al, 2008 (35) | 25.98 | 13.07 | Colorectal | 612 | Blood | PCR-RFLP |
| Royds et al,
2011 (36) | 31.78 | - | GBM | 107 | Blood | Sequencing |
| Thakur et
al, 2012 (37) | 13.64 | - | Cervical | 150 | Fresh tissue | PCR-RFLP |
| Zhang et al,
2011 (38) | - | 17.0 | SCCHN | 1,287 | Blood | PCR-RFLP |
| Zhang et al,
2013 (39) | - | 20.5 | DTC | 303 | Blood | PCR-RFLP |
| - | 20.9 | PTC | 273 | Blood | PCR-RFLP |
| De Giorgi et
al, 2014 (4 0) | 16.67 | - | Melanoma | 12 | Blood | Sequencing |
| Song et al,
2014 (41) | - | 33.88 | SCCOP | 552 | Blood | PCR-RFLP |
| Nascimento et
al, 2015a | 35 | 35 | MTC | 20 | FFPE tissue +
blood | Sequencing |
Using in silico analysis, the current study
identified that the polymorphisms rs11515 and rs3088440 are located
within a transcriptional regulatory region, and the alteration of
nucleotides can affect the binding of potential transcriptional
factors. For example, the presence of the C allele in rs3088440
favors the binding of the transcription factor c-Myb, which
potentially results in the transcriptional repression of the
CDKN2A gene, compromising its normal function in cell cycle
control (42). However, no
association was identified between this polymorphism and the
clinicopathological parameters investigated in the cohort studied
(Table III).
In conclusion, it is suggested that BRAF,
CDKN2A and PI3KCA, listed as potential adjuvants in
the tumorigenesis of MTC, do not participate through somatic
mutations as modulators of oncogenesis. To the best of our
knowledge, the current study is the first to investigate these two
CDKN2A polymorphisms in the pathophysiology of MTC.
Therefore, CDKN2A and its regulatory regions and the
additional genes involved in tumorigenesis warrant further
investigation in MTC.
Acknowledgments
The authors would like to thank the team of the
Laboratory of Molecular and Translational Endocrinology,
particularly Ms. Teresa Kasamatsu, Mr. Gilberto Furuzawa, Dr João
Roberto Martins, Dr Ji Hoon Yang, Dr Fausto Germano Neto and Mr.
Fernando Soares. The authors would additionally like to acknowledge
Mr. Gilmar Miranda from Siratec Ltd. for graphic art design. The
current study was supported grants from the Sao Paulo State
Research Foundation (grant nos. 2012/11036-3, 2012/02465-8,
2012/01628-0, 2009/50575-4, 2012/00079-3 and 2011/20747-8).
References
|
1
|
American Thyroid Association Guidelines
Task Force; Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib
H, Moley JF, Pacini F and Ringel MD: Medullary thyroid cancer:
Management guidelines of the American thyroid association. Thyroid.
19:565–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nosé V: Familial thyroid cancer: A review.
Mod Pathol. 24(Suppl 2): S19–S33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moura MM, Cavaco BM, Pinto AE and Leite V:
High prevalence of RAS mutations in RET-negative sporadic medullary
thyroid carcinomas. J Clin Endocrinol Metab. 96:E863–E868. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciampi R, Mian C, Fugazzola L, Cosci B,
Romei C, Barollo S, Cirello V, Bottici V, Marconcini G, Rosa PM, et
al: Evidence of a low prevalence of RAS mutations in a large
medullary thyroid cancer series. Thyroid. 23:50–57. 2013.
View Article : Google Scholar
|
|
5
|
Agrawal N, Jiao Y, Sausen M, Leary R,
Bettegowda C, Roberts NJ, Bhan S, Ho AS, Khan Z, Bishop J, et al:
Exomic sequencing of medullary thyroid cancer reveals dominant and
mutually exclusive oncogenic mutations in RET and RAS. J Clin
Endocrinol Metab. 98:E364–E369. 2013. View Article : Google Scholar :
|
|
6
|
Tamburrino A, Molinolo AA, Salerno P,
Chernock RD, Raffeld M, Xi L, Gutkind JS, Moley JF, Wells SA Jr and
Santoro M: Activation of the mTOR pathway in primary medullary
thyroid carcinoma and lymph node metastases. Clin Cancer Res.
18:3532–3540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simbolo M, Mian C, Barollo S, Fassan M,
Mafficini A, Neves D, Scardoni M, Pennelli G, Rugge M, Pelizzo MR,
et al: High-throughput mutation profiling improves diagnostic
stratification of sporadic medullary thyroid carcinomas. Virchows
Arch. 465:73–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puppin C, Durante C, Sponziello M,
Verrienti A, Pecce V, Lavarone E, Baldan F, Campese AF, Boichard A,
Lacroix L, et al: Overexpression of genes involved in miRNA
biogenesis in medullary thyroid carcinomas with RET mutation.
Endocrine. 47:528–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rapa I, Saggiorato E, Giachino D,
Palestini N, Orlandi F, Papotti M and Volante M: Mammalian target
of rapamycin pathway activation is associated to RET mutation
status in medullary thyroid carcinoma. J Clin Endocrinol Metab.
96:2146–2153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berrocal A, Cabañas L, Espinosa E,
Fernández-de-Misa R, Martín-Algarra S, Martínez-Cedres JC,
Ríos-Buceta L and Rodríguez-Peralto JL: Melanoma: Diagnosis,
staging and treatment. Consensus group recommendations. Adv Ther.
31:945–960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muscarella P, Bloomston M, Brewer AR,
Mahajan A, Frankel WL, Ellison EC, Farrar WB, Weghorst CM and Li J:
Expression of the p16INK4A/Cdkn2a gene is prevalently downregulated
in human pheochromocytoma tumor specimens. Gene Expr. 14:207–216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Güran S and Tali ET: p53 and p16INK4A
mutations during the progression of glomus tumor. Pathol Oncol Res.
5:41–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nikiforova MN, Kimura ET, Gandhi M,
Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G,
Fusco A, et al: BRAF mutations in thyroid tumors are restricted to
papillary carcinomas and anaplastic or poorly differentiated
carcinomas arising from papillary carcinomas. J Clin Endocrinol
Metab. 88:5399–5404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goutas N, Vlachodimitropoulos D, Bouka M,
Lazaris AC, Nasioulas G and Gazouli M: BRAF and K-RAS mutation in a
Greek papillary and medullary thyroid carcinoma cohort. Anticancer
Res. 28:305–308. 2008.PubMed/NCBI
|
|
15
|
Schulten HJ, Al-Maghrabi J, Al-Ghamdi K,
Salama S, Al-Muhayawi S, Chaudhary A, Hamour O, Abuzenadah A, Gari
M and Al-Qahtani M: Mutational screening of RET, HRAS, KRAS, NRAS,
BRAF, AKT1 and CTNNB1 in medullary thyroid carcinoma. Anticancer
Res. 31:4179–4183. 2011.PubMed/NCBI
|
|
16
|
Boichard A, Croux L, Al Ghuzlan A, Broutin
S, Dupuy C, Leboulleux S, Schlumberger M, Bidart JM and Lacroix L:
Somatic RAS mutations occur in a large proportion of sporadic
RET-negative medullary thyroid carcinomas and extend to a
previously unidentified exon. J Clin Endocrinol Metab.
97:E2031–E2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sauroja I, Smeds J, Vlaykova T, Kumar R,
Talve L, Hahka-Kemppinen M, Punnonen K, Jansèn CT, Hemminki K and
Pyrhönen S: Analysis of G(1)/S checkpoint regulators in metastatic
melanoma. Genes Chromosomes Cancer. 28:404–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasquali D, Circelli L, Faggiano A,
Pancione M, Renzullo A, Elisei R, Romei C, Accardo G, Coppola VR,
De Palma M, et al: CDKN1B V109G polymorphism a new prognostic
factor in sporadic medullary thyroid carcinoma. Eur J Endocrinol.
164:397–404. 2011. View Article : Google Scholar
|
|
19
|
Tian Q, Frierson HF Jr, Krystal GW and
Moskaluk CA: Activating c-kit gene mutations in human germ cell
tumors. Am J Pathol. 154:1643–1647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar
|
|
21
|
Kizys MM, Cardoso MG, Lindsey SC, Harada
MY, Soares FA, Melo MC, Montoya MZ, Kasamatsu TS, Kunii IS,
Giannocco G, et al: Optimizing nucleic acid extraction from thyroid
fine-needle aspiration cells in stained slides,
formalin-fixed/paraffin-embedded tissues and long-term stored blood
samples. Arq Bras Endocrinol Metabol. 56:618–626. 2012. View Article : Google Scholar
|
|
22
|
Venselaar H, Te Beek TA, Kuipers RK,
Hekkelman ML and Vriend G: Protein structure analysis of mutations
causing inheritable diseases. An e-Science approach with life
scientist friendly interfaces. BMC Bioinformatics. 11:5482010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee PH and Shatkay H: F-SNP:
Computationally predicted functional SNPs for disease association
studies. Nucleic Acids Res. 36(Database issue): D820–D824. 2008.
View Article : Google Scholar :
|
|
24
|
Edge SE, Byrd DR, Carducci MA, Compton CC,
Fritz AG, Greene F and Trotti A: AJCC Cancer Staging Manual.
Springer-Verlag; New York: 2009
|
|
25
|
Lindsey SC, Kunii IS, Germano-Neto F,
Sittoni MY, Camacho CP, Valente FO, Yang JH, Signorini PS, Delcelo
R, Cerutti JM, et al: Extended RET gene analysis in patients with
apparently sporadic medullary thyroid cancer: Clinical benefits and
cost. Horm Cancer. 3:181–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar R, Smeds J, Berggren P, Straume O,
Rozell BL, Akslen LA and Hemminki K: A single nucleotide
polymorphism in the 3′untranslated region of the CDKN2A gene is
common in sporadic primary melanomas but mutations in the CDKN2B,
CDKN2C, CDK4 and p53 genes are rare. Int J Cancer. 95:388–393.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chatterjee S and Pal JK: Role of 5′- and
3′-untranslated regions of mRNAs in human diseases. Biol Cell.
101:251–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakano S, Berggren P, Kumar R, Steineck G,
Adolfsson J, Onelöv E, Hemminki K and Larsson P: Clinical course of
bladder neoplasms and single nucleotide polymorphisms in the CDKN2A
gene. Int J Cancer. 104:98–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geddert H, Kiel S, Zotz RB, Zhang J,
Willers R, Gabbert HE and Sarbia M: Polymorphism of p16 INK4A and
cyclin D1 in adenocarcinomas of the upper gastrointestinal tract. J
Cancer Res Clin Oncol. 131:803–808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chansaenroj J, Theamboonlers A,
Junyangdikul P, Swangvaree S, Karalak A, Chinchai T and Poovorawan
Y: Polymorphisms in TP53 (rs1042522), p16 (rs11515 and rs3088440)
and NQO1 (rs1800566) genes in Thai cervical cancer patients with
HPV 16 infection. Asian Pac J Cancer Prev. 14:341–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Straume O, Smeds J, Kumar R, Hemminki K
and Akslen LA: Significant impact of promoter hypermethylation and
the 540 C>T polymorphism of CDKN2A in cutaneous melanoma of the
vertical growth phase. Am J Pathol. 161:229–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boonstra JJ, van Marion R, Tilanus HW and
Dinjens WN: Functional polymorphisms associated with disease-free
survival in resected carcinoma of the esophagus. J Gastrointest
Surg. 15:48–56. 2011. View Article : Google Scholar :
|
|
33
|
Pinheiro UB, de Carvalho Fraga CA, Mendes
DC, Marques-Silva L, Farias LC, de Souza MG, Soares MB, Jones KM,
Santos SH, de Paula AM, et al: p16 (CDKN2A) SNP rs11515 was not
associated with head and neck carcinoma. Tumour Biol. 35:6113–6118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin L, Xu L, Song X, Wei Q, Sturgis EM and
Li G: Genetic variation in MDM2 and p14ARF and susceptibility to
salivary gland carcinoma. PloS One. 7:e493612012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Polakova V, Pardini B, Naccarati A, Landi
S, Slyskova J, Novotny J, Vodickova L, Bermejo JL, Hanova M,
Smerhovsky Z, et al: Genotype and haplotype analysis of cell cycle
genes in sporadic colorectal cancer in the czech republic. Hum
Mutat. 30:661–668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Royds JA, Al Nadaf S, Wiles AK, Chen YJ,
Ahn A, Shaw A, Bowie S, Lam F, Baguley BC, Braithwaite AW, et al:
The CDKN2A G500 allele is more frequent in GBM patients with no
defined telomere maintenance mechanism tumors and is associated
with poorer survival. PloS One. 6:e267372011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thakur N, Hussain S, Nasare V, Das BC,
Basir SF and Bharadwaj M: Association analysis of p16 (CDKN2A) and
RB1 polymorphisms with susceptibility to cervical cancer in Indian
population. Mol Biol Rep. 39:407–414. 2012. View Article : Google Scholar
|
|
38
|
Zhang Y, Sturgis EM, Zafereo ME, Wei Q and
Li G: p14ARF genetic polymorphisms and susceptibility to second
primary malignancy in patients with index squamous cell carcinoma
of the head and neck. Cancer. 117:1227–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang F, Xu L, Wei Q, Song X, Sturgis EM
and Li G: Significance of MDM2 and P14 ARF polymorphisms in
susceptibility to differentiated thyroid carcinoma. Surgery.
153:711–717. 2013. View Article : Google Scholar :
|
|
40
|
De Giorgi V, Savarese I, D'Errico A, Gori
A, Papi F, Colombino M, Cristina Sini M, Stanganelli I, Palmieri G
and Massi D: CDKN2A mutations could influence the dermoscopic
pattern of presentation of multiple primary melanoma: A clinical
dermoscopic genetic study. J Eur Acad Dermatol Venereol.
29:574–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song X, Sturgis EM, Huang Z, Li X, Li C,
Wei Q and Li G: Potentially functional variants of p14ARF are
associated with HPV-positive oropharyngeal cancer patients and
survival after definitive chemoradiotherapy. Carcinogenesis.
35:62–68. 2014. View Article : Google Scholar
|
|
42
|
Stenman G, Andersson MK and Andrén Y: New
tricks from an old oncogene: Gene fusion and copy number
alterations of MYB in human cancer. Cell Cycle. 9:2986–2995. 2010.
View Article : Google Scholar : PubMed/NCBI
|