Introduction
The fibulins are a family of seven extracellular
matrix (ECM) proteins characterized by tandem arrays of epidermal
growth factor-like domains and a C-terminal fibulin-type module
(1). They are widely distributed
and often associated with vasculature and elastic tissues (2). Fibulin-3 has been reported to be
widely distributed in the ECM in vertebrates and is essential for
normal development and maintenance of the microenvironment of
embryonic and adult tissues.
Matrix metalloproteinases (MMPs) have been reported
to serve significant roles in the degradation of ECM components
(3). Tissue inhibitor of
metalloproteinase (TIMP), which binds to MMP with a 1:1 molar
stoichiometry, is an endogenous inhibitor of MMPs and regulates
matrix remodeling by MMPs. Fibulin-3 is able to target endothelial
cellular expression of MMPs and TIMPs, thereby has been suggested
to reduce ECM proteolysis and remodeling (4). Remodeling of ECM is an important
regulator of physiological and pathological processes in the vessel
wall (5). Tumors and cultured
cells overexpressing fibulin-3 have been demonstrated to exhibit
elevated expression levels and activity of MMPs, such as MMP-2 and
MMP-9 (6). A previous study
reported that fibulin-3 negatively modulated the invasiveness of
lung cancer cells via regulation of MMP-7 and -2 (7). An additional study observed that
fibulin-3 was able to reduce MB114 endothelial cell expression of
MMP-2 and -3 while simultaneously increasing the expression of the
MMP inhibitors TIMP-1 and -3 (8).
However, the role of fibulin-3 in hypertensive
vascular remodeling remains unclear. The current study aimed to
elucidate the association between fibulin-3, MMPs and hypertensive
vascular remodeling in a hypertensive animal model. In the current
study, the presence of fibulin-3, MMP-2, MMP-9 and TIMP-3 in the
aortas of spontaneously hypertensive rats (SHR) was analyzed.
Furthermore, the influence of injected fibulin-3 protein was
measured. In addition, the association between fibulin-3 and
oxidative stress in vascular remodeling in hypertension was
investigated.
Materials and methods
Animals and blood pressure
measurement
The experimental protocols were approved by the
Guangdong Pharmaceutical University Animal Care and Use Committee
and were performed in accordance with the Sun Yat-sen University
Guidelines for the Care and Use of Laboratory Animals. Wistar-Kyoto
(WKY) rats (8-week-old; n=10) and spontaneously hypertensive rats
(SHRs) (8-week-old; n=30) weighing 140–160 g were used. The rats
were kept under a 12/12 h light cycle, with specific pathogen free
conditions and 5 g food and 6–7 ml water/100 g body weight. All
rats were purchased from Beijing Vital River Experimental Animal
Technology Co., Ltd. (Beijing, China). The rats were divided into
four groups (n=10/group): Control group, WKY rats with no
treatment; placebo group, SHRs treated with intravenous (I.V.)
physiological saline; FBLN-1 group, SHRs treated with low-dose
fibulin-3 protein (I.V.; 120 ng/kg); and the FBLN-2 group, SHRs
treated with high-dose fibulin-3 protein (I.V.; 240 ng/kg).
Rats were injected with physiological saline or
fibulin-3 protein through the tail vein once per week for 8 weeks.
Blood pressure was serially determined in conscious, trained rats
using a noninvasive tail-cuff device (Anhui Zhenghua Biological
instrument Equipment Co., Ltd., Anhui, China) by a researcher
blinded to the groups. All rats were sacrificed with an overdose of
pentobarbital subsequent to 8 weeks of treatment. The tissue
samples from the aorta were then collected.
Histological analysis
The thoracic aortas were removed and fixed in 4%
paraformaldehyde (pH 7.4; Guangzhou Zhanchen Biological Technology
Co., Ltd., Guangzhou, China) or liquid nitrogen for protein
isolation and western blotting. Fixed tissues were sectioned and
stained with hematoxylin and eosin (Sigma-Aldrich, St. Louis, MO,
USA) for the evaluation of pathological alterations. The
wall-to-lumen (W/L) ratio was evaluated by the ratio of wall
thickness to the internal diameter of the lumen. The wall
thicknesses and internal diameter of lumen were assessed using
Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunohistochemistry
Immunohistochemistry of sections from
paraffin-embedded tissue blocks (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was conducted according to standard protocols in
5 μm-sections of thoracic aorta. Briefly, sections were
deparaffinized in xylene (Guangzhou Zhanchen Biological Technology
Co., Ltd.) and rehydrated using a series of graded alcohols
(Guangzhou Zhanchen Biological Technology Co., Ltd.). The sections
were then treated with 3% hydrogen peroxide (Guangzhou Zhanchen
Biological Technology Co., Ltd.) for 10 min to quench endogenous
peroxidase activity. The antigens were retrieved in 0.01 M sodium
citrate buffer (pH 6.0; Guangzhou Zhanchen Biological Technology
Co., Ltd.) using a microwave oven. The sections were incubated
overnight with the appropriate primary antibody in a humidified
container at 4°C, subsequent to 30 min preincubation in 10% normal
goat serum (Sigma-Aldrich) to prevent nonspecific staining. The
negative control was phosphate-buffered saline (PBS; Guangzhou
Zhanchen Biological Technology Co., Ltd.), used instead of the
primary antibody. The sections were incubated with the rabbit
polyclonal EnVision-horseradish peroxidase (HRP)-conjugated
secondary antibody (K5007; Dako, Glostrup, Denmark) for 30 min at
room temperature. Tthe tissue slides were then treated with a
nonbiotin HRP detection system according to the manufacturer's
instructions. The slides were then counterstained with hematoxylin.
The results were considered positive when a brown precipitate was
observed to have developed in the cytoplasm. The following primary
antibodies were used: Polyclonal rabbit anti-fibulin-3 (AP9095a;
1:50), monoclonal mouse anti-MMP-2 (A-AJ1497b; 1:300), monoclonal
mouse anti-MMP-9 (A-AJ1503b; 1:50) and rabbit polyclonal TIMP-3
(A-AO1052a; 1:50) (all from Abgent, Inc., San Diego, CA, USA). A
total of five positive expression fields from each section were
selected at random in triplicate, and then the positive regions
were analyzed in Image-Pro Plus software, version 6.0, to determine
the integral optical density and area. The average optical density
was calculated, and the average of five optical density values was
determined to represent the expression intensity in the
section.
Protein isolation and western
blotting
Aortas were dissected and harvested (3 pooled aortas
per experiment). Total proteins were isolated from the aorta using
a protein extraction reagent (Nanjing KeyGen Biotech. Co., Ltd.,
Nanjing, China). Samples were lysed on ice for 1 h with lysis
buffer [1X PBS, 1% NP40, 0.1% sodium dodecyl sulfate, 5 mM
ethylenediaminetetraacetic acid, 0.5% sodium deoxycholate and 1 mM
sodium orthoyanadate] containing 1% protease inhibitor
phenylmethanesulfonyl fluoride (Nanjing KeyGen Biotech. Co., Ltd.),
and clarified by centrifugation at 14,000 × g for 10 min at 4°C.
The protein contents of the cleared lysates were determined using a
Bicinchoninic Acid Protein Quantitative Analysis kit (Beijing CoWin
Biotech Co., Ltd., Beijing, China). The protein bands were
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA) which were pretreated with methanol. The
membranes were then incubated with primary antibodies overnight at
4°C and then with the appropriate secondary antibody, as follows:
Rabbit polyclonal EnVision-HRP-conjugated secondary antibody
(K5007), rabbit IgG polyclonal-HRP (ASS1003; 1:5,000; Abgent, Inc.)
or rabbit IgM polyclonal-HRP (ASS1005; 1:5,000; Abgent, Inc.). The
primary antibodies mentioned above against fibulin-3 (1:250), MMP-2
(1:1,000), MMP-9 (1:1,000) and TIMP-3 (1:1,000) were used.
Total RNA extraction and reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR)
A 3 mm-long section of fresh aorta was obtained at
the time of surgical resection and was immediately frozen at −80°C
until required. The section was then prepared for RNA extraction.
Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. For RT-qPCR, SYBR Green Supermix (Roche Diagnostics,
Indianapolis, IN, USA) was used, and standard curves for each
primer set were generated to confirm that only one amplicon was
generated at the same efficiency as the reference gene
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequences for
the primers used for amplifying rat fibulin-3, MMP-9, MMP-2 and the
internal reference GADPH were as described previously (9). RT-qPCR was performed using the
following thermal cycling conditions: 10 min at 95°C; 45 cycles of
denaturation at 95°C for 15 sec, annealing for 25 sec at 62°C and
elongation at 60°C for 1 min; final extension for 10 min at 72°C,
termination of the reaction at 4°C. Target gene expression was
calculated using the ΔΔCq and comparative methods (10) subsequent to normalization to GAPDH
expression.
Detection of oxidative stress
Reactive oxygen species (ROS) production in the
thoracic aortas was detected by dihydroethidium (DHE) staining
(Vigorous Biotechnology Beijing Co., Ltd., Beijing, China). Frozen,
enzymatically intact, 6-μm-thick sections of thoracic aortas
were incubated with 10 μmol/l DHE in PBS for 30 min at 37°C
in a dark, humidified chamber. DHE is oxidized by ROS and converted
to ethidium, which binds to DNA in the nucleus and fluoresces red.
Thus, ROS production was examined under a fluorescent microscope
(BX53; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between groups were analyzed using one-way analysis of
variance, while differences within two groups were assessed using
unpaired Student's t-tests. Statistical analysis was conducted
using SPSS for Windows, version 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
General observations
Systolic blood pressure data are presented in
Table I. No significant
differences in body weight and heart rates were observed between
the four groups. Systolic blood pressures measured in the placebo,
FBLN-1 and FBLN-2 groups were observed to be significantly greater
than those in the control group. Blood pressure in the FBLN-2 group
was significantly reduced compared with the placebo group
(P<0.05).
 | Table IGeneral data of the four groups. |
Table I
General data of the four groups.
| Group | n | Body
weight (g) | SBP
(mmHg) | HR
(beats/min) | Wall
thickness
(μm) | W/L ratio
(%) |
|---|
| Control | 10 | 264.50±15.78 | 122±8 | 289±11 | 81.4±10.8 | 6.34±0.8 |
| Placebo | 10 | 262.68±13.25 | 224±14a | 311±11 | 96.8±10.2b | 7.85±0.6a |
| FBLN-1 | 10 | 260.46±15.08 | 208±11a | 310±8 | 106.9±9.5b,c | 8.03±0.7a |
| FBLN-2 | 10 | 262.78±15.28 | 182±12a,b,c | 302±10 | 124.2±11.8b,c,d | 7.99±0.8a |
| F-value | | 1.774 | 13.014 | 1.523 | 8.658 | 18.06 |
| P-value | | 0.175 | 0.000 | 0.236 | 0.000 | 0.000 |
Histological analysis of aortic
structure
To investigate the effect of hypertensive vascular
remodeling, vascular wall thickness and W/L ratios were
investigated in the thoracic aortas of 16-week-old SHRs and
8-week-old WKY rats. Thoracic aortas were evaluated in sections
stained with hematoxylin and eosin (Fig. 1). The aortas of the SHRs exhibited
wall thickening and hypertrophic vascular smooth muscle cells
(SMCs). The thickness of the vascular wall of all SHRs was observed
to be significantly greater than that in WKY rats (P<0.05).
FBLN-1 and FBLN-2 groups were demonstrated to exhibit significant
increases in aortic wall thickness when compared with the placebo
and control groups (P<0.05), while FBLN-2 group wall thickness
was greater than that of the FBLN-1 group (Table I). The W/L ratios of the placebo,
FBLN-1 and FBLN-2 groups were significantly greater than that of
the control group (P<0.05), while no significant differences
were observed between the FBLN-1, FBLN-2 and placebo groups.
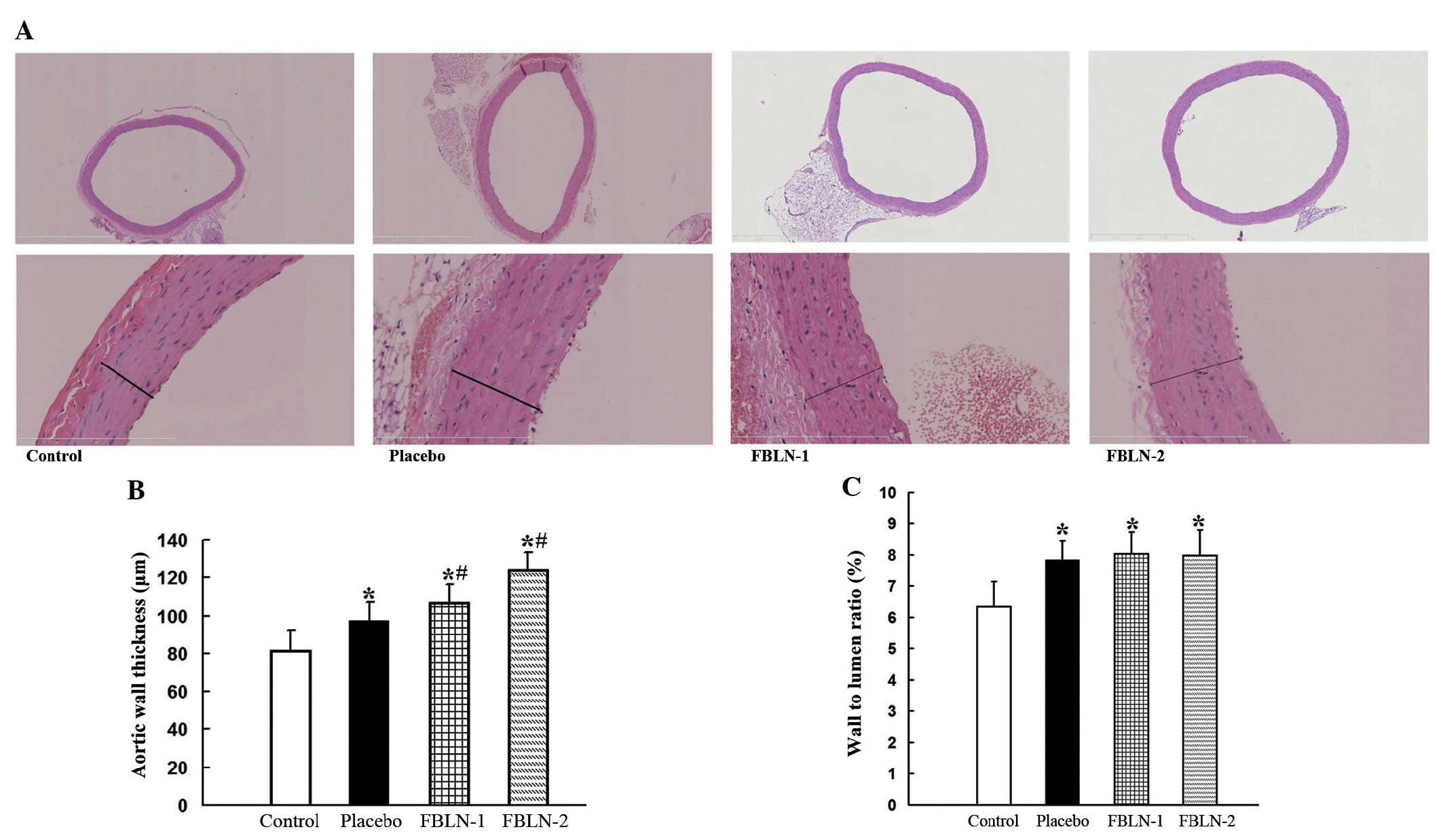 | Figure 1Histological analysis of aortic
structure. (A) The thoracic aorta were stained with hematoxylin and
eosin. Upper panel, magnification of ×50; lower panel,
magnification of ×400. (B) Quantitative analysis of vascular wall
thickness of the control, placebo, FBLN-1 and FBLN-2 groups. (C)
Quantitative analysis for the wall-to-lumen ratios of the control,
placebo, FBLN-1 and FBLN-2 groups. *P<0.05, vs.
control group; #P<0.05, vs. placebo group. FBLN-1,
low-dose fibulin 3 group; FBLN-2, high-dose fibulin 3 group. |
Protein expression of fibulin-3, MMP-2,
MMP-9 and TIMP-3 in rat aortic tissue
The alterations in protein expression levels were
observed by immunohistochemistry (Fig.
2A). Quantitation of the immunohistochemical expression of
fibulin-3, MMP-2, MMP-9 and TIMP-3 were assessed through average
optical density using Image-Pro Plus software, version 6.0, and the
data were presented as the mean ± standard deviation (Fig. 2B). In the placebo group, fibulin-3,
MMP-2 and MMP-9 were significantly increased in the thoracic aortas
compared with the control group (P<0.05). The levels of
fibulin-3 in the FBLN-2 group were greater than that of the placebo
group (P<0.05). The levels of MMP-2 and MMP-9 were observed to
be significantly reduced in the FBLN-2 group compared with the
placebo group (P<0.05). The levels of TIMP-3 however, were not
observed to be significantly different in any of the four
groups.
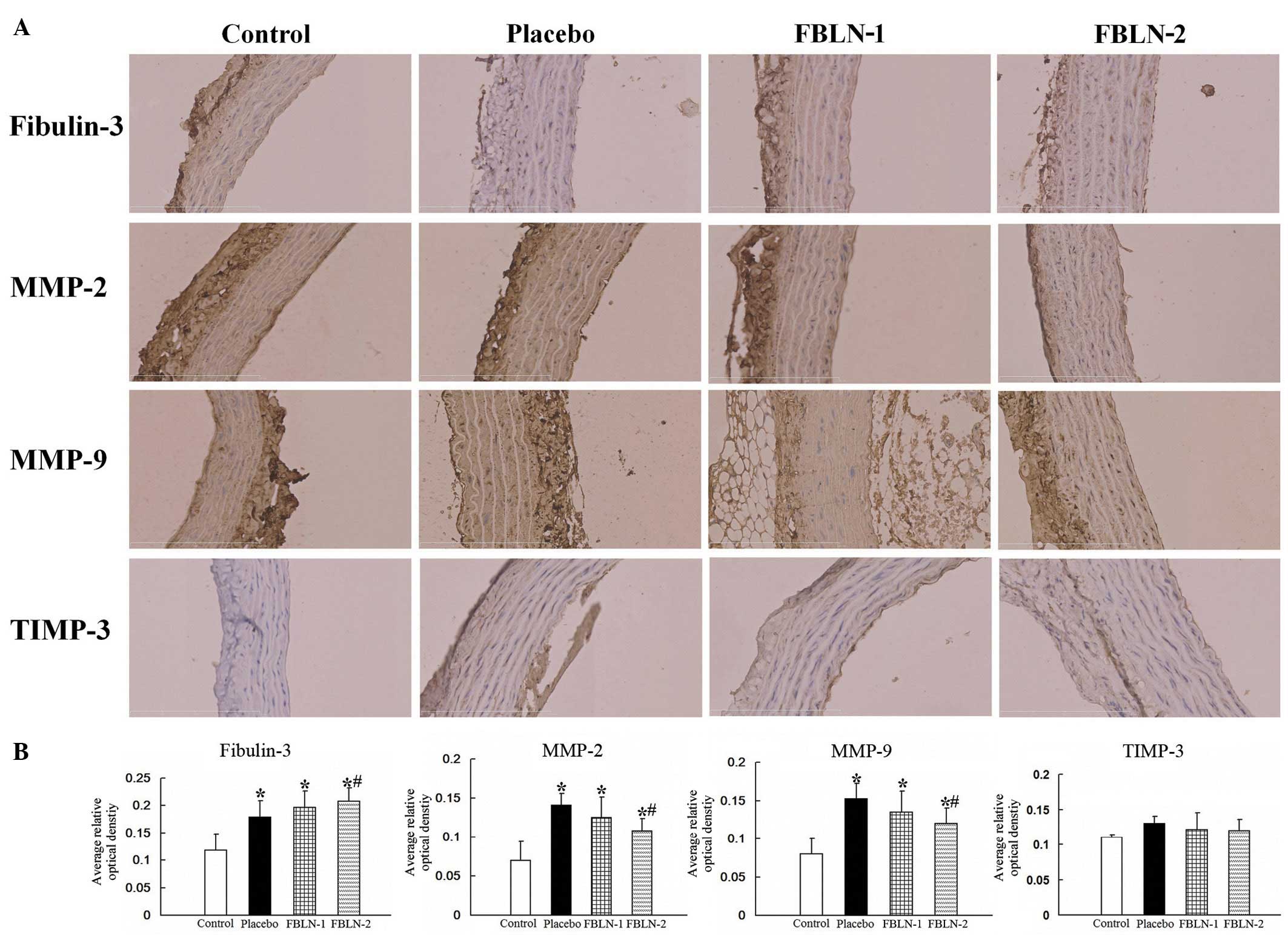 | Figure 2The expression of fibulin-3, MMP-2,
MMP-9 and TIMP-3 by immunohistochemical analysis. (A) Evaluation of
fibulin-3, MMP-2, MMP-9 and TIMP-3 by immunofluorescence microscopy
in rat aortic control, placebo, FBLN-1 and FBLN-2 group tissues
(magnification, ×400). (B) Quantitative analysis of the
immunohistochemical expression of fibulin-3, MMP-2, MMP-9 and
TIMP-3 assessed by the average optical density.
*P<0.05 vs. control group; #P<0.05 vs.
placebo group. MMP, matrix metalloproteinase; TIMP-3, tissue
inhibitor of metalloproteinase 3; FBLN-1, low-dose fibulin 3 group;
FBLN-2, high-dose fibulin 3 group. |
Western blot analysis was also conducted to measure
the protein expression levels of fibulin-3, MMP-2, MMP-9 and
TIMP-3. The expression levels of fibulin-3 were significantly
increased in the placebo, FBLN-1 and FBLN-2 groups, compared with
the control group (Fig. 3A). In
line with the immunohistochemistry results, the expression of MMP-2
and MMP-9 were observd to be increased in the placebo group
compared with the control group, and reduced in the FBLN-treated
groups compared with the placebo group (Fig. 3A). The expression levels of TIMP-3
were not significantly different in any of the four groups, as was
observed in the immunohistochemistry analysis.
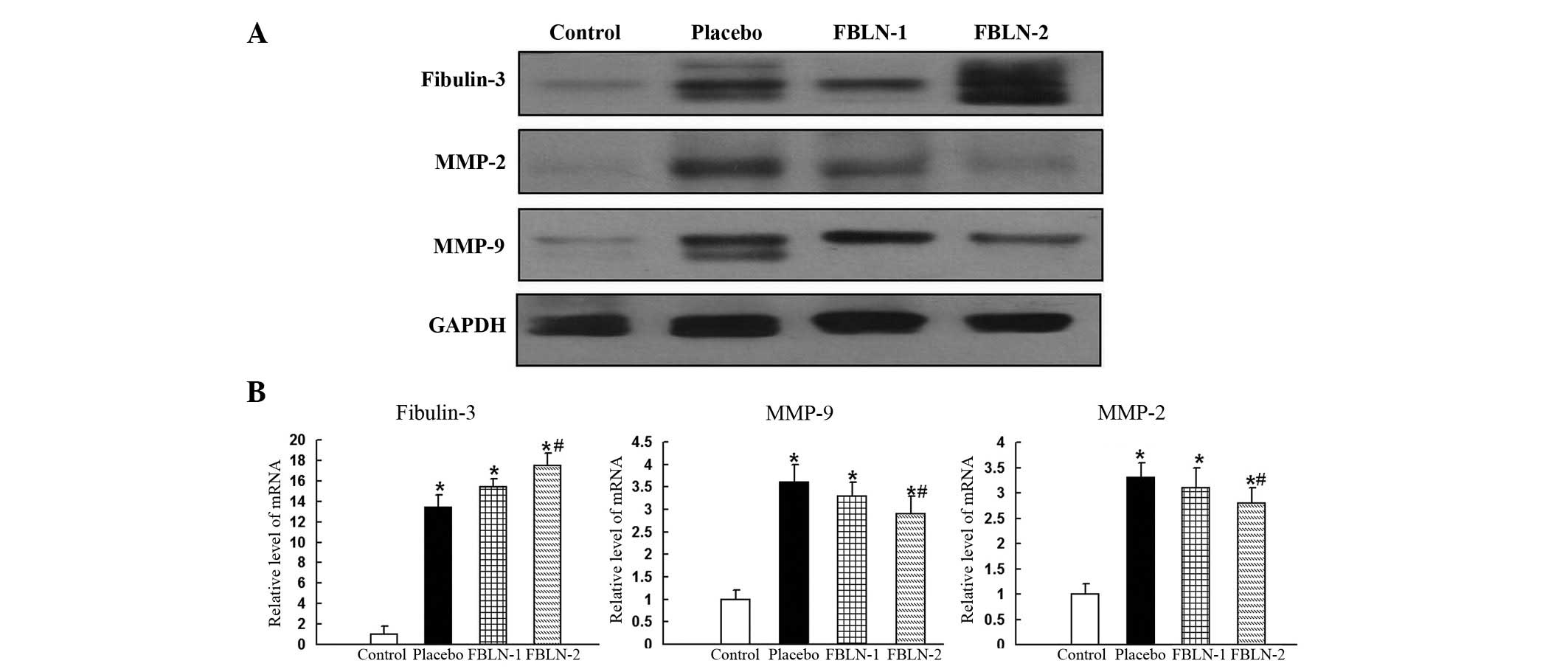 | Figure 3(A) The expression levels of
fibulin-3, MMP-2 and MMP-9 by western blotting in rat aortic
control, placebo, FBLN-1 and FBLN-2 group tissues. The expression
levels of fibulin-3 were sequentially increased in the placebo,
FBLN-1 and FBLN-2 groups, compared with the control group.
Expression of MMP-2 and MMP-9 was increased in the placebo group
compared with the control group. Expression of MMP-2 and MMP-9 was
reduced in the FBLN-treated groups compared with the placebo group.
Protein expression levels were normalized to GADPH. (B) Analysis of
the mRNA expression levels of fibulin-3, MMP-9 and MMP-2 in rat
aortic tissue. In the thoracic aortas, the mRNA levels of
fibulin-3, MMP-2 and MMP-9 were observed to be significantly
increased in the placebo group compared with the control group. The
level of fibulin-3 in the FBLN-2 group was significantly greater
than that of the placebo group, whereas levels of MMP-2 and -9 were
significantly reduced in the FBLN-2 group compared with the placebo
group. The levels of mRNA expression were normalized to GAPDH.
*P<0.05 vs. control group; #P<0.05 vs.
placebo group. MMP, matrix metalloproteinase; FBLN-1, low-dose
fibulin 3 group; FBLN-2, high-dose fibulin 3 group; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
mRNA expression of fibulin-3, MMP-2 and
MMP-9 in rat aortic tissue
In the thoracic aortas, the mRNA levels of
fibulin-3, MMP-2 and MMP-9 were observed to be significantly
increased in the placebo group compared with the control group. The
level of fibulin-3 in the FBLN-2 group was significantly greater
than that of the placebo group (P<0.05). The levels of MMP-2 and
MMP-9 were observed to be significantly reduced in the FBLN-2 group
compared with the placebo group (P<0.05; Fig. 3B).
Oxidative stress in the thoracic
aorta
Dihydroethidium staining indicated clear ROS
generation in the thoracic aortas of the placebo, FBLN-1 and FBLN-2
groups when compared with the control group (Fig. 4). In addition, fibulin-3 protein
was observed to be able to alleviate ROS in the FBLN-1 and FBLN-2
groups compared with the placebo group (Fig. 4). Little or no oxidative stress was
detected in the aortas of the FBLN-1 and FBLN-2 groups (Fig. 4).
Discussion
Fibulins are ECM proteins that serve important roles
in the biology of tissue organogenesis and vasculogenesis, in
addition to the pathology of fibrogenesis, tumorigenesis and
additional genetic diseases. Previous studies have indicated that
fibulin-type modules act to mediate specific ECM binding
interactions (2,11). Of the seven fibulins, aortic
disease-associated functions have reported for fibulin-1, -2, -4
and -5. Fibulin-1 is deposited in human coronary artery
atherosclerotic lesions in association with fibrinogen (12). The capability of fibulin-1 to
inhibit cell motility may influence vascular SMC migration during
vascular remodeling. Fibulin-2 has been observed to be expressed at
a relatively low level in the medial layer of the blood vessels
such as in the aorta (13). By
contrast, the expression of fibulin-2 has been observed to be high
in SMC-rich regions of atherosclerotic aortic lesions (13). Fibulin-2 had additionally been
detected in mechanically injured mouse carotid arteries. These
observations were consistent with a previous study which identified
a fibulin-4 deficiency in mice with enlarged and tortuous aortas
with intramural bleeding, aneurysm formation, aortic stiffening and
aortic dissection (14).
Conditional ablation of fibulin-4 expression in vascular SMCs
indicated that fibulin-4 was critical for SMC differentiation, as
demonstrated by the reduction of smooth muscle myosin heavy chain
and α smooth muscle actin expression (15). Fibulin-5-deficient mice in addition
to humans with a fibulin-5 deficiency have been identified to not
develop aneurysms, however it has been suggested that fibulin-5
serves a role in the vascular injury response. Fibulin-5 was
observed to be upregulated ~28 days following vascular injury
(16). In addition, previous
analysis of vascular SMCs from fibulin-5-deficient mice suggested
that fibulin-5 commonly acts as a negative regulator of
proliferation and migration (17).
Fibulin-3, fibulin-4 and fibulin-5 are small,
monomeric proteins expressed in the ECM of blood vessels and
elastic tissues (2). Fibulin-3
(also termed EFEMP1) was first isolated from the screening of
differentially expressed genes between senescent and quiescent
human fibroblasts. The structure of fibulin-3 is known to be
similar to that of fibulin-4, however, the function of fibulin-3 in
vascular SMCs remains unclear. In the current study, it was
identified that upregulation of the expression of fibulin-3 and
MMPs was associated with hypertensive vascular remodeling in the
thoracic aortas of SHRs compared with those of WKY rats.
Fibulin-3-treated arteries exhibited thicker aortic walls than
those of the placebo group, while the W/L ratio of the aorta was
not significantly different between the FBLN groups and the placebo
group. These results indicated that the increasing level of
fibulin-3 may promote growth of the vascular wall, and did not
aggravate remodeling of the aorta. Therefore, the results of the
current study indicate that fibulin-3 may act as a growth factor in
the arteries, which is similar to the results of a previous study
(17), however the mechanism
remains unclear.
Vascular remodeling may be caused by SMC hypertrophy
or hyperplasia, deposition of ECM elements, or a combination of
these two factors (18). The
current study demonstrated the hyperplasia and hypertrophy of SMCs
in arteries, in addition to observing that fibulin-3, MMP-2 and
MMP-9 were significantly expressed in the SMCs of the aortic wall
in SHRs compared with WKY rats. These observations indicated that
fibulin-3, MMP-2 and MMP-9 serve important roles in hypertensive
vascular remodeling. The function of MMP-2 and MMP-9 in vascular
remodeling was identified to be similar to that observed in
previous studies, which indicated that increased MMP-9 and MMP-2
activity was associated with degradation of the elastic laminae of
hypertensive arteries (19,20).
However, the role of fibulin-3 in vascular remodeling had not, to
the best of our knowledge, been reported previously, thus was
investigated to identify novel results.
Previous studies have demonstrated that the
overexpression of fibulin-3 in tumors may increase the expression
and activity of MMPs, such as MMP-2 and MMP-9, and a disintegrin
and metalloproteinase with thrombospondin motifs 5 (6,7). In
the current study, it was identified that fibulin-3-treated aortas
exhibited reduced levels of MMP-2 and MMP-9. Fibulin-3 negatively
modulated the levels of MMP-2 and MMP-9 in SHRs in vivo. The
result was similar to the previously identified role of fibulin-3
in non-small cell lung cancer (7).
Thus, it was suggested that fibulin-3 was a negative regulator of
MMP-2 and MMP-9 in arteries associated with hypertension.
The mechanisms by which fibulin-3 reduced the levels
of MMPs in hypertension remain to be fully elucidated. A possible
explanation was that activated fibulin-3 in hypertension induces
the expression of TIMP-3, which is an endogenous inhibitor of MMPs
and the binding partner of fibulin-3 (21). However, in the present study, no
alterations in TIMP-3 in the aortic walls of WKY rats and SHRs were
observed. Therefore, it was suggested that the role of fibulin-3 in
MMP expression regulation was not via the TIMP-3 pathway in
hypertension. Thus, further study was required to elucidate the
mechanisms involved in fibulin-3-mediated regulation of MMPs.
Oxidative stress contributes to the pathology of
cardiovascular disorders including hypertension (22). ROS may directly alter vascular
function or cause alterations in vascular tone via several
mechanisms, including altered nitric oxide bioavailability and
signaling (23). Previous studies
have investigated the roles of ROS and cardiovascular remodeling,
which are accompanied by cellular alignment, myocyte elongation and
reconstruction (24). The
inductions of MMP-2, MMP-9 and oxidative stress have been
previously demonstrated to be important in the development of
cardiovascular disease and hypertension (25). Oxidative stress may contribute to
the abnormal structure and function of elastic fibers in
pathological conditions, by reducing cross-linking and interactions
with other proteins required for elastic fiber assembly, including
fibulin-4, fibulin-5, and fibrillin-2 (26). In the current study, the presence
of oxidative stress was identified in the pathogenesis of
hypertension. Furthermore, it was demonstrated that fibulin-3 was
able to reduce MMP-2, MMP-9 and oxidative stress in the FBLN groups
compared with the placebo group. Thus, it was suggested that
increasing fibulin-3 may be beneficial for the improvement of
vascular health, and the reduction of certain cardiovascular risk
factors for hypertension.
In summary, the current study identified that
upregulation of fibulin-3 and MMP expression was associated with
hypertensive vascular remodeling in the thoracic aortas of SHRs.
Fibulin-3 may function as a growth factor in the arteries, however
increasing levels of fibulin-3 did not aggravate vascular
remodeling of hypertensive aortas. In addition, fibulin-3 may
reduce MMP-2, MMP-9 and oxidative stress in vascular remodeling in
hypertension. Thus, it is suggested that increasing fibulin-3 may
be beneficial to improve vascular health and reduce cardiovascular
risk factors for hypertension.
Acknowledgments
The current study was supported in part by the
Foundation of China Guangdong Science and Technology (grant nos.
2013B021800086 and 2013B021800128); the Foundation of Science and
Technology of Bureau of Yuexiu District (Guangzhou, China) (grant
no. 2014-WS-027); the National Natural Science Foundation of China
(grant no. 81402465); and the Natural Science Foundation of
Guangdong, China (grant no. 2015A030313583).
References
|
1
|
Argraves WS, Greene LM, Cooley MA and
Gallagher WM: Fibulins: Physiological and disease perspectives.
EMBO Rep. 4:1127–1131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi N, Kostka G, Garbe JH, Keene DR,
Bächinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML and
Sasaki T: A comparative analysis of the fibulin protein family.
Biochemical characterization, binding interactions and tissue
localization. J Biol Chem. 282:11805–11816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pérez-Rico C, Pascual G, Sotomayor S,
Montes-Mollón MÁ, Trejo C, Sasaki T, Mecham R, Bellón JM and Buján
J: Tropo-elastin and fibulin overexpression in the subepithelial
connective tissue of human pterygium. Am J Ophthalmol. 151:44–52.
2011. View Article : Google Scholar
|
|
5
|
Bergers G and Coussens LM: Extrinsic
regulators of epithelial tumor progression: Metalloproteinases.
Curr Opin Genet Dev. 10:120–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu B, Thirtamara-Rajamani KK, Sim H and
Viapiano MS: Fibulin-3 is uniquely upregulated in malignant gliomas
and promotes tumor cell motility and invasion. Mol Cancer Res.
7:1756–1770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EJ, Lee SY, Woo MK, Choi SI, Kim TR,
Kim MJ, Kim KC, Cho EW and Kim IG: Fibulin-3 promoter methylation
alters the invasive behavior of non-small cell lung cancer cell
lines via MMP-7 and MMP-2 regulation. Int J Oncol. 40:402–408.
2012.
|
|
8
|
Albig AR, Neil JR and Schiemann WP:
Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res.
66:2621–2629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin ZW, Wang Z, Zhu GP, Li BW, Xie WL and
Xiang DC: Hypertensive vascular remodeling was inhibited by
Xuezhikang through the regulation of Fibulin-3 and MMPs in
spontaneously hypertensive rats. Int J Clin Exp Med. 8:2118–2127.
2015.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Muriel JM, Xu X, Kramer JM and Vogel BE:
Selective assembly of fibulin-1 splice variants reveals distinct
extracellular matrix networks and novel functions for
perlecan/UNC-52 splice variants. Dev Dyn. 235:2632–2640. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Argraves WS, Tanaka A, Smith EP, Twal WO,
Argraves KM, Fan D and Haudenschild CC: Fibulin-1 and fibrinogen in
human atherosclerotic lesions. Histochem Cell Biol. 132:559–565.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ström A, Olin AI, Aspberg A and
Hultgårdh-Nilsson A: Fibulin-2 is present in murine vascular
lesions and is important for smooth muscle cell migration.
Cardiovasc Res. 69:755–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanada K, Vermeij M, Garinis GA, de Waard
MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, et
al: Perturbations of vascular homeostasis and aortic valve
abnormalities in fibulin-4 deficient mice. Circ Res. 100:738–746.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J, Davis EC, Chapman SL, Budatha M,
Marmorstein LY, Word RA and Yanagisawa H: Fibulin-4 deficiency
results in ascending aortic aneurysms: A potential link between
abnormal smooth muscle cell phenotype and aneurysm progression.
Circ Res. 106:583–592. 2010. View Article : Google Scholar :
|
|
16
|
Spencer JA, Hacker SL, Davis EC, Mecham
RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN and
Yanagisawa H: Altered vascular remodeling in fibulin-5-deficient
mice reveals a role of fibulin-5 in smooth muscle cell
proliferation and migration. Proc Natl Acad Sci USA. 102:2946–2951.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camaj P, Seeliger H, Ischenko I, Krebs S,
Blum H, De Toni EN, Faktorova D, Jauch KW and Bruns CJ: EFEMP1
binds the EGF receptor and activates MAPK and Akt pathways in
pancreatic carcinoma cells. Biol Chem. 390:1293–1302. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feihl F, Liaudet L, Levy BI and Waeber B:
Hypertension and microvascular remodelling. Cardiovasc Res.
78:274–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Longo GM, Xiong W, Greiner TC, Zhao Y,
Fiotti N and Baxter BT: Matrix metalloproteinases 2 and 9 work in
concert to produce aortic aneurysms. J Clin Invest. 110:625–632.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yasmin SW, McEniery CM, Wallace S, Dakham
Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR and Wilkinson
IB: Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase
activity are associated with systolic hypertension and arterial
stiffness. Arterioscler Thromb Vasc Biol. 25:3722005. View Article : Google Scholar
|
|
21
|
Klenotic PA, Munier FL, Marmorstein LY and
Anand-Apte B: Tissue inhibitor of metalloproteinases-3 (TIMP-3) is
a binding partner of epithelial growth factor-containing
fibulin-like extracellular matrix protein 1 (EFEMP1). Implications
for macular degenerations. J Biol Chem. 279:30469–30473. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jahandideh F, Majumder K, Chakrabarti S,
Morton JS, Panahi S, Kaufman S, Davidge ST and Wu J: Beneficial
effects of simulated gastro-intestinal digests of fried egg and its
fractions on blood pressure, plasma lipids and oxidative stress in
spontaneously hypertensive rats. PLoS One. 9:e1150062014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schulz E, Gori T and Münzel T: Oxidative
stress and endothelial dysfunction in hypertension. Hypertens Res.
34:665–673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsutsui H, Kinugawa S and Matsushima S:
Oxidative stress and heart failure. Am J Physiol Heart Circ
Physiol. 301:H2181–H2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bahrehmand F, Vaisi-Raygani A, Kiani A,
Rahimi Z, Tavilani H, Ardalan M, Vaisi-Raygani H, Shakiba E and
Pourmotabbed T: Matrix metalloproteinase 9 polymorphisms and
systemic lupus erythematosus: Correlation with systemic
inflammatory markers and oxidative stress. Lupus. 24:597–60. 2015.
View Article : Google Scholar
|
|
26
|
Akhtar K, Broekelmann TJ, Miao M, Keeley
FW, Starcher BC, Pierce RA, Mecham RP and Adair-Kirk TL: Oxidative
and nitrosative modifications of tropoelastin prevent elastic fiber
assembly in vitro. J Biol Chem. 285:37396–37404. 2010. View Article : Google Scholar : PubMed/NCBI
|


















