1. Introduction
Substances that convert extracellular signals
received by cell surface receptors to intracellular signals are
known as second messengers (Fig.
1). Extracellular chemical substances (first messengers) cannot
enter cells directly, however translate physical and chemical
signals into adenosine 3′,5′-cyclic monophosphate (cAMP) and cyclic
guanosine monophosphate (cGMP) within the cells via cell surface
receptors. Intracellular second messengers include cAMP, cGMP,
nucleotides, lipids and other small molecules (1). The recognition process between
intracellular second messengers and extracellular receptors gives
rise to a series of biochemical reactions that result in several
physiological effects.
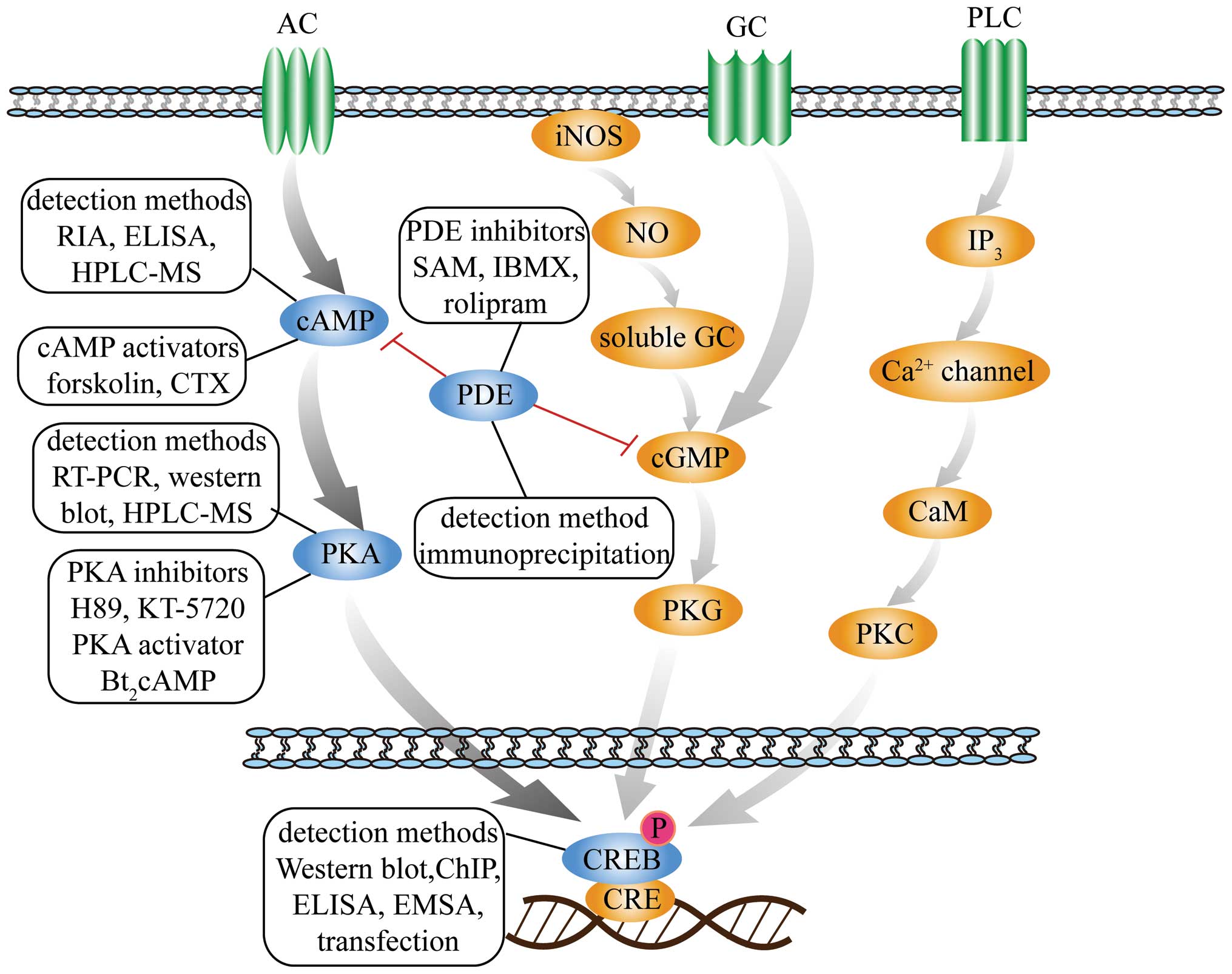 | Figure 1Schematic diagram of second messenger
signaling pathways. The figure presents three second messengers
involved in three signal transduction pathways, including
cAMP-PKA-CREB, NO-cGMP-PKG and IP3-Ca2+-PKC. The
detection methods, inhibitors and activators of cAMP, PKA, PDE and
CREB in the cAMP-PKA-CREB pathway are depicted. cAMP, adenosine
3′,5′-cyclic monophosphate; PKA, protein kinase A; CRE, cAMP
response-element; CREB, CRE binding-protein; NO, nitric oxide;
cGMP, cyclic guanosine monophosphate; PKG, protein kinase G; IP3,
inositol triphosphate; PKC, protein kinase C; PDE,
phosphodiesterase; iNOS, inducible nitric oxide synthase; AC,
adenylate cyclase; GC, guanylyl cyclase; PLC, phospholipase C; CaM,
calmodulin RIA, radioimmunoassay; ELISA, enzyme-linked
immunosorbent assay; HPLC-MS, high performance liquid
chromatography-mass spectrometry; CTX, cholera toxin; RT-PCR,
reverse transcription-quantitative polymerase chain reaction; ChIP,
chromatin immunoprecipitation; EMSA, electrophoretic mobility shift
assay; SAM, S-adenosylmethionine; IBMX,
3-isobutyl-1-methylxanthine. |
Second messengers convert and amplify extracellular
signals by activating protein kinases that serve physiological
roles or by acting on intracellular ligand-gated channels to alter
the membrane potential. The degradation of these second messengers
leads to signal termination. It has been identified that numerous
signaling pathways are triggered by second messengers including
cAMP, diacylglycerol, inositol triphosphate (IP3), cGMP
and Ca2+. This review focuses primarily on reviewing
cAMP, an important second messenger, and the associated cell signal
transduction pathway. Signal response factors associated with cAMP
are discussed below and the current understanding of the cAMP
signaling pathway is presented in Fig.
1.
Adenylate cyclase (AC) converts adenosine
triphosphate (ATP) into cAMP, which stimulates cAMP-dependent
protein kinase A (PKA). Subsequently, specific proteins are
phosphorylated by PKA (2) to evoke
cellular reactions. The phosphorylation of the cAMP
response-element binding-protein (CREB), a transcription factor, is
important in the regulation of gene transcription (3). Extracellular signals activate the
transcription of a variety of target genes via alterations in CREB
phosphorylation, thereby, resulting in multiple physiological
functions (4). Phosphodiesterases
(PDEs) are an enzyme superfamily that have been demonstrated to
catalyze the hydrolysis of intracellular second messenger
molecules, including cAMP and cGMP; therefore, the inactivation of
PDE will indirectly increase the level of cAMP in cells (5).
Second messenger pathways are associated with
numerous conditions and diseases, including inflammation (6,7),
cancer (8,9), myocardial atrophy (2), asynodia (10) and depression (11). All of the conditions and diseases
mentioned above involve the cAMP signaling pathway and its branch
pathway. Due to the importance and varied functions of the cAMP
signaling pathway, Gloerich and Bos (12) and Nakajima et al (13) studied the underlying mechanisms in
detail. The present review discusses the methods used to detect the
cAMP signaling pathway, as well as the diseases associated with the
pathway.
2. Indicators involved in the cAMP signaling
pathway and their detection methods
cAMP is synthesized from ATP via the action of AC
and is inactivated by hydrolysis to AMP by PDE (14). As a result of the degradation of
cAMP by PDE, the catalytic portion of PKA is effectively prevented
from translocating to the nucleus and generating
phosphorylated-CREB (p-CREB) (15). cAMP regulates numerous cellular
functions, including metabolism, transcription and growth, in the
majority of cell types. These cAMP effects, mediated primarily by
cAMP-dependent PKA, are at the root of cAMP-mediated regulation of
various physiological processes, including endocrine,
cardiovascular, neuronal and immune functions (16–18).
Research on cAMP signaling pathways requires the detection of the
signaling system at various levels, including each target
factor.
Methods to detect cAMP
cAMP, as an important messenger involved in the
regulation of metabolism and biological functions in cells,
transfers information regarding cellular status. With functions
including the regulation of neurotransmitter synthesis (19), regulation of membrane protein
activity, participation in ganglion synaptic transmission (20) and regulation of transcription
factors in eukaryotic cells (21),
cAMP may be involved in the prevention and treatment of various
diseases. Therefore, detecting the level of cAMP is important in
the investigation of medically relevant signal transduction
pathways.
An immunochemical assay is a fast and effective
method for detecting cAMP in the field of biomedical research.
Developed in the 1970's, a radioimmunoassay (RIA) (22) is used to detect the concentration
of cAMP. An RIA is a radionuclide-labeled immune analysis method.
The basic principle of an RIA is a competitive binding reaction
between a radioisotope-labeled antigen and an unlabeled antigen for
a specific antibody. An RIA is a method that employs a competitive
inhibition reaction and is characterized by high sensitivity,
strong specificity and low cost. An RIA is convenient for the early
detection of biological samples, however there are concerns with
this assay regarding experimental safety and environmental
protection.
Due to safety considerations, the subsequently
developed enzyme-linked immunosorbent assay (ELISA) has greater
advantages than an RIA. This method is based on an
immuno-competitive binding technique. Currently available ELISA
kits that measure cAMP levels are based on non-affinity-purified
polyclonal anti-cAMP antibodies. Numerous studies have reported on
the use of commercially available ELISA kits for the determination
of cAMP (23,24). This method depends on specific
adsorption and the combination of the antibody and antigen. The
cAMP in the sample or standard competes with a horseradish
peroxidase (HRP)-labeled cAMP conjugate for binding sites on the
anti-cAMP antibodies, and the results are measured with a
multifunctional microplate reader to calculate the antibody or
antigen concentration. To improve the detection sensitivity,
numerous commercial kits suggest pretreating the samples using
acetylation. The substrate system for the ELISA method typically
utilizes the reaction of HRP with tetramethylbenzidine (24). To improve the stability of this
detection method, the Ellman reagent system from Cayman Chemical
Company (Ann Arbor, MI, USA) is helpful. Fluorescent and
chemiluminescent substrates (25),
which are able to greatly improve the sensitivity of detection,
were subsequently developed.
The LANCE-cAMP assay, which was developed by
PerkinElmer Life and Analytical Sciences, Inc. (Shelton, CT, USA)
and is another alternative approach for determining cAMP levels
(26), is a homogeneous
time-resolved fluorescence resonance energy transfer method.
Initially, cell treatment is conducted, after which the samples are
diluted and the intracellular cAMP level is determined using the
LANCE-cAMP kit. The samples are appropriately prepared for
time-resolved fluorescence measurements according to the
manufacturer's instructions. Additionally, there are several other
detection technologies, such as the scintillation proximity assay
(27) and the high performance
liquid chromatography-mass spectrometry (HPLC-MS) analysis
technique.
Methods to detect PKA
By catalyzing phosphorylation in response to
hormonal stimulation, PKA is the primary mediator of cAMP function
and a key regulatory enzyme in pivotal cellular processes, such as
DNA replication (28,29), cell growth and metabolism (30), cell division and rearrangement of
the actin cytoskeleton (31,32).
Due to the fact that PKA is a type of protein kinase, the methods
used in PKA research are divided into three groups: The detection
of kinase activity, mRNA expression levels and protein expression
levels.
Commonly used PKA detection methods include reverse
transcription-polymerase chain reaction (RT-PCR), western blot
analysis and non-radioactive assays. Yang et al (33) obtained the cDNA of rat hepatic
stellate cells by reverse transcription, then used forward
(5′-GCTGGCTTTGATTTACGG-3′) and reverse (5′-GATGTTTCGCTTGAGGATA-3′)
primers for the target gene (505 bp). The RT-PCR products were then
separated by agarose gel electrophoresis, and the results analyzed
with a gel image-analysis system.
Western blot analysis is used to detect the
expression of PKA proteins. In western blot analysis,
polyacrylamide gel electrophoresis (PAGE) is used to separate
proteins, and immunochemical staining or autoradiography is then
used to detect the electrophoretically separated protein expressed
by a specific gene. In general, this method is used to detect p-PKA
with p-(Ser/Thr) PKA-specific antibodies (34–37).
Commercially available kits for the rapid detection
of protein kinases have been used for many years. According to the
characteristics of a phosphate kinase, detection technology was
developed utilizing radioactive phosphorus 32 (32P) as a
marker. Although this method is effective, the large quantity of
32P used makes this assay inconvenient and potentially
hazardous. However, a non-radioactive protein kinase assay
(38) for PKA is now available.
This system is based on the high affinity binding of biotin to
streptavidin. In addition, a fluorescent peptide substrate is used.
This method was designed to be rapid, sensitive and safe.
Due to experimental safety and environmental
protection considerations, researchers have been trying to develop
increased numbers of non-radioactive detection technologies to
detect the activity of PKA. HPLC-MS, which uses liquid
chromatography as the separation system and mass spectrometry as
the detection system, has been suggested to be effective for
measuring PKA activity. Samples are separated by mass in a packed
column under high-pressure flow. The samples are then ionized, and
the mass analyzer separates the ion fragments in accordance with
mass number. The mass spectra are created by the detector. Fujikawa
et al (39) and Kanno et
al (40) used a reversed-phase
HPLC system in which phosphorylated and non-phosphorylated peptides
were detected at an absorbance of 214 nm. The areas of the
non-phosphorylated and phosphorylated substrate peptides (pmol/min)
were used as the index of PKA activity. This method combined the
high separation capability of chromatography with the high
selectivity and high sensitivity of mass spectrometry; therefore,
HPLC-MS has the advantages of rapid analysis and amenability to
automation.
Methods to detect CREB
CREB is a nuclear transcription factor.
Non-phosphorylated CREB is predominantly located in the nucleus.
PKA that is activated by cAMP translocates to the nucleus and
activates CREB through the phosphorylation of the amino terminal
kinase inducible domain, in turn regulating target gene
transcription (41–43). CREB is divided into the C-terminal
and N-terminal domains. The N-terminus is the transcription
activation site, which contains multiple phosphorylation sites,
including serine residue 133 (Ser133), Ser142 and Ser143, which can
be phosphorylated by a variety of protein kinases. Ser133 serves an
important role in the transcriptional activity of CREB (44–47).
The phosphorylation of these sites is associated with downstream
protein expression and function. Therefore, the detection of these
phosphorylated sites is crucial to the study of signal transduction
pathways.
A variety of methods have been developed to detect
CREB. The most widely used is western blot analysis and
transfection together with the luciferase assay. In western
blotting method, the protein samples are separated by PAGE; the
proteins are then transferred to membranes and subsequently probed
with a specific antibody. The antibodies most commonly used for the
above purpose are against CREB (total) (26), p-CREB (Ser133) (26,48)
and p-CREB (Ser129) (49).
Due to the importance of CREB and its
phosphorylation, it has become a focus in research for targets of
novel drug research and development. The ELISA method, which is
suitable for high throughput screening, is well established and is
widely used in drug discovery. Therefore, the cell-based ELISA
method based on the double fluorescent labeling technique has been
widely used previously. Wang et al (50) adopted a cell-based ELISA method for
the detection of p-CREB (Ser133). In this method, an immobilized
capture antibody specific for CREB binds to phosphorylated and
unphosphorylated proteins. Subsequent to washing the unbound
antibodies away, a biotinylated detection antibody that recognizes
p-CREB (Ser133) is used to detect only the phosphorylated protein,
utilizing a standard streptavidin-HRP format. With this ELISA
method, cross-reactivity with unphosphorylated activating
transcription factors/CREB family members is minimal, and peptide
competition demonstrates that the detection antibody is specific
for the Ser133 site of CREB versus other serine phosphorylation
sites.
Due to the fact that CREB is a transcription factor,
the process of CREB activation and transcriptional mediation is
also a focus for research. The traditional detection method is an
electrophoretic mobility shift assay (EMSA). However, due to safety
concerns regarding the use of radioactive isotopes, radioactive
labeling has been replaced by non-radioactive visualization
techniques. Xu et al (51)
and Fang et al (52)
employed an EMSA for the detection of CREB activation. EMSA is a
common technique for studying the interaction between DNA and
protein or RNA and protein levels. This technology is based on the
principle that DNA/protein or RNA/protein complexes have different
mobilities in PAGE. When the nuclear transcription factor combines
with a specific synthetic DNA or RNA, its migration rate in PAGE
will be slower than that of the nuclear transcription factor not
bound to DNA. Therefore, activated protein transcription or
regulatory factors that interact with DNA or RNA can be detected.
The following sequences have been used to study CREB activation:
5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′ and unlabeled
5′-CTAGCTCTCTGACGTCAGGCAATCTCT-3′ (53).
The luciferase reporter gene assay (54) involves the transfection of the
reporter gene plasmid CREB-Luc into cells. The cells undergo
appropriate stimulation and are then lysed, followed by treatment
to detect luciferase activity. This method can gauge the expression
of a reporter gene easily and effectively. The construction of a
reporter gene plasmid is accomplished by cloning the gene
transcription regulatory elements upstream of, or at other
appropriate locations relative to, the luciferase gene. Cells are
transfected with the construct and luciferase activity is detected
following treatment or proper stimulation. The influences of
different treatments on the targeted regulatory elements, or the
differences prior to and subsequent to stimulation, are quantified
using the luciferase activity level.
In addition, chromatin immunoprecipitation (ChIP)
(3) analysis also can be used to
detect the activation of CREB. ChIP is an important method for
investigating the interactions between specific proteins or
modified forms of proteins and a genomic DNA region (55). ChIP is based on the development of
an in vivo analysis method. The basic principle is to
selectively enrich a chromosomal fragment (chromatin), which
contains a specific antigen. An antibody that can identify a
protein or modified protein is used to determine the relative
abundance of the antigen at one or more locations in the
genome.
Methods to detect PDE
PDE hydrolyzes the intracellular second messengers
cAMP and cGMP. PDE terminates the biochemical actions of these
second messenger systems by degrading cAMP or cGMP within cells
(56,57). A complex PDE gene organization and
a great number of PDE splicing variants fine-tune cyclic nucleotide
signals and make PDEs conducive to specificity in the signaling
pathways (1). Inhibitors of PDE
lead to the elevation of cAMP and cGMP levels, which in turn lead
to multifarious cellular effects, including airway smooth muscle
relaxation, inhibitory effects on cellular inflammation and immune
responses (58). The PDE4
inhibitors roflumilast (59,60)
and cilomilast (61) have
indicated the potential of the development of PDE inhibitors into
novel drugs.
Previous studies have used a luminescence-based,
high-throughput screening method in place of the enzyme kinetic
method for measuring cyclic nucleotide PDE activity. In this
method, cNMP (cAMP + cGMP) binds to the inactive PKA holoenzyme,
and the regulatory subunits undergo a conformational change,
resulting in the release of catalytic subunits. The free catalytic
subunits then catalyze the transfer of the terminal phosphate of
ATP to a PKA substrate, consuming ATP in the process. The level of
remaining ATP is then determined. As PDE hydrolyzes the cNMP, PKA
activation is reduced, and increased ATP is available for the
luciferase reaction. As a result, luminescence increases. Thus,
luminescence is directly proportional to the remaining ATP level,
which is directly proportional to the PDE activity (62).
Peter et al (63) used immunoprecipitation and
subsequent activity assays to determine total PDE activity.
Immunoprecipitation is predominantly used for the qualitative
detection of antibodies or antigens. The principle is that the
soluble antigens and antibodies form visible sediments in the
presence of electrolytes according to their abundance.
PDE has various subtypes, and these subtypes have
different functions, the classification of the PDE4 subtype is
important in the research and development of novel drugs. PDE4
inhibitors have been reported to specifically prevent the
hydrolysis of cAMP (58). There
are numerous members of the PDE family, and occasionally, it is
necessary to detect one specific member, for example by conducting
transfections to detect PDE8A1 (2)
or by northern blotting to analyze PDE (A/B/D) (64).
3. Inhibitors and activators involved in
cAMP signaling pathway-associated diseases
The cAMP signaling pathway and multiple activated
factors are involved in regulating numerous physiological
processes, including growth, reproduction, differentiation and
apoptosis (65). Previous studies
have demonstrated that the disruption of the cAMP signaling pathway
or the function of any factor within this pathway can contribute to
the treatment of numerous human diseases (66–70).
For example, by targeting the interruption of the cAMP pathway, a
variety of inhibitors of various factors have been identified, and
as a result, associated drugs for treating various diseases have
been developed.
cAMP-elevating agents
The most widely used inducer of cAMP formation is
forskolin, which is an AC activator. Forskolin increases the
intracellular concentration of cAMP by activating AC. Numerous
studies have demonstrated that forskolin can increase the
expression of lipopolysaccharide (LPS)-induced inflammatory factors
that are modulated via a cAMP-dependent pathway (71) and can enhance the stimulatory
function of these factors, including tumor necrosis factor α
(72). Forskolin additionally acts
as a β-adrenergic agonist (73),
which results in stimulating the transcription of vascular
endothelial growth factors (74).
Alzheimer's disease has been reported to be associated with an
alteration in the activity of AC; therefore, it is suggested that
forskolin may be used as a targeted drug to treat Alzheimer's
disease (75).
Cholera toxin (CTX) has an effect similar to that of
forskolin. Chen et al (23)
used forskolin and CTX to increase cAMP levels, and demonstrated
that CTX and forskolin were able to increase the expression of iNOS
induced by LPS. In addition, dibutyryl-cAMP, a cAMP analog, has
been reported to be able to imitate cAMP activation agents
(48,76–78).
Inhibitors and activators of PKA
The function of the cAMP signaling pathway is
dependent on PKA. H89 is a commonly used PKA inhibitor. H89 is a
selective, potent and cell permeable inhibitor of cAMP-dependent
PKA. Previous studies have indicated that H89 blocks LPS-,
prostaglandin E2 (PGE2)-, and
phospho-ceramide analogue-1-induced cellular secretion of
cyclooxygenase 2 (3), nitric oxide
(NO) (7) and additional
inflammatory factors such as interleukin 6 (26,79).
Cho et al (80)
demonstrated that LPS stimulates the production of inflammatory
factors and the amplification of the immune response via the
mitogen-activated protein kinase (MAPK) pathway. However, H89 can
block the MAPK pathway by inhibiting the CREB-mediated mRNA and
protein expression levels of MAPK phosphatase-1 (80), thereby alleviating the inflammatory
reaction induced by LPS. Furthermore, PGE2 promotes the
proliferation of cholangiocarcinoma cells (CCLP1) through the
activation of the cAMP-PKA-CREB pathway, which can be inhibited by
H89 (81).
In the process of gastrointestinal inflammation,
muscularis macrophages produce NO to induce resident intestinal
macrophage dysfunction. PGE2 activates EP2 and EP4
receptors through the activation of the cAMP/extracellular
signal-related kinase pathway, leading to the expression of iNOS.
However, EP2 or EP4-mediated iNOS expression can be attenuated by
KT-5720 (76). Therefore,
inhibitors of PKA can be used in the treatment of gastrointestinal
inflammation. Similarly, H89 and KT-5720 can be used to block the
production of NO and the expression of iNOS induced by LPS
(23).
The only PKA activator commonly used is
Bt2cAMP, also known as dibutyryl-cAMP. Chen et al
(23) demonstrated that
Bt2cAMP directly activates PKA, accelerates
LPS-stimulated expression of iNOS in a concentration-dependent
manner, and leads to the activation of nuclear factor (NF)-κB in
the nucleus.
Inhibitors of PDE
PDE is the unique intracellular hydrolase for cAMP.
The intracellular cAMP concentration is regulated via the
stimulation of adenyl and guanyl cyclases in response to
extracellular signaling (82). The
PDEs are a superfamily of enzymes; there are a minimum of 100
different PDE enzymes, which degrade cyclic nucleotides (1). PDE inhibitors cause an increase in
the intracellular concentration of cAMP and have an impact on a
variety of cells (58). PDE
inhibitors have become a research focus.
PDE inhibitors have the potential to treat
incontinence, regulate heart rate disorders, prevent heart failure
(62), and antagonize malignant
tumors in myeloid and lymphoid tissue and in the prostate (83,84).
In addition, PDE isozymes participate in several pathological
processes in kidney cells. Therefore, it is suggested that PDE
inhibitors can be used for the treatment of nephritis and renal
failure (85).
PDE4 inhibitors have been most extensively applied;
for example, these inhibitors are used to treat chronic obstructive
pulmonary disease (86),
inflammation (87), asthma
(61), autoimmune diseases
(88), and depression (64), in addition to learning and memory
disorders (82). The most well
known PDE4 inhibitor is rolipram. Rolipram has been demonstrated to
significantly increase cAMP levels (79,89),
strengthen arginine enzyme activity (90), treat depression (91), ameliorate memory and intelligence
(22) and suppress several types
of inflammation (92). Rolipram
acts by inhibiting PDE4 and reducing cAMP hydrolysis. Due to the
fact that rolipram is not highly selective for the PDE4 subtype,
this drug has strong side effects, such as inducing vomiting.
Another commonly used inhibitor is
3-isobutyl-1-meth-ylxanthine (93). In addition, a variety of other
inhibitors have been developed based on S-adenosylmethionine (SAM).
SAM functions as an anti-inflammatory drug and has been
demonstrated to act as an effective PDE4B inhibitor for the
treatment of chronic inflammatory diseases (94).
Another PDE inhibitor, pentoxifylline, increases
intracellular cAMP, acts as an immunosuppressant, has anti-fibrotic
activity, and improves hemodynamics. In recent years,
pentoxifylline and rolipram have been increasingly used in clinical
settings (95). These drugs have
been observed to be able to increase bone mass in mice and are thus
used in the treatment of osteoporosis. Pentoxifylline and rolipram
can block macrophage activation and the production of NO in
vivo and in vitro (5).
Furthermore, pyrazolopyridines (96), as novel PDE4 inhibitors, have the
capacity to treat chronic obstructive pulmonary disease, chronic
bronchitis and emphysema.
Cilostazol, an inhibitor of PDE3, not only has
strong anti-inflammatory effects but also inhibits platelet
aggregation and leads to vasodilation (97).
The research and development of PDE5 inhibitors,
such as Viagra, Levitra and Cialis, has triggered interest in the
function of PDEs in the central nervous system (10). It has been demonstrated that PDE8
serves a decisive role in modulating the concentration of steroids,
T-cell adhesion and heart rhythm (2). In addition, Dong et al
(98) demonstrated that
dipyridamole, an inhibitor of PDE8, strongly inhibited the
migration of unstimulated and stimulated splenocytes.
4. Cross-talk of the cAMP signaling pathway
with other pathways
The presence of several second messengers in
addition to cAMP, including IP3, cGMP and
Ca2+ was mentioned above. Numerous second
messenger-mediated signaling pathways have been observed, for
example, the NO-cGMP-protein kinase G pathway (99) and the
IP3-diacyl-glycerol-Ca2+ double messenger
system (the protein kinase C pathway) (100). The physiological processes that
occur in vivo and in vitro frequently involve a
signaling network, rather than one pathway alone. Various signaling
pathways work together to generate the corresponding cellular
effects, such as the interaction with the NF-κB pathway (101).
Association with the cGMP pathway
A high concentration of one type of nucleotide, cAMP
or cGMP, will prevent the generation, metabolism or degradation of
the other cyclic nucleotide (102); there is an antagonistic
association between the physiological effects. For example,
isoproterenol (103) promotes
myocardial contraction and increases the concentration of cAMP,
while the concentration of cGMP is simultaneously reduced. Liou
et al (95) reported that
KMUP-1, a xanthine derivative, has osteoclastogenic effects via the
cAMP and cGMP pathways with associated inhibitory effects on NF-κB,
MAPKs, and additional factors and pathways. KMUP-1 is able to
increase intracellular cAMP and cGMP; therefore, KMUP-1 may
potentially be used in the treatment of osteoporosis. From a
previous study focussing upon the antidepressant medications
fluoxetine and amitriptyline (104), it is known that the cGMP and cAMP
signaling pathways are able to function simultaneously. Evidence
also indicates that cAMP and cGMP can function in combination.
Association with the NF-κB pathway
The cAMP-PKA and NF-κB pathways are involved in a
variety of physiological functions, such as the anti-inflammatory
response (105). The activation
of IκB kinase (IKK) is the key step at the beginning of the NF-κB
pathway (106). Notably, Chen
et al (107) identified
that the activation of the PKA pathway can initiate the
phosphorylation of IKK α/β and NF-κB p65. SAM acts as an
anti-inflammatory drug, the mechanism of which is predominantly
attributed to increasing the levels of intracellular cAMP and
reducing the activity of NF-κB. Studies have indicated that the
cAMP pathway is associated with the transcription of NF-κB
(94). Ollivier et al
(108) additionally reported that
cAMP inhibits NF-κB-mediated transcription in human monocytes and
endothelial cells. An additional study demonstrated that SN50, an
inhibitor of NF-κB, is able to inhibit the activation of AC induced
by LPS (71). AC is the most
important enzyme producing cAMP; therefore, AC influences the
activation of the cAMP pathway (71). Taken together, this suggested that
cAMP is associated with the NF-κB pathway.
Association with the Ca2+
pathway
cAMP and Ca2+ are two important second
messengers, which mediate the intracellular effects of cell surface
receptors (109). These two
second messengers regulate a variety of cellular functions,
including protein synthesis, protein phosphorylation, the
regulation of enzymatic activity (110) and gene expression. In eukaryotic
cells, the cAMP and Ca2+ signaling pathways are
cooperative. Landa et al (111) identified that the cAMP and
Ca2+ signaling pathways cooperate to regulate insulin
secretion in MIN6 β-cells. Previous studies have demonstrated that
cAMP levels can influence the release of Ca2+ (112). Henley et al (113) demonstrated that the
Ca2+ signaling pathway is modulated by cAMP. These
authors noted that cAMP and Ca2+ regulated the nerve
growth cone steering response induced by a variety of channels, and
that enhanced Ca2+ signaling was induced by
myelin-associated glycoprotein through increasing the activity of
the cAMP signaling pathway. Vajanaphanich et al (114) previously suggested that there was
cross-talk between the cAMP and Ca2+ second messenger
pathways in secreting cells. This cross-talk may regulate secretion
in cells, and cells treated with drugs that simultaneously increase
the levels of cAMP and Ca2+ may lead to a synergistic
reaction.
5. Future directions
The efficiency of the research methods commonly used
for elucidating the cAMP signaling pathway must be improved. The
high-throughput and high-content screening technologies developed
in recent years may be applied to increase the speed of screening
for inhibitors and agonists of the cAMP signaling pathway, and may
also improve the efficiency of novel drug research and development
(115,116). For instance, chIP was shown to
markedly shorten the early drug discovery process (115), and recent innovations in flow
cytometry have allowed up to 30-fold faster serial processing of
samples (116). The methods of
disease treatment described in the present review predominantly
focus on blocking or reducing signaling messengers using pathway
inhibitors. Conversely, few drugs exert curative effects by
increasing the concentration of cAMP. Therefore, further
elucidating the role of the cAMP signaling pathway in diseases
associated with signal dysfunction and interruption may aid in the
development of a therapeutic strategy based on pathway
activation.
Acknowledgments
This review was supported by the National Natural
Science Foundation of China (grant no. 81173469) and the National
Key Basic Research Program (973 project; grant no.
2014CB542902).
References
|
1
|
Conti M and Beavo J: Biochemistry and
physiology of cyclic nucleotide phosphodiesterases: essential
components in cyclic nucleotide signaling. Annu Rev Biochem.
76:481–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown KM, Lee LC, Findlay JE, Day JP and
Baillie GS: Cyclic AMP-specific phosphodiesterase, PDE8A1, is
activated by protein kinase A-mediated phosphorylation. FEBS Lett.
586:1631–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diaz-Muñoz MD, Osma-García IC, Fresno M
and Iñiguez MA: Involvement of PGE2 and the cAMP signalling pathway
in the up-regulation of COX-2 and mPGES-1 expression in
LPS-activated macrophages. Biochem J. 443:451–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jhala US, Canettieri G, Screaton RA,
Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M and
Montminy M: cAMP promotes pancreatic beta-cell survival via
CREB-mediated induction of IRS2. Genes Dev. 17:1575–1580. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beshay E, Croze F and Prud'homme GJ: The
phosphodiesterase inhibitors pentoxifylline and rolipram suppress
macrophage activation and nitric oxide production in vitro and in
vivo. Clin Immunol. 98:272–279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park PH, Huang H, McMullen MR, Bryan K and
Nagy LE: Activation of cyclic-AMP response element binding protein
contributes to adiponectin-stimulated interleukin-10 expression in
RAW 264.7 macrophages. J Leukoc Biol. 83:1258–1266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang SY, Kim DB, Ryu GR, Ko SH, Jeong IK,
Ahn YB, Jo YH and Kim MJ: Exendin-4 inhibits iNOS expression at the
protein level in LPS-stimulated Raw264.7 macrophage by the
activation of cAMP/PKA pathway. J Cell Biochem. 114:844–853. 2013.
View Article : Google Scholar
|
|
8
|
Rosethorne EM, Nahorski SR and Challiss
RA: Regulation of cyclic AMP response-element binding-protein
(CREB) by Gq/11-protein-coupled receptors in human SH-SY5Y
neuro-blastoma cells. Biochem Pharmacol. 75:942–955. 2008.
View Article : Google Scholar :
|
|
9
|
Burdyga A, Conant A, Haynes L, Zhang J,
Jalink K, Sutton R, Neoptolemos J, Costello E and Tepikin A: cAMP
inhibits migration, ruffling and paxillin accumulation in focal
adhesions of pancreatic ductal adenocarcinoma cells: Effects of PKA
and EPAC. Biochim Biophys Acta. 1833.2664–2672. 2013.
|
|
10
|
Menniti FS, Faraci WS and Schmidt CJ:
Phosphodiesterases in the CNS: Targets for drug development. Nat
Rev Drug Discov. 5:660–670. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang IS, Kang UG, Kim YS, Ahn YM, Park JB
and Juhnn YS: Isoform-specific changes of adenylate cyclase mRNA
expression in rat brains following chronic electroconvulsive shock.
Prog Neuropsychopharmacol Biol Psychiatry. 25:1571–1581. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gloerich M and Bos JL: Epac: Defining a
new mechanism for cAMP action. Annu Rev Pharmacol Toxicol.
50:355–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakajima T, Uchida C, Anderson SF, Parvin
JD and Montminy M: Analysis of a cAMP-responsive activator reveals
a two-component mechanism for transcriptional induction via
signal-dependent factors. Genes Dev. 11:738–747. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maurice DH, Palmer D, Tilley DG, Dunkerley
HA, Netherton SJ, Raymond DR, Elbatarny HS and Jimmo SL: Cyclic
nucleotide phosphodiesterase activity, expression and targeting in
cells of the cardiovascular system. Mol Pharmacol. 64:533–546.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McLean JH, Smith A, Rogers S, Clarke K,
Darby-King A and Harley CW: A phosphodiesterase inhibitor,
cilomilast, enhances cAMP activity to restore conditioned odor
preference memory after serotonergic depletion in the neonate rat.
Neurobiol Learn Mem. 92:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jackson EK and Dubey RK: Role of the
extracellular cAMP-adenosine pathway in renal physiology. Am J
Physiol Renal Physiol. 281:F597–F612. 2001.PubMed/NCBI
|
|
17
|
Seino S and Shibasaki T: PKA-dependent and
PKA-independent pathways for cAMP-regulated exocytosis. Physiol
Rev. 85:1303–1342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Richards JS: New Signaling pathways for
hormones and cyclic adenosine 3′,5′-monophosphate action in
endocrine cells. Mol Endocrinol. 15:209–218. 2001.PubMed/NCBI
|
|
19
|
Guseva D, Wirth A and Ponimaskin E:
Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front
Behav Neurosci. 8:3062014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patterson SL, Abel T, Deuel TA, Martin KC,
Rose JC and Kandel ER: Recombinant BDNF rescues deficits in basal
synaptic transmission and hippocampal LTP in BDNF knockout mice.
Neuron. 16:1137–1145. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Metz R and Ziff E: cAMP stimulates the
C/EBP-related transcription factor rNFIL-6 to translocate to the
nucleus and induce c-fos transcription. Genes Dev. 5:1754–1766.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barad M, Bourtchouladze R, Winder DG,
Golan H and Kandel E: Rolipram, a type IV-specific
phosphodiesterase inhibitor, facilitates the establishment of
long-lasting long-term potentiation and improves memory. Proc Natl
Acad Sci USA. 95:15020–15025. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CC, Chiu KT, Sun YT and Chen WC: Role
of the cyclic AMP-protein kinase a pathway in
lipopolysaccharide-induced nitric oxide synthase expression in RAW
264.7 macrophages. J Biol Chem. 274:31559–331564. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moon EY, Lee JH, Lee JW, Song JH and Pyo
S: ROS/Epac1-mediated Rap1/NF-kappaB activation is required for the
expression of BAFF in Raw264.7 murine macrophages. Cell Signal.
23:1479–1488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DiPilato LM, Cheng X and Zhang J:
Fluorescent indicators of cAMP and Epac activation reveal
differential dynamics of cAMP signaling within discrete subcellular
compartments. Proc Natl Acad Sci USA. 101:16513–16518. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Avni D, Ernst O, Philosoph A and Zor T:
Role of CREB in modulation of TNFalpha and IL-10 expression in
LPS-stimulated RAW264.7 macrophages. Mol Immunol. 47:1396–1403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horton JK and Baxendale PM: Mass
measurements of cyclic AMP formation by radioimmunoassay, enzyme
immunoassay and scintillation proximity assay. Methods Mol Biol.
41:91–105. 1995.
|
|
28
|
Costanzo V, Robertson K, Ying CY, Kim E,
Avvedimento E, Gottesman M, Grieco D and Gautier J: Reconstitution
of an ATM-dependent checkpoint that inhibits chromosomal DNA
replication following DNA damage. Mol Cell. 6:649–659. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Costanzo V, Avvedimento EV, Gottesman ME,
Gautier J and Grieco D: Protein kinase A is required for
chromosomal DNA replication. Curr Biol. 9:903–906. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith A, Ward MP and Garrett S: Yeast PKA
represses Msn2p/Msn4p-dependent gene expression to regulate growth,
stress response and glycogen accumulation. EMBO J. 17:3556–3564.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Verin AD, Borbiev T and Garcia JG:
Role of cAMP-dependent protein kinase A activity in endothelial
cell cytoskeleton rearrangement. Am J Physiol Lung Cell Mol
Physiol. 280:L1309–L1317. 2001.PubMed/NCBI
|
|
32
|
Gerits N, Mikalsen T, Kostenko S, Shiryaev
A, Johannessen M and Moens U: Modulation of F-actin rearrangement
by the cyclic AMP/cAMP-dependent protein kinase (PKA) pathway is
mediated by MAPK-activated protein kinase 5 and requires
PKA-induced nuclear export of MK5. J Biol Chem. 282:37232–37243.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang W, LV X, Yu S, Guan W, Di D, Wang H
and Li J: Effect of cAMP-PKA-CREB signal pathway in the model of
alcoholic hepatic fibrosis stellate cells isolated from rats. Anhui
Med Pharm J. 16:729–731. 2012.
|
|
34
|
Bruce JI, Shuttleworth TJ, Giovannucci DR
and Yule DI: Phosphorylation of inositol 1, 4,5-trisphosphate
receptors in parotid acinar cells. A mechanism for the synergistic
effects of cAMP on Ca2+ signaling. J Biol Chem. 277:1340–1348.
2002. View Article : Google Scholar
|
|
35
|
Grønborg M, Kristiansen TZ, Stensballe A,
Andersen JS, Ohara O, Mann M, Jensen ON and Pandey A: A mass
spectrometry-based proteomic approach for identification of
serine/threonine-phosphorylated proteins by enrichment with
phospho-specific antibodies: Identification of a novel protein,
Frigg, as a protein kinase a substrate. Mol Cell Proteomics.
1:517–527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmitt A and Nebreda AR: Inhibition of
Xenopus oocyte meiotic maturation by catalytically inactive protein
kinase A. Proc Natl Acad Sci USA. 99:4361–4366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lei H, Venkatakrishnan A, Yu S and
Kazlauskas A: Protein kinase A-dependent translocation of Hsp90
alpha impairs endothelial nitric-oxide synthase activity in high
glucose and diabetes. J Biol Chem. 282:9364–9371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goueli BS, Hsiao K and Goueli ASA: A novel
and simple method to assay the activity of individual protein
kinases in a crude tissue extract. Methods Mol Med. 39:633–644.
2001.PubMed/NCBI
|
|
39
|
Fujikawa H, Kanno T, Nagata T and
Nishizaki T: The phosphodiesterase III inhibitor olprinone inhibits
hippocampal glutamate release via a cGMP/PKG pathway. Neurosci
Lett. 448:208–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanno T, Yamamoto H, Yaguchi T, Hi R,
Mukasa T, Fujikawa H, Nagata T, Yamamoto S, Tanaka A and Nishizaki
T: The linoleic acid derivative DCP-LA selectively activates
PKC-epsilon, possibly binding to the phosphatidylserine binding
site. J Lipid Res. 47:1146–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brindlet P, Nakajima T and Montminy M:
Multiple protein kinase A-regulated events are required for
transcriptional induction by cAMP (cAMP response element-binding
protein). Proc Natl Acad Sci USA. 92:10521–10525. 1995. View Article : Google Scholar
|
|
42
|
Shih HM, Goldman PS, DeMaggio AJ,
Hollenberg SM, Goodman RH and Hoekstra MF: A positive genetic
selection for disrupting protein-protein interactions:
Identification of CREB mutations that prevent association with the
coactivator CBP. Proc Natl Acad Sci USA. 93:13896–13901. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferreri K, Gillt G and Montminy M: The
cAMP-regulated transcription factor CREB interacts with a component
of the TFIID complex (glutamine-rich activator/TATA binding
protein-associated factor dTAF11O). Proc Natl Acad Sci USA.
91:1210–1213. 1994. View Article : Google Scholar
|
|
44
|
Ginty DD: Calcium regulation of gene
expression: Isn't that spatial? Neuron. 18:183–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Parker D, Ferreri K, Nakajima T, LaMorte
VJ, Evans R, Koerber SC, Hoeger C and Montminy MR: Phosphorylation
of CREB at Ser-133 induces complex formation with CREB-binding
protein via a direct mechanism. Mol Cell Biol. 16:694–703. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Silva AJ, Kogan JH, Frankland PW and Kida
S: CREB and memory. Neurosci. 21:127–148. 1998.
|
|
47
|
Riccio A, Alvania RS, Lonze BE, Ramanan N,
Kim T, Huang Y, Dawson TM, Snyder SH and Ginty DD: A nitric oxide
signaling pathway controls CREB-mediated gene expression in
neurons. Mol Cell. 21:283–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moon EY, Lee YS, Choi WS and Lee MH:
Toll-like receptor 4-mediated cAMP production up-regulates B-cell
activating factor expression in Raw264.7 macrophages. Exp Cell Res.
317:2447–2455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deng H, Zhang N, Wang Y, Chen J, Shen J,
Wang Z, Xu R, Zhang J, Song D and Li D: S632A3, a new glutarimide
antibiotic, suppresses lipopolysaccharide-induced pro-inflammatory
responses via inhibiting the activation of glycogen synthase kinase
3β. Exp Cell Res. 318:2592–2603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang QS, Tian JS, Cui YL and Gao S:
Genipin is active via modulating monoaminergic transmission and
levels of brain-derived neurotrophic factor (BDNF) in rat model of
depression. Neuroscience. 275:365–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu G, Tu W and Qin AS: The relationship
between deiodinase activity and inflammatory responses under the
stimulation of uremic toxins. J Transl Med. 12:2392014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fang JQ, Jun JF, Liang Y and Du JY:
Electroacupuncture mediates extracellular signalregulated kinase
1/2 pathways in the spinal cord of rats with inflammatory pain. BMC
Complement Altern Med. 14:2852014. View Article : Google Scholar
|
|
53
|
Guan CX, Cui YR, Sun GY, Yu F, Tang CY, Li
YC, Liu HJ and Fang X: Role of CREB in vasoactive intestinal
peptide-mediated wound healing in human bronchial epithelial cells.
Regul Pept. 153:64–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang Y, Yu T, Lee YG, Yang WS, Oh J, Jeong
D, Lee S, Kim TW, Park YC, Sung GH and Cho JY: Methanol extract of
Hopea odorata suppresses inflammatory responses via the direct
inhibition of multiple kinases. J Ethnopharmacol. 145:598–607.
2013. View Article : Google Scholar
|
|
55
|
Carey MF, Peterson CL and Smale ST:
Chromatin immunoprecipitation (ChIP). Cold Spring Harb Protoc.
2009.pdb prot52792009.
|
|
56
|
Andreeva SG, Dikkes P, Epstein PM and
Rosenberg PA: Expression of cGMP-Specific Phosphodiesterase 9A mRNA
in the rat brain. J Neurosci. 21:9068–9076. 2001.PubMed/NCBI
|
|
57
|
Lugnier C: Cyclic nucleotide
phosphodiesterase (PDE) superfamily: A new target for the
development of specific therapeutic agents. Pharmacol Ther.
109:366–398. 2006. View Article : Google Scholar
|
|
58
|
Nanda K, Chatterjee M, Arya R, Mukherjee
S, Saini KS, Dastidar S and Ray A: Optimization and validation of a
reporter gene assay for screening of phosphodiesterase inhibitors
in a high throughput system. Biotechnol J. 3:1276–1279. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Page CP and Spina D: Selective PDE
inhibitors as novel treatments for respiratory diseases. Curr Opin
Pharmacol. 12:275–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pinner NA, Hamilton LA and Hughes A:
Roflumilast: A phosphodiesterase-4 inhibitor for the treatment of
severe chronic obstructive pulmonary disease. Clin Ther. 34:56–66.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Spina D: PDE4 inhibitors: Current status.
Br J Pharmacol. 155:308–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lehnart SE, Wehrens XH, Reiken S, Warrier
S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M and Marks AR:
Phosphodiesterase 4D deficiency in the ryanodine-receptor complex
promotes heart failure and arrhythmias. Cell. 123:25–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peter D, Jin SL, Conti M, Hatzelmann A and
Zitt C: Differential expression and function of phosphodiesterase 4
(PDE4) subtypes in human primary CD4+ T cells: Predominant role of
PDE4D. J Immunol. 178:4820–4831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Takahashi M, Terwilliger R, Lane C, Mezes
PS, Conti M and Duman RS: Chronic antidepressant administration
increases the expression of cAMP-specific phosphodiesterase 4A and
4B isoforms. J Neurosci. 19:610–618. 1999.PubMed/NCBI
|
|
65
|
Jin L, Hill KK, Filak H, Mogan J, Knowles
H, Zhang B, Perraud AL, Cambier JC and Lenz LL: MPYS is required
for IFN response factor 3 activation and type I IFN production in
the response of cultured phagocytes to bacterial second messengers
cyclic-di-AMP and cyclic-di-GMP. J Immunol. 187:2595–2601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu X, Guo H, Sayed MD, Lu Y, Yang T, Zhou
D, Chen Z, Wang H, Wang C and Xu J: cAMP/PKA/CREB/GLT1 signaling
involved in the antidepressant-like effects of phosphodiesterase 4D
inhibitor (GEBR-7b) in rats. Neuropsychiatr Dis Treat. 12:219–227.
2016.PubMed/NCBI
|
|
67
|
Kono Y and Hülsmann S: Presynaptic
facilitation of glycinergic mIPSC is reduced in mice lacking α3
glycine receptor subunits. Neuroscience. 320:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ramakrishnan SK, Zhang H, Takahashi S,
Centofanti B, Periyasamy S, Weisz K, Chen Z, Uhler MD, Rui L,
Gonzalez FJ and Shah YM: HIF2α Is an Essential Molecular Brake for
Postprandial Hepatic Glucagon Response Independent of Insulin
Signaling. Cell Metab. Feb 3–2016.Epub ahead of print. View Article : Google Scholar
|
|
69
|
Pal S, Khan K, China SP, Mittal M,
Shrivastava R, Taneja I, Hossain Z, Mandalapu D, Gayen JR, et al:
Theophylline, a methylxanthine drug induces osteopenia and alters
calciotropic hormones, and prophylactic vitamin D treatment
protects against these changes in rats. Toxicol Appl Pharmacol. Feb
3–2016.Epub ahead of print. View Article : Google Scholar
|
|
70
|
Bobin P, Varin A, Lefebvre F, Fischmeister
R, Vandecasteele G and Leroy J: Calmodulin kinase II inhibition
limits the pro-arrhythmic Ca2+ waves induced by
cAMP-phosphodiesterase inhibitors. Cardiovasc Res. Feb 4–2016.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Osawa Y, Lee HT, Hirshman CA, Xu D and
Emala CW: Lipopolysaccharide-induced sensitization of adenylyl
cyclase activity in murine macrophages. Am J Physiol Cell Physiol.
290:C143–C151. 2006. View Article : Google Scholar
|
|
72
|
Kobayashi Y, Mizoguchi T, Take I, Kurihara
S, Udagawa N and Takahashi N: Prostaglandin E2 enhances
osteoclastic differentiation of precursor cells through protein
kinase A-dependent phosphorylation of TAK1. J Biol Chem.
280:11395–11403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Malbon CC and Graziano MP: Adenosine
deaminase normalizes cyclic AMP responses of hypothyroid rat fat
cells to forskolin, but not beta-adrenergic agonists. FEBS Lett.
155:35–38. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW,
Chun GT, Yie SW, Eom SH and Kim PH: The PKA/CREB Pathway is closely
involved in VEGF expression in mouse macrophages. Mol Cells.
23:23–29. 2007.PubMed/NCBI
|
|
75
|
Burgos-Ramos E, Hervás-Aguilar A,
Puebla-Jiménez L, Boyano-Adánez MC and Arilla-Ferreiro E: Chronic
but not acute intracerebroventricular administration of amyloid
beta-peptide 25–35 decreases somatostatin content, adenylate
cyclase activity, somatostatin-induced inhibition of adenylate
cyclase activity and adenylate cyclase I levels in the rat
hippocampus. J Neurosci Res. 85:433–442. 2007. View Article : Google Scholar
|
|
76
|
Tajima T, Murata T, Aritake K, Urade Y,
Michishita M, Matsuoka T, Narumiya S, Ozaki H and Hori M: EP2 and
EP4 receptors on muscularis resident macrophages mediate
LPS-induced intestinal dysmotility via iNOS upregulation through
cAMP/ERK signals. Am J Physiol Gastrointest Liver Physiol.
302:G524–G534. 2012. View Article : Google Scholar :
|
|
77
|
Okado-Matsumoto A, Matsumoto A, Fujii J
and Taniguchi N: Effect of cAMP on inducible nitric oxide synthase
gene expression: Its dual and cell-specific functions. Antioxid
Redox Signal. 2:631–642. 2000. View Article : Google Scholar
|
|
78
|
Mukhopadhyay S, Das S, Williams EA, Moore
D, Jones JD, Zahm DS, Ndengele MM, Lechner AJ and Howlett AC:
Lipopolysaccharide and cyclic AMP regulation of CB(2) cannabinoid
receptor levels in rat brain and mouse RAW 264.7 macrophages. J
Neuroimmunol. 181:82–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Goldsmith M, Avni D, Ernst O, Glucksam Y,
Levy-Rimler G, Meijler MM and Zor T: Synergistic IL-10 induction by
LPS and the ceramide-1-phosphate analog PCERA-1 is mediated by the
cAMP and p38 MAP kinase pathways. Mol Immunol. 46:1979–1987. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cho IJ, Woo NR, Shin IC and Kim SG: H89,
an inhibitor of PKA and MSK, inhibits cyclic-AMP response element
binding protein-mediated MAPK phosphatase-1 induction by
lipopolysaccharide. Inflamm Res. 58:863–872. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ma J, Chen M, Xia SK, Shu W, Guo Y, Wang
YH, Xu Y, Bai XM, Zhang L, Zhang H, et al: Prostaglandin E2
promotes liver cancer cell growth by the upregulation of
FUSE-binding protein 1 expression. Int J Oncol. 42:1093–1104.
2013.PubMed/NCBI
|
|
82
|
Kotomi F, Kotera J, Michibata H, Yuasa K,
Takebayashi S, Okumura K and Omori K: Cloning and Characterization
of a novel human phosphodiesterase that hydrolyzes both cAMP and
cGMP (PDE10A). J Biol Chem. 274:18438–18445. 1999. View Article : Google Scholar
|
|
83
|
Wheeler MA, Ayyagari RR, Wheeler GL and
Weiss RM: Regulation of cyclic nucleotides in the urinary tract. J
Smooth Muscle Res. 41:1–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lerner A and Epstein PM: Cyclic nucleotide
phosphodiesterases as targets for treatment of haematological
malignancies. Biochem J. 393:21–41. 2006. View Article : Google Scholar :
|
|
85
|
Dousa TP: Cyclic-3′,5′-nucleotide
phosphodiesterase isozymes in cell biology and pathophysiology of
the kidney. Kidney Int. 55:29–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lipworth BJ: Phosphodiesterase-4
inhibitors for asthma and chronic obstructive pulmonary disease.
Lancet. 365:167–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dastidar SG, Rajagopal D and Ray A:
Therapeutic benefit of PDE4 inhibitors in inflammatory diseases.
Curr Opin Investig Drugs. 8:364–372. 2007.PubMed/NCBI
|
|
88
|
Bielekova B, Lincoln A, McFarland H and
Martin R: Therapeutic potential of phosphodiesterase-4 and-3
inhibitors in Th1-mediated autoimmune diseases. J Immunol.
164:1117–1124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Avni D, Philosoph A, Meijler MM and Zor T:
The ceramide-1-phosphate analogue PCERA-1 modulates tumour necrosis
factor-alpha and interleukin-10 production in macrophages via the
cAMP-PKA-CREB pathway in a GTP-dependent manner. Immunology.
129:375–385. 2010. View Article : Google Scholar :
|
|
90
|
Sosroseno W, Musa M, Ravichandran M, Fikri
Ibrahim M, Bird PS and Seymour GJ: The role of cyclic-AMP on
arginase activity by a murine macrophage cell line (RAW264.7)
stimulated with lipopolysaccharide from Actinobacillus
actinomycetemcomitans. Oral Microbiol Immunol. 21:347–352. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Navakkode S, Sajikumar S and Frey JU:
Mitogen-activated protein kinase-mediated reinforcement of
hippocampal early long-term depression by the type IV-specific
phosphodies-terase inhibitor rolipram and its effect on synaptic
tagging. J Neurosci. 25:10664–10670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chi ZL, Hayasaka S, Zhang XY, Hayasaka Y
and Cui HS: Effects of rolipram, a selective inhibitor of type 4
phosphodiesterase, on lipopolysaccharide-induced uveitis in rats.
Invest Ophthalmol Vis Sci. 45:2497–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yoshimura K, Hiramatsu Y and Murakami M:
Cyclic AMP potentiates substance P-induced amylase secretion by
augmenting the effect of calcium in the rat parotid acinar cells.
Biochim Biophys Acta. 1402:171–187. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gobejishvili L, Avila DV, Barker DF, Ghare
S, Henderson D, Brock GN, Kirpich IA, Joshi-Barve S, Mokshagundam
SP, McClain CJ and Barve S: S-adenosylmethionine decreases
lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates
tumor necrosis factor expression via cAMP/protein kinase A pathway.
J Pharmacol Exp Ther. 337:433–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liou SF, Hsu JH, Lin IL, Ho ML, Hsu PC,
Chen LW, Chen IJ and Yeh JL: KMUP-1 suppresses RANKL-induced
osteoclastogenesis and prevents ovariectomy-induced bone loss:
Roles of MAPKs, Akt, NF-kB and calcium/calcineurin/NFATc1 pathways.
PLoS One. 8:e694682013. View Article : Google Scholar
|
|
96
|
Hamblin JN, Angell TD, Ballantine SP, Cook
CM, Cooper AW, Dawson J, Delves CJ, Jones PS, Lindvall M, Lucas FS,
et al: Pyrazolopyridines as a novel structural class of potent and
selective PDE4 inhibitors. Bioorg Med Chem Lett. 18:4237–4241.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Park WS, Jung WK, Lee DY, Moon C, Yea SS,
Park SG, Seo SK, Park C, Choi YH, Kim GY, et al: Cilostazol
protects mice against endotoxin shock and attenuates LPS-induced
cytokine expression in RAW 264.7 macrophages via MAPK inhibition
and NF-kappaB inactivation: Not involved in cAMP mechanisms. Int
Immunopharmacol. 10:1077–1085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dong H, Osmanova V, Epstein PM and Brocke
S: Phosphodiesterase 8 (PDE8) regulates chemotaxis of activated
lymphocytes. Biochem Biophys Res Commun. 345:713–719. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wunder F, Stasch JP, Hütter J,
Alonso-Alija C, Hüser J and Lohrmann E: A cell-based cGMP assay
useful for ultra-high-throughput screening and identification of
modulators of the nitric oxide/cGMP pathway. Anal Biochem.
339:104–112. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lallemend F, Lefebvre PP, Hans G, Rigo JM,
Van de Water TR, Moonen G and Malgrange B: Substance P protects
spiral ganglion neurons from apoptosis via
PKC-Ca2+-MAPK/ERK pathways. J Neurochem. 87:508–521.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Denninger JW and Marletta MA: Guanylate
cyclase and the. NO/cGMP signaling pathway. Biochim Biophys Acta.
1411:334–350. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Edgar VA, Cremaschi GA, Sterin-Borda L and
Genaro AM: Altered expression of autonomic neurotransmitter
receptors and proliferative responses in lymphocytes from a chronic
mild stress model of depression: Effects of fluoxetine. Brain Behav
Immun. 16:333–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Reierson GW, Mastronardi CA, Licinio J and
Wong ML: Repeated antidepressant therapy increases cyclic GMP
signaling in rat hippocampus. Neurosci Lett. 466:149–153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lee AK, Sung SH, Kim YC and Kim SG:
Inhibition of lipopolysaccharide-inducible nitric oxide synthase,
TNF-alpha and COX-2 expression by sauchinone effects on
I-kappaBalpha phosphorylation, C/EBP and AP-1 activation. Br J
Pharmacol. 139:11–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zandi E, Rothwarf DM, Delhase M, Hayakawa
M and Karin M: The IkB kinase complex (IKK) contains two kinase
subunits, IKKalpha and IKKbeta, Necessary for IkappaB
Phosphorylation and NF-kappaB activation. Cell. 91:243–252. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen BC, Liao CC, Hsu MJ, Liao YT, Lin CC,
Sheu JR and Lin CH: Peptidoglycan-induced IL-6 production in RAW
264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4
receptors, protein kinase A, I kappa B Kinase and NF-kappa B. J
Immunol. 177:681–693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ollivier V, Parry GC, Cobb RR, de Prost D
and Mackman N: Elevated cyclic AMP inhibits NF-kappaB-mediated
transcription in human monocytic cells and endothelial cells. J
Biol Chem. 271:20828–20835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hofer AM and Lefkimmiatis K: Extracellular
calcium and cAMP: Second messengers as 'third messengers'?
Physiology (Bethesda). 22:320–327. 2007. View Article : Google Scholar
|
|
110
|
Bhalla US and Iyengar R: Emergent
properties of networks of biological signaling pathways. Science.
283:381–387. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Landa LR Jr, Harbeck M, Kaihara K,
Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ,
Holz GG and Roe MW: Interplay of Ca2+ and cAMP signaling in the
insulin-secreting MIN6 beta-cell line. J Biol Chem.
280:31294–31302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Moore TM, Chetham PM, Kelly JJ and Stevens
T: Signal transduction and regulation of lung endothelial cell
permeability. Interaction between calcium and cAMP. Am J Physiol.
275:L203–L222. 1998.PubMed/NCBI
|
|
113
|
Henley JR, Huang KH, Wang D and Poo MM:
Calcium mediates bidirectional growth cone turning induced by
myelin-associated glycoprotein. Neuron. 44:909–916. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Vajanaphanich M, Schultz C, Tsien RY,
Traynor-Kaplan AE, Pandol SJ and Barrett KE: Cross-talk between
calcium and cAMP-dependent intracellular signaling pathways.
Implications for synergistic secretion in T84 colonic epithelial
cells and rat pancreatic acinar cells. J Clin Invest. 96:386–393.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kapur R, Giuliano KA, Campana M, Adams T,
Olson K, Jung D, Mrksich M, Chandrasekaran V and Taylor DL:
Streamlining the drug discovery process by integrating
miniaturization, high throughput screening, high content screening,
and automation on the CellChip™ system. Biomed Microdevices.
2:99–109. 1999. View Article : Google Scholar
|
|
116
|
Edwards BS, Oprea T, Prossnitz ER and
Sklar LA: Flow cytometry for high-throughput, high-content
screening. Curr Opin Chem Biol. 8:392–398. 2004. View Article : Google Scholar : PubMed/NCBI
|















