Introduction
Breast cancer is a common types of malignancy among
females, worldwide (1). Genetic
and epigenetic alterations are involved in the underlying
mechanisms associated with breast cancer development (2–4).
Although therapeutic and diagnostic methods have improved, this
type of cancer remains the primary cause of cancer-associated
mortality among females (5). It is
estimated that there are 464,000 cases of breast cancer, accounting
for 13.5% of all cancer cases in Europe in 2012, and the number of
breast cancer-associated mortalities is 131,000 (6). Furthermore, breast cancer is the most
common cause of cancer-associated mortality in females. Therefore,
it is essential to understand its molecular mechanism and develop
more effective therapeutic methods for breast cancer treatment.
The estrogen receptor (ER) is critical in
determining the phenotype of human breast cancers and is one of the
most important therapeutic targets (7). Furthermore, certain studies have
suggested that activation of ER is responsible for various
biological processes, including cell growth and differentiation,
and programmed cell death (8,9). It
is reported that the response of ER to estrogen is critical in
controlling specific protein synthesis (10). ER-mediated transcription has been
extensively investigated on a small number of endogenous target
promoters (11,12). Carroll et al (13) identified various novel features of
ER transcription, including an involvement of distal
cis-regulatory enhancer regions, and a requirement for the
Forkhead protein, FoxA1, in facilitating ER binding to chromatin
and subsequent gene transcription. However, the mechanisms
underlying estrogen-associated gene expression changes in breast
cancer remain poorly understood.
The human cancer cell line, MCF7, contains ER and
demonstrates an estrogen response (14). In the present study, the microarray
data was obtained from the Gene Expression Omnibus (GEO) database,
which was developed using MCF7 cells stimulated with estrogen for
different durations. The differentially expressed genes (DEGs)
between the control and estrogen treatment groups were analyzed.
The ER binding sites were identified and motif analysis was
performed. The aim was to investigate the regulatory mechanism of
ER in the progression of breast cancer.
Materials and methods
Affymetrix microarray data
The mRNA microarray datasets (accession no.
GSE11324) were obtained from the GEO database (http://www.ncbi.nlm.nih.gov/geo/), which was
deposited by Carroll et al (13). The gene expression profiles were
developed from 12 batches of MCF7 cells that had been treated with
100 nM estrogen for 0, 3, 6 or 12 h. The experiment had been
repeated three times.
Data preprocessing and DEG
Identification
The raw Affymetrix CEL data were downloaded based on
the platform of GPL570 (HG-U133_Plus_2) Affymetrix
GeneChip® Human Genome U133 Plus 2.0 Array (Affymetrix,
Santa Clara, CA, USA). To obtain the expression matrix, mRNA
expression data were first preprocessed using the Affy Package in R
language (http://bioconductor.org/packages/release/bioc/html/affy.html)
(15). When multiple probes mapped
to the same Entrez Gene ID, the mean expression value of these
probes was calculated for the gene.
The DEGs between the ER-stimulated MCF7 cells were
screened by limma package in R language (16) and genes with an adjusted P-value
<0.05 were considered to be DEGs. Subsequently, the DEGs in the
3 vs. 0 h, 6 vs. 0 h and 12 vs. 0 h groups underwent hierarchical
clustering analysis (17) using
the pheatmap package in R language (R Core Team, Vienna,
Austria).
ER binding site analysis
Chromatin immunoprecipitation with high-throughput
sequencing (ChIP-seq) data was obtained from the GEO repository
(accession no. GSE25710; ChIP-seq for forkhead box A1, ER and
CCCTC-binding factor in breast cancer cell lines). The ChIP-seq
data were treated with ER antibody (Ab-10; Neomarkers, Lab Vision;
Fremont, CA, USA) and mapped to the whole genome sequence using
BowTie software version 2.1.0 (https://sourceforge.net/projects/bowtie-bio/files/bowtie2/)
(18). To identify the possible ER
binding sites, peak calling was performed by model-based analysis
of ChIP-Seq (MACS; version 1.4.2) (19). The q-value was set at <0.01 and
served as the cut-off to improve the ChIP-seq peak detection. The
DNA sequence fragments that interacted with ER in different regions
of the whole genome were evaluated using the Cis-regulatory Element
Annotation System software (http://liulab.dfci.harvard.edu/CEAS) and a graph of
the results was constructed (20,21).
Binding and expression target analysis
(BETA)
BETA version 1.0.7 (http://cistrome.org/BETA/) (22) is a software package that predicts
target genes through the analysis of ChIP-seq data and DEGs. The ER
target genes were screened, using BETA software, based on the
ER-associated ChIP-seq data and ER-stimulated DEG data. Briefly,
the genes within a 100-kb distance of the significant top 10,000
peaks were collected. These genes were ranked based on their
distance from the peak and the significance of their differential
expression. The genes ranked at the top exhibited the greatest
possibility of being regulated by ER.
Subsequently, the regulatory function (activation or
inhibition) of ER among the top 500 DEGs in the 12 vs. 0 h group,
which neared the top 10,000 peaks were evaluated by BETA analysis.
The motifs of the ER binding sites were obtained and the factors
that interacted with ER were predicted (23).
Results
Identification of DEGs
The DEGs with adjusted P<0.05 in the 3 vs. 0 h, 6
vs. 0 h and 12 vs. 0 h groups were screened out. Subsequently, the
gene expression profiles of DEGs at different time-points were
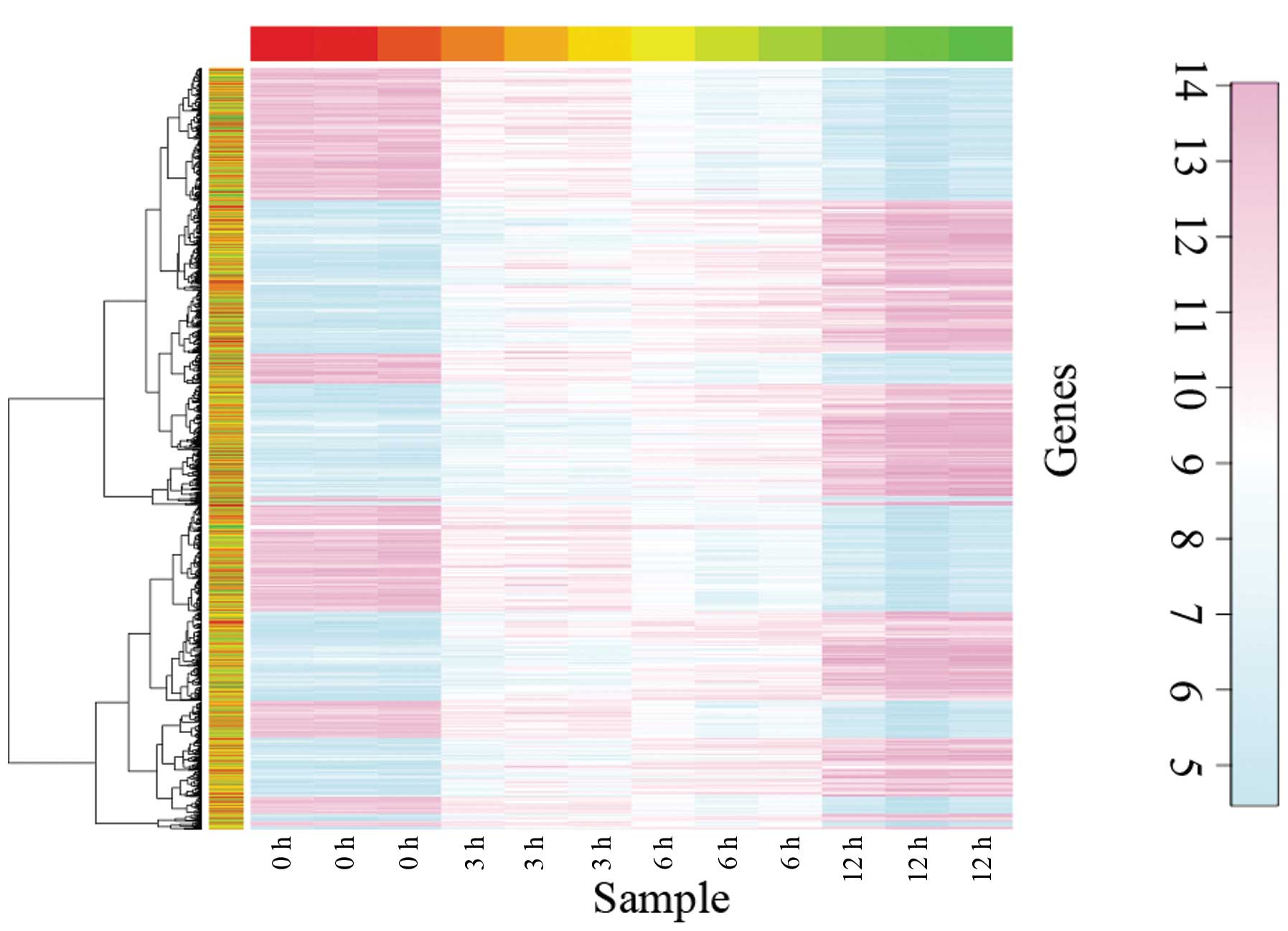
analyzed. Hierarchical clustering indicated that the DEGs were
clearly separated and the difference in gene expression became more
pronounced with increasing duration of estrogen treatment (Fig. 1). In order to obtain more reliable
results, a total of 3,122 DEGs with adjusted P<0.01 in the 12
vs. 0 h group were selected for further analysis, including 1,755
upregulated and 1,366 downregulated genes.
Identification of ER-specific binding
sites
Based on the ChIP-seq peaks determined by MACS and
the cut-off value of q<0.01, a total of 10,058 peaks were
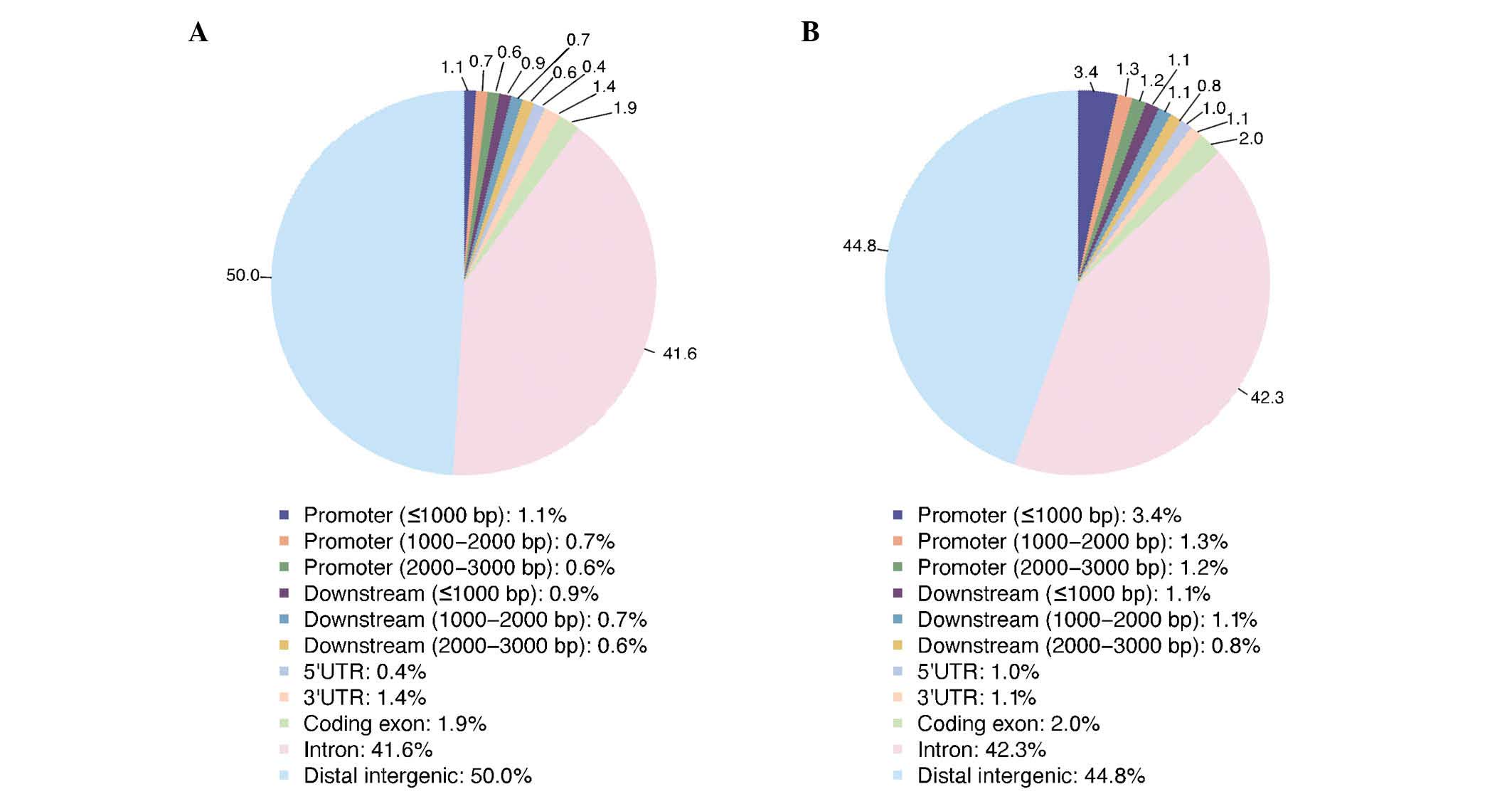
obtained. Based on the ChIP-seq data analysis, the distribution of
ER binding sites in the whole genome was determined. Fig. 2 demonstrates that the DNA sequence
fragments that interact with the ER are located in different
regions of the whole genome, such as promoter, downstream and
intergenic regions.
BETA analysis
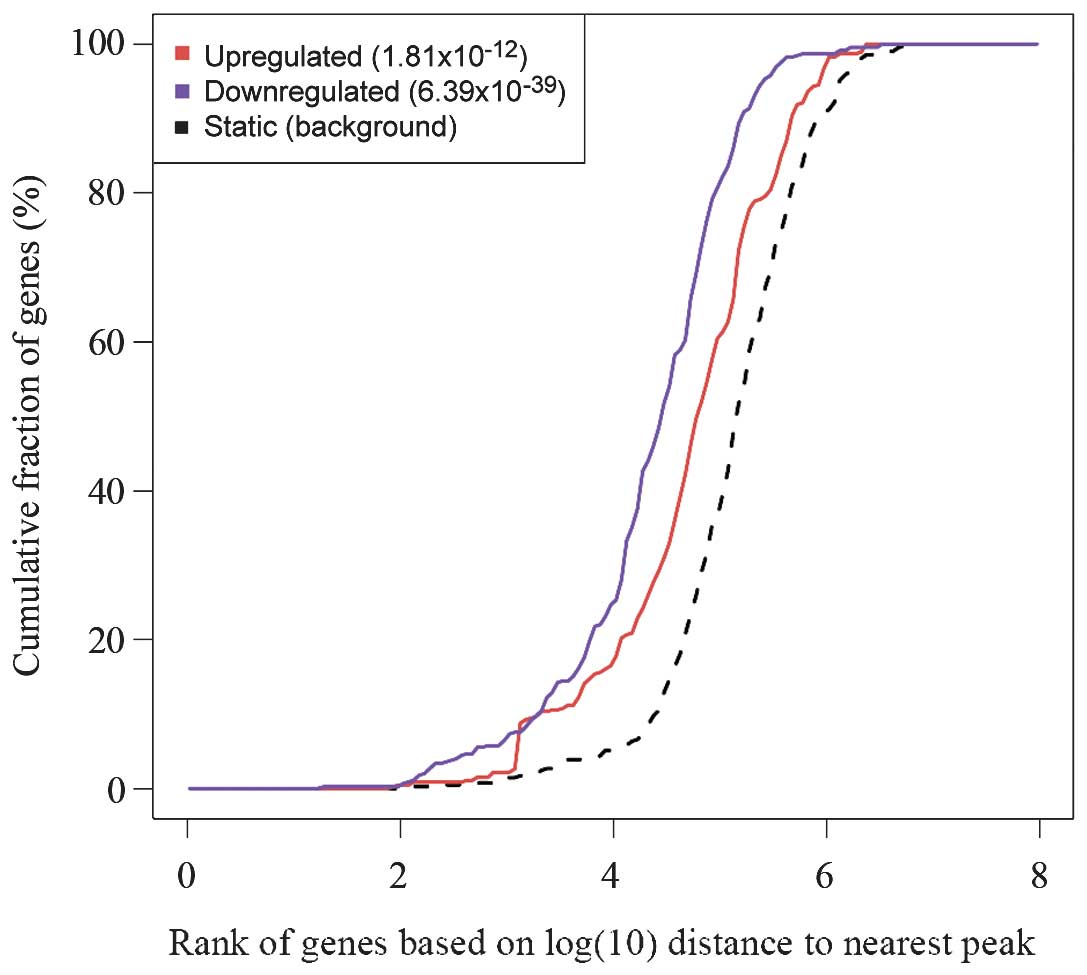
In order to analyze the regulatory effects of ER on
its target genes, the 10,000 most significant peaks and the 500
most significant DEGs in the 12 vs. 0 h group were selected for
BETA analysis. Results of the BETA analysis indicated that the ER
exerts an inhibitory role in addition to an activating role
regarding the regulation of its target genes (Fig. 3). In addition, motif analysis
revealed that ER may exert an activation role in gene expression by
interacting with MEIS1 and FOXP3 (Table I, Part A) and inhibit gene
expression by interacting with THRB and GRHL1
(Table I, Part B).
 | Table IMotifs in the target genes. |
Table I
Motifs in the target genes.
A, Upregulated
target genes
|
|---|
| Symbol | DNA-binding
domain | Species | P-value
(t-test) | T-score |
|---|
| ESR1 | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| ESRRB | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| ESRRA | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| ESRRG | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| RARA | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| PPARG | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| NR2F1 | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| ESR2 | Nuclear hormone
receptor family | Homo
sapiens |
2.24×10−40 | 14.50 |
| MEIS1 | Homeo domain
family | Homo
sapiens |
1.90×10−18 | 8.95 |
| FOXP3 | Forkhead domain
family | Homo
sapiens |
2.52×10−9 | 5.93 |
B, Downregulated
target genes
|
|---|
| Symbol | DNA-binding
domain | Species | P-value
(t-test) | T-score |
|---|
| ESR1 | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| ESRRB | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| ESRRA | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| ESRRG | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| RARA | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| PPARG | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| NR2F1 | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| ESR2 | Nuclear hormone
receptor family | Homo
sapiens |
1.12×10−43 | 14.58 |
| THRB | Nuclear hormone
receptor family | Homo
sapiens |
7.83×10−12 | 6.82 |
| GRHL1 | CP2 transcription
factor domain family | Homo
sapiens |
1.28×10−11 | 6.75 |
Discussion
The ER is recognized as the master transcriptional
regulator of the breast cancer phenotype and is critical in
predicting the early recurrence of breast cancer. However, to the
best of our knowledge, the role of ER in breast cancer cell gene
expression has not been clearly clarified. In the present study,
DEGs in the MCF7 breast cancer cell line that had been stimulated
by estrogen for different durations were analyzed. Using a
combination of the ChIP-seq dataset and the identified DEGs, the ER
target and response genes were predicted. A set of
cis-acting targets across the whole genome of the ER were
identified.
The present results demonstrated that 3,122 genes
were differentially expressed as a result of estrogen stimulation.
The hierarchical clustering analysis for the DEGs indicated that
the long-term stimulation by estrogen improved the differential
gene expression in breast cancer cells. Using motif analysis, ER
was identified to inhibit and stimulate target gene expression,
which was demonstrated by interactions between MEIS1,
FOXP3, THRB and GRHL1, and ER.
In the present study, ER was found to activate gene
expression by interacting with MEIS1 and FOXP3.
MEIS1 is a homeobox gene, and encodes the homeobox protein,
MEIS1 (24). In addition, certain
homeobox proteins are associated with tumor formation; Mahmoud
et al (25) proposed
MEIS1 as a critical transcriptional regulator of
cardiomyocyte proliferation and as a potential therapeutic target
for heart regeneration. It was previously reported that
MEIS1 is a prognostic and predictive biomarker for breast
cancer (26) and a recent study
indicated that MEIS1, as a HOX gene, is associated with
decreased proliferation in the mesenchymal stem-like subtype of
breast cancer (27). Furthermore,
the androgen/estrogen metabolism pathway is responsible for
ER-negative breast cancer (27).
Therefore, whether the MEIS1 response to ER is responsible
for the ER-positive breast cancer subtype requires further
investigation.
FOXP3, a member of the FOX protein family, is
involved in the immune system response. FOXP3 controls the
expression of numerous genes and has recently been reported to be
expressed in tumor cells (28).
FOXP3 expression was reported to be enhanced in
estrogen-treated mice (29). Fox
et al (30) showed that
expression of the transcription factor, FOXP1 is associated
with ERα and improved survival in patients with primary breast
carcinomas. Merlo et al (28) suggested that FOXP3
expression was a novel independent prognostic factor for breast
carcinoma. Thus, ER may interact with MEIS1 and FOXP3
to activate gene expression in breast cancer. In addition, the
results of the present study showed that ER suppressed gene
expression via THRB and GRHL1. THRB is
considered to be a potential cancer suppressor (31) and THRB gene silencing by
aberrant methylation is highly prevalent in breast cancer patients
(31). Furthermore, the loss of
THRB expression as a result of methylation may be a plasma
biomarker for the prognosis of breast cancer patients (31). Baniahmad et al (32) identified that the interaction of
THRB with transcription factors may mediate the activation
of the target gene. GRHL1 inhibits tumorigenicity and is a
prognostic marker in neuroblastoma (33,34).
Tao et al (35) found that
Xenopus GRHL1 is essential for epidermal differentiation. de
la Garza et al (36)
indicated that interferon regulatory factor 6 promoted
differentiation of the periderm by stimulating the expression of
grainyhead-like 3 (36). Although
the evidence for the interaction between ER and THRB and
GRHL1 is insufficient, THRB and GRHL may be
potential targets to analyze the function of ER in breast
cancer.
In conclusion, ER may activate or suppress gene
expression by interacting with MEIS1 and FOXP3, or
THRB and GRHL1, respectively. These data may be
useful for identifying novel therapeutic agents and designing
clinical trials; however, further experiments are required to
confirm the results.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dworkin AM, Huang TH and Toland AE:
Epigenetic alterations in the breast: Implications for breast
cancer detection, prognosis and treatment. Semin Cancer Biol.
19:165–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jovanovic J, Rønneberg JA, Tost J and
Kristensen V: The epigenetics of breast cancer. Mol Oncol.
4:242–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polyak K: Breast cancer: Origins and
evolution. J Clin Invest. 117:3155–3163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding LH, Ye QN, Lu QJ, Zhu JH, Yan JH,
Wang ZH and Huang CF: Expression of XBP-1 in breast cancer cell
lines and its role in ERalpha signaling. Yi Chuan Xue Bao.
31:380–384. 2004.In Chinese. PubMed/NCBI
|
|
8
|
Katzenellenbogen BS: Estrogen receptors:
Bioactivities and interactions with cell signaling pathways. Biol
Reprod. 54:287–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katzenellenbogen BS, Montano MM, Ekena K,
Herman ME, McInerney EM and William L: McGuire Memorial Lecture.
Antiestrogens: Mechanisms of action and resistance in breast
cancer. Breast Cancer Res Treat. 44:23–38. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horwitz KB and McGuire WL: Estrogen
control of progesterone receptor in human breast cancer:
Correlation with nuclear processing of estrogen receptor. J Biol
Chem. 253:2223–2228. 1978.PubMed/NCBI
|
|
11
|
Shang Y, Hu X, DiRenzo J, Lazar MA and
Brown M: Cofactor dynamics and sufficiency in estrogen
receptor-regulated transcription. Cell. 103:843–852. 2000.
View Article : Google Scholar
|
|
12
|
Shang Y and Brown M: Molecular
determinants for the tissue specificity of SERMs. Science.
295:2465–2468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carroll JS, Meyer CA, Song J, Li W,
Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC,
Hall GF, et al: Genome-wide analysis of estrogen receptor binding
sites. Nat Genet. 38:1289–1297. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lippman M, Bolan G and Huff K: The effects
of estrogens and antiestrogens on hormone-responsive human breast
cancer in long-term tissue culture. Cancer Res. 36:4595–4601.
1976.PubMed/NCBI
|
|
15
|
Irizarry R, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smyth GK: limma: Linear models for
microarray data. Bioinformatics and Computational Biology Solutions
Using R and Bioconductor. Gentleman R, Carey VJ, Huber W, Irizarry
RA and Dudoit S: 1st edition. Springer; New York, NY, USA: pp.
397–420. 2005, View Article : Google Scholar
|
|
17
|
Langfelder P and Horvath S: Fast R
functions for robust correlations and hierarchical clustering. J
Stat Softw. 46:pii: i11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng J, Liu T and Zhang Y: Using MACS to
identify peaks from ChIP-Seq data. Curr Protoc Bioinformatics
Chapter 2: Unit 2. 14:2011. View Article : Google Scholar
|
|
20
|
Zhang Y, Liu T, Meyer CA, Eeckhoute J,
Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W and
Liu XS: Model-based analysis of ChIP-Seq (MACS). Genome Biol.
9:R1372008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin H, Liu T, Manrai AK and Liu XS: CEAS:
Cis-regulatory element annotation system. Bioinformatics.
25:2605–2606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grober OM, Mutarelli M, Giurato G, Ravo M,
Cicatiello L, De Filippo MR, Ferraro L, Nassa G, Papa MF, Paris O,
et al: Global analysis of estrogen receptor beta binding to breast
cancer cell genome reveals an extensive interplay with estrogen
receptor alpha for target gene regulation. BMC Genomics. 12:362011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Sun H, Ma J, Zang C, Wang C, Wang
J, Tang Q, Meyer CA, Zhang Y and Liu XS: Target analysis by
integration of transcriptome and ChIP-seq data with BETA. Nat
Protoc. 8:2502–2515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moskow JJ, Bullrich F, Huebner K, Daar IO
and Buchberg AM: Meis1, a PBX1-related homeobox gene involved in
myeloid leukemia in BXH-2 mice. Mol Cell Biol. 15:5434–5443. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahmoud AI, Kocabas F, Muralidhar SA,
Kimura W, Koura AS, Thet S, Porrello ER and Sadek HA: Meis1
regulates postnatal cardiomyocyte cell cycle arrest. Nature.
497:249–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doolan P, Clynes M, Kennedy S, Mehta JP,
Germano S, Ehrhardt C, Crown J and O'Driscoll L: TMEM25, REPS2 and
Meis 1: Favourable prognostic and predictive biomarkers for breast
cancer. Tumour Biol. 30:200–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Merlo A, Casalini P, Carcangiu ML,
Malventano C, Triulzi T, Mènard S, Tagliabue E and Balsari A: FOXP3
expression and overall survival in breast cancer. J Clin Oncol.
27:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polanczyk MJ, Hopke C, Huan J, Vandenbark
AA and Offner H: Enhanced FoxP3 expression and Treg cell function
in pregnant and estrogen-treated mice. J Neuroimmunol. 170:85–92.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fox SB, Brown P, Han C, Ashe S, Leek RD,
Harris AL and Banham AH: Expression of the forkhead transcription
factor FOXP1 is associated with estrogen receptor alpha and
improved survival in primary human breast carcinomas. Clin Cancer
Res. 10:3521–3527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ling Y, Xu X, Hao J, Ling X, Du X, Liu X
and Zhao X: Aberrant methylation of the THRB gene in tissue and
plasma of breast cancer patients. Cancer Genet Cytogenet.
196:140–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baniahmad A, Ha I, Reinberg D, Tsai S,
Tsai MJ and O'Malley BW: Interaction of human thyroid hormone
receptor beta with transcription factor TFIIB may mediate target
gene derepression and activation by thyroid hormone. Proc Natl Acad
Sci USA. 90:8832–8836. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fabian J, Lodrini M, Schier M, Thole T,
Kopp-Schneider A, Capper D, von Deimling A, Oehme I, Wiegand I,
Milde T, et al: GRHL1 inhibits tumorigenicity and is a prognostic
marker in neuroblastoma. Klin Padiatr. 225:A292013. View Article : Google Scholar
|
|
34
|
Fabian J, Lodrini M, Oehme I, Schier MC,
Thole TM, Hielscher T, Kopp-Schneider A, Opitz L, Capper D, von
Deimling A, et al: GRHL1 acts as tumor suppressor in neuroblastoma
and is negatively regulated by MYCN and HDAC3. Cancer Res.
74:2604–2616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tao J, Kuliyev E, Wang X, Li X, Wilanowski
T, Jane SM, Mead PE and Cunningham JM: BMP4-dependent expression of
Xenopus Grainyhead-like 1 is essential for epidermal
differentiation. Development. 132:1021–1034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de la Garza G, Schleiffarth JR, Dunnwald
M, Mankad A, Weirather JL, Bonde G, Butcher S, Mansour TA, Kousa
YA, Fukazawa CF, et al: Interferon regulatory factor 6 promotes
differentiation of the periderm by activating expression of
grainyhead-like 3. J Invest Dermatol. 133:68–77. 2013. View Article : Google Scholar :
|