Introduction
Alcoholic liver disease (ALD), caused by long-term
alcohol consumption, is the broad term used to identify a number of
alcohol-associated health problems, including mild alcoholic liver
injury, alcoholic fatty liver, alcoholic hepatitis, alcoholic
hepatic fibrosis (fatty liver) and alcoholic cirrhosis (1). The pathogenesis of the disease is
complex, multifactorial and remains to be fully elucidated.
Previous studies have found that endoplasmic reticulum (ER) stress,
(ERS) which mediates liver cell apoptosis, is important in several
liver diseases, including those associated with alcohol consumption
(2–4).
The ER is one of the important organelles in the
eukaryotic cell, which is responsible for protein synthesis,
folding, assembly, transportation, lipid synthesis and calcium
storage (5). Furthermore, when any
of these processes are disrupted, it causes an accumulation of
unfolded or misfolded proteins in the ER, and the cells trigger a
stress response in the ER, known as the unfolded protein response
(UPR) (6). This type of ERS is a
self-protective mechanism, and appropriate levels are necessary to
maintain ER and cellular homeostasis (7). However, severe or prolonged ERS can
lead to the activation of lipogenesis, inflammation and apoptosis
(8). A variety of factors can
cause ERS, including viruses, oxidative stress and high levels of
homocysteine (9). Notably, the
mechanism of ERS during acute liver injury remains to be fully
elucidated, rendering the identification of novel pharmacological
treatment options difficult. Therefore, further examination of the
association between ERS and ALD is necessary in order to develop
efficient clinical drugs to treat this disease.
As oxidative stress is one of the predominant causes
of ERS, it is necessary to screen the antioxidants of plant
extracts prior to clinical use. Several medicinal plants contain
antioxidants, which can be used to remove free radicals and protect
cells during oxidative stress. Therefore, these types of plants may
be particularly useful to treat the cellular stress observed in
patients with ALD (10). One
traditional Chinese medicinal plant of interest is Scutellariae
baicalensis Georgi, known as Huang qin in Chinese, which is a
perennial herb belonging to the Lamiaceae family (11). The peeled and dried root of this
plant, known as Scutellariae Radix (SR), has a variety of
therapeutic uses, and 295 compounds have been isolated from this
antioxidant-rich herb to date, including flavonoids, phenylethanoid
glycosides, and diterpenes among others (12). Furthermore, baicalin, which has two
adjacent phenolic hydroxyl structures, is one of the major
flavonoid components found in SR (13). The structure of baicalin indicates
a high level of antioxidant activity, therefore, it may be critical
during free radical scavenging and other activities involved in
protecting cells against oxidative damage (14). In addition, SR extract (SRE) has
been used as a traditional medicine to protect the liver during
acute and chronic liver injury, including that caused by carbon
tetrachloride, iron overload, acetyl ammonia chemicals and sword
bean element A (15–18). However, the biological effects of
SRE during alcohol-associated liver injury remain to be elucidated.
In the present study, the effects of SRE on acute alcohol-induced
liver injury were investigated in mice, and the mechanism
underlying the protective effects of this and similar medicinal
plants during ALD-associated ERS were investigated, providing
theoretical and practical support for the development of novel
protective therapeutic agents for the liver.
Materials and methods
Chemicals
Ethanol, methanol and acetic acid, of high
performance liquid chromatography (HPLC) grade, were purchased from
Nanjing Chemical Reagent Co., Ltd. (Nanjing China). Water was
prepared using redistilled water equipment (Milli-Q Advantage A10;
EMD Millipore, Billerica, MA, USA). All other chemicals used in the
present study were of analytical grade and from Nanjing Chemical
Reagent Co., Ltd. Mouse glutathione (GSH) and malondialdehyde (MDA)
kits were obtained from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). The glucose regulated protein 78 kD (Grp78)
enzyme-linked immunosorbent assay (ELISA) kit was purchased from
Suzhou Calvin Biotechnology Co., Ltd. (Suzhou, China), the
Terminal-deoxynucleoitidyl Transferase Mediated Nick End Labeling
(TUNEL) kit was purchased from Nanjing KeyGEN Biotech Co., Ltd.
(Nanjing China). Tiopronin was provided by He'nan New Yi
Pharmaceutical Co., Ltd. (Xinxiang, China). Tunicamycin (TM) was
obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibody against
GRP78 was purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA; cat. no. sc-13968). Baicalin with a purity of >98% was
used for the quantitative analysis of SRE, which was purchased from
Dalian Meilun Biotech Co., Ltd (Dalian China).
Plant materials
Scutellartiae Radix methanol extract was provided by
the Department of Pharmacy of Bengbu Medical College (Bengbu,
China).
Analysis of SRE
The standard, baicalin, was used for the
quantitative analysis of SRE. Baicalin (9.1 mg) and Scutellariae
Radix extract (10.1 mg), were dissolved in grade methanol with a
constant volume of 10 ml. The HPLC apparatus (Agilent 1200 Series;
Agilent Technologies, Santa Clara, CA, USA) was used for analysis.
The analytical column was a Kromasil C18 column (250×4.6 mm i.d; 5
μm particle size; AkzoNobel, Brewster, NY, USA), and the
column temperature was maintained at 22°C. The column eluent was
monitored at ultraviolet 278 nm. The chromatography was performed
at room temperature with a flow rate of 1.0 ml/min, and a 20
μl volume was analyzed. The mobile phase comprised
methanol:water (containing 2% acetic acid) at a ratio of 50:50.
Animals
Male Kunming mice (n=60; weight, 20±2 g; age, 4–5
weeks) were obtained from the Experimental Animal Center of Anhui
medical University (Hefei, China). The animals were acclimatized
for 1 week in standard conditions, with a temperature of 22°C
[standard deviation (SD)=2], relative humidity of 55% (SD=5%) and a
12/12 h light/dark cycle, with access to food and water ad
libitum, prior to the experiments. All procedures were
performed in strict accordance with the Use and Care of Laboratory
Animals (National Research Council of USA, 1996) (19,20),
and the current study was approved by the associated ethical
regulations of Bengbu Medical College.
Acute alcohol-induced liver injury
The male mice were divided into the following six
groups, each containing 10 mice: Normal control group; model group,
group treated with 30 mg/kg tiopronin as a positive control; group
treated with 40 mg/kg SRE; group treated with 80 mg/kg SRE; and
group treated with 160 mg/kg SRE. The SRE and tiopronin were
prepared by dissolving the extracts in 0.5% sodium carboxymethyl
cellulose (CMC-Na; Shanghai Shenguang Food Chemicals Co., Ltd.,
Shanghai, China). The mice in normal control group and the model
group were provided with equal volumes of the 0.5% CMC-Na solution.
According to previous associated literature with minor adjustments
(21–23), the mice were treated with the
tiopronin (30 mg/kg) or SRE (40, 80 or 160 mg/kg) for 14
consecutive days via intragastric administration. At ~4 h following
each treatment, 50% alcohol (12 ml/kg) was orally administered to
induce acute alcohol liver injury, whereas the mice in the normal
(non-alcohol treated) group were orally administered with equal
volumes of redistilled water.
TM-induced liver injury
TM is a common drug used to induce ERS. In order to
further examine the association between the protective function of
SRE in ERS in the liver, the present study also induced TM-induced
liver injury in mice. Male mice were again divided into six groups
(10 mice in each) in the aforementioned treatment groups. However,
rather than alcohol administration following SRE treatment, the
mice were orally administered with 1 mg/kg TM on the 14th day.
Blood samples (~1 ml) were collected 16 h following the final
treat-ment by eye removal under ether (Nanjing Chemical Reagent
Co., Ltd.) anesthesia, and these samples were used to detect the
following serum biomarkers: Aspartate transaminase (AST), alanine
transferase (ALT) and triglyceride (TG). All animals were
subsequently sacrificed by cervical dislocation, and liver tissue
was isolated for GSH and MDA marker, and histological analysis.
Serum biochemical measurements
The aforementioned blood samples were centrifuged at
1,125 × g for 15 min at room temperature. The levels of AST, ALT
and TG in the serum samples were detected using an automatic
biochemical analyzer (Hitachi 7100; Hitachi, Tokyo, Japan).
Evaluation of hepatic levels of MDA and
GSH
In order to detect the hepatic lipid per oxidation
and antioxidant capacity, accurately weighed liver tissue samples
(0.3 g) were homogenized in nine volumes of physiological saline to
obtain 10% (w/v) homogenates. The homogenates were then centrifuged
at 2,500 × g, for 15 min at 4°C and the supernatants were obtained,
which were used for the assessment of MDA and GSH. Corresponding
kits (Nanjing Jiancheng Bioengineering Institute) were used,
according to the manufacturer's protocols.
Histopathological observation
Small sections of liver tissues were removed from
the edges of the left liver lobe (0.5 cm) and fixed with 10%
formaldehyde (BioSharp, Hefei, China). Following routine
dehydration, transparency and paraffin embedding (Shanghai Yuanye
Bio-Technology Co., Ltd., Shanghai, China), the sections (5
μm thick) were stained with hematoxylin and eosin (H&E;
Nanjing Jiancheng Bioengineering Institute), and the
histomorphology was observed under a light microscope (Olympus
CX21; Olympus Corporation, Tokyo, Japan).
ELISA
The reagents were provided in the Grp78 ELISA kit,
and standard wells and testing sample wells were used. The kits
were used according to the manufacturer's protocols. The standard
(50 μl) was added to the standard well. The mouse serum
testing sample (10 μl) and sample diluent (40 μl)
were added to the testing sample well. A blank well, with no
additions, was included. HRP-conjugate reagent (100 μl) was
then added to each well, covered with an adhesive strip and
incubated for 60 min at 37°C. Each well was aspirated and washed,
which was repeated four times for a total of five washes. Washing
was performed by filling each well with wash solution (400
μl) using a squirt bottle, manifold dispenser or
auto-washer. The complete removal of liquid at each step is
essential for optimal performance. Following the final wash, any
remaining wash solution was removed by aspirating or decanting. The
plate was inverted and blotted against clean paper towels.
Chromogen solution A (50 μl) and chromogen solution B (50
μl) were added to each well, gently mixed and incubated for
15 min at 37°C in the dark. Stop solution (50 μl) was then
added to each well. The color in the wells are expected to change
from blue to yellow. If the color in the wells is green or the
color change does not appear uniform, the plate is tapped gently to
ensure thorough mixing. The optical density (OD) at 450 nm was
measured using a microplate reader (Synergy HT; Bio-Tek
Instruments, Inc. Winooski, VT, USA) within 15 min.
Immunohistochemical assessment
In order to observe the dynamic changes in GRP78
distribution mice with acute liver injury, liver samples were
paraffin-embedded and slices (5 μm thick) were prepared
using a microtome (CM1950; Leica Microsystems GmbH, Wetzlar,
Germany). Following dewaxing and hydration, antigen retrieval was
conducted. The sections were immersed in 0.01 mol/l citrate salt
buffer (pH 7.0; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China). The sections were then heated four times in
a microwave oven for 6 min each time and then soaked in a 3% (v/v)
hydrogen peroxide solution (Nanjing Jiancheng Bioengineering
Institute) for 30 min to block endogenous peroxidase activity.
Following washing of the sections with phosphate-buffered saline
(PBS) three times for 5 min, rabbit serum was added to block any
nonspecific binding sites. Subsequently, the slices were incubated
with monoclonal rabbit anti-mouse GRP78 antibody (1:100) at room
temperature overnight. Following washing with PBS, the slices were
incubated with monoclonal biotinylated goat anti-rabbit IgG (1:50;
BioSharp) for 30 min 37°C, and were visualized using
diaminobenzidine (DAB; Nanjing Jiancheng Bioengineering Institute).
The cell nuclei were then stained using hematoxylin, and the
sections underwent gradient alcohol dehydration and xylene
(BioSharp) transparency prior to mounting. The liver sections were
then observed under a light microscope (Olympus Corporation).
Terminal-deoxynucleotidyl transferase
mediated nick end labeling (TUNEL)
In order to identify apoptotic cells, TUNEL was
performed on the dewaxed, hydrated, transparent liver sections (5
μm thick) using TUNEL reaction mixture (Shanghai Beyotime
Institute of Biotechnology, Shanghai, China) at 37°C for 1 h. The
slices were then immersed in 2X saline sodium citrate (Nanjing
KeyGEN Biotech Co., Ltd.) for 15 min to terminate the reaction.
Endogenous peroxidase activity was also blocked in the slices, and
they were then stained with DAB and hematoxylin. The sections were
then washed with ddH2O for 6 min, rehydrated with a
gradient of ethanol and soaked in xylene. Following mounting, the
sections were observed under a light microscope (Olympus
Corporation).
Statistical analysis
All experiments were repeated at least three times.
Data are expressed as the mean ± SD. The difference of data between
groups was analyzed by the two-tailed Student's t-test and one-way
analysis of variance using SPSS 17.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
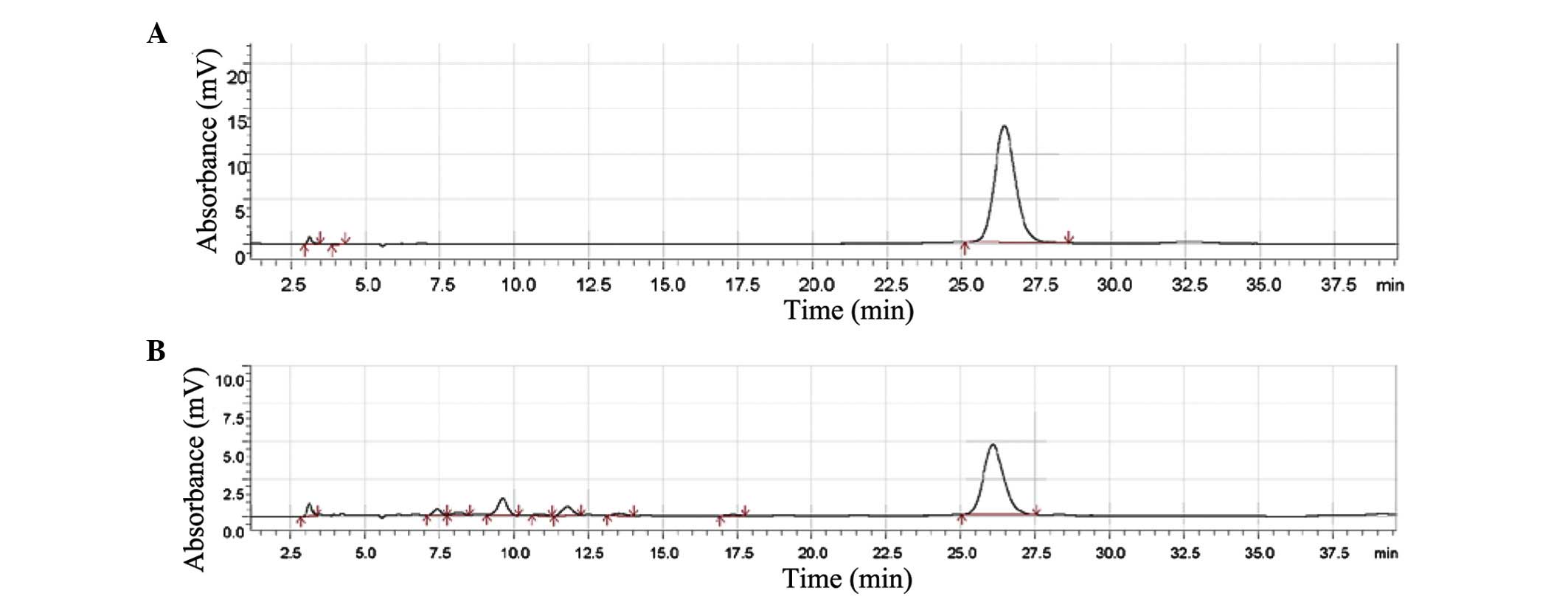
HPLC profiles of SRE
Baicalin is one of the primary active
ingredients of Scutellaria baicalensis Georgi. Baicalin is a
quality control indicator in several traditional Chinese medicine
preparations that contain Scutellaria baicalensis Georgi.
The present study analyzed the primary ingredient of SRE by HPLC,
and the peak area in the chromatogram (Fig. 1) indicated that 100 mg SRE
contained ~31.2 mg baicalin.
ERS and the effects of acute
alcohol-induced liver injury in mice
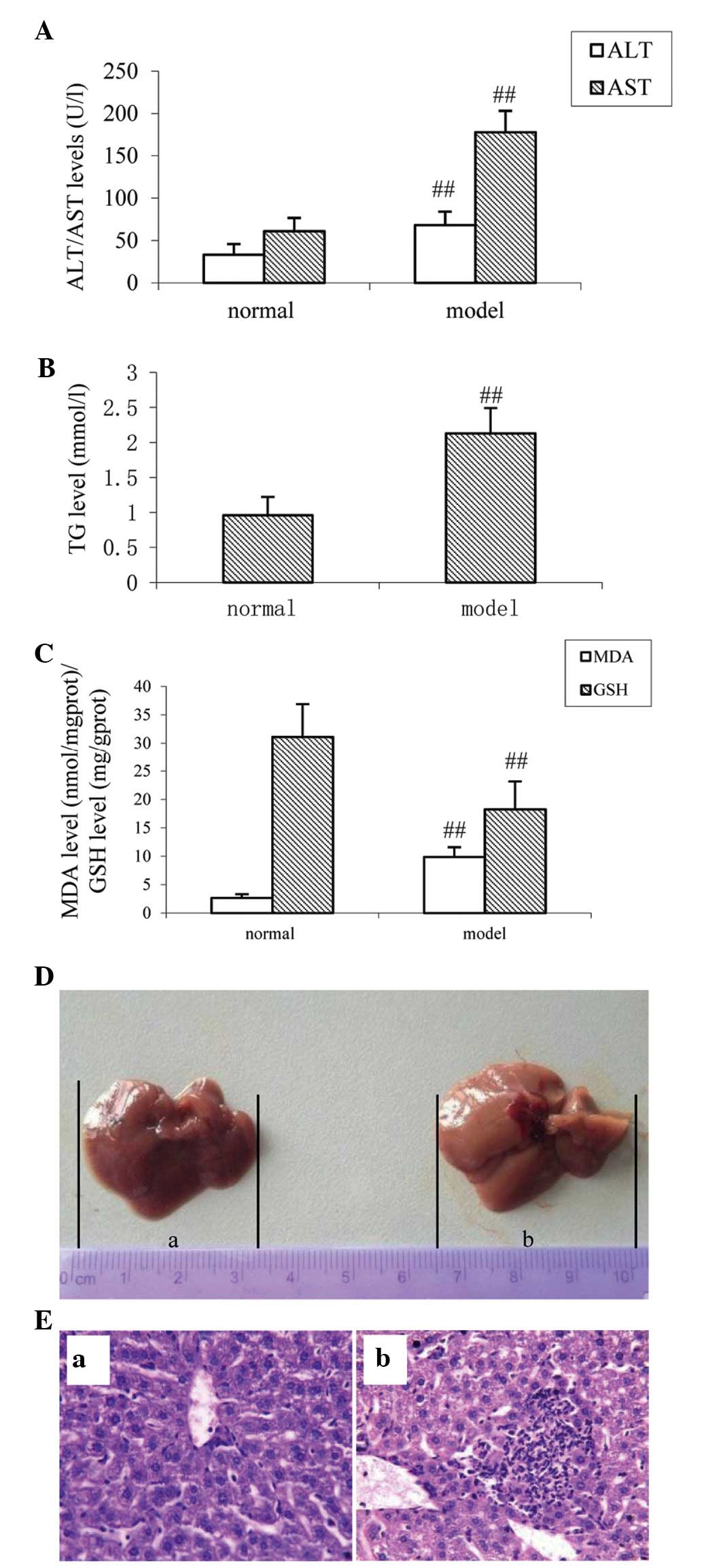
The serum levels of ALT and AST are important
indicators of liver cell damage (24). The accumulation of fat is also a
major pathological change observed in ALD, therefore, TG was used
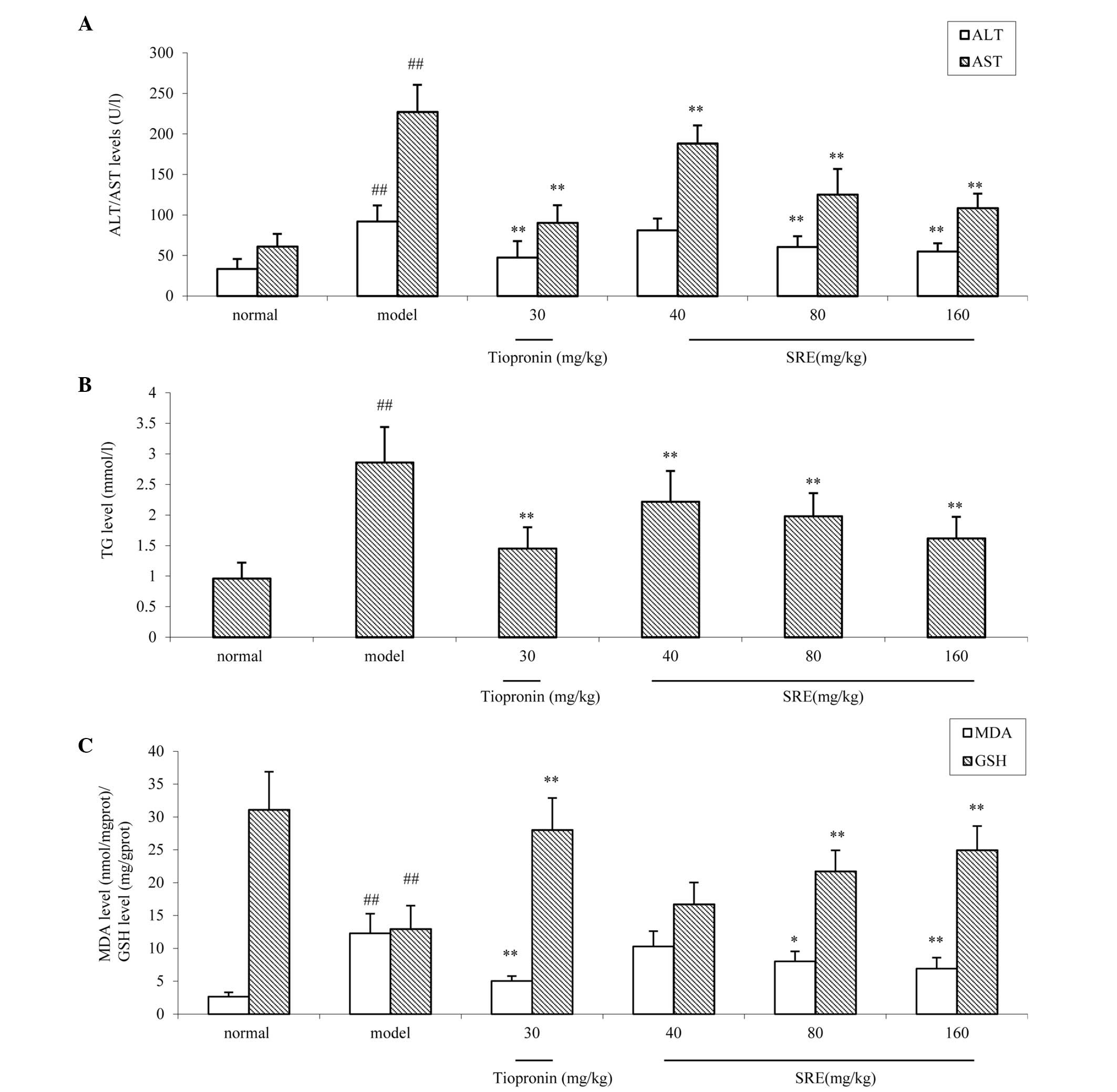
as a significant liver damage marker in the present study (25). As shown in Fig. 2A and B, compared with the normal
group, the levels of serum ALT, AST and TG were increased following
the administration of alcohol for the 14 days (all P<0.01). MDA
is an end product of lipid peroxidation, which has potent
biological toxicity and can seriously damage the structure of the
cell membrane, leading to cell swelling and necrosis (26). The level of MDA directly reflects
the degree of organ oxidative damage. As shown in Fig. 2C, compared with the normal group,
the levels of hepatic MDA were significantly increased in the
alcohol-induced injury group (P<0.01). Furthermore, the levels
of GSH, an endogenous oxygen free radical scavenger (27) were examined. Compared with the
normal group, the levels of GSH were decreased in the
alcohol-induced injury group (P<0.01; Fig. 2C). On observation with the naked
eye, the livers isolated from mice in the control group were bright
red with sharp edges and a glossy surface (Fig. 2D). However, the livers isolated
from the mice in the alcohol-injured group had reduced luster and
elasticity. The liver volume and capsular tension increased
gradually, which were in accordance with the results of the
biochemical analysis described above. Observed under a light
microscope, the liver lobular structures were clear, and
hepatocytes were arranged regularly in the control mice. Apparent
damage was inspected in the alcohol-injured group, which had large
areas of cell necrosis, mass inflammatory cell infiltration and fat
vacuolization in the liver cells (Fig.
2E).
Effects of acute alcohol-induced ERS in
mice
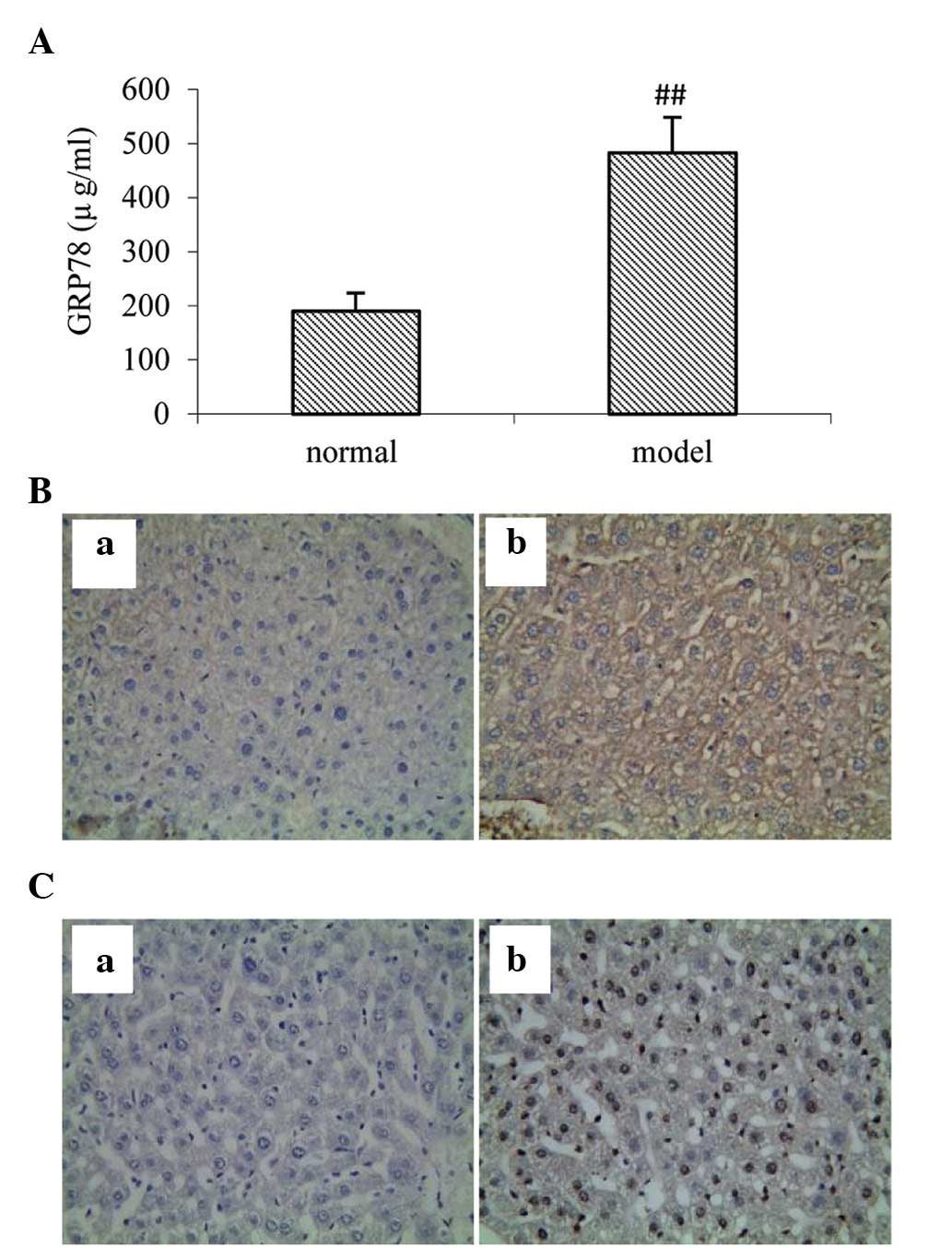
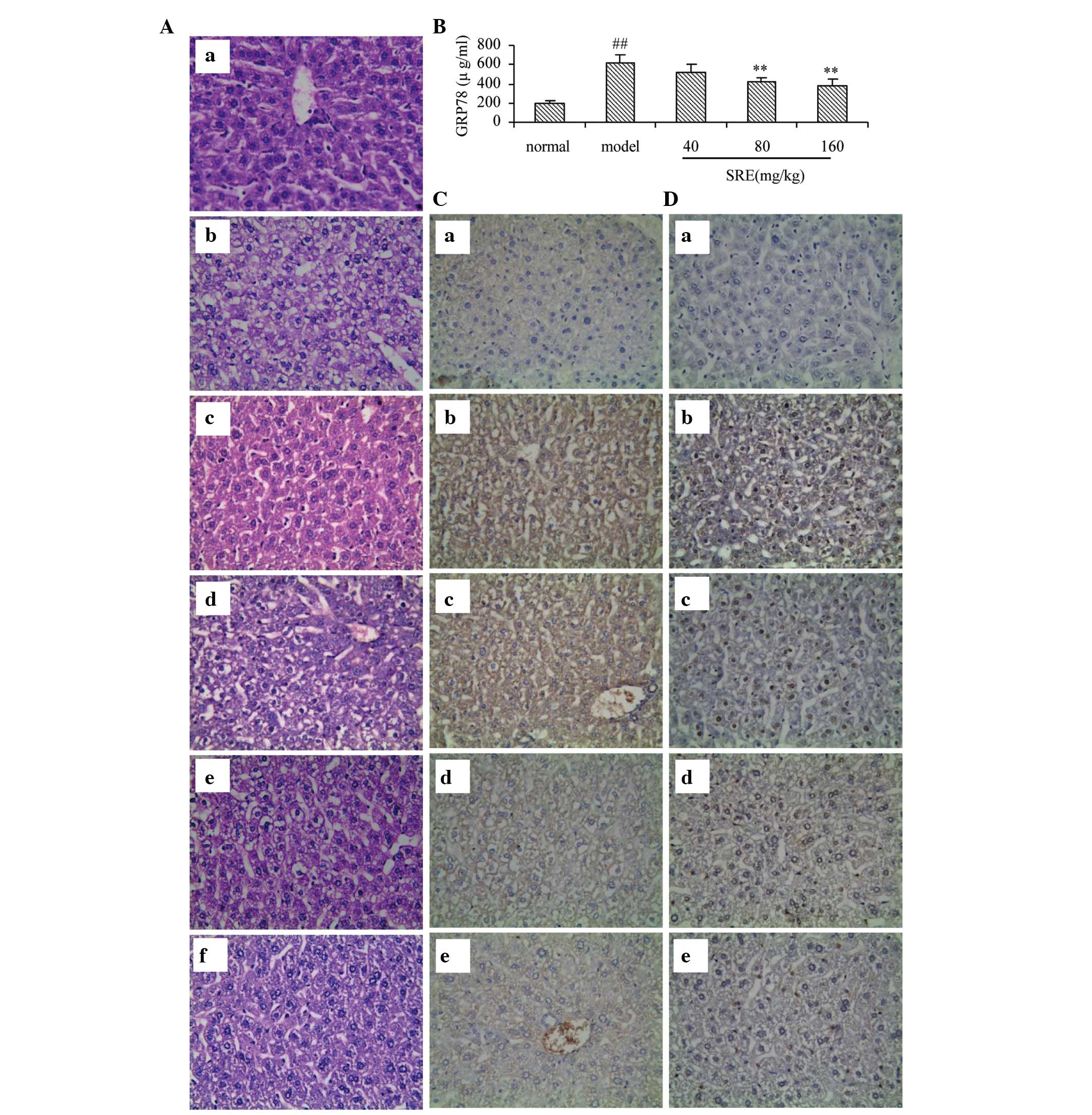
GRP78 is a well-known marker of ERS. To investigate
the effects of acute alcohol-induced ERS in the mice, ELISA and
immunohistochemistry were used for the determination of ERS. A
single dose of alcohol administration significantly increased the
level of serum GRP78 in the ELISA (P<0.01) and in the
hepatocytes, determined by immunohistochemistry (Fig. 3A and B). As shown in Fig. 3C, compared with the normal control
group, the number of TUNEL-positive cells were elevated in the mice
following the administration of alcohol. The above results
suggested that ERS is important in alcohol-induced liver injury in
mice, which mediated liver cell apoptosis.
Effects of SRE pretreatment on acute
alcohol-induced liver injury in mice
As shown in Fig. 4A and
B, compared with the normal group, the levels of serum ALT, AST
and TG were all increased following administration of alcohol, on
the 14th day of treatment. Pretreatment with 80 and 160 mg/kg SRE
significantly inhibited the increases in ALT, AST and TG (P<0.05
and P<0.01, respectively) in a dose-dependent manner. Tiopronin
pretreatment also exerted an analogous effect against acute alcohol
liver injury mice by reducing the elevations of ALT, AST and TG
(P<0.01; Fig. 4A). As shown in
Fig. 4C, compared with the normal
group, the levels of hepatic MDA significantly increased in the
alcohol-injured group (P<0.01). However, the tissue
concentrations of MDA were decreased significantly, in a
dose-dependent manner, following pretreating with 40, 80 or 160
mg/kg SRE (all P<0.01). Furthermore, the levels of GSH, an
endogenous oxygen free radical scavenger were examined. Compared
with the normal group, the levels of GSH were decreased in the
alcohol-injured group (P<0.01). The concentrations of GSH in the
liver were increased following treatment with 80 (P<0.05) and
160 mg/kg SRE (P<0.01) and 30 mg/kg tiopronin (P<0.01). On
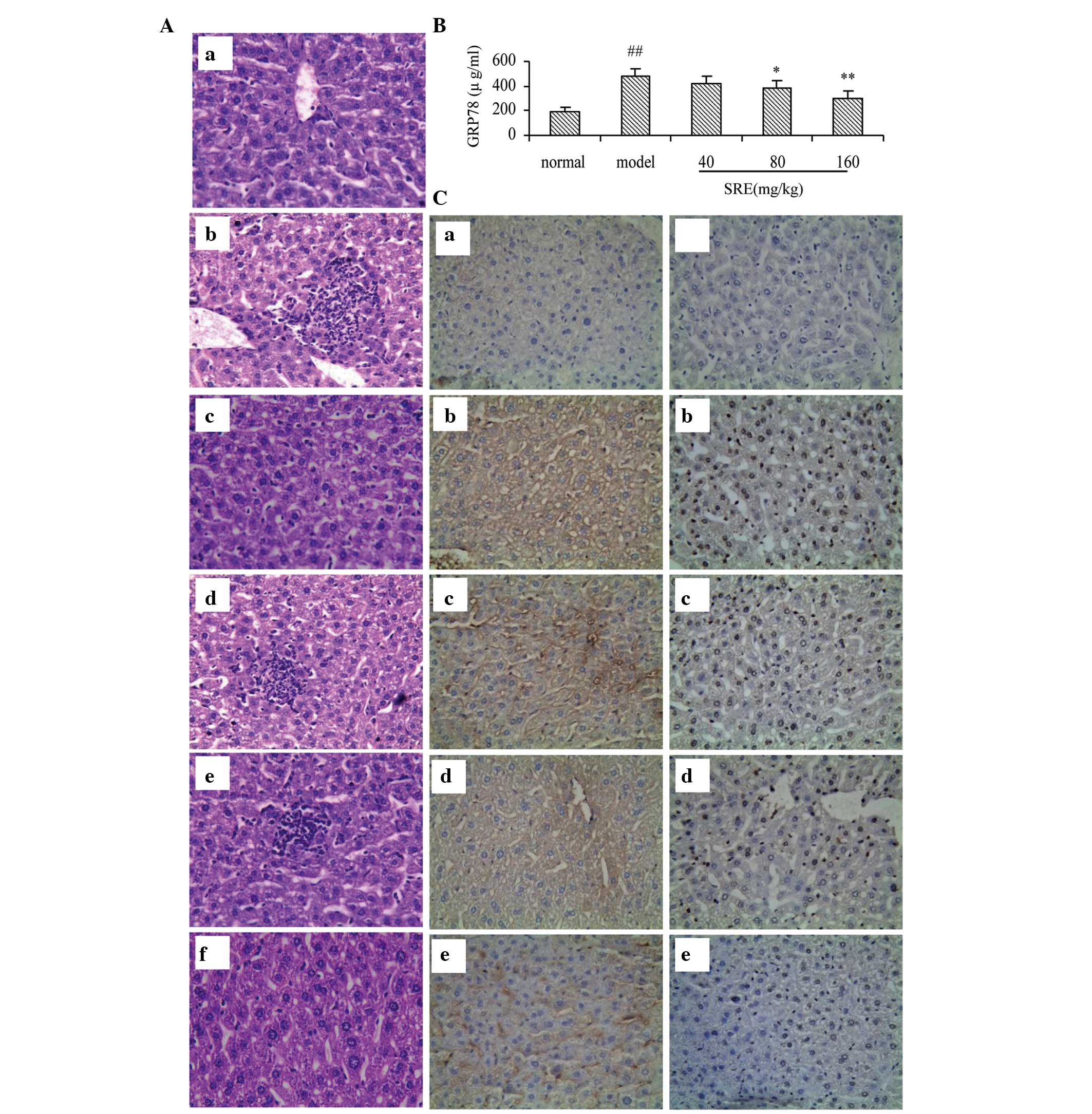
observation under a light microscope, the liver lobular structures
were clear and hepatocytes were arranged regularly in the control
mice. Apparent damage was observed in the alcohol-injured group,
which had large areas of cell necrosis, mass inflam-matory cell
infiltration and fat vacuolization in the liver cells (Fig. 5A). These histopathological changes
were reduced in the SRE-treated mice, in a dose-dependent manner.
Furthermore, the highest dose of SRE improved the hepatic injury to
a level similar to the effect observed in the tiopronin group.
SRE has a protective effect on acute
alcohol-induced liver injury in mice by reducing ERS
The present study detected GRP78 in mice using ELISA
and immunohistochemical examination. The serum levels of GRP78 were
investigated using ELISA (Fig.
5B). Compared with the normal group, GRP78 was elevated
significantly in the mice treated with alcohol alone (P<0.01),
whereas pretreatment with SRE (80 or 160 mg/kg) reduced this
increase significantly (P<0.05 and P<0.01, respectively). For
a comprehensive assessment of the effects of SRE against
alcohol-induced acute liver injury, the present study also observed
trace quantities of GRP78 in the normal control group (Fig. 5C), whereas this expression was
enhanced in the alcohol group. Following pretreatment with SRE (40,
80 or 160 mg/kg), the expression levels of GRP78 decreased in a
dose-dependent manner. These results suggested that SRE protected
against acute alcohol-induced liver injury by downregulating GRP78.
The TUNEL technique was used to observe changes in liver cell
apoptosis in acute alcohol-induced liver injury in mice following
SRE pretreatment. As shown in Fig.
5D, the number of TUNEL-positive cells were elevated in the
mice following the administration of alcohol, whereas SRE
pretreatment markedly inhibited the alcohol-induced increase in
apoptosis, in a dose-dependent manner.
Effects of SRE pretreatment on acute
TM-induced liver injury in mice
To confirm the protective effects of SRE on acute
alcohol-induced liver injury, and further examine the involved
mechanism, the present study also examined the effects of this
extract on acute TM-induced liver injury. The results revealed that
SRE decreased the serum concentrations of ALT, AST and TG (Fig. 6A and B), as well as the tissue
levels of MDA (Fig. 6C), compared
with the untreated TM-induced model group. Histological examination
revealed that pretreatment with SRE also alleviated the central
vein necrosis, hepatocyte lesions and inflammatory infiltrates
observed in the TM-induced mice (Fig.
7A). The protein concentrations of GRP78 (Fig. 7B and C) also increased in the
TM-induced mice, which were notably decreased in the groups
pretreated with SRE. Finally, similar to the alcohol-induced liver
injury, TUNEL staining of the TM-induced samples indicated that the
level of apoptosis was increased (Fig.
7D). However, pretreatment with SRE was again observed to limit
these levels, suggesting that the effects of SRE on TM-induced ERS
mimicked those observed during alcohol-induced liver injury.
Discussion
The incidence of ALD is gradually increasing yearly,
indicating how relatively small changes in lifestyle and diet can
result in serious health and social development issues. However,
the pathogenesis of ALD is complex and remains to be fully
elucidated. The upregulation of ERS-associated signaling pathways
likely indicates that apoptosis is also involved in alcohol-induced
liver injury (28). The ER is an
important net-like apparatus in cells, which functions to fold
proteins, synthesize lipids and release calcium. The disruption of
these physiological ER functions by oxidative stress, nutritional
deficiency, viruses or free radicals, increases the number of
unfolded proteins in the ER lumen, triggering the UPR (29). The UPR is a type of protective
stress reaction of eukaryotic cells. When the number of unfolded
proteins in the ER is too high, a series of pathological reactions,
including fat formation, inflammation and apoptosis, are activated,
which is often termed the ERS response (30). ERS can result in the expression and
activation of sterol regulatory element binding protein and cause
problems in lipid synthesis, leading to the increase of TG
biosynthesis and excess fat deposit in the liver (31). It is currently hypothesized that,
within mammalian cells, GRP78 functions to detect the accumulation
of misfolded/unfolded proteins in the ER lumen, and when these
levels exceed a certain threshold, GRP78 detaches from the
chaperoned proteins and launches a series of complex reactions.
Therefore, GRP78 is a crucial marker of ERS (32). This mechanism allows the cells to
survive and maintain homeostasis. However, when UPR cannot maintain
cell survival, ERS can result in the upregulation of a series of
detrimental signaling pathways, including apoptosis (33). Therefore, in the present study,
GRP78 was selected as an index reflecting ERS. The results of the
present study demonstrated that the levels of serum ALT, AST and TG
were increased in alcohol-induced injury model group. On
observation under a light microscope, there were large areas of
cell necrosis, mass inflammatory cell infiltration and fat
vacuolization in the liver cells of the model mice livers. The
above results suggested the successful construction of an
alcohol-induced liver injury mouse model. Compared with the normal
group, the protein expression levels of GRP78, determined by
immunohistochemical examination and ELISA, were significantly
enhanced in the alcohol-induced injury group. Compared with the
normal control group, the number of TUNEL-positive cells was
elevated in the mice following the administration of alcohol. In
conclusion, ERS, which mediated liver cell apoptosis, was involved
in the physiological and pathological processes of alcohol-induced
liver injury, as one aspect of the pathogenesis.
There are currently a number of drugs available to
treat ALD, each with its own distinct effects. However, to date,
there is no single drug that can halt or reverse this process.
Thus, more attention has been paid to the pathogenesis of ALD, and
emphasis has been placed on screening for a reliable, curative drug
to protect against alcohol-induced liver injury by reducing liver
cell ERS. Evidence also indicates that ALD may cause liver cell
apoptosis by inducing ERS with high levels of homocysteine and
added oxidative stress (34).
Therefore, it is necessary to screen antioxidants from plant
extracts prior to clinical use. SR is a commonly used Chinese
traditional medicine, and reports have also suggested that SR has
antioxidant properties. However, whether SR can protect the liver
from alcohol abuse-associated injury has not been established.
Therefore, in the present study, the hepatoprotective effect and
underlying molecular mechanism of SRE in acute alcohol-induced
liver injury were investigated in a mouse model.
In the present study, a number of markers were used
to measure liver function. ALT and AST are usually localized in the
liver cells, however, during liver injury, these markers leak from
the damaged cells into the blood (35). The decreases in AST and ALT in mice
pretreated with SRE provide an indication of improved liver
function, demonstrating that SRE likely stabilizes the cytomembrane
and prevents the damage of hepatic tissue (36). MDA is the final product of lipid
peroxidation, the levels of which indirectly reflect the degree of
free radical damage. MDA is also an important index of liver cell
recovery following treatment. By contrast, GSH is a scavenger of
low molecular weight components, including free radicals and lipid
peroxide radicals, and is the substrate of GSH peroxidase.
Therefore, GSH is a direct measure of liver antioxidant capacity in
the body (37).
Using these markers, the present study found that
oxidative stress was a primary factor during acute alcohol-induced
liver injury in mice. Furthermore, the protective abilities of SRE
were demonstrated, as shown by the decreasing levels of each of the
serum markers and MDA, and inhibiting the decrease of GSH activity.
In addition, compared with the liver sections from the
alcohol-induced injury group, the sections obtained from the mice
treated with alcohol and SRE had lower levels of liver cell
swelling, inflammation and fat vacuole formation.
In the present study, compared with the normal
group, liver tissue isolated from the alcohol group showed
pathological damage, and protein levels in the liver were elevated,
confirming the involvement of ERS in acute alcohol-induced liver
injury. These changes, and thus overall ERS, were also prevented
following SRE treatment. Therefore the results of the present study
confirmed that the protective effect of SRE against alcohol-induced
liver injury may occur through the maintenance of ER homeostasis
and the protection against ERS mediated hepatocyte apoptosis. In
order to further evaluate the effect of SRE in acute
alcohol-induced apoptosis, TUNEL assays were performed. These
results indicated that treatment with SRE effectively prevented
alcohol-induced DNA damage. In addition, a similar protective
effect to that of SRE by inhibiting ERS was observed in mice with
TM-induced injury.
In conclusion, the present study demonstrated that
SRE exerted a hepatoprotective effect against acute alcohol-induced
liver injury in mice through the downregulation of GRP78 and
inhibition of ERS, resulting in regulated blood lipid levels and
normal levels of apoptosis. However, the complete mechanism of SRE
in the double-edged function of ERS in protecting liver cells
requires additional investigation. The results of the present study
suggest that believe that SRE may be a suitable candidate for the
pharmacological treatment of ALD and warrants further
investigation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81372899), the Natural
Science Foundation of Anhui Province (grant no. 090413135) and the
Key Project of Natural Science Research of the Education Department
of Anhui Province, China (grant no. KJ2012A202).
References
|
1
|
Chen P, Stärkel P, Turner JR, Ho SB and
Schnabl B: Dysbiosis-induced intestinal inflammation activates
tumor necrosis factor receptor I and mediates alcoholic liver
disease in mice. Hepatology. 61:883–894. 2015. View Article : Google Scholar :
|
|
2
|
Galligan JJ, Fritz KS, Backos DS, Shearn
CT, Smathers RL, Jiang H, MacLean KN, Reigan PR and Petersen DR:
Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse
model of alcoholic liver disease: Functional independence of ATPase
activity and chaperone function. Free Radic Biol Med. 73:411–420.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen L, Ren F, Zhang H, et al: Inhibition
of glycogen synthase kinase 3β ameliorates D-GalN/LPS-induced liver
injury by reducing endoplasmic reticulum stress-triggered
apoptosis. PloS one. 7:e452022012. View Article : Google Scholar
|
|
4
|
Fredriksson L, Wink S, Herpers B,
Benedetti G, Hadi M, de Bont H, Groothius G, Luijten M, Danen E, de
Graauw M, et al: Drug-induced endoplasmic reticulum and oxidative
stress responses independently sensitize toward TNFα-mediated
hepatotoxicity. Toxicol Sci. 140:144–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanjore H, Cheng DS, Degryse AL, Zoz DF,
Abdolrasulnia R, Lawson WE and Blackwell TS: Alveolar epithelial
cells undergo epithelial-to-mesenchymal transition in response to
endoplasmic reticulum stress. J Biol Chem. 286:30972–30980. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng Q, Zhou Y, Liu Z, Zhang L, Song G,
Guo Z, Wang W, Qu X, Zhu Y and Yang D: An alternatively spliced
heat shock transcription factor, OsHSFA2dI, functions in the heat
stress-induced unfolded protein response in rice. Plant Biol
(Stuttg). 17:419–429. 2015. View Article : Google Scholar
|
|
7
|
Baek HA, Kim do S, Park HS, Jang KY, Kang
MJ, Lee DG, Moon WS, Chae HJ and Chung MJ: Involvement of
endoplasmic reticulum stress in myofibroblastic differentiation of
lung fibroblasts. Am J Respir Cell Mol Biol. 46:731–739. 2012.
View Article : Google Scholar
|
|
8
|
Tan TC, Crawford DH, Jaskowski LA,
Subramaniam VN, Clouston AD, Crane DI, Bridle KR, Anderson GJ and
Fletcher LM: Excess iron modulates endoplasmic reticulum
stress-associated pathways in a mouse model of alcohol and high-fat
diet-induced liver injury. Lab Invest. 93:1295–1312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao B, Cui LM, Ma DJ, Liu SP and Zhang
XW: Endoplasmic reticulum stress in diethylnitrosamine-induced rat
liver cancer. Oncol Lett. 7:23–27. 2014.
|
|
10
|
Zhu XY, Zhang ZL, Li P, Liang WY, Feng XR
and Liu ML: Shenyuan, an extract of American Ginseng and Corydalis
Tuber formula, attenuates cardiomyocyte apoptosis via inhibition of
endoplasmic reticulum stress and oxidative stress in a porcine
model of acute myocardial infarction. J Ethnopharmacol.
150:672–681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Cao J, Xu S, Gu D, Wang Y and Xiao
S: Depletion of high-abundance flavonoids by metal complexation and
identification of low-abundance flavonoids in Scutellaria
baicalensis. Georgi J Chromatogr A. 1315:107–117. 2013. View Article : Google Scholar
|
|
12
|
Tsai CL, Lin YC, Wang HM and Chou TC:
Baicalein, an active component of Scutellaria baicalensis, protects
against lipopolysaccharide-induced acute lung injury in rats. J
Ethnopharmacol. 153:197–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orzechowska B, Chaber R, Wiśniewska A,
Pajtasz-Piasecka E, Jatczak B, Siemieniec I, Gulanowski B, Chybicka
A and Błach-Olszewska Z: Baicalin from the extract of Scutellaria
baicalensis affects the innate immunity and apoptosis in leukocytes
of children with acute lymphocytic leukemia. Int Immunopharmacol.
23:558–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JK, Kim YS, Kim Y, Uddin MR, Kim YB,
Kim HH, Park SY, Lee MY, Chung SO and Park SU: Comparative analysis
of flavonoids and polar metabolites from hairy roots of Scutellaria
baicalensis and Scutellaria lateriflora. World J Microbiol
Biotechnol. 30:887–892. 2014. View Article : Google Scholar
|
|
15
|
Sun H, Che QM, Zhao X and Pu XP:
Antifibrotic effects of chronic baicalein administration in a CCl4
liver fibrosis model in rats. Eur J Pharmacol. 631:53–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Li H, Gao Z and Xu H: Effects of
dietary baicalin supplementation on iron overload-induced mouse
liver oxidative injury. Eur J Pharmacol. 509:195–200. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang SI, Kim HJ, Hwang KM, Jekal SJ, Pae
HO, Choi BM, Yun YG, Kwon TO, Chung HT and Kim YC: Hepatoprotective
effect of baicalin, a major flavone from Scutellaria radix, on
acetaminophen-induced liver injury in mice. Immunopharmacol
Immunotoxicol. 25:585–594. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu LL, Gong LK, Wang H, Xiao Y, Wu XF,
Zhang YH, Xue X, Qi M and Ren J: Baicalin protects mouse from
concanavalin A-induced liver injury through inhibition of cytokine
production and hepatocyte apoptosis. Liver Int. 27:582–591. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bayne K: Revised guide for the care and
use of laboratory animals available American Physiological Society.
Physiologist. 39:119208–111. 1996.
|
|
20
|
Clark J, Gebhart GF, Gonder JC, Keeling ME
and Kohn DF: The 1996 guide for the care and use of laboratory
animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar
|
|
21
|
Yi J, Xia W, Wu J, Yuan L, Wu J, Tu D,
Fang J and Tan Z: Betulinic acid prevents alcohol-induced liver
damage by improving antioxidant system in mice. J Vet Sci.
15:141–148. 2014. View Article : Google Scholar :
|
|
22
|
Tang CC, Huang HP, Lee YJ, Tang YH and
Wang CJ: Hepatoprotective effect of mulberry water extracts on
ethanol-induced liver injury via anti-inflammation and inhibition
of lipogenesis in C57BL/6 J mice. Food Chem Toxicol. 62:786–796.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao HY, Huang J, Wang HY, Du XW, Cheng SM,
Han Y, Wang LF, Li GY and Wang JH: Protective effect of Zhuyeqing
liquor, a Chinese traditional health liquor, on acute
alcohol-induced liver injury in mice. J Inflamm (Lond). 10:302013.
View Article : Google Scholar
|
|
24
|
Al-Sayed E, El-Lakkany NM, Seif El-Din SH,
Sabra AN and Hammam OA: Hepatoprotective and antioxidant activity
of Melaleuca styphelioides on carbon tetrachloride-induced
hepatotoxicity in mice. Pharm Biol. 52:1581–1590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu KH, Tseng HC, Liu CT, Huang CJ, Chyuan
JH and Sheen LY: Wild bitter gourd protects against alcoholic fatty
liver in mice by attenuating oxidative stress and inflammatory
responses. Food Funct. 5:1027–1037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Qi X, Cao S and Li P: Protective
effect of flavonoid extract from Chinese bayberry (Myrica rubra
Sieb. et Zucc.) fruit on alcoholic liver oxidative injury in mice.
J Nat Med. 68:521–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui Y, Ye Q, Wang H, Li Y, Yao W and Qian
H: Hepatoprotective potential of Aloe vera polysaccharides against
chronic alcohol-induced hepatotoxicity in mice. J Sci Food Agric.
94:1764–1771. 2014. View Article : Google Scholar
|
|
28
|
Wu D and Cederbaum AI: Inhibition of
autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via
elevated oxidative stress, mitochondria dysfunction and activation
of p38 and JNK MAPK. Redox Biol. 1:552–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandizzi F, Frigerio L, Howell SH and
Schäfer P: Endoplasmic reticulum-shape and function in stress
translation. Front Plant Sci. 5:4252014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamada H, Nakajima T, Domon H, Honda T and
Yamazaki K: Endoplasmic reticulum stress response and bone loss in
experimental periodontitis in mice. J Periodontal Res. 50:500–508.
2015. View Article : Google Scholar
|
|
31
|
Sun LN, Zhou DF, Zhou JY, Zhao CY and Zhen
Z: Role of endoplasmic reticulum stress in alcoholic liver
disease-related hepatocyte apoptosis. Zhonghua Gan Zang Bing Za
Zhi. 20:35–39. 2012.In Chinese. PubMed/NCBI
|
|
32
|
Kavitha CV, Jain AK, Agarwal C, Pierce A,
Keating A, Huber KM, Serkova NJ, Wempe MF, Agarwal R and Deep G:
Asiatic acid induces endoplasmic reticulum stress and apoptotic
death in glioblastoma multiforme cells both in vitro and in vivo.
Mol Carcinog. 2014.PubMed/NCBI
|
|
33
|
Petrasek J, Iracheta-Vellve A, Csak T,
Satishchandran A, Kodys K, Kurt-Jones EA, Fitzgerald KA and Szabo
G: STING-IRF3 pathway links endoplasmic reticulum stress with
hepatocyte apoptosis in early alcoholic liver disease. Proc Natl
Acad Sci USA. 110:16544–16549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei J, Huang Q, Huang R, Chen Y, Lv S, Wei
L, Liang C, Liang S, Zhuo L and Lin X: Asiatic acid from Potentilla
chinensis attenuate ethanol-induced hepatic injury via suppression
of oxidative stress and Kupffer cell activation. Biol Pharm Bull.
36:1980–1989. 2013. View Article : Google Scholar
|
|
35
|
Li SQ, Wang DM, Shu YJ, Wan XD, Xu ZS and
Li EZ: Proper heat shock pretreatment reduces acute liver injury
induced by carbon tetrachloride and accelerates liver repair in
mice. J Toxicol Pathol. 26:365–373. 2013. View Article : Google Scholar
|
|
36
|
Lu XX, Wang SQ, Zhang Z, Xu HR, Liu B and
Huangfu CS: Protective effects of sodium nitrite preconditioning
against alcohol-induced acute liver injury in mice. Sheng Li Xue
Bao. 64:313–320. 2012.In Chinese. PubMed/NCBI
|
|
37
|
Xie Q, Guo FF and Zhou W: Protective
effects of cassia seed ethanol extract against carbon
tetrachloride-induced liver injury in mice. Acta Biochim Pol.
59:265–270. 2012.PubMed/NCBI
|