Introduction
Cordyceps sinensis, a Chinese caterpillar
fungus, is known to be one of the most valuable and effective
Chinese medicinal herbs, which possesses potential antioxidant,
immunomodulatory, antitumor and anti-inflammatory properties
(1). Paecilomyces hepiali,
a parasitic fungus generally found in Cordyceps sinensis,
contains similar chemical constituents and exhibits similar
bioactivities (2).
Polysaccharide-enriched extract, separated from Paecilomyces
hepiali (PHC), has been reported as the major active element,
which exhibit anti-oxidant activity (3), limit A549 cell proliferation and
induce apoptosis (4). In our
previous experiments, Cordyceps militaris polysaccharides
were confirmed to possess antidiabetic, anti-nephropathic and
antihypoxic activities (5,6). However, the antifatigue and
antihypoxic effects of Paecilomyces hepiali mycelium remain
to be elucidated.
Fatigue is characterized by a physical and/or mental
weariness, which results in negative effects on work performance
and exercise intensity, family life and social relationships
(7). Physical fatigue, a complex
condition, is described as a time-dependent, exercise-induced
reduction in the maximal force-generating capacity of a muscle
(8). Intense exercise results in
the accumulation of reactive free radicals and leads to the
consumption of adenosine triphosphate (ATP) and glycogen (9). Energy metabolism is involved in the
pathophysiology of fatigue, and hypoxia, which occurs during acute
and chronic vascular disease, cancer and stroke, is defined as a
decrease in the normal level of tissue oxygen tension (10). As reported previously, hypoxia is
also associated with energy metabolism (11). 5′-AMP-activated protein kinase
(AMPK) is a key regulator of cellular and whole body energy balance
(12). It acts to suppress
anabolic ATP-consumption pathways, and stimulates catabolic
ATP-generating pathways (13). In
addition, the antioxidant enzyme system protects against excessive
or exhaustive exercise-induced oxidative damage, and is associated
with physical status in athletes (14). Enhanced antioxidant enzyme activity
prolongs exercise performance, and reduces physical fatigue and
hypoxia (15,16). The identification of natural
antioxidants originating from plants has been an area of increased
attention (17).
Pharmacological drugs or therapies used for treating
fatigue and hypoxia remain unsatisfactory to meet individual
requirements effectively. Additionally, the majority of the
broad-spectrum drugs exhibit adverse effects (18). Delaying the occurrence of fatigue
and hypoxia, and promoting rapid recovery are current foci of
medical investigations (7). The
prevalence of potential alternative medicines derived from herbs
have been increasing worldwide, which can be used not only for
medicinal purposes, but also for food preservation, as dietary
supplements or functional foods, and in cosmetics (17). Herba rhodiolae, a traditional
Chinese herb, is commonly used by the Tibetan population for the
treatment of hypoxia (19), which
also leads to the enhancement of fatigue-associated movements and
levels of key metabolites of glycolysis, including ATP (20). Based on previous evidence, the
present study hypothesized that PHC-enriched extraction may possess
antifatigue and antihypoxic activities. To confirm this hypothesis,
the present study aimed to investigate the associated biological
activities of Paecilomyces hepiali using a mouse model. In
addition, ATP metabolism and antioxidant enzyme activities were
detected in the serum and liver tissues. To further analyze its
underlying mechanism, the phosphorylation of protein kinase B
(AKT), mammalian target of rapamycin (mTOR) and AMPK in liver were
determined via western blot analysis. The present study aimed to
elucidate understanding of the anti-fatigue and anti-hypoxia
effects of P. hepiali
Materials and methods
Strain culture
Paecilomyces hepiali, purchased from Anhui
Agricultural University (Anhui, China; RCEF1429), was cultured in a
100 liter full-automatic fermenter (Biotech-100JS; Baoxing
Bioscience Company, Shanghai, China) at 26°C for 5 days using a
defined liquid medium containing 25 g/l sucrose, 10 g/l peptone, 18
g/l yeast extract powder, 3 g/l KH2PO4, 3 g/l
MgSO4·7H2O, 10 g/l
(NH4)2SO4, 0.01 g/l
ZnCl2 and 0.24 g/l vitamin B1 (all obtained
from Sigma-Aldrich, St. Louis, MO, USA). The mycelia were harvested
by centrifugation at 2,667 × g for 10 min at 4°C, and were
lyophilized for further use in a Genesis SQ 25ES lyophilizer (SP
Industries, Inc., Warminster, PA, USA) (6). All chemical reagents used in the
submerged fermentation were obtained from Sigma-Aldrich.
Crude extract preparation
The aqueous extract from the Paecilomyces
hepiali was extracted at 80°C for 4 h, which was performed
twice. Following centrifugation at 2,667 × g for 10 min at 4°C, the
supernatant was sequentially concentrated in an evaporator (R1002B;
Shanghai SENCO Technology Co., Ltd., Shanghai, China) under reduced
pressure (0.09 mPa at 80°C), and was then freeze-dried to produce
the solid aqueous extract, PHC (21).
Animal care
The experimental animal protocol used in the present
study was approved by the Lab Animal Centre of Jilin University
[Changchun, China; SCXK (JI)-2011-0003] and the present study was
approved by the ethics committee of Jilin University. KunMing (KM)
mice (6-week-old; 18–22 g, 1:1 male: female ratio, n=20/group),
purchased from Norman Bethune University of Medical Science, Jilin
University, were maintained in a 12-h light/dark cycle (lights on
07:00–19:00) at 23±1°C with water and food available ad
libitum. At 8 h prior to initiation of the experiment, the
animals were deprived of food, with free access to water. All
experiments were performed in a quiet room, and each animal (total,
600) was used only once.
Anti-fatigue resistance assessment
The KM mice were randomly divided into five groups
(n=20/group; 1:1 male to female ratio), and orally administered
with double distilled (D.D.) water (vehicle group), 0.6 g/kg
rhodiola capsule (positive group) (22) and PHC at doses of 0.04, 0.2 and 1.0
g/kg once per day for 7 days. At the end of drug administration,
the following experiments were performed.
Autonomic activity assessment
The mice were placed in a multichannel activity box
(ZZ-6; Taimeng Science Technology, Ltd., Chengdu, China) and
locomotor activities were measured for 5 min. The use of an
infrared sensor with multiple Fresnel lenses (component of ZZ-6)
enabled vertical movements, including jumping, as well as
horizontal movements, including walking and running, to be counted.
Measurements were performed between 12:00 and 16:00 (23).
Forced running assessment
The endurance of the mice was assessed on a
treadmill (FT-200; Taimeng Science Technology, Ltd.), which allowed
them to run at a set speed of 20 mph for 1 min. Following three
training exercises, the mice were placed on the treadmill at the 20
mph speed. The number of shocks received from an electrode, touched
when the mice cannot run at the set speed, in a 5 min period was
used to evaluate running performance (23).
Rotating rod assessment
A fatigue turning device (ZB-200; Taimeng Science
Technology, Ltd.) was used to determine mouse performance following
PHC administration for 7 days. Prior to formal assessment, the mice
were allowed three training exercises, in which a speed of 20 rpm
was applied for 1 min. For subsequent fatigue analysis, the mice
were placed on the turning device at a speed of 20 rpm, and the
total duration for which the mouse remained on the rod was
recorded.
Weight-loaded forced swimming
assessment
Following the 7 day PHC administration, a
weight-loaded forced swimming assessment was performed to evaluate
the endurance and performance of each mouse, using a method
described previously, with minor modifications (24). The mice were monitored swimming in
water when loaded with a weight equivalent to 10% of their body
weight. The temperature and depth of the water were 22±1°C and 30
cm, respectively. Exhaustion duration was determined from the
beginning of swimming to the point at which the mice failed to
return to the water surface within 15 sec (12).
Antihypoxic capacity assessment
As with the assessment of antifatigue, the KM mice
were randomly divided into five groups (n=20/group; 1:1 male:
female ratio), and orally administered with either D.D. water
(vehicle group), 0.6 g/kg rhodiola capsule (positive group)
(22) or PHC at doses of 0.04 0.2
and 1.0 g/kg once a day for 7 days.
Normobaric hypoxia assessment
At 60 min following the final administration, each
mouse was placed into a 250 ml airtight container containing
medical soda lime (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China). The duration of survival in oxygen deprivation was
recorded.
Sodium nitrite toxicosis assessment
At 60 min following the final administration, each
mouse was injected with 240 mg/kg sodium nitrite (Sinopharm
Chemical Reagent Co., Ltd.) intraperitoneally, and the duration of
survival was recorded.
Acute cerebral ischemia assessment
At 60 min following the final administration, each
mouse was sacrificed immediately by decapitation. The duration of
time between decapitation and the final gasp was recorded.
Sample collection
Following overnight fasting, the mice (n=20/group;
1:1 male: female ratio) were orally administered with either D.D.
water as the vehicle group, 0.6 g/kg rhodiola capsule as the
positive group or PHC, at doses of 0.04, 0.2 and 1.0 g/kg, once a
day for 7 days. At 60 min following the final treatment, 10 mice in
each group were forced to swim for 60 min and recess for 10 min,
following which 0.2 ml blood samples were collected from the caudal
vein of the mice. At the end of the experiment, the mice were
sacrificed by injection of 200 mg/kg pentobarbital (Beijing
Chemical Reagent Company, Beijing, China) and liver tissues were
dissected, washed with ice-cold physiological saline, and
homogenized in D.D. water.
Parameter determination
The levels of ATP, superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px) in the serum and liver tissues were
determined according to the manufacturer's protocol of the
associated assay kits, Superoxide Dismutase assay kit (WST-1
method) and the Glutathione Peroxidase assay kit (colorimetric
method; Nanjing Jiangcheng Bioengineering Institute, Nanjing,
China).
Western blot analysis
The liver tissue samples were homogenized using 5–10
volumes of lysis buffer containing 1 mM phenylmethanesulfonyl
fluoride (Sigma-Aldrich) and 1X protease inhibitor cocktail
(Sigma-Aldrich). The homogenate was centrifuged at 9,588 × g for 10
min at 4°C, and the resulting supernatants were used as the whole
protein extract. The total protein was estimated using a
Bicinchoninic Acid Assay kit (Nanjing Biotechnology Co., Ltd.), and
40 µg protein was separated by 10% SDS-PAGE [30% acrylamide
(Solarbio Science and Technology Co., Ltd., Beijing, China), SDS
(Sinopharm Chemical Reagent Co., Ltd.), ammonium persulfate
(Sinopharm Chemical Reagent Co., Ltd.), tetramethylethylenediamine
(Beijing Dingguo Changsheng Biotechnology Co., Ltd, Beijing, China)
and buffer solution] and transferred onto a nitrocellulose membrane
(0.45 µm; Bio Basic, Inc, Markham, ON, Canada) using an
electroblotting apparatus (PowerPac™ power supply and
Mini-PROTEAN® Tetra Cell; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 100 V for 120 min. The transferred membranes
were then blotted with the following primary antibodies at 4°C
overnight, at dilutions of 1:1,000: Rabbit anti-mouse monoclonal
phosphorylated (p)-mTOR (Abcam, Cambridge, MA, USA; cat. no
ab109268); rabbit anti-mouse polyclonal total (t)-mTOR (Abcam; cat.
no. ab83495); mouse anti-mouse monoclonal p-AKT (EMD Millipore,
Billerica, MA, USA; cat. no. 05-1003); rabbit anti-mouse polyclonal
t-AKT (Abcam; cat. no. ab126811); rabbit anti-mouse polyclonal
p-AMPK (EMD Millipore; cat. no. 07-681); rabbit anti-mouse
polyclonal t-AMPK (EMD Millipore; cat. no. 07-181); and rabbit
anti-mouse polyclonal glyceraldehyde-3-phosphate dehydrogenase (EMD
Millipore; cat. no. ABS16). The membranes were subsequently
incubated with horseradish peroxidase-conjugated mouse anti-rabbit
IgG (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no.
sc-2357; 1:2,000) and goat anti-mouse IgG (Santa Cruz
Biotechnology, Inc.; cat. no. sc-2005; 1:2,000) secondary
antibodies at 4°C for 4 h. Chemiluminescence was detected using an
ECL detection kit (GE Healthcare Life Sciences, Chalfont, UK). The
intensity of the bands was quantified by scanning densitometry
using Quantity One-4.5.0 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All values are expressed as the mean ± standard
deviation. One-way analysis of variance was use to detect
statistical significance, followed by post-hoc multiple comparison
using Dunn's test. Statistical analysis was conducted using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
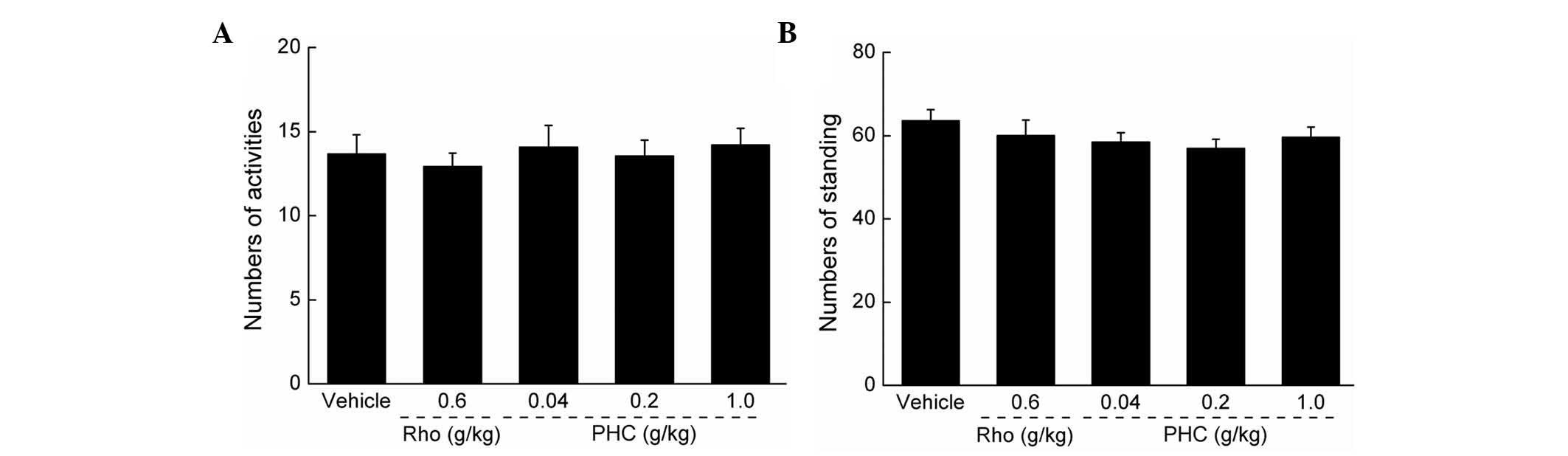
Effects of PHC on autonomic activity
No significant effects on mouse autonomic activity
were observed following PHC treatment, indicating that PHC was a
safe agent for use in the subsequent experiments (P>0.05;
Fig. 1).
Antifatigue activities of PHC
The antifatigue activities of PHC were detected via
forced swimming, forced running and rotating rod assessments.
Similar to previous findings in Herba rhodiolae (19), PHC treatment significantly enhanced
swimming duration, with a maximum recording of 8.26 min, compared
with the duration of 3.01 min in the control group (P<0.001;
Fig. 2A). In the forced running
assessment, the number of shocks were significantly reduced
following the administration of 0.2 and 1 g/kg PHC for 7 days,
compared with the control (P<0.01; Fig. 2B). The duration for which the mice
remained on the rotating rod were recorded to evaluate the
antifatigue activities of PHC. Compared with the mice in the
control group, 0.2 and 1 g/kg PHC treatment enhanced the duration
remaining on the rod by almost 18.91 and 66.17%, respectively
(P<0.05; Fig. 2C).
Antihypoxic activities of PHC
In the sodium nitrite toxicosis assessment, 1 g/kg
PHC administration extended the survival duration by 16.5%,
compared with the vehicle-treated mice, and by almost 10.3%,
compared with the rhodiola capsule (P<0.01; Fig. 3A). In the normobaric hypoxia
assessment, as with Herba rhodiolae, PHC dose-dependently increased
survival duration in the mice exposed to hypoxia (P<0.05;
Fig. 3B). Compared with the
control group, treatment with 1 g/kg PHC enhanced survival duration
by almost 59.07% (P<0.001). Additionally, in the acute cerebral
ischemia assessment, 1 g/kg PHC and 0.6 g/kg rhodiola capsule
improved survival duration by 89.64 and 78.60%, compared with the
vehicle-treated and 0.6 g/kg rhodiola capsule-treated groups
(P<0.05; Fig. 3C).
PHC increases the levels of ATP, SOD and
GSH-Px in the serum and liver
Following treatment for 7 days, prior to swimming, 1
g/kg PHC led to an increase of 99.28% in serum ATP concentration,
compared with the control group (P<0.05; Fig. 4A). A similar trend of PHC was
observed following 60 min swimming (Fig. 4A). In the liver, the ATP
concentration was significantly higher, compared with that prior to
swimming. Treatment with 1 g/kg PHC resulted in 47.45 and 67.48%
increases prior to and following swimming, respectively (P<0.05;
Fig. 4B).
Treatment with 1 g/kg PHC increased serum SOD levels
by 37.58% following swimming, compared with the vehicle-treated
mice (P<0.05; Fig. 5A).
Additionally, 7-day treatment with 1 g/kg PHC enhanced the levels
of SOD in the liver by 17.13 and 21.54% prior to and following
swimming, respectively (P<0.05; Fig. 5B).
 | Figure 5PHC increases levels of SOD and
GSH-Px. Mice were treated with PHC (0.04, 0.2 and 1 g/kg) or
rhodiola capsule (0.6 g/kg) for 7 days, prior to and following
60-min swimming, the activities of SOD activities in the (A) serum
and (B) liver, and levels of GSH-Px in the (C) serum and (D) liver
were determined, respectively. Data are expressed as the mean ±
standard deviation (n=10) and analyzed using one-way analysis of
variance followed by Dunn's test. *P<0.05,
**P<0.01, and ***P<0.001 vs.
vehicle-treated mice. PHC, Paecilomyces hepiali extract;
Rho, rhodiola capsule; SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase. |
On determining the levels of GSH-Px prior to and
following swimming, the same trend was noted in the serum and liver
tissues. In the serum, 1 g/kg PHC treatment resulted in increases
of 43.34 and 32.94% prior to and following swimming, respectively
(P<0.05; Fig. 5C). In the
liver, increases in 35.97 and 23.08% prior to and following
swimming were observed in the 1 g/kg PHC-treated mice, respectively
(P<0.05; Fig. 5D).
Effects of PHC on the activation of
p-AKT, p-AMPK and p-Mtor
The activation of AKT, AMPK and mTOR were further
analyzed in the liver tissues to investigate the underlying
mechanism. In the rhodiola capsule-treated group, no significant
effects on the expression levels of p-AKT, p-AMPK or p-mTOR were
observed (Fig. 6). Treatment for 7
days with 1 g/kg PHC enhanced the expression levels of p-AKT,
p-AMPK and p-mTOR in the liver by 31.37, 35.91 and 16.94%, compared
with the vehicle-treated mice (P<0.05; Fig. 6).
 | Figure 6Mice were treated with PHC (0.04, 0.2
and 1 g/kg) or rhodiola capsule (0.6 g/kg) for 7 days, following
60-min swimming, and the activation of AKT, AMPK and mTOR in the
liver were analyzed using Western blot analysis. Quantification of
the expression levels of p-AKT, p-AMPK and p-mTOR were normalized
by corresponding levels of t-AKT, t-AMPK and t-mTOR. Data are
expressed as the mean ± standard deviation (n=10) and analyzed
using one-way analysis of variance followed by Dunn's test.
*P<0.05, vs. vehicle-treated mice. PHC,
Paecilomyces hepiali extract; Rho, rhodiola capsule;
p-phosphorylated; t-, total; AKT, protein kinase B; AMPK,
5′-monophosphate-activated protein kinase; mTOR, mammalian target
of rapamycin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Discussion
Overexercise and acute mountain sickness, which
leads to the production of increased oxygen radicals, lead to
irreversible tissue damage (25).
In clinical trials, various herbs have been used to alleviate the
symptoms of fatigue and hypoxia (10,22,26).
The aim of the present study was to investigate the antifatigue and
antihypoxic effects of PHC and examine the underlying mechanisms.
Preliminary determination showed that PHC contained 27.22%
polysaccharides, 20.6% total proteins, 43.06% organic acid and
1.56% adenosine.
The physiological effect of fatigue can be
attributable to energy metabolism, metabolite accumulation, and
muscle glycogen depletion, which are also associated with hypoxia
(27). The enhancement of ATP
levels in the serum and liver following 7-day administration may
contribute, in part, to PHC-mediated fatigue recovery.
Additionally, PHC treatment increased the levels of SOD and GSH-Px
in the serum and liver, prior to and following exercise for 60 min.
SOD catalyzes the conversion of superoxide into hydrogen peroxide
and oxygen; whereas GSH-Px scavenges hydroxyl radicals (28). As reported previously, antioxidant
enzymes are important in preventing oxidative injury in an in
vivo mice model (29). Our
previous experiments confirmed that Cordyceps militaris
polysaccharides upregulated the levels of SOD and GSH-Px in
diabetic rats (5). Cordyceps
sinensis scavenges ROS, superoxide anions and hydroxyl radicals
by inhibiting malondialdehyde formation (30). In high intensity or exhaustive
exercise, the overproduction of ROS is observed (31). Supporting endogenous antioxidant
systems with additional oral antioxidants has been demonstrated to
prevent or reduce oxidative stress, decrease muscle damage and
improve exercise performance (32). The Ucp2 gene has been shown to have
an antifatigue effect, efficiently improving endurance in sedentary
mice, which subsequently increases the expression of antioxidant
enzymes and reduces ROS levels (33). The activation of SOD and GSH leads
to TiO2 removing ROS, improving the survival of B.
mori larvae under phoxim-induced toxicity (34), alleviates fatigue (35) and enhances antihypoxic effects
(36). Taken together, the
regulation of oxidation-associated factors may be responsible for
PHC-mediated antifatigue and antihypoxic effects.
In the present study, PHC was also found to improve
the activities of AMPK, AKT and mTOR in the mouse liver tissues
following 60-min swimming. AKT phosphorylation is generally
considered to enhance the activity of mTOR, which further sense
cellular nutrients, oxygen and energy levels (37). AMPK is known to be important in
energy homeostasis, and is considered a major switch, regulating
glucose and lipid metabolism (38). In abnormal conditions, including
starvation, hypoxia and oxidative stress, activated AMPK promotes
cell survival (39). In the liver,
AMPK switches on ATP-producing processes and inhibits ATP-consuming
anabolic processes (40), and it
has been reported that treatment with the AMPK agonist,
5-aminoimidazole carboxamide ribonucleotide, can induce the
expression levels of metabolic genes and enhance running endurance
(41). Once activated by falling
cellular energy status, AMPK activates catabolic pathways, which
generate ATP whilst inhibiting anabolic pathways and other cellular
processes that consume ATP (42).
In the PHC-treated mice in the present study, the enhanced ATP
concentration in the serum and liver following 60-min swimming may
have combined with AMPK phosphorylation. Furthermore, as an axis of
energy metabolism, AMPK activation counteracts oxidative stress by
inhibiting NAD(P)H oxidase-derived ROS accumulation (43). Via activation of the AMPK-sterol
regulatory element-binding protein signaling pathway, the levels of
SOD and GSH-Px in the liver are enhanced (44). The results of the present study
suggested that the antifatigue and antihypoxic effects of PHC
treatment were predominantly through modulation of the AMPK
pathway.
A limitation of the present study was that the data
did not permit investigation of the association between the AMPK
and AKT/mTOR pathways. In previous investigations performed in in
cancer cells or brain tissue, an increase in p-AKT and a decrease
in p-AMPK has been demonstrated to lead to the increased
phosphorylation of mTOR (45).
However, PHC treatment enhanced the phosphorylation of AMPK and
mTOR in the liver tissue. Further investigations are required to
elucidate the underlying mechanism in more details.
In conclusion, the present study demonstrated that
PHC induced recovery from fatigue and hypoxia in mice, at least
partially via the activation of the AMPK and AKT/mTOR pathways. PHC
treatment resulted in increases in the levels of ATP, SOD and
GSH-Px in the serum and liver tissues. These data provide
experimental evidence supporting the clinical use of PHC as an
effective agent against fatigue and hypoxia.
Acknowledgments
This study was supported by the National Science and
Technology Support Program of P.R. China (grant no. 2012BAL29B05),
the National Natural Science Foundation of P.R. China (grant no.
81402955), and the Science and Technology Key Project in Jilin
Province (grant no. 20130201006ZY).
References
|
1
|
Chen PX, Wang SN, Nie SP and Marcone M:
Properties of Cordyceps sinensis: A review. J Funct Foods.
5:550–569. 2013. View Article : Google Scholar
|
|
2
|
Wu Z, Yang Z, Gan D, Fan J, Dai Z, Wang X,
Hu B, Ye H, Abid M and Zeng X: Influences of carbon sources on the
biomass, production and compositions of exopolysaccharides from
Paecilomyces hepiali HN1. Biomass Bioenergy. 67:260–269. 2014.
View Article : Google Scholar
|
|
3
|
Yu SJ, Zhang Y, Li CR, Zhang Q, Ma ZY and
Fan MZ: Optimization of ultrasonic extraction of mycelial
polysaccharides from Paecilomyces hepiali using response surface
methodology and its antioxidant activity. African Journal of
Biotechnology. 10:17241–17250. 2011.
|
|
4
|
Thakur A, Hui R, Hongyan Z, Tian Y,
Tianjun C and Mingwei C: Pro-apoptotic effects of Paecilomyces
hepiali, a Cordyceps sinensis extract on human lung adenocarcinoma
A549 cells in vitro. J Cancer Res Ther. 7:421–426. 2011. View Article : Google Scholar
|
|
5
|
Dong Y, Jing T, Meng Q, Liu C, Hu S, Ma Y,
Liu Y, Lu J, Cheng Y, Wang D and Teng L: Studies on the
antidiabetic activities of Cordyceps militaris extract in
diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed
Res Int. 2014:1609802014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong Y, Hu S, Liu C, Meng Q, Song J, Lu J,
Cheng Y, Gao C, Liu Y, Wang D and Teng L: Purification of
polysaccharides from Cordyceps militaris and their anti-hypoxic
effect. Mol Med Rep. 11:1312–1317. 2015.
|
|
7
|
Zhang W, Wu SZ, Cao JL, Li HM, Li Y, He JG
and Zhang LB: A preliminary study on anti-hypoxia activity of yak
milk powder in vivo. Dairy Science & Technology. 94:633–639.
2014. View Article : Google Scholar
|
|
8
|
You L, Ren J, Yang B, Regenstein J and
Zhao M: Antifatigue activities of loach protein hydrolysates with
different antioxidant activities. J Agric Food Chem.
60:12324–12331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu C, Lv J, Lo YM, Cui SW, Hu X and Fan M:
Effects of oat β-glucan on endurance exercise and its anti-fatigue
properties in trained rats. Carbohydr Polym. 92:1159–1165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie Y, Jiang S, Su D, Pi N, Ma C and Gao
P: Composition analysis and anti-hypoxia activity of polysaccharide
from Brassica rapa L. Int J Biol Macromol. 47:528–533. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou D, Chen K, Liu P, Chang H, Zhu J and
Mi M: Dihydromyricetin improves physical performance under
simulated high altitude. Med Sci Sports Exerc. 46:2077–2084. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu RM, Sun YY, Zhou TT, Zhu ZY, Zhuang JJ,
Tang X, Chen J, Hu LH and Shen X: Arctigenin enhances swimming
endurance of sedentary rats partially by regulation of antioxidant
pathways. Acta Pharmacol Sin. 35:1274–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bijland S, Mancini SJ and Salt IP: Role of
AMP-activated protein kinase in adipose tissue metabolism and
inflammation. Clin Sci (Lond). 124:491–507. 2013. View Article : Google Scholar
|
|
14
|
Dekany M, Nemeskéri V, Györe I, Harbula I,
Malomsoki J and Pucsok J: Antioxidant status of interval-trained
athletes in various sports. Int J Sports Med. 27:112–116. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bogdanis GC, Stavrinou P, Fatouros IG,
Philippou A, Chatzinikolaou A, Draganidis D, Ermidis G and Maridaki
M: Short-term high-intensity interval exercise training attenuates
oxidative stress responses and improves antioxidant status in
healthy humans. Food Chem Toxicol. 61:171–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandareesh MD and Anand T: Ergogenic
effect of dietary L-carnitine and fat supplementation against
exercise induced physical fatigue in Wistar rats. J Physiol
Biochem. 69:799–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao H, Long Y, Jiang X, Liu Z, Wang D,
Zhao Y, Li D and Sun BL: Beneficial effects of Yerba Mate tea (Ilex
paraguariensis) on hyperlipidemia in high-fat-fed hamsters. Exp
Gerontol. 48:572–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ni W, Gao T, Wang H, Du Y, Li J, Li C, Wei
L and Bi H: Anti-fatigue activity of polysaccharides from the
fruits of four Tibetan plateau indigenous medicinal plants. J
Ethnopharmacol. 150:529–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Zhu W, Guan S, Feng R, Zhang H, Liu
Q, Sun P, Lin D, Zhang N and Shen J: Metabolomic analysis of
anti-hypoxia and anti-anxiety effects of Fu Fang Jin Jing Oral
Liquid. PLoS One. 8:e782812013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panossian A and Wagner H: Stimulating
effect of adaptogens: An overview with particular reference to
their efficacy following single dose administration. Phytother Res.
19:819–838. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du LN, Song J, Wang HB, Li P, Yang ZZ,
Meng LJ, Meng FQ, Lu JH and Teng LR: Optimization of the
fermentation medium for Paecilomyces tenuipes N45 using statistical
approach. African Journal of Microbiology Research. 6:6130–6141.
2012. View Article : Google Scholar
|
|
22
|
Zhang CX and Dai ZR: Anti-hypoxia activity
of a polysaccharide extracted from the Sipunculus nudus L. Int J
Biol Macromol. 49:523–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagawasai O, Yamada K, Nemoto W,
Fukahori M, Tadano T and Tan-No K: Liver hydrolysate assists in the
recovery from physical fatigue in a mouse model. J Pharmacol Sci.
123:328–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nallamuthu I, Tamatam A and Khanum F:
Effect of hydroalcoholic extract of Aegle marmelos fruit on radical
scavenging activity and exercise-endurance capacity in mice. Pharm
Biol. 52:551–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu C, Chen R, Wang XS, Shen B, Yue W and
Wu Q: Antioxidant and anti-fatigue activities of phenolic extract
from the seed coat of Euryale ferox Salisb. and identification of
three phenolic compounds by LC-ESI-MS/MS. Molecules.
18:11003–11021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai Y, Lu Y, Chen R, Wei Q and Lu X:
Anti-hypoxia activity and related components of Rhodobryum
giganteum par. Phytomedicine. 18:224–229. 2011. View Article : Google Scholar
|
|
27
|
Bowtell JL, Cooke K, Turner R, Mileva KN
and Sumners DP: Acute physiological and performance responses to
repeated sprints in varying degrees of hypoxia. J Sci Med Sport.
17:399–403. 2014. View Article : Google Scholar
|
|
28
|
Borges P, Oliveira B, Casal S, Dias J,
Conceição L and Valente L: Dietary lipid level affects growth
performance and nutrient utilisation of Senegalese sole (Solea
senegalensis) juveniles. Br J Nutr. 102:1007–1014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Virmani A, Gaetani F, Imam S, Binienda Z
and Ali S: The protective role of L-carnitine against neurotoxicity
evoked by drug of abuse, methamphetamine, could be related to
mitochondrial dysfunction. Ann NY Acad Sci. 965:225–232. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, E Q, Zuo J, Tao Y and Liu W:
Protective effect of Cordyceps polysaccharide on hydrogen
peroxide-induced mitochondrial dysfunction in HL-7702 cells. Mol
Med Rep. 7:747–754. 2013.
|
|
31
|
Sureda A, Ferrer MD, Tauler P, Romaguera
D, Drobnic F, Pujol P, Tur JA and Pons A: Effects of exercise
intensity on lymphocyte H2O2 production and antioxidant defences in
soccer players. Br J Sports Med. 43:186–190. 2009. View Article : Google Scholar
|
|
32
|
Peternelj TT and Coombes JS: Antioxidant
supplementation during exercise training: Beneficial or
detrimental? Sports Med. 41:1043–1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lortz S, Gurgul-Convey E, Naujok O and
Lenzen S: Overexpression of the antioxidant enzyme catalase does
not interfere with the glucose responsiveness of insulin-secreting
INS-1E cells and rat islets. Diabetologia. 56:774–782. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su J, Li B, Cheng S, Zhu Z, Sang X, Gui S,
Xie Y, Sun Q, Cheng Z, Cheng J, et al: Phoxim-induced damages of
Bombyx mori larval midgut and titanium dioxide nanoparticles
protective role under phoxim-induced toxicity. Environ Toxicol.
29:1355–1366. 2014. View Article : Google Scholar
|
|
35
|
Wang X, Xing R, Chen Z, Yu H, Li R and Li
P: Effect and mechanism of mackerel (Pneumatophorus japonicus)
peptides for anti-fatigue. Food Funct. 5:2113–2119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou TB, Ou C, Rong L and Drummen GP:
Effect of all-trans retinoic acid treatment on prohibitin and
renin-angiotensin-aldosterone system expression in hypoxia-induced
renal tubular epithelial cell injury. J Renin Angiotensin
Aldosterone Syst. 15:243–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokunaga C, Yoshino K and Yonezawa K: mTOR
integrates amino acid- and energy-sensing pathways. Biochem Biophys
Res Commun. 313:443–446. 2004. View Article : Google Scholar
|
|
38
|
Ceddia RB: The role of AMP-activated
protein kinase in regulating white adipose tissue metabolism. Mol
Cell Endocrinol. 366:194–203. 2013. View Article : Google Scholar
|
|
39
|
Bonini MG and Gantner BN: The multifaceted
activities of AMPK in tumor progression-why the 'one size fits all'
definition does not fit at all? IUBMB Life. 65:889–896. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grahame Hardie D: AMP-activated protein
kinase: A key regulator of energy balance with many roles in human
disease. J Intern Med. 276:543–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narkar VA, Downes M, Yu RT, Embler E, Wang
YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al:
AMPK and PPARdelta agonists are exercise mimetics. Cell.
134:405–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rios M, Foretz M, Viollet B, Prieto A,
Fraga M, García-Caballero T, Costoya JA and Senaris R: Lipoprotein
internalisation induced by oncogenic AMPK activation is essential
to maintain glioblastoma cell growth. Eur J Cancer. 50:3187–3197.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
St-Pierre J, Drori S, Uldry M, Silvaggi
JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al:
Suppression of reactive oxygen species and neurodegeneration by the
PGC-1 transcriptional coactivators. Cell. 127:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee HI, Yun KW, Seo KI, Kim MJ and Lee MK:
Scopoletin prevents alcohol-induced hepatic lipid accumulation by
modulating the AMPK-SREBP pathway in diet-induced obese mice.
Metabolism. 63:593–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Russo E, Andreozzi F, Iuliano R, Dattilo
V, Procopio T, Fiume G, Mimmi S, Perrotti N, Citraro R, Sesti G, et
al: Early molecular and behavioral response to lipopolysaccharide
in the WAG/Rij rat model of absence epilepsy and depressive-like
behavior, involves interplay between AMPK, AKT/mTOR pathways and
neuroinflammatory cytokine release. Brain Behav Immun. 42:157–168.
2014. View Article : Google Scholar : PubMed/NCBI
|