Introduction
Paraquat (1,1′-dimethyl-4,4′-bipyridinium
dichloride; PQ) is an effective, widely used, nonselective
herbicide. It is toxic to humans and is associated with high
mortality, mainly as a consequence of respiratory failure (1). Upon entering the cell, PQ undergoes
cyclic single-electron reduction/oxidation through its quaternary
ammonium nitrogen atoms and bipyridyl ring, producing reactive
oxygen species (ROS) and PQ radicals (2). Redox cycling is considered to serve
an important role in the initiation of the lung damage and fibrosis
resulting from PQ exposure (2,3). In
view of this, antioxidant therapy is considered an important
strategy for the treatment of PQ poisoning (2,3).
Sirtuin 1 (SIRT1) is an NAD+-dependent
deacetylase (4). SIRT1 regulates a
wide variety of cellular processes by deacetylating histones and
numerous other crucial transcription factors, including factors in
control of ROS production (5). The
nuclear factor, erythroid 2-like 2 (NRF2) transcription factor is a
member of the cap 'n' collar basic-region leucine zipper
transcription factors, and is considered an effective target for
antioxidant therapy for PQ poisoning (3,6).
Previous studies have demonstrated that the SIRT1-mediated
deacetylation of NRF2 terminated the transcription of antioxidant
genes (7). By contrast, SIRT1 is
known to protect cells from oxidative stress injury, and silencing
its activity results in decreased NRF2 expression levels (8,9).
However, the association between SIRT1 and NRF2 and their effect in
PQ-induced oxidative stress remains unclear.
Resveratrol (3,4′,5-trihydroxystilbene; Res) is a
natural polyphenol present in grapes, berries, peanuts and other
plants (10). Previous studies
have reported the beneficial effects of Res in numerous diseases,
including cancer, cardiovascular diseases, ischemic injuries and
acute poisoning (11–13). This wide range of biological
effects may be explained in part by the antioxidant properties of
Res, and the activation and expression of SIRT1 is postulated to be
a key event in the pathophysiology of Res (10,11).
Based on these data, the current study investigated the effects of
Res on PQ-induced oxidative stress and lung injury. Furthermore,
the potential roles of SIRT1 and NRF2 in this progress were also
illustrated.
Materials and methods
Animals and reagents
Male Institute of Cancer Research mice (age, 6–8
weeks; weight, 18–20 g) were provided by the Animal Experimental
Center of Wenzhou Medical University (Wenzhou, China). The study
was approved by the Laboratory Animal Ethics Committee of Wenzhou
Medical University & Laboratory Animal Centre of Wenzhou
Medical University (Wenzhou, China). Animals were housed in a room
with a 12-h light/dark cycle and allowed free access to tap water
and standard laboratory food. The PQ and Res used in the experiment
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit
anti-mouse SIRT1 monoclonal antibody (cat. no. 3931) and rabbit
anti-mouse Nrf2 polyclonal antibody (cat. no. ab31163) were
obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA)
and Abcam (Cambridge, MA, USA), respectively. Polyclonal mouse
anti-β actin antibody (cat. no. BS1002) was purchased from Bioworld
Technology (St. Louis Park, MN, USA). Formaldehyde, Hematoxylin and
Eosin (H&E) Staining kit, agarose gel, SDS-PAGE and Nuclear and
Cytoplasmic Protein Extraction kit (no. P0028) were purchased from
Beyotime Institute of Biotechnology (Haimen, China)
Experimental design
The dose- and time-dependent effects of Res on SIRT1
expression and lung injury after PQ exposure were examined by two
independent experiments. A) Mice were randomly divided into four
groups as follows: i) The control group, mice received saline
solution by intraperitoneal (i.p.) injection (n=6); ii) The Res
group, mice received Res (30 mg/kg i.p.; n=6); iii) The PQ group,
mice received PQ (20 mg/kg i.p.; n=6); iv) The PQ + Res group, mice
received PQ (20 mg/kg i.p.) and Res (15, 30 or 60 mg/kg i.p.; n=6
for each condition). PQ was dissolved in saline solution and
injected intraperitoneally in a single toxic dose of 20 mg/kg of
body weight based on preliminary experiments. Mice were
anesthetized with 50 mg/kg pentobarbital (Hanlim Pharm Co., Ltd.,
Seoul, Korea). Pulmonary samples were collected at 24 h subsequent
to PQ injection. B) Mice were randomly divided into three groups as
follows: i) Control group (n=18); ii) The PQ group, mice received
PQ (20 mg/kg i.p.; n=18); iii) The PQ + Res group, mice received PQ
(20 mg/kg i.p.) and Res (30 mg/kg i.p.; n=18). Pulmonary samples
were collected at 6, 24 and 72 h subsequent to PQ exposure, and
tissues from the same mice were used for histopathological, PCR and
western blot analyses.
Histopathological assessment of pulmonary
tissue
The lower lobes of the right lungs of six mice pre
group were removed and then transferred to 4% formaldehyde for 24
h. The lungs were embedded in paraffin (Leica Microsystems,
Wetzlar, Germany), and 2–3 butterfly-shaped sections of 4-µm
thickness were cut per sample using a microtome (Leica RM2016;
Leica Microsystems) and placed on glass microscope slides stained
with hematoxylin and eosin (H&E) for histopathological
analysis. Lung histopathology images were acquired using a
microscope (Nikon Eclipse 80i; Nikon, Tokyo, Japan). The severity
of lung injury was determined by a histopathologist blinded to the
protocol design.
Wet/dry (W/D) weight ratio
To quantify the magnitude of pulmonary edema, the
lung W/D weight ratio was evaluated. The middle lobe of right lung
of six animals was excised and the wet weight was determined,
following which the lung was placed in a drying oven at 60°C for 72
h to stabilize the dry weight. The ratio of W/D was calculated.
RNA isolation and reverse
transcription-semiquantitative polymerase chain reaction
(RT-sqPCR)
The total RNA of the upper right lung lobe of six
mice per group was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA USA). The cDNA was
prepared from 2 µg RNA using a PCR master mix (Promega
Corp., Madison, WI, USA). The primers used were as follows: SIRT1,
F 5′-ACGCTGTGGCAGATTGTTATTA-3′ and R 5′-TTGAAGAATGGTCTTGGGTCTT-3′;
NRF2, F 5′-ATTCTTTCAGCAGCATCCTCTC-3′ and R
5′-ACACTTCCAGGGGCACTATCTA-3′, resulting in PCR products of 278 and
403 bp, respectively. The primers for mouse β-actin were F
5′-ATATCGCTGCGCTGGTCGTC-3′ and R 5′-AGGATGGCGTGAGGGAGAGC-3′,
resulting in a PCR product of 517 bp. Amplified fragments of
expected size were analyzed using a 2% agarose gel and images were
captured under ultraviolet (UV) light (302 nm) (Tianneng Co.,
Shanghai, China). Gels were imaged using the ChemiDoc™ MP Imaging
System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
quantified by densitometry using Quantity One 4.52 software
(Bio-Rad Laboratories, Inc.). Data are presented as fold changes in
gene expression normalized to β-actin.
Western blot analysis
The upper parts of the left lung from six animals
per group were harvested and homogenized immediately. Total
proteins were extracted from the lungs using the Nuclear and
Cytoplasmic Protein Extraction kit according to the manufacturer's
protocol. Equal amounts of total proteins (40 µg) per lane
were separated by 8% SDS-PAGE (80 V, 30 min; 120 V, 90 min) and
then transferred to polyvinylidene fluoride membranes (Solarbio,
Beijing, China). Membranes were incubated with rabbit anti-mouse
SIRT1 monoclonal antibody (1:1,000 dilution) or rabbit anti-mouse
Nrf2 polyclonal antibody (1:500 dilution) at 4°C for 24 h and
washed with Tris-buffered saline containing Tween 20 (Solarbio).
Subsequent to washing, samples were incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary
antibody (cat. no. sc-2030; 1:2,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature
and visualized using Western Blotting Luminol reagent (cat. no.
sc-2048; Santa Cruz Biotechnology, Inc.). Images of the blots were
captured using the ChemiDoc™ MP Imaging System and quantified by
densitometry using Quantity One 4.52 software.
Enzyme-linked immunosorbent assay
(ELISA)
The heme oxygenase-1 (HO-1) activity in samples of
the lower part of the left lung of six mice was measured by
commercially available ELISA kits (Westang Biotechnology Co.,
Shanghai, China). ELISA was performed following the protocols
provided by the manufacturer.
Detection of superoxide dismutase (SOD)
and catalase (CAT) activity and protein levels of glutathione (GSH)
and malondialdehyde (MDA)
The MDA and GSH contents in samples of the lower
part of the left lung of six mice were detected according to the
instructions of the MDA and GSH assay kits purchased from Nanjing
Jiancheng Bioengineering Research Institute (Nanjing, China). The
SOD and CAT activities were detected according to the instructions
of the SOD and CAT assay kits (Nanjing Keygen Biotech Co., Ltd.,
Nanjing, China).
Statistical analysis
All data are described as the mean ± standard
deviation and analyzed using SPSS 19.0 software (International
Business Machines, Armonk, NY, USA). The differences between groups
were analyzed by one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Res upregulated the expression of SIRT1
in PQ-exposed lung tissue
To assess the potential role of SIRT1 in PQ-induced
lung injury, the SIRT1 levels in lung tissue prior and following
Res injection were determined. As demonstrated in Fig. 1A and B, the expression of SIRT1
mRNA and protein levels were elevated at 6 and 24 h after PQ
exposure compared with the saline group (P<0.05). At 72 h, the
SIRT1 mRNA and protein levels were significantly decreased compared
with the saline group (P<0.05). Additionally, administration of
30 mg/kg Res significantly increased the mRNA and protein levels of
SIRT1 at all time points compared with the PQ group (P<0.05).
Administration of 15, 30 or 60 mg/kg Res upregulated SIRT1
expression levels in the lung at 24 h compared with the PQ group
(P<0.05; Fig. 1C and D).
Res attenuates PQ-induced lung injury in
mice
Histopathological and W/D weight ratio analyses were
performed to determine the effect of Res on PQ-induced lung injury.
Compared with the control group, PQ administration caused marked
lung hemorrhage, edema, alveolar septal thickening, influx of
inflammatory cells and fibrin deposition (Fig. 2A). In the PQ+Res group, similar
changes were identified, however to a lesser degree. Additionally,
the W/D weight ratios of the lung tissues were significantly
increased following PQ administration compared with the ratios in
the NS group (P<0.05; Fig. 2B).
In the PQ+Res group, the lung W/D weight ratio was significantly
lower than that in the PQ group at 24 and 72 h subsequent to PQ
exposure (P<0.05; Fig. 2B).
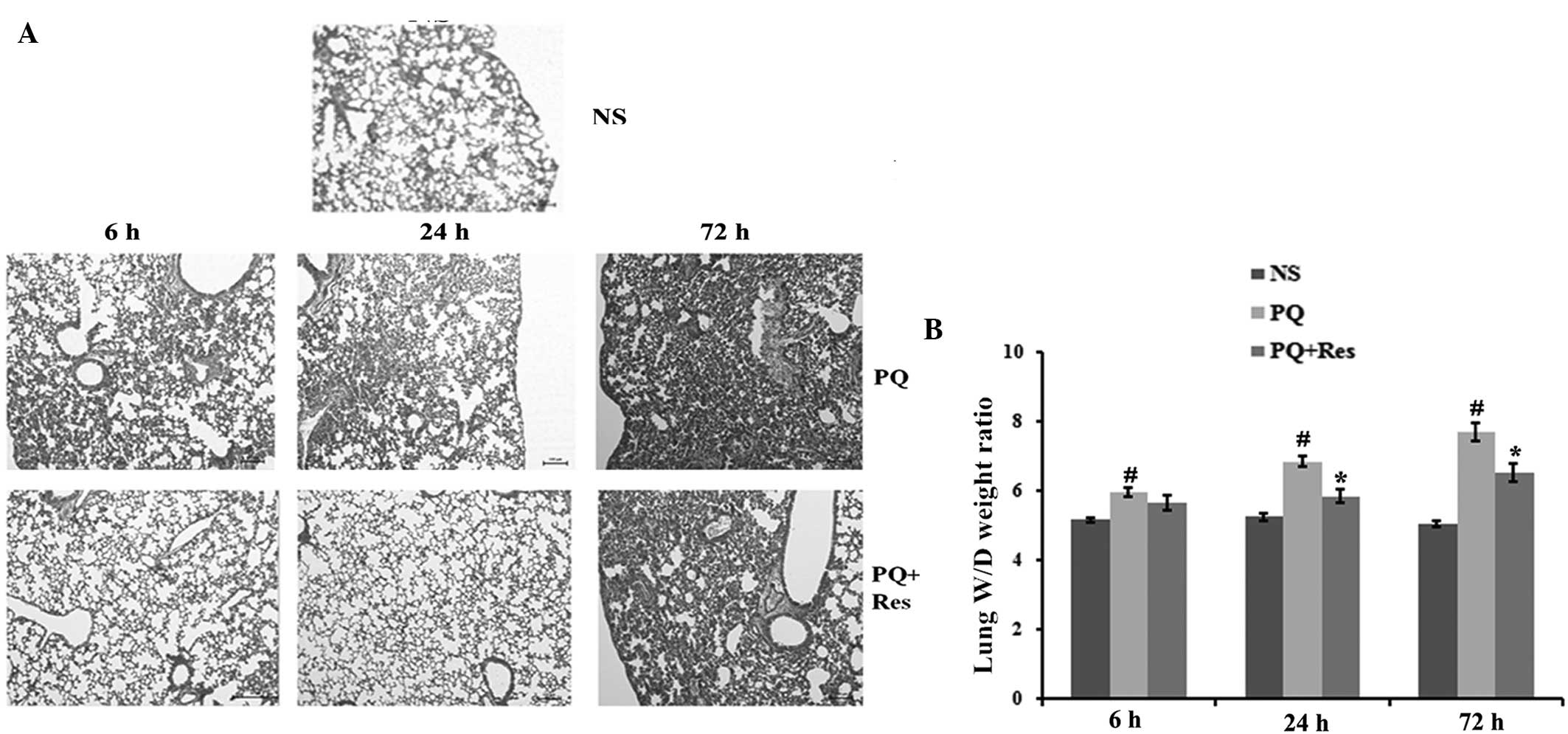 | Figure 2Res attenuates PQ-induced lung injury
in mice. (A) Histological examination of mouse lung tissue was
performed at 6, 24 and 72 h post-PQ treatment. Tissue was fixed and
stained with hematoxylin and eosin (magnification, ×100). In the he
NS group, parenchyma and lung airways are normal, whereas the PQ
groups show marked lung hemorrhage, edema, alveolar septal
thickening, influx of inflammatory cells and fibrin deposition.
These changes were ameliorated by Res treatment. (B) The lung W/D
ratio was determined at 6, 24 and 72 h after PQ administration.
Data are presented as the mean ± standard deviation (n=6).
#P<0.05 vs. the NS group, *P<0.05 vs.
the PQ group. Res, resveratrol; PQ, paraquat, W/D, wet/dry; NS,
normal saline. |
Effects of Res on NRF2 expression in the
lung following PQ exposure
To investigate whether the protective role of Res
was mediated through NRF2, the NRF2 expression levels in the mouse
lung tissues were determined. NRF2 protein and mRNA expression
levels were significantly elevated at 6 and 24 h after PQ
administration but were decreased at 72 h, compared with the NS
group (P<0.05; Fig. 3).
However, Res administration significantly increased NRF2 protein
expression levels compared with the PQ group at all time points
(P<0.05; Fig. 3).
 | Figure 3Effects of Res on NRF2 expression
levels in mouse lung tissue following PQ exposure. The protein and
mRNA expression levels of NRF2 were determined by (A) western blot
and (B) semiquantitative polymerase chain reaction analyses,
respectively, at 6, 24 and 72 h after PQ administration. Data are
presented as the mean ± standard deviation (n=6).
#P<0.05 vs. the NS group, *P<0.05 vs.
the PQ group. Res, resveratrol; NRF2, nuclear factor, erythroid
2-like 2; PQ, paraquat, NS, normal saline. |
Effects of Res on HO-1 activity in mouse
lung tissue following PQ exposure
Compared with the NS group, HO-1 activity in the
lung tissue was upregulated markedly at 6 and 24 h after PQ
administration, but was decreased at 72 h (P<0.05; Fig. 4). In the PQ+Res group, HO-1
activity was upregulated compared with that in the PQ group
(P<0.05; Fig. 4).
Effects of Res and PQ on SOD and CAT
activity levels in mouse lung tissues
SOD and CAT activity levels in the mouse lung tissue
were measured. Compared with the NS group, the PQ group exhibited a
significant decrease in SOD and CAT activity levels at all time
points (P<0.05; Fig. 5).
Following Res administration, SOD and CAT activity levels
significantly increased in the lung tissue, compared with the NS
group (P<0.05; Fig. 5).
Effects of Res and PQ on GSH and MDA
levels in mouse lung tissues
Following PQ administration, GSH protein levels were
decreased in the lung tissue compared with the NS group (P<0.05;
Fig. 6A). The GSH protein levels
were significantly increased in the PQ+Res group compared with
those in the PQ group (P<0.05; Fig.
6A). By contrast, PQ administration led to an increase in MDA
activity levels compared with the NS group (P<0.05; Fig. 6B). Res administration decreased MDA
activity levels compared with those in the PQ group (P<0.05;
Fig. 6B).
Discussion
The lung is targeted in PQ poisoning through the
pulmonary polyamine uptake system that recruits PQ. This results in
a 6-10-fold increase in the lung PQ levels compared with plasma
levels. Based on its induction of redox cycling, PQ in the lung
results in oxidative stress-associated cell death and lung injury
(14,15).
Sirtuins are a unique class of type III histone
deacetylases with seven isoforms, SIRT1-7 (16). SIRT1 is the best characterized
member among the mammalian sirtuins (16). Previous studies have revealed a
crosstalk between the SIRT1 expression levels and oxidative stress
(5,17). Oxidative stress can inhibit the
SIRT1 expression, and the overexpression of SIRT1 has been
demonstrated to inhibit apoptosis induced by oxidative stress
(18,19). The current study demonstrated
increased SIRT1 expression levels in mouse lung tissue at 6 and 24
h after PQ administration. However, long-term exposure to PQ
significantly decreased the expression of SIRT1. Treatment with
Res, a known allosteric activator of SIRT1, upregulated the
expression of SIRT1, accompanied by decreased oxidative stress and
lung injury. This demonstrated that the SIRT1 agonist in Res
treatment has a protective role in PQ-induced lung injury by
decreasing oxidative stress.
A major mechanism in the cellular defense against
oxidative stress is the activation of the NRF2 antioxidant response
element signaling pathway (20).
Previous studies have demonstrated that NRF2 is important in
PQ-induced lung injury; PQ can inhibit the NRF2 expression in
vitro and in vivo (6,21).
Additionally, overexpression of NRF2 can ameliorate PQ-induced lung
injury and cell apoptosis (21,22).
The function of the NRF2-antioxidant pathway is controlled by
multiple factors, including the acetylation-deacetylation of NRF2.
Kawai et al (7)
demonstrated that SIRT1-mediated deacetylation of the NRF2 protein
terminated the transcription of antioxidant genes in vitro.
However, other studies have demonstrated that SIRT1 overexpression
significantly promoted the nuclear accumulation, DNA binding and
transcriptional activity of NRF2 and NRF2-mediated gene expression
(8,9,23).
In the current study, an increase in the SIRT1 expression levels by
Res was associated with a high level of NRF2. In addition, the
HO-1, SOD and CAT activity and the levels of GSH were upregulated
following Res administration. Indeed, previous studies indicated
that the acetylation of NRF2 can reduce NRF2 stability and impaired
antioxidant defenses (24).
Therefore, as a protein deacetylase, SIRT1 may be important for
NRF2 protein stability and expression.
Taken together, the results of the current study
demonstrated that Res, an SIRT1 activator, can reduce PQ-induced
lung injury by regulating SIRT1 expression in combination with the
NRF2 antioxidant pathway. The present study indicated that SIRT1
and NRF2 serve a critical role in PQ-induced lung injury. However,
the mechanism of regulation of NRF2 expression levels by SIRT1
requires further investigation.
Acknowledgments
This work was supported by the State Key Program of
the Natural Science Foundation of Zhejiang province (no.
LZ12H26001) and the Medical and Health Research Program of Zhejiang
province (no. 2012ZDA034).
References
|
1
|
Gawarammana IB and Buckley NA: Medical
management of paraquat ingestion. Br J Clin Pharmacol. 72:745–757.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blanco-Ayala T, Andérica-Romero AC and
Pedraza-Chaverri J: New insights into antioxidant strategies
against paraquat toxicity. Free Radic Res. 48:623–640. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong GL, Liu JM, Zhao GJ, Wang L, Liang G,
Wu B, Li MF, Qiu QM and Lu ZQ: The reversal of paraquat-induced
mitochondria-mediated apoptosis by cycloartenyl ferulate, the
important role of Nrf2 pathway. Exp Cell Res. 319:2845–2855. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caito S, Rajendrasozhan S, Cook S, Chung
S, Yao H, Friedman AE, Brookes PS and Rahman I: SIRT1 is a
redox-sensitive deacetylase that is post-translationally modified
by oxidants and carbonyl stress. FASEB J. 24:3145–3159. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salminen A, Kaarniranta K and Kauppinen A:
Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging
Process. Int J Mol Sci. 14:3834–3859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong GL, Cai QQ, Tan JP, Jiang XZ, Zhao
GJ, Wu B, Li MF, Qiu QM and Lu ZQ: Mifepristone-inducible
recombinant adenovirus attenuates paraquat-induced lung injury in
rats. Hum Exp Toxicol. 34:32–43. 2015. View Article : Google Scholar
|
|
7
|
Kawai Y, Garduño L, Theodore M, Yang J and
Arinze IJ: Acetylation-deacetylation of the transcription factor
Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its
transcriptional activity and nucleocytoplasmic localization. J Biol
Chem. 286:7629–7640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang K, Huang J, Xie X, Wang S, Chen C,
Shen X, Liu P and Huang H: Sirt1 resists advanced glycation end
products-induced expressions of fibronectin and TGF-β1 by
activating the Nrf2/ARE pathway in glomerular mesangial cells. Free
Radic Biol Med. 65:528–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang K, Chen C, Hao J, Huang J, Wang S,
Liu P and Huang H: Polydatin promotes Nrf2-ARE anti-oxidative
pathway through activating Sirt1 to resist AGEs-induced
upregulation of fibronetin and transforming growth factor-β1 in rat
glomerular messangial cells. Mol Cell Endocrinol. 399:178–189.
2015. View Article : Google Scholar
|
|
10
|
Leonard SS, Xia C, Jiang BH, Stinefelt B,
Klandorf H, Harris GK and Shi X: Resveratrol scavenges reactive
oxygen species and effects radical-induced cellular responses.
Biochem Biophys Res Commun. 309:1017–1026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borra MT, Smith BC and Denu JM: Mechanism
of human SIRT1 activation by resveratrol. J Biol Chem.
280:17187–17195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Novelle MG, Wahl D, Diéguez C, Bernier M
and de Cabo R: Resveratrol supplementation: Where are we now and
where should we go? Ageing Res Rev. 21:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rivera H, Shibayama M, Tsutsumi V,
Perez-Alvarez V and Muriel P: Resveratrol and trimethylated
resveratrol protect from acute liver damage induced by CCl4 in the
rat. J Appl Toxicol. 28:147–155. 2008. View
Article : Google Scholar
|
|
14
|
Hoet PHM and Nemery B: Polyamines in the
lung: Polyamine uptake and polyamine-linked pathological or
toxicological conditions. Am J Physiol Lung Cell Mol Physiol.
278:L417–L433. 2000.PubMed/NCBI
|
|
15
|
Dinis-Oliveira RJ, Pontes H, Bastos ML,
Remião F, Duarte JA and Carvalho F: An effective antidote for
paraquat poisonings: The treatment with lysine acetylsalicylate.
Toxicology. 255:187–193. 2009. View Article : Google Scholar
|
|
16
|
Cantó C and Auwerx J: Targeting sirtuin 1
to improve metabolism: All you need is NAD(+)? Pharmacol Rev.
64:166–187. 2012. View Article : Google Scholar
|
|
17
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao C, Lu S, Kivlin R, Wallin B, Card E,
Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, et al: SIRT1
confers protection against UVB- and H2O2-induced cell death via
modulation of p53 and JNK in cultured skin keratinocytes. J Cell
Mol Med. 13(9B): 3632–3643. 2009. View Article : Google Scholar
|
|
19
|
Hasegawa K, Wakino S, Yoshioka K,
Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K
and Itoh H: Sirt1 protects against oxidative stress-induced renal
tubular cell apoptosis by the bidirectional regulation of catalase
expression. Biochem Biophys Res Commun. 372:51–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho HY, Reddy SP and Kleeberger SR: Nrf2
defends the lung from oxidative stress. Antioxid Redox Signal.
8:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He XY, Zhao GJ, Lu ZQ, Hong GL, He F,
Liang H, Qiu QM and Li JR: Oxidative stress of acute paraquat
poisoned rats and sodium dimercaptopropane sulfonate intervention.
Chin J Ind Hyg Occup Dis. 27:476–479. 2009.
|
|
22
|
Jiang XZ, Song Q, Xu XP, Cai QQ, Hong GL,
Liang H and Lu ZQ: The effects of Nrf2 gene expression induced by
RU486 at different doses on A549 cell damage induced by paraquat.
Chin J Ind Hyg Occup Dis. 30:268–272. 2012.
|
|
23
|
Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q,
Li J, Zhang Q, Gao Y, Gao L, et al: Alpha-lipoic acid improves
high-fat diet-induced hepatic steatosis by modulating the
transcription factors SREBP-1, FoxO1 and Nrf2 via the
SIRT1/LKB1/AMPK pathway. J Nutr Biochem. 25:1207–1217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mercado N, Thimmulappa R, Thomas CM,
Fenwick PS, Chana KK, Donnelly LE, Biswal S, Ito K and Barnes PJ:
Decreased histone deacetylase 2 impairs Nrf2 activation by
oxidative stress. Biochem Biophys Res Commun. 406:292–298. 2011.
View Article : Google Scholar : PubMed/NCBI
|




















