Introduction
Random flap is widely used in the repair and
reconstruction of tissue defect due to its convenience and
flexibility (1). However, since no
known blood vessels are found in the random flap, the distal end is
prone to necrosis, which greatly limits its application in clinical
practice (2). The ischemic injury
mechanism of random flap remains to be fully elucidated (3).
Several growth factors, including basic fibroblast
growth factor (bFGF), are used to improve the survival rate of flap
and are applied to the research of promoting angiogenesis (4). bFGF is susceptible to proteolytic
degradation in vivo, which is destroyed and degraded rapidly
in the body due to its short half-life, and the biological effect
of partial use remains to be elucidated (5). Using a microsphere system to control
the local delivery of growth factor may be an effective method for
solving the above problem (6).
Hypoxic inducible factor (HIF)-1 is a transcription
factor widely present in the body of mammals and humans under
hypoxic conditions, which is highly sensitive to the concentration
of oxygen in the environment (7).
With a prolonged hypoxic duration, the quantity of HIF-1α protein
also increases, and once hypoxia is improved, HIF-1α levels decline
rapidly (8). Previous studies have
shown that the gene expression of HIF in the ischemic area is
upregulated, suggesting that the gene expression of HIF may be
associated with local hypoxia following ischemia, endothelial
progenitor cell (EPC) amplification and improvement of ischemia by
promoting angiogenesis (9,10). The present study aimed to
investigate whether bFGF combined with HIF-1 improved random skin
flap survival of rats.
Materials and methods
Materials
bFGF, HIF-1, superoxide dismutase (SOD) and
malondialdehyde (MDA) were obtained from Nanjing KeyGen Biotech.
Co., Ltd., (Nanjing, China). Anti-cyclooxygenase (COX)-2,
anti-vascular endothelial growth factor (VEGF) and anti-β-actin
antibodies were obtained from Bioworld Technology (Nanjing,
China).
Animals
Male Sprague-Dawley rats (weighing, 260–300 g) were
obtained from Laboratory Animals of Wenzhou Medical University
(Wenzhou, China) and were treated in accordance with the Guide for
the Care and Use of Laboratory Animals of The 2nd Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China). The rats
were housed in separate clean cages at a temperature of 20–24°C
under 12 h light/dark cycles, with chow pellets and tap water
available ad libitum.
Flap model and experimental design
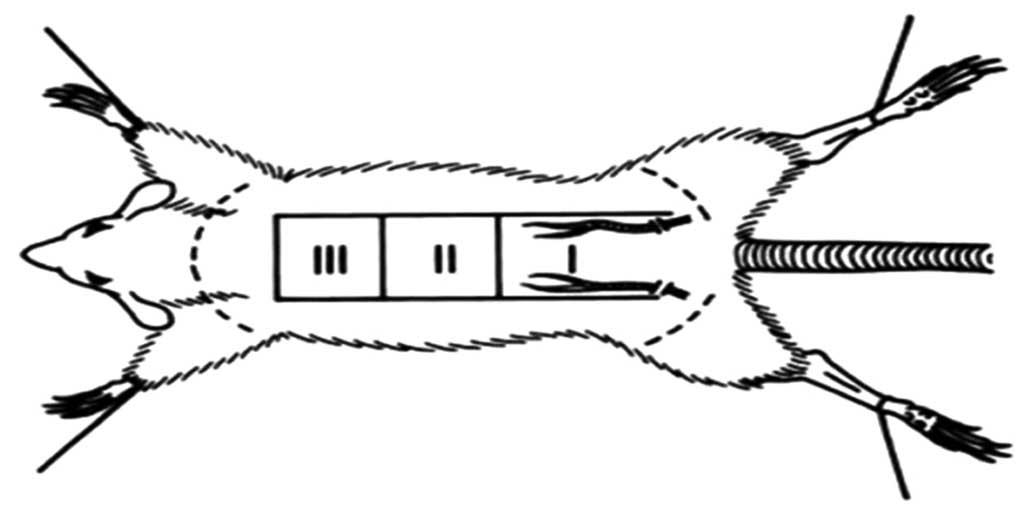
Random dorsal skin flaps were elevated using the
previously described model (11).
The rats were anesthetized with sodium pentobarbitone (40 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injections.
The dorsal skin was shaved and the rats were placed into the prone
position by securing their limbs with adhesive tape. Povidone
iodine solution (Shandong Lierkang Disinfection Technology Co.,
Ltd., Shandong, China) was used to disinfect the skin and all
surgical procedures were performed under sterile conditions.
Full-thickness rectangular skin incisions were made on the dorsum
of each animal, and were immediately sutured with interrupted 4-0
nylon sutures (Fig. 1).
All rats were randomly divided into four groups
(n=10/group): (i) Control; (ii) Nano-microcapsule-bFGF; (iii)
Nano-microcapsule-HIF-1; and (iv) bFGF combined with HIF-1
(combined). In the control group, the model rats were treated with
intraperitoneal injections of physiological saline (0.1 ml/100 g).
The bFGF group rats were treated with 2.5 µg/day bFGF for 5
days by intra-peritoneal injection and the HIF-1 group rats were
treated with 1.0 µg/day HIF-1 for 5 days by intraperitoneal
injection. In the combined treatment group, the rats were treated
with bFGF and HIF-1 at the same concentration as single treatment
groups for 5 days by intraperitoneal injections.
Assessment of the expression levels of
bFGF, HIF-1 MDA, SOD, TNF-α and IL-1β
Following combined treatment, blood sample were
acquired from the vein of flap tissues at room temperature. The
serum levels of bFGF, HIF-1, MDA, SOD, TNF-α and IL-1β were
analyzed using enzyme linked immunosorbent assay kits, according to
the manufacturer's protocols (KeyGen Biotech. Co., Ltd,. Nanjing,
China).
Assessment of survival areas
Following combined treatment, the flap tissues were
biopsied for histological assessment and immunohistochemical
staining of VEGF. The flaps and surviving areas were measured and
images were captured with superimposition of photographs on graph
paper. The images were exposed in a ChemiDoc MP Imaging System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) The computational
formula was calculated as follows: Extent of viable area × 100 /
total area.
Western blot assay for COX-2 and
VEGF
Following combined treatment, the total cellular
protein was extracted from the flap tissues. The flap tissues were
homogenized using a radioimmunoprecipitation lysis buffer (Nanjing
Sunshine Biotechnology, Co., Ltd., Nanjing, China). The homogenized
tissue samples were centrifuged at 12,000 × g for 10 min at 4°C and
the supernatant liquor was subsequently absorbed into the new tube.
The concentration of the total cellular protein was quantified
using a BCA Protein Assay (Pierce Biotechnology Inc., Rockford, IL,
USA). Equal quantities of protein (50 µg) were separated by
electrophoresis using 12% SDS-PAGE gels (Beyotime Institute of
Biotechnology, Haimen, China) and were transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Following protein transfer, the membranes were blocked with
5% non-fat milk and incubated with the corresponding primary
antibodies, anti-COX-2 (1:1,000 dilution; cat. no. sc-514489, Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-VEGF (1:1,000;
cat. no. sc-48835; Santa Cruz Biotechnology, Inc.) and rabbit
anti-mouse anti-β-actin (1:500 dilution; cat. no. AA132; Beyotime
Institute of Biotechnology, Haimen, China) overnight at 4°C. The
membranes were incubated with secondary antibodies (1:5,000;
C520011-0100; Sangon Biotech Co., Ltd., Shanghai, China) for 2–3 h
at 37°C and were subsequently incubated with chemiluminescence
detection reagent (P0203, Beyotime Institute of Biotechnology).
Images were captured using Image-Pro Plus software, version 6.0
(Media Cybernetics, Rockville, MD, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed by one-way analysis of variance using
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
P< 0.05 was considered to indicate a statistically significant
difference.
Results
bFGF expression
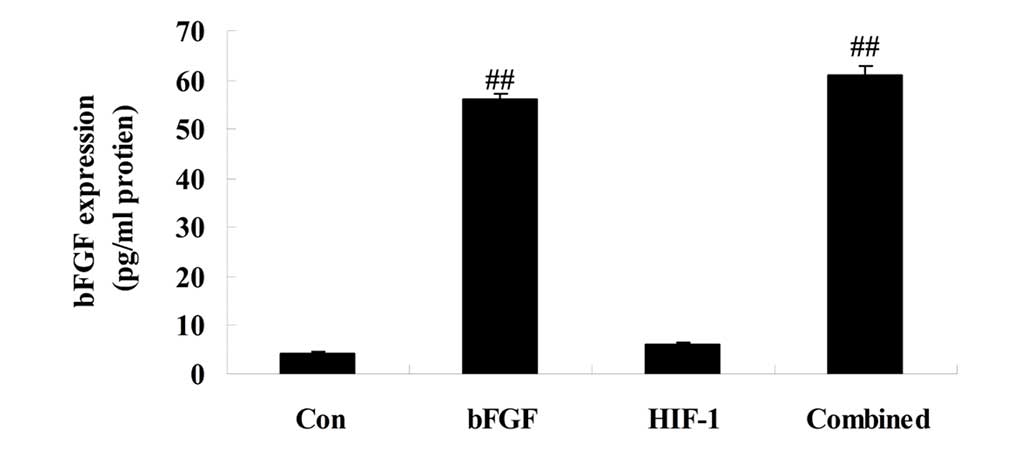
On day 5 following treatment, the expression of bFGF
was assessed. As compared with control, the expression of bFGF was
significantly advanced in bFGF group (Fig. 2). Additionally, the expression of
bFGF in the HIF-1 group was similar to that of the control group
(Fig. 2). In the combined
treatment group, the expression of bFGF was similar to that of bFGF
group (Fig. 2).
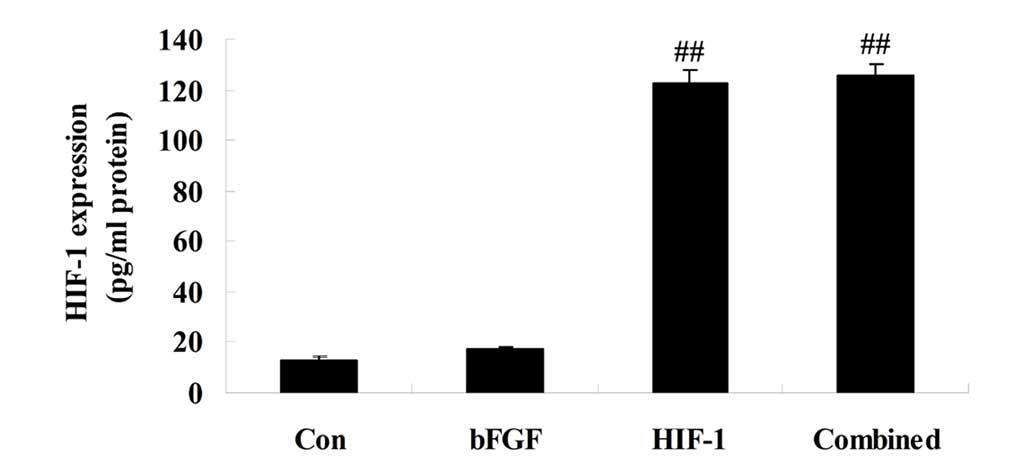
HIF-1 expression
On day 5 following treatment, the expression of
HIF-1 was detected. The expression of HIF-1 in the bFGF group was
similar to that of the control group, and significantly lower
compared with that of the HIF-1 group (Fig. 3). The expression of HIF-1 in the
HIF-1 and combined treatment groups exhibited similar changes and
was significantly higher compared with the control or bFGF groups
(Fig. 3).
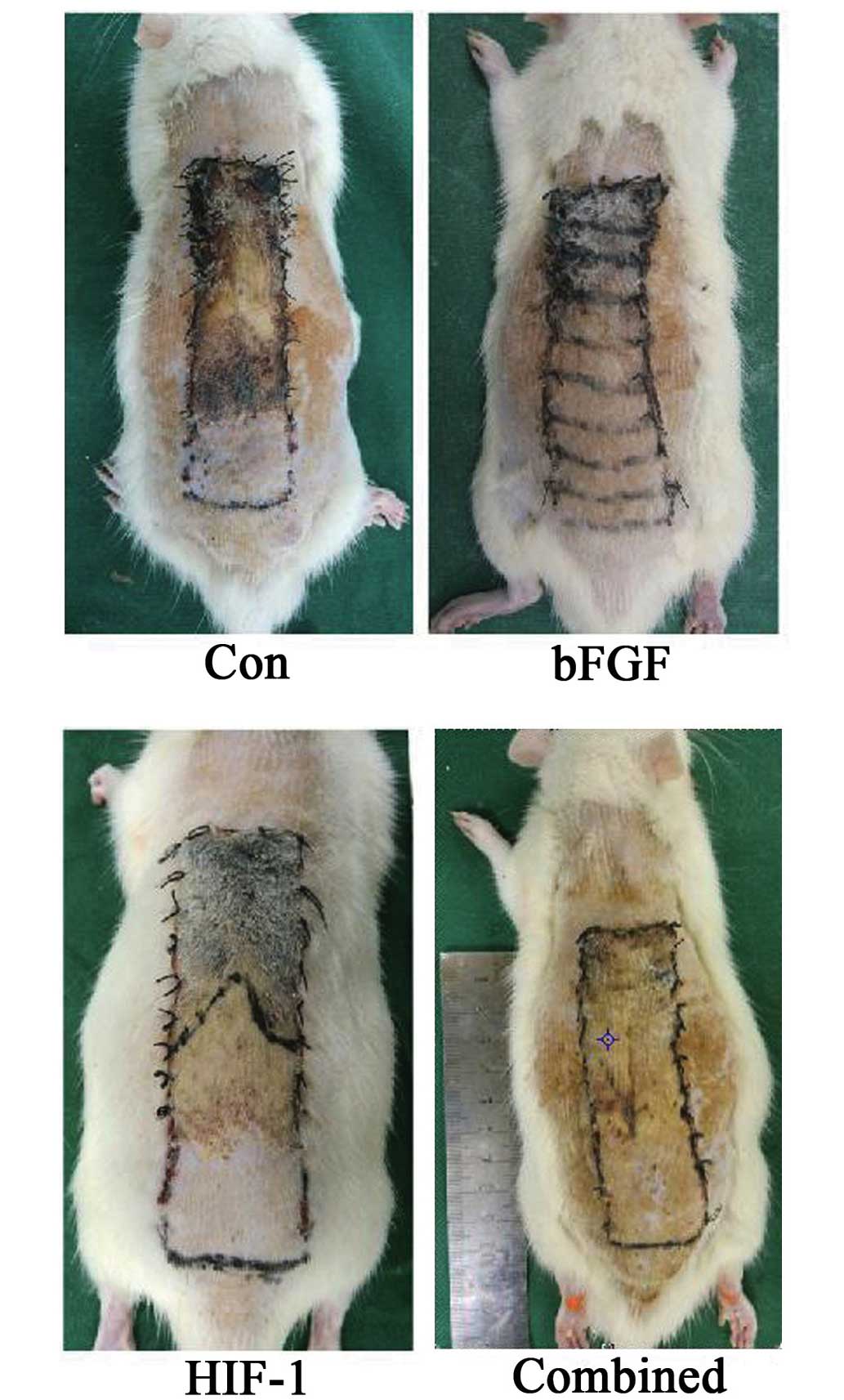
Combined treatment improves flap
survival
On day 5 following treatment, whether the
combination treatment improved flap survival was assessed. As shown
in Fig. 4, the boundaries between
necrotic and surviving regions were highest in the control group.
In the bFGF and the HIF-1 groups, the boundaries between necrotic
and surviving regions were significantly inhibited compared with
those of the control group (Fig.
4). These necrotic and surviving regions in the combined
treatment group were lower compared with those of the bFGF or HIF-1
groups, and the difference were statistically significant (Fig. 4).
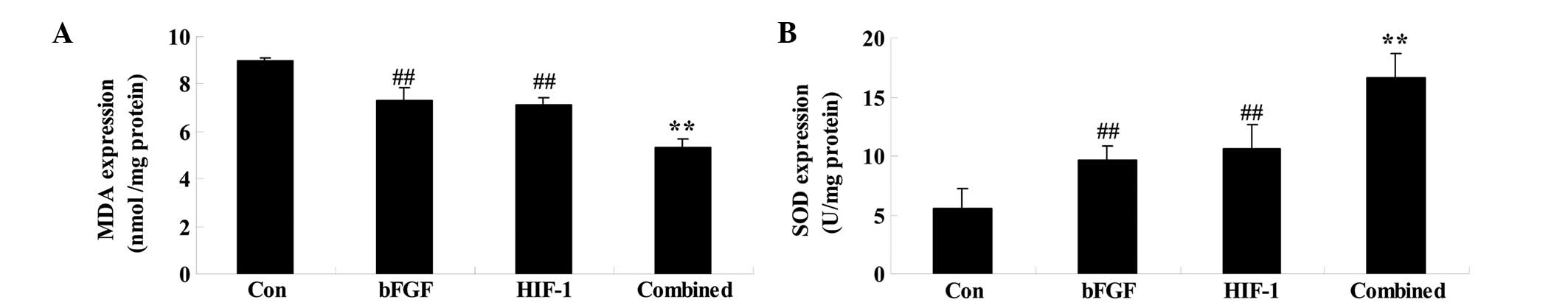
Combined treatment affects the levels of
MDA and SOD
On day 5 following treatment, whether combined
treatment affected the MDA and SOD levels was assessed. Firstly,
bFGF treatment significantly reduced and increased the levels of
MDA and SOD, respectively, compared with those of control group
(Fig. 5). The HIF-1 group
exhibited similar changes to the control group (Fig. 5). Finally, these oxidative stresses
were significantly receded by combined treatment compared with
those of the bFGF or HIF-1 groups (Fig. 5).
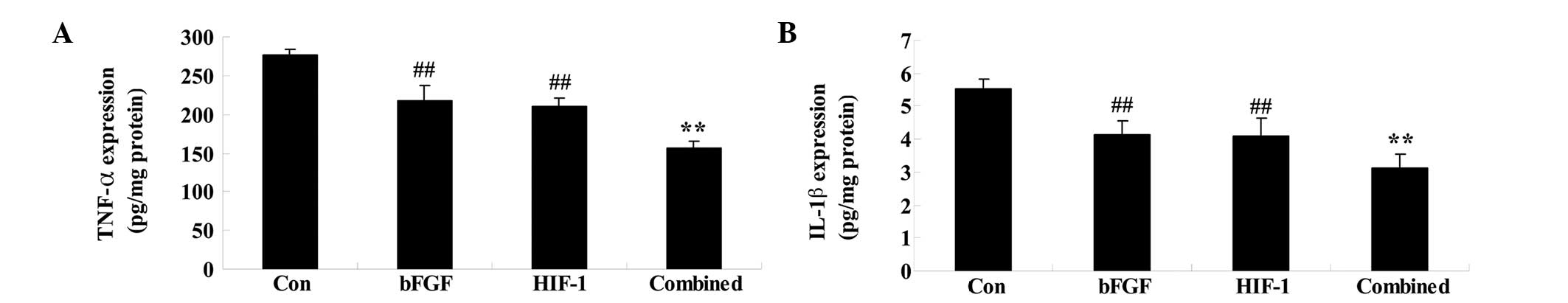
Combined treatment affects the levels of
TNF-α and IL-1β
On day 5 following treatment, whether combined
treatment affected the MDA and SOD levels was determined. The TNF-α
and IL-1β levels in the bFGF or HIF-1 groups were significantly
weakened compared with those of the control group (Fig. 6). However, these inflammatory
factors were significantly weakened in the combined treatment group
compared with those of the bFGF or HIF-1 groups (Fig. 6).
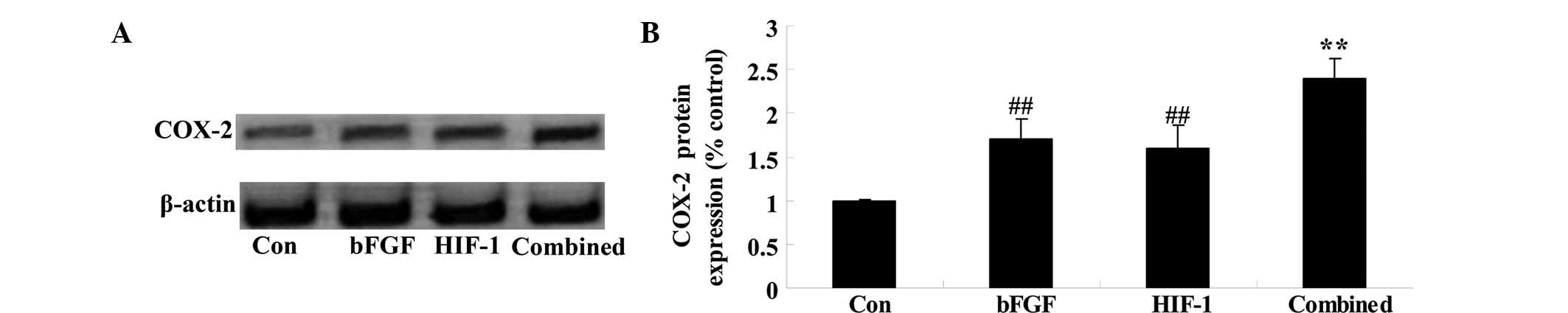
Combined treatment affects the expression
of COX-2
To check whether combined treatment affects the
expression of COX-2 following 5 days of treatment, the protein
expression of COX-2 was analyzed by western blotting. A significant
difference in the protein expression of COX-2 was observed between
the bFGF and HIF-1 groups (Fig.
7). When compared with that of the bFGF or HIF-1 groups, the
protein expression of COX-2 in the combined treatment group was
significantly increased (Fig.
7).
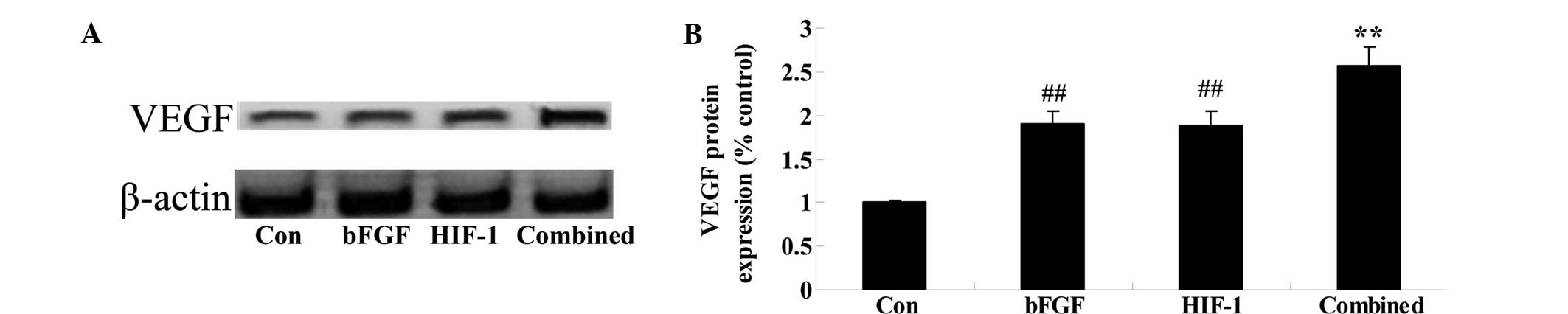
Combined treatment affects the expression
of VEGF
The present study next determined whether combined
treatment affected the expression of COX-2 following 5 days of
treatment. As shown in Fig. 7, the
protein expression of COX-2 was significantly promoted in the bFGF
and HIF-1 groups, as compared with that of the control group
(Fig. 8). Notably, the combined
treatment significantly increased the protein expression of COX-2,
as compared with that of the control group (Fig. 8).
Discussion
bFGF is composed of 155 amino acids and has a high
affinity with dextran, in addition to heparan sulfate
proteoglycans. bFGF has no signal peptide, however, exists in a
paracrine or autocrine form outside the cell, the explanation of
how remains to be elucidated (12). Due to the increase of bFGF in
wounded tissue, the secretion of bFGF is assumed to be based on
cell damage and exocytosis (13).
However, domestic and foreign studies revealed that a large number
of bFGFs are secreted following the effect of mitogens, including
phorbol myristate acetate (14).
In the present study, the effect of nano-microcapsule bFGF combined
with HIF-1 on the random skin flap survival of rats was
investigated. Fayazzadeh et al (15) reported that recombinant human bFGF
and erythropoietin protected skin flap ischemic necrosis.
Therefore, nano-microcapsule bFGF combined with HIF-1 may be a
beneficial pattern for random skin flap survival.
HIF-1α protein is markedly unstable in normoxic
condition, likely to be decomposed by the ubiquitin-proteasome
system, of which the half-life is >5 min. Therefore HIF-1α
protein is difficult to detect under normoxic conditions (16). The stability of HIF1α under
normoxic conditions is not enough to activate HIF-1, and only
inhibiting the degradation of ubiquitin and the proteasome is not
enough to produce the transcriptional activity of HIF-1, therefore,
it should be transformed into exogenous HIF-1α, to promote its
combination with structural ARNT in vivo to generate active
HIF-1. By transfecting exogenous HIF-1α into peripheral HIF-1α EPC
of humans, the previous study aimed to observe its effect on the
EPC directed differentiation and vascular activity in normoxic
condition (17). In the present
study, nano-microcapsule bFGF combined with HIF-1 significantly
weakened oxidative stresses and inflammatory factors in the random
skin flap survival of rats. A previous study has demonstrated that
bFGF reduces bisphosphonate-induced oxidative in oral epithelium of
rat (18). Wang et al also
demonstrated bFGF ischemic oxidative injury through the
upregulation of the ERK1/2 pathways (19). Tasso et al (20) reported that the inflammatory
response on the ability of mesenchymal stem cells to activate
endogenous regenerative mechanisms is reduced by bFGF.
Rodriguez-Miguelez et al (21) indicated that HIF-1α modulates
inflammation and the expression of VEGF. However, the detailed
mechanisms on how nano-microcapsule bFGF combined with HIF-1
influences oxidative stresses and inflammatory factors in random
skin flap survival of rats remain to be elucidated, and further
clarification is required in a future study.
COX is a rate-limiting enzyme synthesized by
prostaglandin, and there are three known isomers, COX-1, COX-2 and
COX-3, in which the expression of COX- is induced, and it is
undetectable in most normal tissues, however the expression is
significantly upregulated under various stimuli, including
cytokines, growth factors, oncogenes and tumor-promoting agents,
involved in inflammation, angiogenesis, cell proliferation and
differentiation processes (22).
The expression of COX-2 may promote an angiogenesis effect via VEGF
(23). In the present study,
nano-microcapsule bFGF combined with HIF-1 increased the protein
expression of COX-2 in the random skin flap survival of rats. Erdem
et al (24) showed that the
association between bFGF and astrocyte elevated gene-1 increased
COX-2 markers with prognostic factors in prostate carcinomas. The
results of Seo et al (25)
suggested that activation of HIF-1α upregulates COX-2 and matrix
metalloproteinase-9, and prevents acinar cell death. However, the
specific mechanisms of nano-microcapsule bFGF combined with HIF-1
on the expression of COX-2 remains unclear in the random skin flap
survival of rats, and further clarification is required in a future
study.
VEGF is also known as vascular permeability factor
or angiogenic factor, which is a glycoprotein that can specifically
act on the surface receptors in vascular endothelial cells, with
several biological effects, including pro-vascular endothelial cell
proliferation and migration, angiogenesis, vascular endothelial
cell life extension and vascular permeability increasing (26,27).
The present study revealed that nano-microcapsule bFGF combined
with HIF-1 also increased the protein expression of VEGF in the
random skin flap survival of rats. Chen et al (28) indicated that inhibiting HIF-1
reduced VEGF in human colorectal cancer cells. Tzeng et al
(29) also demonstrated that bFGF
promotes endothelial progenitor cells and induces VEGF expression
in chondrosarcoma cells. These results implied that the effect of
nano-microcapsule bFGF combined with HIF-1 on random skin flap
survival was associated with the expression of VEGF, and further
clarification is required in a future study.
In conclusion, nano-microcapsule bFGF combined with
HIF-1 prevents random skin flap survival in rats. The present
study, therefore, confirmed that nano-microcapsule bFGF combined
with HIF-1 may be a protective effect in terms of oxidative
stresses and inflammatory activity and activates COX-2 and VEGF
signal channel.
Acknowledgments
The present study was supported by the Zhejiang
Provincial Medical Science Research Foundation (no. 2009A146) and
the Wenzhou Science & Technology Foundation of China (no.
2013S0430).
References
|
1
|
Cao B, Wang L, Lin D, Cai L and Gao W:
Effects of lidocaine on random skin flap survival in rats. Dermatol
Surg. 41:53–58. 2015. View Article : Google Scholar
|
|
2
|
Ghavami A, Nutt MP and Hardy SP: Heat
shock protein and high-dose aspirin: Effects on random skin flap
survival in a rat model. Ann Plast Surg. 48:60–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huemer GM, Froschauer SM, Pachinger T,
Kwasny O and Schoffl H: A comparison of pretreatment with a topical
combination of nonivamide and nicoboxil and surgical delay in a
random pattern skin flap model. J Plast Reconstr Aesthet Surg.
62:914–919. 2009. View Article : Google Scholar
|
|
4
|
Li Q, Gao S, Yu Y, Wang W, Chen X, Wang R,
Li T, Wang C, Li X and Wu X: A novel bFGF antagonist peptide
inhibits breast cancer cell growth. Mol Med Rep. 6:210–214.
2012.PubMed/NCBI
|
|
5
|
Zhang L, Mai HM, Zheng J, Zheng JW, Wang
YA, Qin ZP and Li KL: Propranolol inhibits angiogenesis via
downregulating the expression of vascular endothelial growth factor
in hemangioma derived stem cell. Int J Clin Exp Pathol. 7:48–55.
2014.
|
|
6
|
Cui K, Zhou X, Luo J, et al: Dual gene
transfer of bFGF and PDGF in a single plasmid for the treatment of
myocardial infarction. Exp Ther Med. 7:691–696. 2014.PubMed/NCBI
|
|
7
|
Kim DS, Ko JH, Jeon YD, Han YH, Kim HJ,
Poudel A, Jung HJ, Ku SK, Kim SJ, Park SH, et al: Ixeris dentata
NAKAI Reduces Clinical Score and HIF-1 Expression in Experimental
Colitis in Mice. Evid Based Complement Alternat Med.
2013:6712812013.PubMed/NCBI
|
|
8
|
Koukourakis MI, Giatromanolaki A, Chong W,
et al: Amifostine induces anaerobic metabolism and
hypoxia-inducible factor 1 alpha. Cancer Chemother Pharmacol.
53:8–14. 2004. View Article : Google Scholar
|
|
9
|
Weidemann A, Klanke B, Wagner M, Volk T,
Willam C, Wiesener MS, Eckardt KU and Warnecke C: Hypoxia, via
stabilization of the hypoxia-inducible factor HIF-1alpha, is a
direct and sufficient stimulus for brain-type natriuretic peptide
induction. Biochem J. 409:233–242. 2008. View Article : Google Scholar
|
|
10
|
Lee SH, Lee JH, Yoo SY, Hur J, Kim HS and
Kwon SM: Hypoxia inhibits cellular senescence to restore the
therapeutic potential of old human endothelial progenitor cells via
the hypoxia-inducible factor-1α-TWIST-p21 axis. Arterioscler Thromb
Vasc Biol. 33:2407–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren H, Lin D, Mou Z and Dong P: The
adverse effect of selective cyclooxygenase-2 inhibitor on random
skin flap survival in rats. PLoS One. 8:e828022013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura M, Oda M, Inoue J, Ito T, Akiba
Y, Kitajima M, Tsuchiya M and Ishii H: Plasticity of myofibroblasts
appearing in granulation tissues after acetic acid treatment.
Effect of bFGF Dig Dis Sci. 40:2477–2480. 1995. View Article : Google Scholar
|
|
13
|
Wang TT, Yuan Y, Kang Y, Yuan WL, Zhang
HT, Wu LY and Feng ZT: Effects of acupuncture on the expression of
glial cell line-derived neurotrophic factor (GDNF) and basic
fibroblast growth factor (FGF-2/bFGF) in the left sixth lumbar
dorsal root ganglion following removal of adjacent dorsal root
ganglia. Neurosci Lett. 382:236–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohideen MA, Hruska-Hageman A and
Nilsen-Hamilton M: A unique bFGF-responsive transcriptional
element. Gene. 237:81–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fayazzadeh E, Ahmadi SH, Rabbani S,
Boroumand MA, Salavati A and Anvari MS: A comparative study of
recombinant human basic fibroblast growth factor (bFGF) and
erythropoietin (EPO) in prevention of skin flap ischemic necrosis
in rats. Arch Iran Med. 15:553–556. 2012.PubMed/NCBI
|
|
16
|
Wang F, Chang M, Shi Y, Jiang L, Zhao J,
Hai L, Sharen G and Du H: Downregulation of hypoxia-inducible
factor-1 suppresses malignant biological behavior of
triple-negative breast cancer cells. Int J Clin Exp Med.
7:3933–3940. 2014.
|
|
17
|
Nanduri J, Vaddi DR, Khan SA, Wang N,
Makarenko V, Semenza GL and Prabhakar NR: HIF-1α activation by
intermittent hypoxia requires NADPH oxidase stimulation by xanthine
oxidase. PLoS One. 10:e01197622015. View Article : Google Scholar
|
|
18
|
Koçer G, Nazıroğlu M, Çelik Ö, Önal L,
Özçelik D, Koçer M and Sönmez TT: Basic fibroblast growth factor
attenuates bisphosphonate-induced oxidative injury but decreases
zinc and copper levels in oral epithelium of rat. Biol Trace Elem
Res. 153:251–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang
X, Yan Y, Fu X, Hu H, Li X, et al: bFGF inhibits ER stress induced
by ischemic oxidative injury via activation of the PI3K/Akt and
ERK1/2 pathways. Toxicol Lett. 212:137–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tasso R, Gaetani M, Molino E, Cattaneo A,
Monticone M, Bachi A and Cancedda R: The role of bFGF on the
ability of MSC to activate endogenous regenerative mechanisms in an
ectopic bone formation model. Biomaterials. 33:2086–2096. 2012.
View Article : Google Scholar
|
|
21
|
Rodriguez-Miguelez P, Lima-Cabello E,
Martínez-Flórez S, Almar M, Cuevas MJ and González-Gallego J:
Hypoxia-inducible factor-1 modulates the expression of vascular
endothelial growth factor and endothelial nitric oxide synthase
induced by eccentric exercise. J Appl Physiol (1985).
118:1075–1083. 2015. View Article : Google Scholar
|
|
22
|
Liu L, Zhang J, Li M, Zhang X, Zhang J, Li
Z, Wang L, Wu J and Luo C: Inhibition of HepG2 cell proliferation
by ursolic acid and polysaccharides via the downregulation of
cyclooxygenase-2. Mol Med Rep. 9:2505–2511. 2014.PubMed/NCBI
|
|
23
|
Gallindo RM, Gonçalves FL, Figueira RL,
Pereira LA, Simões AL, Schmidt AF and Sbragia L: Ventilation causes
pulmonary vascular dilation and modulates the NOS and VEGF pathway
on newborn rats with CDH. J Pediatr Surg. 50:842–848. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erdem H, Yildirim U, Uzunlar AK, Cam K,
Tekin A, Kayikci MA, Sahiner C, Oktay M, Ankarali H and Aydin LY:
Relationship among expression of basic-fibroblast growth factor,
MTDH/astrocyte elevated gene-1, adenomatous polyposis coli, matrix
metalloproteinase 9, and COX-2 markers with prognostic factors in
prostate carcinomas. Niger J Clin Pract. 16:418–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seo Y, Ji YW, Lee SM, Shim J, Noh H, Yeo
A, Park C, Park MS and Chang EJ: Lee HKL Activation of HIF-1α
(hypoxia inducible factor-1α) prevents dry eye-induced acinar cell
death in the lacrimal gland. Cell Death Dis. 5:e13092014.
View Article : Google Scholar
|
|
26
|
Hong JP, Li XM, Li MX and Zheng FL: VEGF
suppresses epithelial-mesenchymal transition by inhibiting the
expression of Smad3 and miR192, a Smad3-dependent microRNA. Int J
Mol Med. 31:1436–1442. 2013.PubMed/NCBI
|
|
27
|
Kranz A, Rau C, Kochs M and Waltenberger
J: Elevation of vascular endothelial growth factor-A serum levels
following acute myocardial infarction. Evidence for its origin and
functional significance. J Mol Cell Cardiol. 32:65–72. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Ren Z, Ye LC, Zhou PH, Xu JM, Shi
Q, Yao LQ and Zhong YS: Factor inhibiting HIF1α (FIH-1) functions
as a tumor suppressor in human colorectal cancer by repressing
HIF1α pathway. Cancer Biol Ther. 16:244–252. 2015. View Article : Google Scholar
|
|
29
|
Tzeng HE, Chen PC, Lin KW, Lin CY, Tsai
CH, Han SM, Teng CL, Hwang WL, Wang SW and Tang CH: Basic
fibroblast growth factor induces VEGF expression in chondrosarcoma
cells and subsequently promotes endothelial progenitor cells-primed
angiogenesis. Clin Sci (Lond). 129:147–158. 2015. View Article : Google Scholar
|