Introduction
Non-small-cell lung cancer (NSCLC) is one of the
most common types of malignancy worldwide (1). With >1,000,000 novel cases per
year, lung cancer represents the most frequent lethal neoplasm in
males, while its incidence increases progressively in females
(2). Despite considerable efforts,
only 5–10% of patients survive 5 years after diagnosis and the
majority of these long-term survivors undergo surgery (3). Therefore, tumor angiogenesis and
tumor metastasis to multiple organs is a critical problem for
patients with lung cancer. The prevention and treatment of tumor
angiogenesis and tumor metastasis is clinically important (4).
Interleukin (IL)-17 is produced predominantly by
activated CD4 T cells (5). It has
pleiotropic biological activities including induction of IL-6,
CXCL8/IL-8 and vascular endothelial growth factor-A (VEGF-A)
(5–14). IL-17R is a type 1 transmembrane
protein with an extraordinarily long intracellular domain (5,15).
Although the expression of IL-17 mRNA is restricted to activated T
cells, the expression of IL-17R mRNA has been detected in nearly
all cells and tissues (5,15). In its role as a proinflammatory
cytokine, IL-17 levels have been found to significantly increase in
rheumatoid arthritis synovium and in other chronic inflammatory
diseases, including multiple sclerosis and psoriasis (16–20).
IL-17 has also been implicated in certain tumors, affecting
tumorigenesis, proliferation, angiogenesis and metastasis. This
allows the tumor to adapt and to confer immune and chemotherapy
resistance (21). Accumulating
evidence has shown that IL-17-positive cells are frequently present
in multiple inflammation-associated cancers and that IL-17 promotes
angiogenesis in tumor models (22). Expression of IL-17 is correlated
with the number of blood vessels in human ovarian cancer (23), hepatocellular carcinoma (24) and NSCLC (25), and with poor survival in
hepatocellular carcinoma (24).
Although IL-17 is central in endothelial
proliferation and tumor growth in several types of cancer (23), its possible effects on lung cancer
and the precise mechanism underlying its biologic function remain
unclear (24,25). To further address the roles of
IL-17 in lung cancer, Lewis lung carcinoma (LLC) cells were
transplanted into C57BL/6 mice to examine the roles of IL-17 in the
processes of tumorigenesis and metastasis in vivo. Tumor
size, lymph node weight and angiogenic factors, which were
expressed in tumor mass, as indicated at the time point following
implantation were detected and compared. The present study provided
definitive evidence of a critical role of IL-17 in LLC
cell-implanted tumor growth and metastasis.
Materials and methods
Reagents and antibodies
Mouse recombinant protein IL-17 was purchased from
Biochem Technologies (Shanghai, China). Rat anti-mouse CD34
monoclonal antibody (mAb) (MEC 14.7, cat no. NB600-1071),
phycoerythrin (PE)-conjugated rat anti-mouse F4/80 mAb (cat no.
NBP2-22134), rat anti-mouse vascular endothelial growth factor
(VEGF) mAb (RM0009-2G02, cat no. NB110-61025), rabbit anti-mouse
matrix metalloproteinase (MMP)2 mAb (cat no. NB200-193), rabbit
anti-mouse MMP9 mAb (cat no. NBP1-39597), rabbit anti-mouse tumor
necrosis factor (TNF)-α mAb (cat no. NB100-57620) and mouse
anti-mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mAb
(cat no. NB300-328H) were purchased from Novus Biologicals
(Littleton, CO, USA). Allophycocyanin-conjugated rat anti-mouse
CCR2 mAb (cat no. LS-C127284-100) was purchased from LifeSpan
BioSciences (Seattle, WA, USA). Rat anti-mouse migration inhibitory
factor (MIF) mAb (cat no. MAB1978) was purchased from R&D
Systems (Minneapolis, MI, USA). Rabbit anti-mouse thrombospondin
(TSP)-1 mAb (cat no. orb7127) was purchased from Biorbyt (San
Francisco, CA, USA).
Cell line and mice
LLC cells, a murine non-small cell lung carcinoma
cell line, were purchased from Shanghai Biotechnology Corporation
(Shanghai, China). Cells were maintained in Dulbecco's modified
Eagle's medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% fetal calf serum (Thermo Fisher Scientific, Waltham, MA,
USA). C57BL/6 mice (n=192) weighing 20–25 g were supplied by the
Immunology Laboratory of Nanjing Medical University (Nanjing,
China) and were kept in our animal facility under specific
pathogen-free conditions. All animal experiments were approved by
the Guideline for the Care and Use of Laboratory Animals on the
Chinese Medical Academy and the Nanjing Medical University Care
Committee. Animals were kept in groups of 5 and fed regular lab
chow and water ad libitum. A 12-h light/dark cycle was
maintained.
Cell culture and tumor implantation
model
LLC cells were grown in RPMI-1640 (Thermo Fisher
Scientific) culture medium containing 10% fetal bovine serum, 2 mM
L-glutamine, 2 mM sodium pyrovate, 20 mM HEPES, 1% non-essential
amino acid, 100 µg/ml streptomycin and 100 U/ml penicillin.
Cells were maintained at 37°C with 5% CO2. Cells were
progressively passed to larger plates and allowed to reach ~90%
confluence. Cells were harvested by trypsinization, washed with
HBSS buffer (Life Technologies, Grand Island, NY, USA) three times
and resuspended at 1.0×107 cells per ml in serum-free
HBSS buffer. LLC cells (1×106) in 100 µl HBSS
buffer were injected subcutaneously into 8-week-old male C57BL/6
mice (n=144). Tumor growth was assessed on day 6, 8, 10, 14, 16 and
18 after LLC cell injection by the measurement of two bisecting
diameters in each tumor using calipers. The size of the tumor was
determined by direct measurement of the tumor dimensions. The
volume was calculated according to the equation:
V=(L×W2) × 0.5, where V=volume, L=length and W=width
(22). On day 14 after tumor
implantation, the mice were anaesthetized using 1.2%, 0.2 ml/10 mg
2-Tribro moethanol (Sigma-Aldrich) and sacrificed by dislocation of
the cervical spine, and the tumor tissues were dissected and
weighed.
Macroscopic assessments of cervical lymph
node metastasis
The mice were sacrificed on day 21 after the
implantation of LLC cells, and cervical tissues surrounding tumor
masses were excised en bloc. The nearby cervical lymph nodes were
removed and their weights were measured immediately.
Flow cytometric analysis of CD34-, CCR2-
and F4/80-positive cells
Tumor tissues dissected from C57BL/6 mice (n=48)
were minced with scissors, and were homogenized in RPMI-1640
medium. Cell suspensions were then passed over a nylon filter with
a 100-µm pore size. The resultant cells were further stained
with rat anti-mouse CD34 mAb followed by staining with
PE-conjugated swine anti-rat IgG mAb. In an assay to detect
CCR2+/F4/80+ cells, cells were stained with
PE-conjugated rat anti-mouse F4/80 mAb and
allophycocyanin-conjugated rat anti-mouse CCR2 mAb. Fluorescence
intensities were determined using a FACS Calibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA), together with samples
stained with non-immunized swine IgG (cat. no. DAGIC1433; Creative
Diagnostics, Shirley, NY, USA) or rat IgG (cat. no. DAGIC1336;
Creative Diagnostics) as an isotype control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from LLC cells from mice
(n=48) with the use of the RNeasy Mini kit (Qiagen, Tokyo, Japan).
The resultant RNA preparations were further treated with
ribonuclease-free deoxyribonuclease (DNase) I (Life Technologies
Inc., Gaithersburg, MD, USA) to remove genomic DNA. In total, 2
µg total RNA was reverse transcribed at 42°C for 1 h in 20
µl of reaction mixture containing mouse Moloney leukemia
virus reverse transcriptase and hexanucleotide random primers
(Qiagen). The PCR solution contained 2 µl cDNA, the specific
primer set (0.2 µM final concentration), and 12.5 µl
SYBR Premix Ex Taq (SYBR Premix Ex Taq Perfect Real Time PCR Kit,
Takara, Bio, Inc., Shiga, Japan) in a final volume of 25 µl.
Quantitative PCR was performed on iCycler iQ Multi-Color Real Time
PCR Detection system (170-8740, Bio-Rad, Hercules, CA, USA). PCR
parameters were as follows: Initial denaturation at 95°C for 1 min,
followed by 40 circles of 95°C for 5 sec, and 60°C for 30 sec. The
relative gene expression levels were calculated using the
2−ΔΔCq method, where Cq represents the threshold cycle,
and GAPDH was used as a reference gene. The primers sequences are
shown in Table I. All primers used
were purchased from Genescript (Nanjing, China)
 | Table ISequences of the primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction.
| Primer | Sequence
(5′→3′) | Product Size
(bp) | Annealing
Temperature (°C) | PCR cycles |
|---|
| MIF | F:
CCATGCCTATGTTCATCGTG | | | |
| R:
AGGCCACACAGCAGCTTACT | 250 | 57 | 40 |
| VEGF | F:
CTGCTGTACCTCCACCATGCCAAGT | | | |
| R:
CTGCAAGTACGTTCGTTTAACTCA | 254 | 57 | 40 |
| MMP-2 | F:
GAGTTGGCAGTGCAATACCT | | | |
| R:
GCCATCCTTCTCAAAGTTGT | 333 | 57 | 40 |
| MMP-9 | F:
AGTTTGGTGTCGCGGAGCAC | | | |
| R:
TACATGAGCGCTTCCGGCAC | 380 | 57 | 40 |
| TNF-α | F:
CAGCCTCTTCTCATTCCTGCTTGTG | | | |
| R:
CTGGAAGACTCCTCCCAGGTATAT | 256 | 57 | 40 |
| TSP-1 | F:
ACCAAAGCCTGCAAGAAAGA | | | |
| R:
ATGCCATTTCCACTGTAGCC | 156 | 57 | 40 |
| GAPDH | F:
ACCACAGTCCATGCCATCAC | | | |
| R:
TCCACCACCCTGTTGCTGTA | 452 | 57 | 40 |
Western blot analysis
Cell lysates from dissected tumor tissue (n=48 mice)
were prepared 14 days after injection. Briefly, protein samples
were dissolved in Laemmli buffer (Bio-Rad Laboratories, Hercules,
CA, USA), boiled for 5–10 min, and centrifuged for 2 min at 10,000
× g to remove insoluble materials. In total, 30 µg of
protein per lane were separated by SDS/PAGE (12%, Bio-Rad
Laboratories) and transferred onto Immobilon-P membranes
(Millipore, Bedford, MA). The membranes were blocked with
phosphate-buffered saline (Thermo Fisher Scientific), containing 5%
non-fat dry milk, for non-specific binding for 1 h at room
temperature. The blocked membranes were subsequently probed
overnight at 4°C overnight with rat anti mouse MIF or VEGF
antibodies (1:250) or rabbit anti-mouse MMP2, MMP-9, TNF-α or TSP-1
antibodies (1:200) respectively and mouse anti-mouse GAPDH.
Membranes were subsequently washed three times for 15 min with
phosphate-buffered saline containing Tween-20. Subsequently, the
membranes were incubated with secondary horseradish
peroxidase-conjugated antibody (cat. no. 611-1302; Rockland
Immunochemicals, Inc., Limerick, PA, USA), and immunoreactive bands
were visualized using enhanced chemiluminescence reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA). Immunoreactive bands
corresponding to target proteins were quantified by Image J
analysis (http://rsb.info.nih.gov/ij/download.html) and
normalized to those of GAPDH. Blots are representative of at least
three experiments.
Wound scratch assays
In order to investigate the effects of IL-17 on LLC
cell migration, wound scratch assays were performed. The assay is
simple and inexpensive, and the experimental conditions can be
easily modified for different purposes. In brief, cells were seeded
into 6-well plates in a density that, after 24 h of growth, reached
70–80% confluence as a monolayer. The monolayer was gently, slowly
and perpendicularly scratched with a new 1 ml pipette tip across
the center of the well. The resulting gap distance therefore equals
to the outer diameter of the end of the tip. After being scratched,
the wells were gently washed twice with HBSS medium (Thermo Fisher
Scientific) to remove the detached cells. Then the wells were
replenished with fresh medium. Cells were treated with 1
µg/ml human recombinant IL-17 (cat. no. NBP2-35040; Novus
Biologicals) in experimental wells and phosphate-buffered saline in
control wells. Cells were grown for an additional 48 h and cells
were photographed on a BX43 microscope (Oympus, Tokyo, Japan) at 0,
12, 24, 48 and 72 h. The gap distance was quantitatively evaluated
using Image J software. Each experimental group was repeated three
times.
Determination of the rate of cell
proliferation
The rate of proliferation was determined using the
Cell Counting kit 8 (CCK8, Dojindo Molecular Technologies,
Kumamoto, Japan). Cells (5×103 cells per well) were
incubated in 96-well plates and maintained in complete medium 12 h
after IL-17 stimulation. After 24, 48 and 72 h, 10 µl
sterile CCK8 dye was added to the cells and incubated for 4 h at
37°C. Spectrometric absorbance at a wavelength of 450 nm was
measured on an enzyme immunoassay analyzer (ELx-800; Molecular
Devices, Sunnyvale, CA, USA) after 4 h incubation. Experiments were
performed at least three times, with six replicate measurements,
and data are presented as the mean optical density ± standard
deviation.
Statistical analysis
The mean ± standard error of the mean were
calculated for all parameters determined in the study. Data were
analyzed statistically using one-way analysis of variance, or
two-tailed Student's t-test. SPSS 18.0 software (IBM SPSS, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
IL-17 significantly promotes in vivo
tumor growth and angiogenesis
It was hypothesized that IL-17 promotes tumor growth
and angiogenesis of lung cancer subcutaneously implanted into
C57/B6 mice. To examine this hypothesis, LLC cells were grafted
subcutaneously into recombinant IL-17 protein injected groups and
control groups, and the development of solid tumors around the
injection sites was analyzed. Tumors in IL-17-injected groups were
significantly increased in size compared with those in control
groups 10 days following implantation. On day 14 after
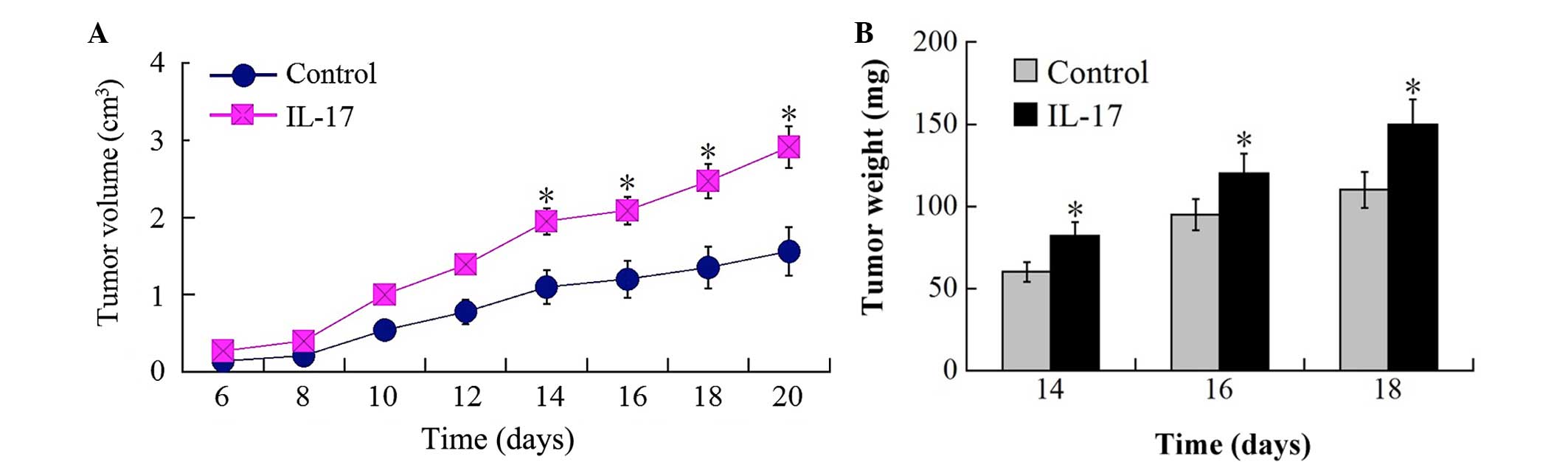
implantation, the tumor masses in IL-17 injected groups were
increased to ~1.5 fold of that in control mice (Fig. 1A and B). Flow cytometry examination
of xenografts revealed that the vascularization of tumor tissues
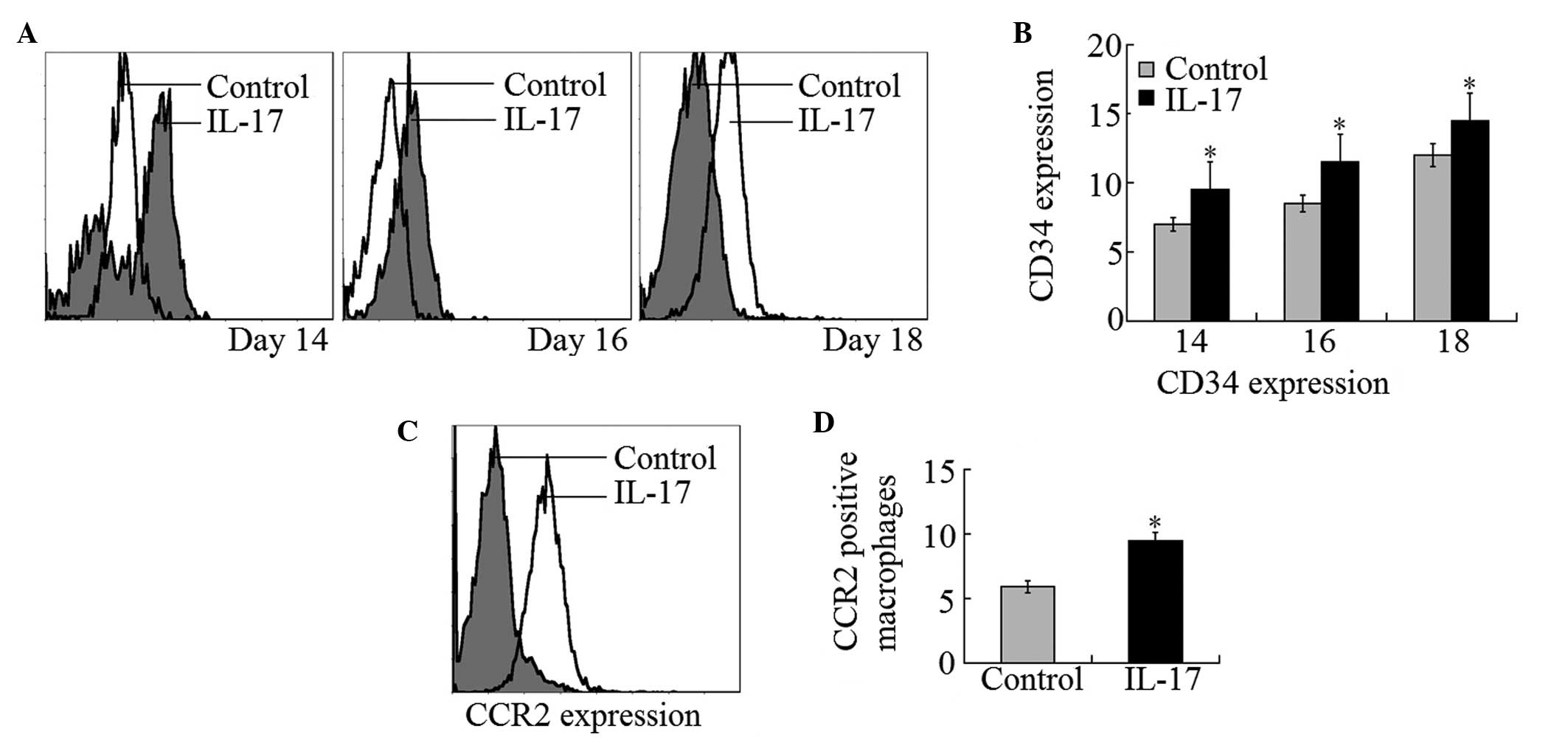
was markedly increased by intraperitoneal injection of IL-17. CD34
expression of vascular endothelial cells was ~20, 18 and 15% higher
in tumors grafted in IL-17 injected groups than those grafted in
control groups at day 14, 16 and 18 after implantation,
respectively (Fig. 2A and B).
IL-17 enhances CCR2-positive macrophage
infiltration in tumor masses
It was reported that F4/80-positive macrophages
infiltrated tumor masses and that they were associated with tumor
angiogenesis (26). CCR2-positive
macrophages were considered to have positive effects on tumor
angiogenesis by inducing tumor cell secretion of VEGF and bFGF
(27). In the present study, CCR2
expression was detected in F4/80-positive macrophages that had
infiltrated into tumor masses. It was demonstrated that
CCR2-positive macrophages were markedly increased in IL-17-treated
mice compared with control mice (Fig.
2C and D). These observations indicate that IL-17 treatment
enhanced tumor mass infiltration of CCR2-positive macrophages.
IL-17 significantly enhances tumor
metastasis in IL-17-injected mice
Secondly, the effects of IL-17 in tumor metastasis
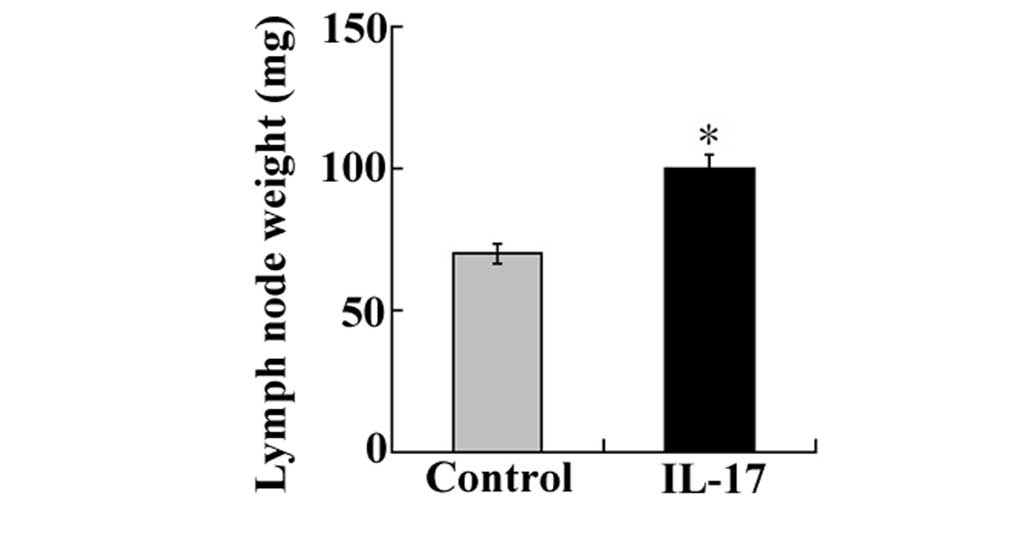
were examined by dissecting and weighing cervical lymph nodes
surrounding implanted tumor masses. The results showed that the
weight of the cervical lymph nodes was markedly increased in
IL-17-injected groups implanted with LLC cells compared with those
of the nodes in control mice (Fig.
3A). The subcutaneous implantation of LLC cells in the
IL-17-injected groups was shown to result in enhanced metastatic
growth in the cervical lymph nodes as well as increased tumor
volume at the implanted site when compared with the control
group.
Angiogenetic factor expression is
increased but MIF and TSP-1 expression is decreased in the tumors
of IL-17 mice
The increased tumor angiogenesis resulted in an
increase in the size of tumor foci in the xenografts of the IL-17
group, but not in the control group. It was demonstrated that the
mRNA expression levels of the VEGF, MMP-2, MMP-9 and TNF-α in tumor
tissues were markedly higher in the IL-17 group compared with the
control mice (Fig. 4). In
addition, the expression levels of MIF and TSP-1 mRNA were lower in
IL-17 groups than in control mice. Western blot analysis
demonstrated the same trend in the protein levels of these proteins
(Fig. 5). These results suggest
that IL-17 is pivotal in tumor-associated VEGF, MMP-2, MMP-9 and
TNF-α production, and accompanying angiogenesis.
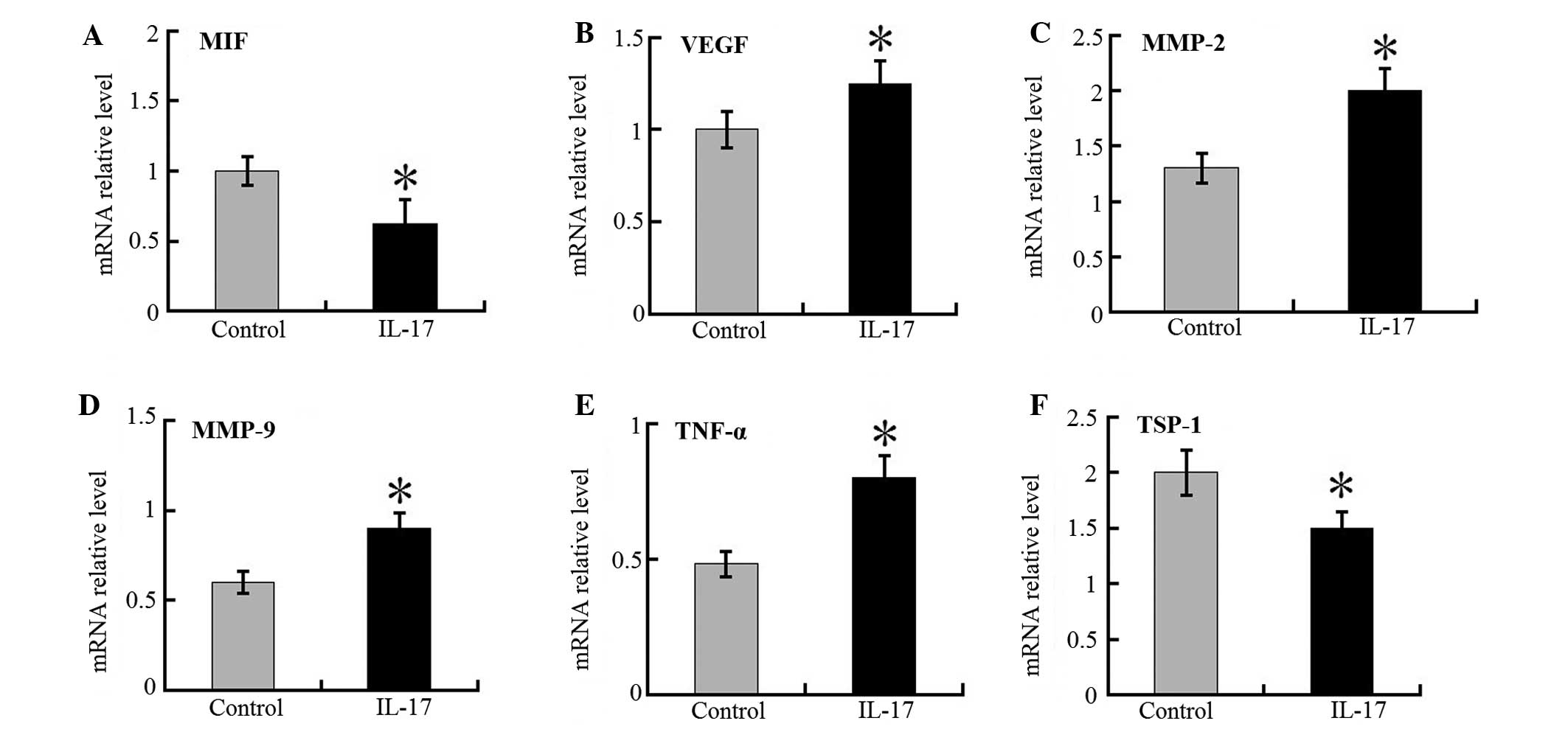 | Figure 4RT-qPCR detection of relative
intra-tumor target gene expression in IL-17 groups and control
mice. mRNA ratios of (A) MIF, (B) VEGF, (C) MMP-2, (D) MMP-9, (E)
TNF-α and (F) TSP-1 to glyceraldehyde 3-phosphate dehydrogenase in
tumor masses of IL-17 and control groups were determined. Each
value represents the mean ± standard error of the mean (n=5–8).
*P<0.05 compared with control mice. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; IL,
interleukin; MIF, migration inhibitory factor; VEGF, vascular
endothelial growth factor; MMP, matrix metalloproteinase; TNF,
tumor necrosis factor; TSP, thrombospondin. |
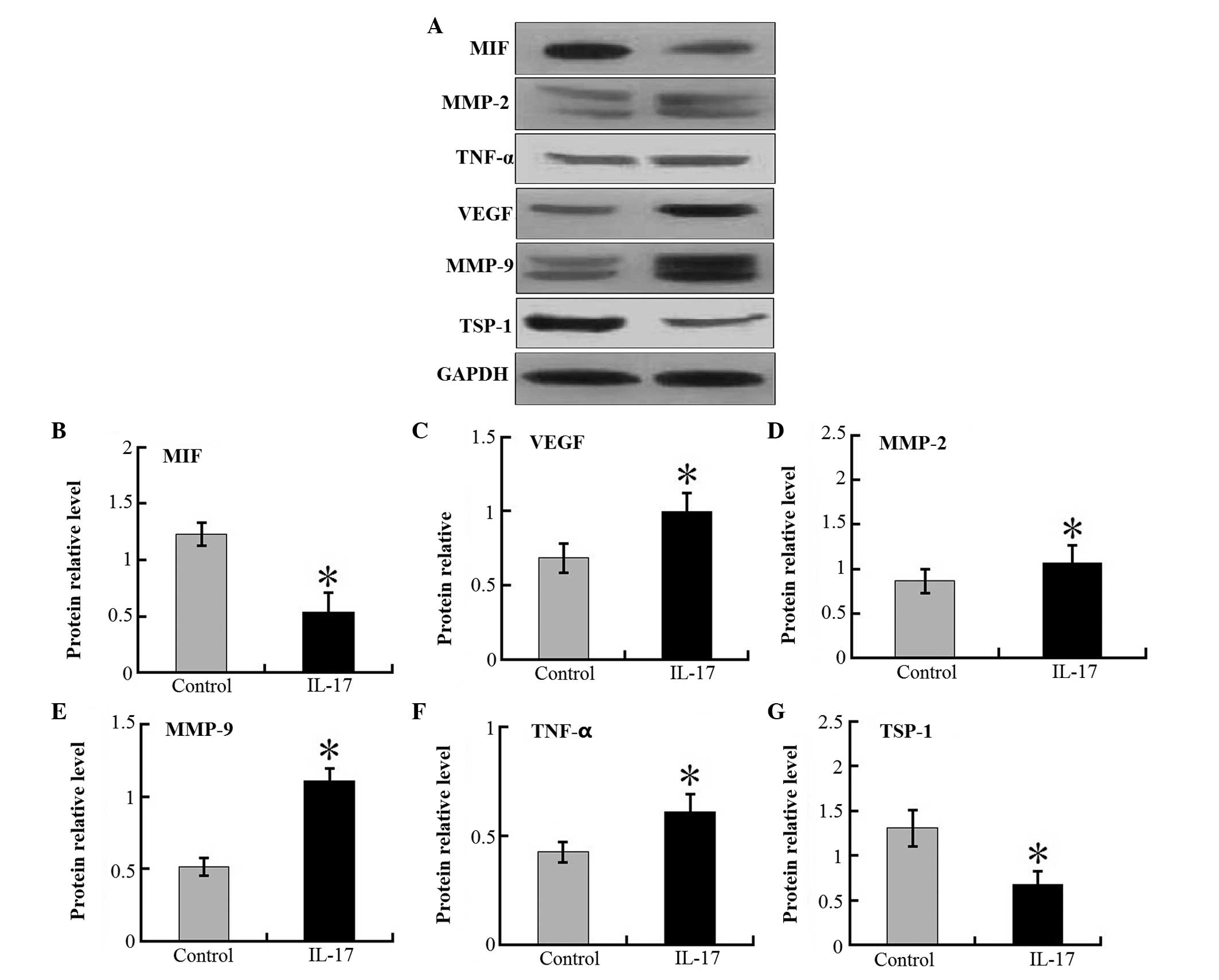 | Figure 5Western blot analysis of relative
intra-tumor target protein expression in IL-17 groups and control
mice. (A) Representative results of western blotting from three
independent experiments. Protein ratios of (B) MIF, (C) VEGF, (D)
MMP-2, (E) MMP-9, (F) TNF-α and (G) TSP-1 against
glyceralde-hyde-3-phosphate dehydrogenase in tumor masses of IL-17
and control groups were determined. Each value represents the mean
± standard error of the mean (n=5–8). *P<0.05;
compared with control mice. IL, interleukin; MIF, migration
inhibitory factor; VEGF, vascular endothelial growth factor; MMP,
matrix metalloproteinase; TNF, tumor necrosis factor; TSP,
thrombospondin; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
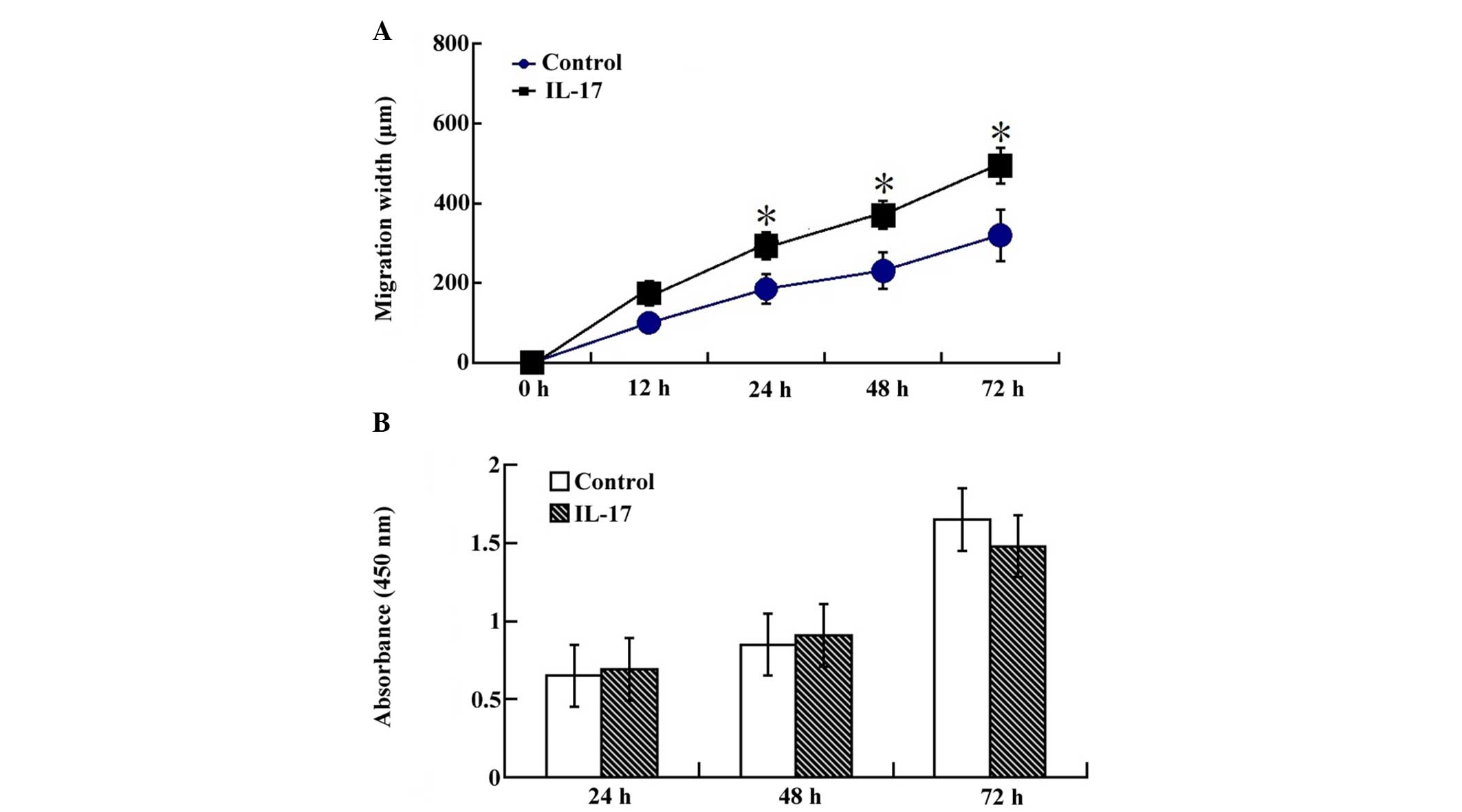
IL-17 stimulation increases migration in
a wound scratch assay
In order to further delineate the effects of IL-17
on the biologic function of LLCs, the role of IL-17 was examined in
cell migration. LLCs efficiently seal linear scratch wounds after
48 h culture, and this migratory process was increased following
IL-17 supplementation in the culture medium. Cells migrated into
the wounded area, which was markedly increased after 24 h in the
IL-17 stimulating group (Fig. 6A).
This data indicated that an increase in the migration of LLCs after
IL-17 stimulation was responsible for the attenuated wound healing
response.
Effects on LLC proliferation
The effect of IL-17 of LLC proliferation was also
investigated. Following stimulation with IL-17, LLC proliferation
was determined by an MTT assay. IL-17 was not identified to
increase cell proliferation, as determined by an MTT assay
performed with cells up to 24 h after stimulation with IL-17
(Fig. 6B).
Discussion
IL-17 has recently been described as a
pro-inflammatory cytokine predominantly secreted by activated T
lymphocytes, such as Th17 cells (28), which is capable of promoting
angiogenesis (29,30). A recent study by Reppert et
al (31) addressed the
expression of Th17 markers in NSCLC. Induction of IL-17A mRNA
expression was identified in lung samples derived from patients
affected by lung adenocarcinoma as compared with controls, which
suggested local induction of Th17 responses in the lung in NSCLC.
Additional studies addressed the functional role of Th17 cells in
lung cancer (32). These
experiments suggested that the key cytokine IL-17 produced by lung
CD4+ Th17 cells is important in experimental NSCLC. In
the present study, a pivotal role and novel mechanism for IL-17 in
facilitating LLC tumor growth was observed by measuring the tumor
volume in vivo. IL-17 was also observed to increase the
expression of VEGF, MMP-2, MMP-9 and TNF-α in LLCs in vivo.
This indicates that LLC tumor growth was correlated with IL-17
protein expression, suggesting a possible novel biological function
of IL-17 protein in tumors. This suggests that a possible
therapeutic approach for lung cancer may be to target IL-17.
IL-17A has been found to favor development of tumors
in certain tumor models (33,34).
In fact, it has previously been reported that IL-17A boosts tumor
development by inducing a tumor promoting microenvironment
(33,35). However, it has not been
investigated whether IL-17 promotes lymphatic metastasis. A
previous study reported that nodal micrometastases were found in up
to 36% of resected lungs from the patients with peripheral NSCLC,
and the presence of metastases to the lymph nodes significantly
reduces patient survival rates (36–38).
Thus, the development of a strategy to suppress lymphatic
metastasis is critical in the treatment of patients with lung
cancer patients. In the present study, implanted tumor volume and
the number of cervical lymph nodes surrounding tumor masses were
measured. The results showed that cervical lymph nodes surrounding
tumor masses in the IL-17 group were markedly higher in weight than
those in control mice. Notably, tumor volume in the groups treated
with IL-17 was increased compared with those in control mice. These
findings confirm that IL-17 is crucial in promoting the metastasis
of implanted LLC tumor cells in vivo and concomitantly
suggest that they also induce the proliferation of tumor cells.
Several lines of evidence indicate that
CCR2-positive macrophages have the ability to promote secretion of
angiogenic factors, resulting in tumor growth (39,40).
It was assumed that IL-17 may promote tumor growth by inducing
CCR2-positive macrophage recruitment. Consistent with this
hypothesis, intraperitoneal injection of IL-17 enhanced
CCR2-positive macrophage infiltration into tumors as detected by
flow cytometry. Furthermore, the IL-17/IL-17R interactions also
have profound effects on the macrophage infiltration, a rich source
of angiogenic factors, such as VEGF, MMP-2, MMP-9 and TNF-α they
were all enhanced, while the expression of anti-angiogenic factor,
TSP-1, was reduced in IL-17 groups. Furthermore, MIF, an inhibitor
for macrophage infiltration, mRNA and protein expression was
analyzed in tumor masses. RT-qPCR and western blot analysis
demonstrated that MIF mRNA and protein expression levels were lower
in IL-17 groups than in control groups. These observations indicate
that the IL-17/IL-17R axis is crucial to tumor growth by promoting
macrophage infiltration and inducing expression of VEGF, MMP-2,
MMP-9 and TNF-α in CCR2-expressing macrophages.
A previous study revealed that IL-17 may prompt cell
migration and proliferation (41).
In the present study, the effects of IL-17 on LLC cell migration
and cell proliferation were observed in vitro by wound
scratch and CCK-8 assays, respectively. Results showed that LLC
migration and proliferation were promoted by IL-17 stimulation.
This suggests that IL-17 exhibits a critical role in tumor growth
by multiple pathways, including promotion of angiogenesis factors
expression, tumor cell migration, tumor cell proliferation and
CCR2-positive macrophage infiltration into tumor masses. The exact
mechanism of IL-17 in lung tumor pathogenesis remains to be
elucidated, however, the preliminary results from the present study
suggested that IL-17 can be used as an effective intervention
target against tumor growth.
In particular, recent observations highlight a
functional role of IL-17 in lung tumor progression. Targeting of
cytokine-dependent molecular signaling events in tumor cells is
likely to lead to innovative tailored immunotherapies for patients
with lung cancer. In the present study, it was also identified that
IL-17 is critically involved in LLC-implanted tumor growth, tumor
metastasis and tumor angiogenesis. Furthermore, the levels of VEGF,
MMP-2, MMP-9 and TNF-α, key pro-angiogenesis factors, were
increased compared with that in the control mice. Furthermore,
levels of MIF and TSP-1 were decreased compared with that in the
control mice. These findings suggest that IL-17 enhances cancer
growth via multiple pathways. Thus, targeting IL-17 is likely to
have beneficial clinical effects not only by inhibiting
inflammation and tumor blood vessel formation but also by
inhibiting CCR2-positive macrophage infiltration and
tumor-promoting angiogenesis pathways.
Acknowledgments
This study was supported by the Suzhou Natural
Science Foundation.
References
|
1
|
Machtay M and Jeremic B: Complex and
controversial issues in locally advanced non-small cell lung
carcinoma. Semin Surg Oncol. 21:128–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanley K and Stjernswärd J: Lung cancer-a
worldwide health problem. Chest. 96(Suppl 1): S1–S5. 1989.
View Article : Google Scholar
|
|
3
|
Thatcher N: New perspectives in lung
cancer. 4 Haematopoietic growth factors and lung cancer treatment.
Thorax. 47:119–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weynants P, Marchandise FX and Sibille Y:
Pulmonary perspective: Immunology in diagnosis and treatment of
lung cancer. Eur Respir J. 10:1703–1719. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao Z, Fanslow WC, Seldin MF, Rousseau AM,
Painter SL, Comeau MR, Cohen JI and Spriggs MK: Herpes virus
Saimiri encodes a new cytokine, IL-17, which binds to a novel
cytokine receptor. Immunity. 3:811–821. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Starnes T, Robertson MJ, Sledge G, Kelich
S, Nakshatri H, Broxmeyer HE and Hromas R: IL-17F, a novel cytokine
selectively expressed in activated T cells and monocytes, regulates
angiogenesis and endothelial cell cytokine production. J Immunol.
167:4137–4140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hymowitz SG, Filvaroff EH, Yin JP, Lee J,
Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, et al:
IL-17s adopt a cystine knot fold: Structure and activity of a novel
cytokine, IL-17F and implications for receptor binding. EMBO J.
20:5332–5341. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonysamy MA and Numasaki M:
Interleukin-17 (IL-17, IL-25) In The Cytokine Handbook, 4th Ed A W
Thomson and M T Lotze, eds. Academic Press; London: pp. 475–502.
2003
|
|
9
|
Fossiez F, Djossou O, Chomarat P,
Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E,
Saeland S, et al: T cell interleukin-17 induces stromal cells to
produce proinflammatory and hematopoietic cytokines. J Exp Med.
183:2593–2603. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao Z, Painter SL, Fanslow WC, Ulrich D,
Macduff BM, Spriggs MK and Armitage RJ: Human IL-17: A novel
cytokine derived from T cells. J Immunol. 155:5483–5486.
1995.PubMed/NCBI
|
|
11
|
Numasaki M, Tomioka Y, Takahashi H and
Sasaki H: IL-17 and IL-17F modulate GM-CSF production by lung
microvascular endothelial cells stimulated with IL-1beta and/or
TNF-alpha. Immunol Lett. 95:175–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Numasaki M, Takahashi H, Tomioka Y and
Sasaki H: Regulatory roles of IL-17 and IL-17F in G-CSF production
by lung microvascular endothelial cells stimulated with IL-1beta
and/or TNF-alpha. Immunol Lett. 95:97–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Numasaki M, Lotze MT and Sasaki H:
Interleukin-17 augments tumor necrosis factor-alpha-induced
elaboration of proangiogenic factors from fibroblasts. Immunol
Lett. 93:39–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aarvak T, Chabaud M, Miossec P and Natvig
JB: IL-17 is produced by some proinflammatory Th1/Th0 cells but not
by Th2 cells. J Immunol. 162:1246–1251. 1999.PubMed/NCBI
|
|
15
|
Yao Z, Spriggs MK, Derry JM, Strockbine L,
Park LS, VandenBos T, Zappone JD, Painter SL and Armitage RJ:
Molecular characterization of the human interleukin (IL)-17
receptor. Cytokine. 9:794–800. 1997. View Article : Google Scholar
|
|
16
|
Kotake S, Udagawa N, Takahashi N,
Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N,
Gillespie MT, et al: IL-17 in synovial fluids from patients with
rheumatoid arthritis is a potent stimulator of osteoclastgenesis. J
Clin Invest. 103:1345–1352. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chabaud M, Lubberts E, Joosten L, van Den
Berg W and Miossec P: IL-17 derived from juxta-articular bone and
synovium contributes to joint degradation in rheumatoid arthritis.
Arthritis Res. 3:168–177. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonysamy MA, Fanslow WC, Fu F, Li W,
Qian S, Troutt AB and Thomson AW: Evidence for a role of IL-17 in
organ allograft rejection: IL-17 promotes the functional
differentiation of dendritic cell progenitors. J Immunol.
162:577–584. 1999.PubMed/NCBI
|
|
19
|
Teunissen MB, Koomen CW, de Waal Malefyt
R, Wierenga EA and Bos JD: Interleukin-17 and interferon-gamma
synergize in the enhancement of proinflammatory cytokine production
by human keratinocytes. J Invest Dermatol. 111:645–649. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molet S, Hamid Q, Davoine F, Nutku E, Taha
R, Pagé N, Olivenstein R, Elias J and Chakir J: IL-17 is increased
in asthmatic airways and induces human bronchial fibroblasts to
produce cytokines. J Allergy Clin Immunol. 108:430–438. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murugaiyan G and Saha B: Protumor vs
antitumor functions of IL-17. J Immunol. 183:4169–4175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human CD4+
IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA.
105:15505–15510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Numasaki M, Watanabe M, Suzuki T,
Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze
MT, Kolls JK and Sasaki H: IL-17 enhances the net angiogenic
activity and in vivo growth of human non-small cell lung cancer in
SCID mice through promoting CXCR-2-dependent angiogenesis. J
Immunol. 175:6177–6189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin L, Chen YS, Yao YD, Chen JQ, Chen JN,
Huang SY, Zeng YJ, Yao HR, Zeng SH, Fu YS and Song EW: CCL18 from
tumor-associated macrophages promotes angiogenesis in breast
cancer. Oncotarget. 6:34758–34773. 2015.PubMed/NCBI
|
|
27
|
Dobrzycka B, Mackowiak-Matejczyk B,
Kinalski M and Terlikowski SJ: Pretreatment serum levels of bFGF
and VEGF and its clinical significance in endometrial carcinoma.
Gynecol Oncol. 128:454–460. 2013. View Article : Google Scholar
|
|
28
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar
|
|
30
|
Kato T, Furumoto H, Ogura T, Onishi Y,
Irahara M, Yamano S, Kamada M and Aono T: Expression of IL-17 mRNA
in ovarian cancer. Biochem Biophys Res Commun. 282:735–738. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reppert S, Boross I, Koslowski M, Türeci
Ö, Koch S, Lehr HA and Finotto S: A role for T-bet-mediated tumour
immune surveillance in anti-IL-17A treatment of lung cancer. Nat
Commun. 2:6002011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan MC, Zhong XN, Liu GN and Wei JR: The
Treg/Th17 paradigm in lung cancer. J Immunol Res. 2014:7303802014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iida T, Iwahashi M, Katsuda M, Ishida K,
Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K, et al:
Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor
microenvironment and promote tumor progression in human gastric
cancer. Oncol Rep. 25:1271–1277. 2011.PubMed/NCBI
|
|
34
|
Chae WJ, Gibson TF, Zelterman D, Hao L,
Henegariu O and Bothwell AL: Ablation of IL-17A abrogates
progression of spontaneous intestinal tumorigenesis. Proc Natl Acad
Sci USA. 107:5540–5544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Charles KA, Kulbe H, Soper R,
Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P,
Thompson RG, Kollias G, Smyth JF, et al: The tumor-promoting
actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in
mice and humans. J Clin Invest. 119:3011–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ichinose Y, Yano T, Asoh H, Yokoyama H,
Yoshino I and Katsuda Y: Prognostic factors obtained by a
pathologic examination in completely resected non-small-cell lung
cancer. An analysis in each pathologic stage. J Thorac Cardiovasc
Surg. 110:601–605. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Velzen E, Snijder RJ, Brutel de la
Rivière A, Elbers HJ and van den Bosch JM: Type of lymph node
involvement influences survival rates in T1N1M0 non-small cell lung
carcinoma. Lymph node involvement by direct extension compared with
lobar and hilar node metastases. Chest. 110:1469–1473. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mountain CF and Dresler CM: Regional lymph
node classification for lung cancer staging. Chest. 111:1718–1723.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sierra-Filardi E, Nieto C, Domínguez-Soto
A, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven
J, Ardavín C, Rodríguez-Fernández JL, et al: CCL2 shapes macrophage
polarization by GM-CSF and M-CSF: Identification of
CCL2/CCR2-dependent gene expression profile. J Immunol.
192:3858–3867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mitchem JB, Brennan DJ, Knolhoff BL, Belt
BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L,
Piwnica-Worms D, et al: Targeting tumor-infiltrating macrophages
decreases tumor-initiating cells, relieves immunosuppression, and
improves chemotherapeutic responses. Cancer Res. 73:1128–1141.
2013. View Article : Google Scholar :
|
|
41
|
Chen X, Wan J, Liu J, Xie W, Diao X, Xu J,
Zhu B and Chen Z: Increased IL-17-producing cells correlate with
poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer.
69:348–354. 2010. View Article : Google Scholar
|