Introduction
The incidence of diabetes has notably increased in
recent years, and it is estimated that ~380 million patients will
be diagnosed worldwide by 2025 (1). Insulin resistance (IR), a key feature
of type 2 diabetes, is a state in which insulin has a reduced
ability to mediate glucose homeostasis in its major target tissues,
resulting in compensatory hyperinsulinemia. Previous studies have
indicated that various signaling pathways, particularly insulin
receptor substrate-1 (IRS-1)/phosphatidylinositol 3-kinase
(PI3K)/Akt, are key in mediating the occurrence of IR (2,3).
Insulin action involves a series of signaling
transduction pathways, initiated by insulin binding to its
receptor, which initiates receptor autophosphorylation and
activation of the receptor tyrosine kinase, resulting in tyrosine
phosphorylation of IRS-1, a substrate of the insulin receptor
(4,5). Subsequently, phosphorylation of IRS-1
leads to activation of PI3K and Akt and its downstream signaling
molecules, all of which are important in the promotion of the
synthesis of glycogen and regulation of glucose homeostasis
(6). Thus, a state of IR often
suggests the attenuation or failure of insulin signaling
transduction by inhibiting the phosphorylation of key
molecules.
Bisphenol A (BPA) is widely used as a plasticizer
and stabilizer in the manufacture of consumer products, however, it
is considered an endocrine disrupting chemical (EDC). Exposure to
EDCs is proposed to be involved in the etiology of IR and
associated metabolic disorders (7). Data from the U.S. National Health and
Nutritional Examination Survey 2003–2008 reported a positive
association between urinary BPA levels and an increased prevalence
of diabetes mellitus independent of traditional diabetes risk
factors (8), consistent with
research findings in China (9). In
addition, previous studies have elucidated the mechanisms by which
BPA provoked IR by affecting glucose transport (10,11),
adiponectin secretion (12),
adipocyte differentiation and lipid accumulation (13).
Furthermore, suppressor of cytokine signaling 3
(SOCS-3), a negative regulator of insulin signaling, is known to be
associated with IR. A previous study demonstrated resistin induced
IR in HepG2 cells via induction of SOCS-3 expression (14). It has also been previously reported
that the expression levels of SOCS-3 were markedly increased in
mice with IR induced by a high fat diet (15), which may contribute to increased
serine phosphorylation of IRS-1. Insulin signaling transduction is
impaired by inhibiting the activation of tyrosine phosphorylation
of IRS-1 (16). However, to the
best of our knowledge, no investigation into the effect of BPA on
SOCS-3 has been conducted. The aim of the current study was to
investigate whether BPA modulates SOCS-3 production and affects
insulin signaling transduction in 3T3-L1 adipocytes.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). BPA, dexamethasone, insulin
and 3-isobuty-1-methylxanthine (IBMX) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-SOCS-3
antibody (cat. no. 2923), rabbit monoclonal anti-IRS-1 antibody
(cat. no. 2390), rabbit polyclonal anti-Akt antibody (cat. no.
9272) and rabbit polyclonal anti-phosphorylated (p)-Akt (Ser473)
antibody (cat. no. 9271) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA; dilution, 1:1,000 for western
blotting). Goat polyclonal anti-p-IRS-1 (Tyr 632) antibody (cat.
no. sc-17196) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA; dilution, 1:200). Mouse monoclonal antibodies
against β-actin (cat. no. ab8226; dilution, 1:1000), horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG)
(cat. no. ab6721; dilution 1:200), horseradish peroxidase goat
anti-mouse IgG (cat. no. ab6789; dilution, 1:200), horseradish
peroxidase-conjugated donkey anti-goat IgG (cat. no. ab6885;
dilution, 1:100), fluorescein isothiocyanate (FITC)-conjugated
polyclonal donkey anti-goat IgG (cat. no. ab6881; dilution, 1:200),
FITC-conjugated polyclonal goat anti-rabbit IgG (cat. no. ab6717;
dilution, 1:100) and cyanine 3 (Cy3)-conjugated goat anti-rabbit
IgG (cat. no. 97075; dilution, 1:100) secondary antibodies were
purchased from Abcam (Cambridge, MA, USA).
Cell culture and treatment
The 3T3-L1 mouse preadipocyte cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA), and
the cells were cultured and differentiated into adipocytes as
described previously (17).
Briefly, the cells were grown in DMEM containing 10% FBS, 100 U/ml
penicillin (Beyotime Institute of Biotechnology, Haimen, China) and
100 mg/ml streptomycin (Beyotime Institute of Biotechnology) at
37°C in a humidified atmosphere of 5% CO2. The cells
were exposed to standard differentiation inducers 48 h after
confluency was reached. The inducer used was DMEM containing 0.5 mM
IBMX, 1 µM dexamethasone and 10 µg/ml insulin for 48
h (from day 0 to 2). The medium was then changed and supplemented
with 10 µg/ml insulin only for the following 48 h (from day
2 to 4). Thereafter, the medium was replaced by growth medium and
changed every 2 days. At 10 days after the induction of
differentiation, >80% of cells exhibited typical morphology and
biochemical properties of adipocytes. Following overnight
incubation in serum-free DMEM, 3T3-L1 adipocytes were treated with
80 µM BPA diluted in DMEM for 0, 2, 6, 12 and 24 h
respectively.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from 3T3-L1 adipocytes treated with BPA
was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols and quantified
spectrophotometrically by measuring absorbance at wavelengths 260
and 280 nm on a BioPhotometer spectrophotometer (Eppendorf,
Hamburg, Germany). RT was conducted using Transcriptor First Strand
cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland) from 1
µg RNA as described by the manufacturer's protocols. The
temperature protocol for the reaction was 25°C for 10 min, 55°C for
30 min and 85°C for 5 min. qPCR was performed to determine the
relative mRNA expression levels of SOCS-3. β-actin served as an
internal control for normalization. Specific mRNAs were amplified
in FastStart Universal SYBR Green Master mix (Roche Diagnostics)
using the ABI Prism 7500 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) sequence detector with the following
thermocycling conditions: 50°C for 2 min; 95°C for 10 min; 40
cycles of 95°C for 15 sec and 60°C for 1 min. The primers
(Invitrogen; Thermo Fisher Scientific, Inc.) were as follows:
Forward, 5′-ATGGTCACCCACAGCAAGTTT-3′ and reverse,
5′-TCCAGTAGAATCCGCTCTCCT-3′ for SOCS-3; and forward,
5′-CTACAATGAGCTGCGTGTGG-3′ and reverse, 5′-AAGGAAGGCTGGAAGAGTGC-3′
for β-actin. Three data points were used and the experiment was
replicated three times and the data was analyzed using the
2−ΔΔCq method (18).
Protein extraction and western blot
analysis
At 0, 2, 6, 12 and 24 h, the cells treated with BPA
were washed twice with ice-cold phosphate-buffered saline (PBS;
Thermo Fisher Scientific, Inc.) and lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) supplemented with 1% protease inhibitor (GeneChem
Co., Ltd., Shanghai, China) and 1% phosphatase inhibitor (GeneChem
Co., Ltd.). Total and phosphorylated proteins were extracted and
concentration was determined using the BCA Protein assay kit
(Beyotime Institute of Biotechnology). Following 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis for 30 min at 80 V
followed by 120 V, the protein bands (20 µg/lane) were
electrophoretically transferred onto polyvinyl difluoride membranes
(GE Healthcare Life Sciences, Chalfont, UK). The blots were blocked
for 2 h at room temperature in Tris-buffered saline (Beyotime
Institute of Biotechnology), with 0.1% Tween 20 (Sigma-Aldrich;
TBS-T) and 5% non-fat dried milk and subsequently incubated
overnight at 4°C with the primary antibodies against SOCS3, IRS-1,
p-IRS-1, Akt, p-Akt and β-actin. Following washing in TBS-T buffer,
the membranes were incubated with HRP-conjugated secondary
antibodies at room temperature for 1 h. Following washing in TBS-T,
antigen-antibody complexes were detected using Amersham ECL Western
Blotting Detection reagent (GE Healthcare Life Sciences) and
visualized with the ChemiDoc XRS imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and quantified using the
Quantity One image software (version 4.31; Bio-Rad Laboratories,
Inc.).
Immunocytochemistry
After 6 h treatment with BPA, 3T3-L1 adipocytes were
fixed for 30 min with 4% paraformaldehyde (Sigma-Aldrich) at room
temperature, and then rinsed three times for 5 min with PBS. The
cells were permeabilized for 10 min in 0.1% Triton X-100
(Sigma-Aldrich), then again rinsed twice for 5 min in PBS, and
blocked for 1 h in PBS with 5% bovine serum albumin (Sigma-Aldrich)
at room temperature. Antibodies against p-IRS-1 (dilution, 1:200),
Akt (dilution, 1:200) and anti-p-Akt (dilution, 1:200) were
incubated with the cells at 4°C overnight. The cells were then
incubated with FITC-conjugated goat anti-rabbit IgG (1:500) or
Cy3-conjugated goat anti-rabbit IgG (1:500) at room temperature for
2 h, followed by washing in PBS. The cells were stained with DAPI
(Sigma-Aldrich) for 3 min and images were captured using a Nikon
Eclipse Ti-S fluorescent inverted microscope (Nikon Corporation,
Tokyo, Japan) at magnification ×200.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences among 3–5 independent groups were
statistically evaluated using one-way ANOVA, while significant
differences between 2 independent groups were analyzed using
Student's t-test. Statistical analysis was conducted using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
BPA increases SOCS-3 expression levels in
3T3-L1 adipocytes
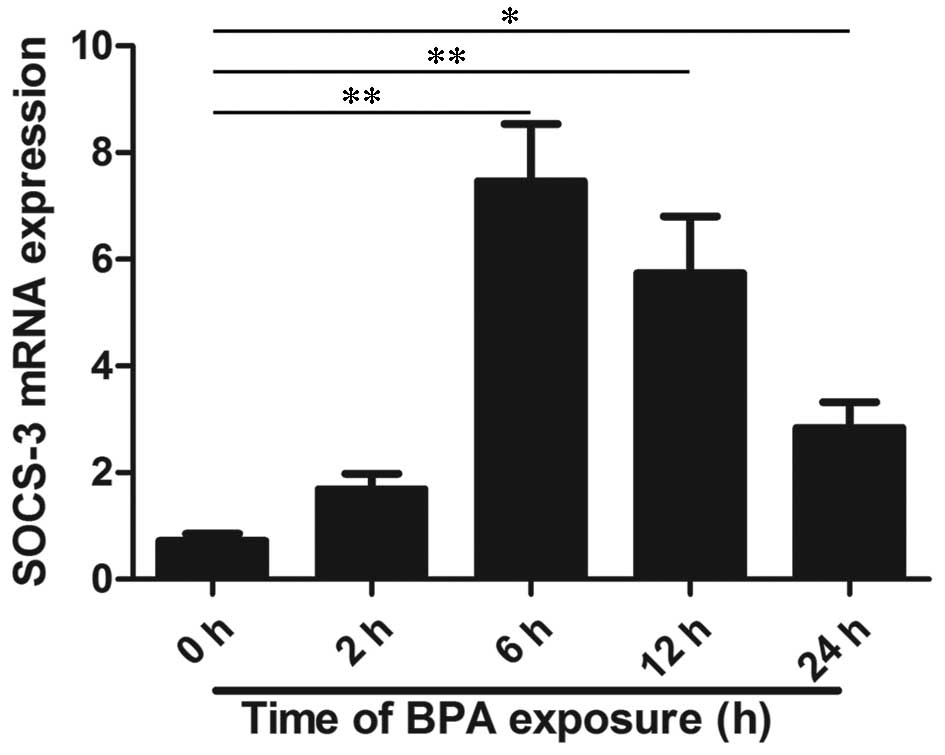
The mRNA expression levels of SOCS-3 were analyzed
using RT-qPCR and the results indicated that BPA treatment at 80
µM significantly increased mRNA expression levels in
time-dependent manners (P<0.01 at 2–12 h and P<0.05 at 24 h;
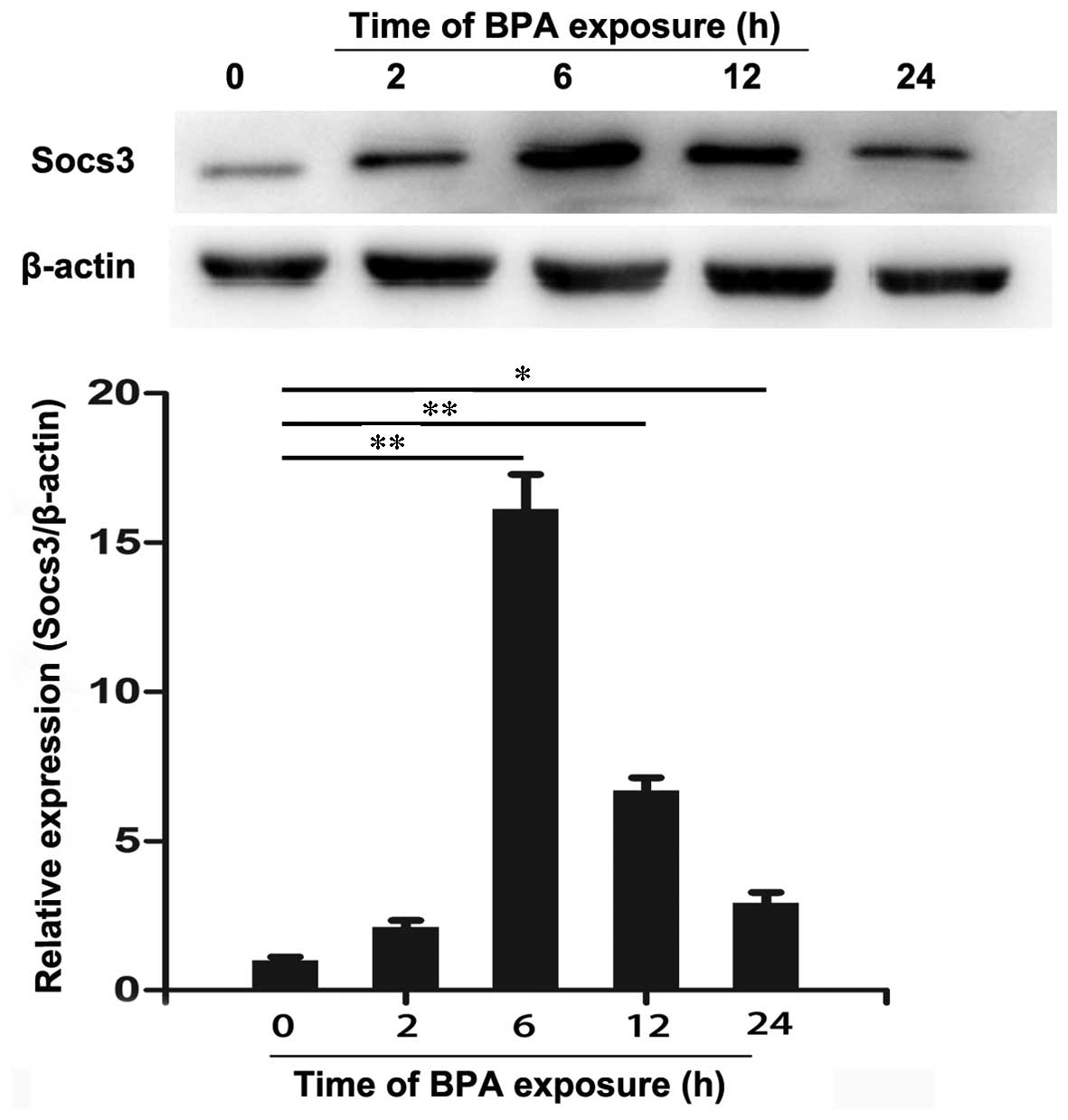
Fig. 1). In order to further
investigate the protein expression of SOCS-3, western blotting was
performed and the result is presented in Fig. 2. RT-qPCR and western blot analysis
indicated that BPA significantly increased SOCS-3 mRNA and protein
expression levels after 6 and 12 h of treatment compared to 0 h
(P<0.01). In addition, the expression levels of SOCS-3 mRNA and
protein were overexpressed after 24 h of treatment with BPA
(P<0.05).
BPA alters protein expression levels of
insulin signaling molecules in 3T3-L1 adipocytes
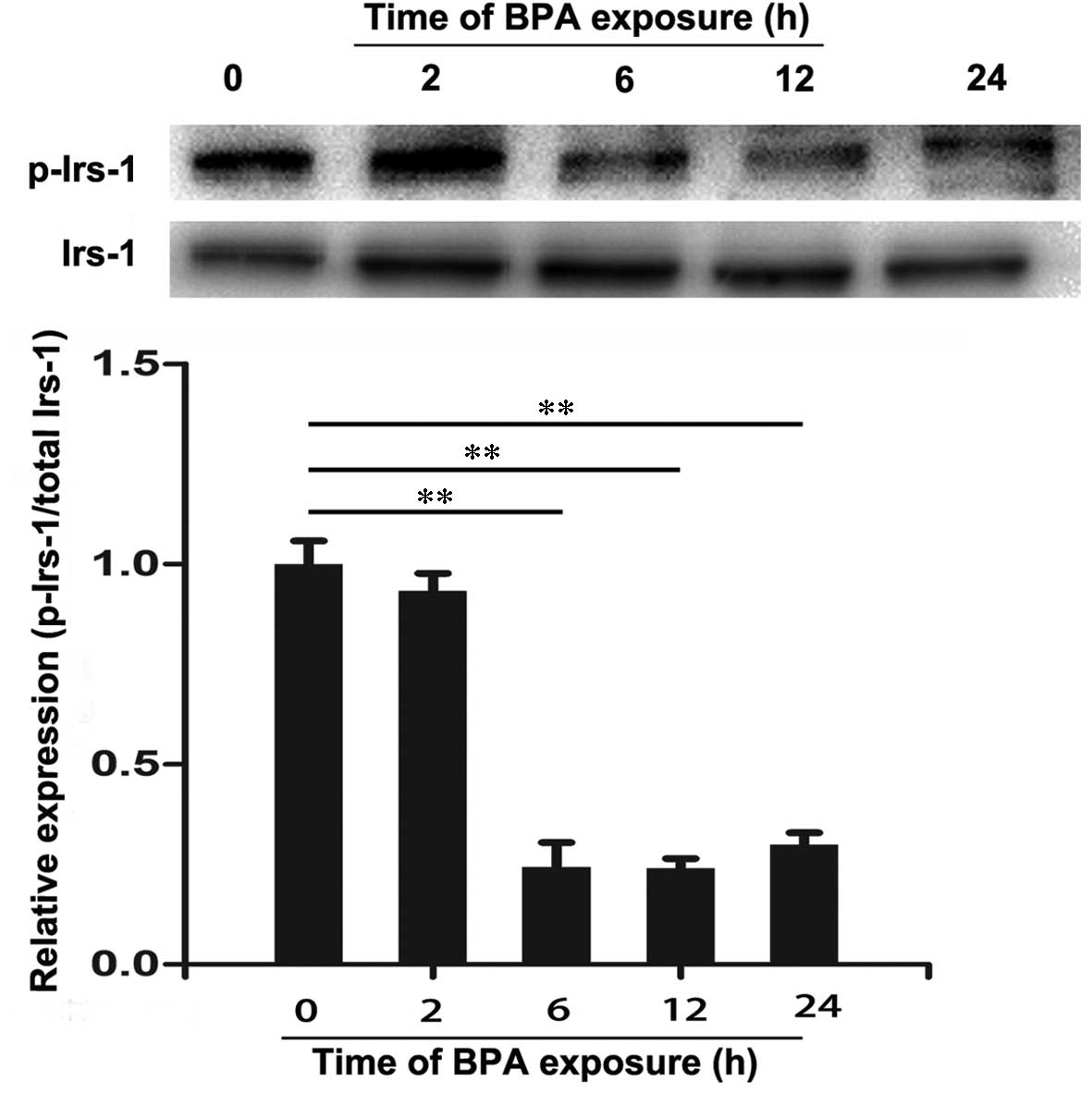
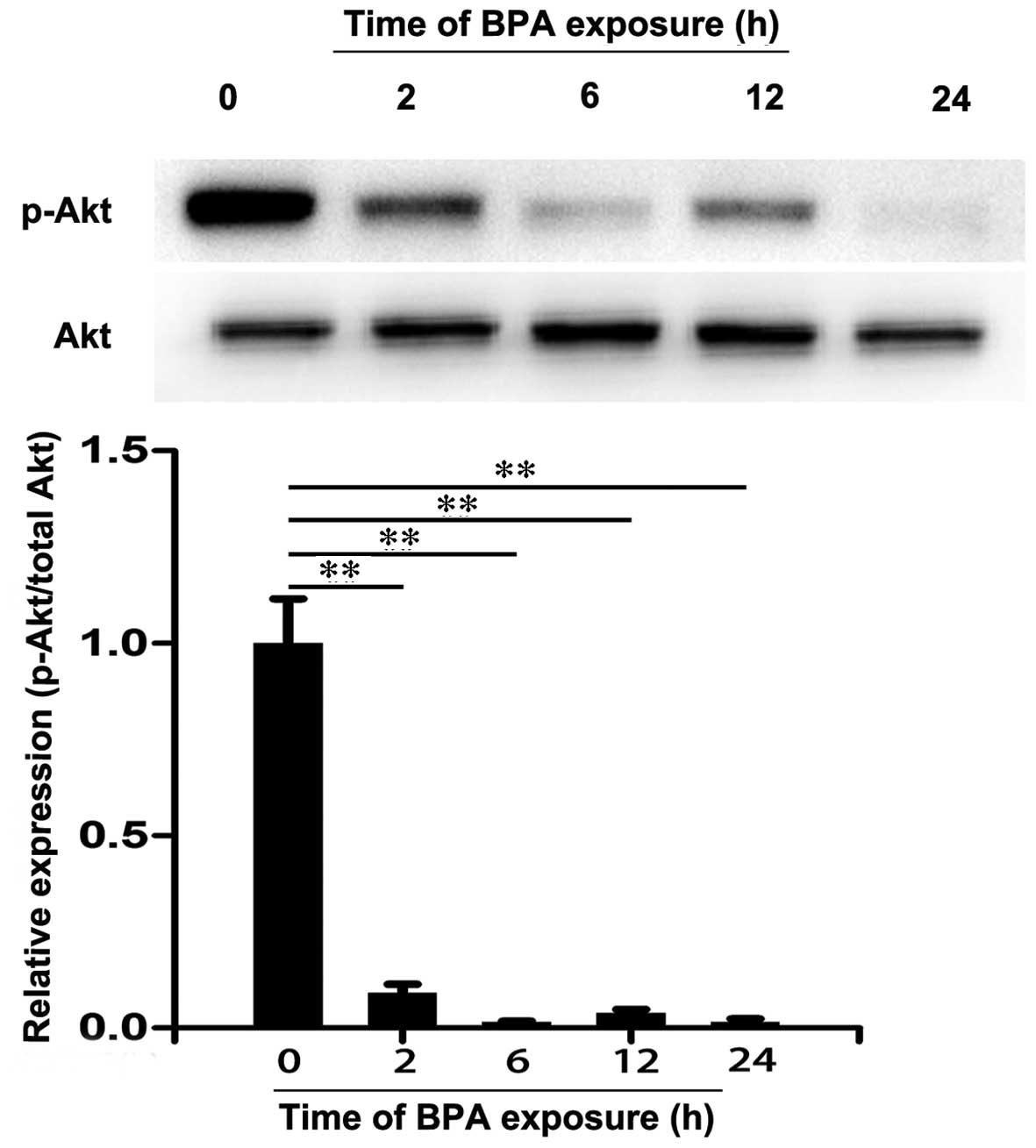
3T3-L1 adipocytes were treated with 80 µM BPA
for 0, 2, 6, 12 and 24 h and the expression levels of IRS-1,
p-IRS-1, Akt and p-Akt were analyzed by western blotting. As
presented in Fig. 3, there were no
significant differences in the protein expression levels of IRS-1
among these groups. However, BPA decreased the expression levels of
p-IRS-1 at 6 h of treatment compared with 0 h (P<0.01). Similar
effects were also observed in the level of Akt (Fig. 4). However, a significant decrease
in expression levels of p-Akt was observed following treatment with
BPA for 2 to 24 h (P<0.01; Fig.
4). These results suggest that BPA markedly decreased the
expression levels of insulin signaling molecules in 3T3-L1
adipocytes.
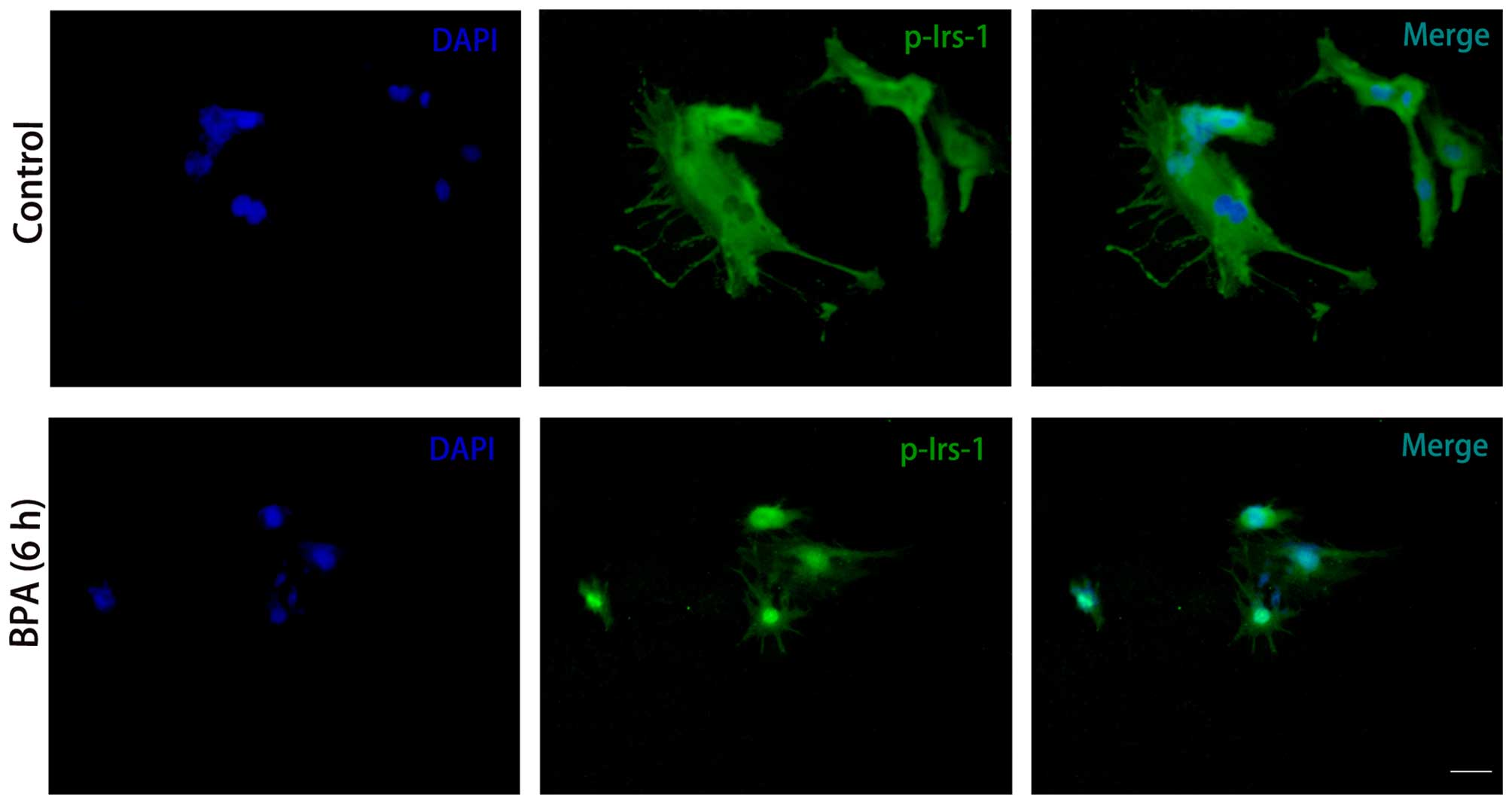
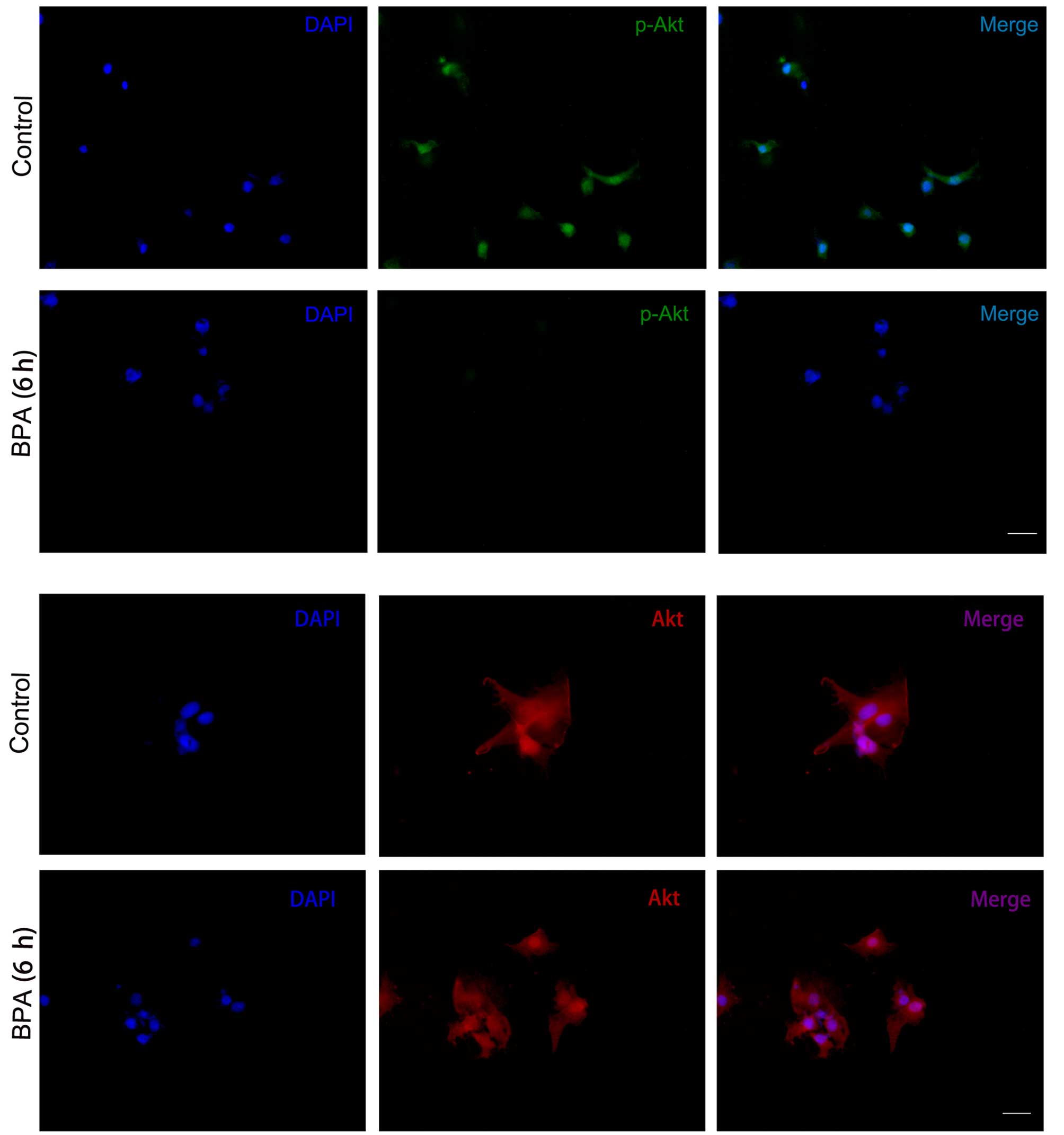
BPA decreased expression levels of
p-IRS-1 and p-Akt
To further elucidate the effect of BPA,
immunocytochemistry was conducted to investigate the expression
levels of the insulin signaling molecules. Consistent with the
results from the western blotting, p-IRS-1 (Fig. 5) and p-Akt (Fig. 6) expression levels were markedly
decreased in BPA-treated cells compared with control cells, while
expression levels of Akt did not exhibit a marked change (Fig. 6).
Discussion
Adipose tissue is important in insulin sensitivity
and basal metabolic rate. Thus, 3T3-L1 adipocytes were selected to
investigate the effects of BPA on SOCS-3 and insulin signaling
transduction. In the current study, it was observed that BPA
significantly increased SOCS-3 secretion in 3T3-L1 adipocytes
(P<0.01) and decreased the expression of key molecules involved
in the IRS-1/PI3K/Akt signaling pathway.
BPA, in addition to other environmental estrogens,
has become a public health concern due to deleterious effects on
energy balance and glucose homeostasis (19). The present study indicates that BPA
exposure impairs insulin signaling in peripheral tissues and may be
a risk factor for the development of type 2 diabetes (20). During the early stages of life, BPA
exposure may impair pancreatic development and result in adults
susceptible to diabetes (21). In
epidemiological studies in humans, >93% of US adults have
detectable BPA levels in urine, higher levels are particularly
observed in the population with diabetes, hypertension and obesity
(22). In animal models, BPA
exposure in pregnant rats increased their offspring's body weight,
and the levels of fasting blood glucose and serum insulin, which
may predispose them to IR (23).
Data from a previous study demonstrated that BPA exhibited
estrogen-like activities via binding to estrogen receptors (ERs),
non-classical membrane ERs, G-protein-coupled receptor 30 and
estrogen-related receptors (24).
There were, however, few studies that had investigated the effect
of BPA on insulin signal transduction, thus, the present study
aimed to investigate the association between BPA and the
IRS-1/PI3K/Akt signaling pathway.
As previously described, IR may be induced by the
inhibition of insulin signaling transduction. In the current study,
the results of the western blotting indicated that BPA
significantly decreased the expression levels of p-IRS-1 and p-Akt
(P<0.01), which are key in insulin-stimulated glucose transport
(25). The decrease in protein
expression levels of p-IRS-1 and p-Akt were further shown by
immunocytochemistry. In vitro, the cellular uptake of
glucose into the cells by glucose transporters requires insulin and
receptor-mediated tyrosine phosphorylation of IRS-1 (26), which is key in insulin signal
transduction and affects insulin signaling by regulating protein
presentation, post-translational modification and subcellular
localization of proteins, particularly in
phosphorylation/dephosphorylation of post-translational
modification (27). IRS-1 is
closely associated with PI3K activation, which is responsible for
activation of the Akt signaling cascade (28). It is generally accepted that
impaired tyrosine phosphorylation of IRS-1 is responsible for
reduced insulin signaling and impaired downstream PI3K/Akt signal
transduction (29). The
downregulated phosphorylation of Akt resulting from attenuated
tyrosine phosphorylation of IRS-1 may impair GLUT4 translocation
and glucose uptake. A previous study has indicated that
insulin-stimulated Akt phosphorylation was suppressed in skeletal
muscle and livers of BPA-treated pregnant mice, these mice then
suffered from metabolic disorders associated with glucose
homeo-stasis (30). The present
study suggests that 80 µM BPA may inhibit the IRS-1/PI3K/Akt
signaling pathway, which results in IR.
To further investigate the underlying mechanisms of
BPA induced impairment of insulin signaling transduction, the mRNA
and protein expression levels of SOCS-3 were investigated by
RT-qPCR and western blotting. The results demonstrated that BPA
markedly increased SOCS-3 mRNA and protein expression levels in a
time-dependent manner. In addition, it was observed that tyrosine
phosphorylation of IRS-1 and serine phosphorylation of Akt was
decreased as demonstrated by a decrease in the expression levels of
these proteins following the treatment with BPA. This was
consistent with the increased expression levels of SOCS-3 at the
same time points.
SOCS-3 is one member of the SOCS protein family,
which is overexpressed in insulin-sensitive tissues from patients
with type 2 diabetes and IR and animal models of the conditions
(31,32). Previous studies have demonstrated
SOCS-3 binds via the SH2 domain to tyrosine phosphorylation sites
on cytokine receptors to inhibit inflammatory signal transduction
(33). In the skeletal muscle of
obese Zucker rats, SOCS-3 protein concentration and co-localization
of SOCS-3 with IRS-1 is notably increased, while tyrosine
phosphorylation of IRS-1 was decreased and serine phosphorylation
of IRS-1 was increased (34).
Furthermore, mice with muscle-specific deletion of SOCS-3 were
protected against the development of hyperinsulinemia and IR due to
enhanced skeletal muscle IRS-1 and Akt phosphorylation (16). Similarly, genetic deletion of
SOCS-3 from mouse liver also results in enhanced insulin signaling
due to increased IRS-1 phosphorylation (35). These studies suggest SOCS-3
interferes with insulin signaling and results in IR by inhibiting
tyrosine phosphorylation of IRS-1.
In conclusion, BPA significantly increases mRNA and
protein expression levels of SOCS-3 and decreases the
phosphorylation of IRS-1 and Akt. Based on these results, the
present study hypothesizes that BPA may inhibit insulin signal
transmission and lead to the development of IR via promoting the
expression of SOCS-3 and preventing tyrosine phosphorylation of
IRS-1. The present study provides a novel insight into the
mechanism by which BPA induces IR.
Acknowledgments
The present study was supported by grants from the
National Key Basic Research Program of China (grant no.
2013CB530604), the National Natural Science Foundation of China
(grant nos. 30973231 and 81270928), the Talent Foundation of
Jiangsu Province, China (grant no. QRX11051), and the Science and
Technology Development Foundation of Nanjing Medical University
(grant no. 2014NLMU144).
References
|
1
|
Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y,
Liang D, Zhang R, Zhang S, Wang H and Cao F: Apelin stimulates
glucose uptake through the PI3K/Akt pathway and improves insulin
resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 353:305–313.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Avogaro A, de Kreutzenberg SV and Fadini
GP: Oxidative stress and vascular disease in diabetes: Is the
dichotomization of insulin signaling still valid? Free Radic Biol
Med. 44:1209–1215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galadari S, Rahman A, Pallichankandy S,
Galadari A and Thayyullathil F: Role of ceramide in diabetes
mellitus: Evidence and mechanisms. Lipids Health Dis. 12:982013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esposito DL, Li Y, Cama A and Quon MJ:
Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are
important for full activation of insulin-stimulated
phosphatidylinositol 3-kinase activity and translocation of GLUT4
in adipose cells. Endocrinology. 142:2833–2840. 2001.PubMed/NCBI
|
|
5
|
Tsai CW, Liu KL, Lin YR and Kuo WC: The
mechanisms of carnosic acid attenuates tumor necrosis
factor-α-mediated inflammation and insulin resistance in 3T3-L1
adipocytes. Mol Nutr Food Res. 58:654–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi K and Kim YB: Molecular mechanism of
insulin resistance in obesity and type 2 diabetes. Korean J Intern
Med. 25:119–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padmanabhan V, Sarma HN, Savabieasfahani
M, Steckler TL and Veiga-Lopez A: Developmental reprogramming of
reproductive and metabolic dysfunction in sheep: Native steroids
vs. environmental steroid receptor modulators. Int J Androl.
33:394–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shankar A and Teppala S: Relationship
between urinary bisphenol A levels and diabetes mellitus. J Clin
Endocrinol Metab. 96:3822–3826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y,
Lu J, Chen Y, Wang W, Li X, et al: Urinary bisphenol A (BPA)
concentration associates with obesity and insulin resistance. J
Clin Endocrinol Metab. 97:E223–E227. 2012. View Article : Google Scholar
|
|
10
|
Ropero AB, Alonso-Magdalena P,
Garcia-Garcia E, Ripoll C, Fuentes E and Nadal A: Bisphenol-A
disruption of the endocrine pancreas and blood glucose homeostasis.
Int J Androl. 31:194–200. 2008. View Article : Google Scholar
|
|
11
|
Sakurai K, Kawazuma M, Adachi T, Harigaya
T, Saito Y, Hashimoto N and Mori C: Bisphenol A affects glucose
transport in mouse 3T3-F442A adipocytes. Br J Pharmacol.
141:209–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kidani T, Kamei S, Miyawaki J, Aizawa J,
Sakayama K and Masuno H: Bisphenol A downregulates Akt signaling
and inhibits adiponectin production and secretion in 3T3-L1
adipocytes. J Atheroscler Thromb. 17:834–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sargis RM, Johnson DN, Choudhury RA and
Brady MJ: Environmental endocrine disruptors promote adipogenesis
in the 3T3-L1 cell line through glucocorticoid receptor activation.
Obesity (Silver Spring). 18:1283–1288. 2010. View Article : Google Scholar
|
|
14
|
Luo Z, Zhang Y, Li F, He J, Ding H, Yan L
and Cheng H: Resistin induces insulin resistance by both
AMPK-dependent and AMPK-independent mechanisms in HepG2 cells.
Endocrine. 36:60–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghanim H, Abuaysheh S, Sia CL,
Korzeniewski K, Chaudhuri A, Fernandez-Real JM and Dandona P:
Increase in plasma endotoxin concentrations and the expression of
toll-like receptors and suppressor of cytokine signaling-3 in
mononuclear cells after a high-fat, high-carbohydrate meal:
Implications for insulin resistance. Diabetes Care. 32:2281–2287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jorgensen SB, O'Neill HM, Sylow L,
Honeyman J, Hewitt KA, Palanivel R, Fullerton MD, Öberg L,
Balendran A, Galic S, et al: Deletion of skeletal muscle SOCS3
prevents insulin resistance in obesity. Diabetes. 62:56–64. 2013.
View Article : Google Scholar
|
|
17
|
Xie XY, Kong PR, Wu JF, Li Y and Li YX:
Curcumin attenuates lipolysis stimulated by tumor necrosis factor-α
or isoproterenol in 3T3-L1 adipocytes. Phytomedicine. 20:3–8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Wei J, Lin Y, Li Y, Ying C, Chen J, Song
L, Zhou Z, Lv Z, Xia W, Chen X and Xu S: Perinatal exposure to
bisphenol A at reference dose predisposes offspring to metabolic
syndrome in adult rats on a high-fat diet. Endocrinology.
152:3049–3061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batista TM, Alonso-Magdalena P, Vieira E,
Amaral ME, Cederroth CR, Nef S, Quesada I, Carneiro EM and Nadal A:
Short-term treatment with bisphenol-A leads to metabolic
abnormalities in adult male mice. PloS One. 7:e338142012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F,
Wang J and Xiao H: Perinatal bisphenol A exposure and adult glucose
homeostasis: Identifying critical windows of exposure. PloS One.
8:e641432013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shankar A and Teppala S: Urinary bisphenol
A and hypertension in a multiethnic sample of US adults. J Environ
Public Health. 2012:4816412012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Ma C, Wen Z, Zhang L, Zhang Z and
Jia L: Effect of bisphenol A exposure during early development on
body weight and glucose metabolism of female filial rats. Wei Sheng
Yan Jiu. 41:543–545. 2012.In Chinese.
|
|
24
|
Ben-Jonathan N, Hugo ER and Brandebourg
TD: Effects of bisphenol A on adipokine release from human adipose
tissue: Implications for the metabolic syndrome. Mol Cell
Endocrinol. 304:49–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jayashree S, Indumathi D, Akilavalli N,
Sathish S, Selvaraj J and Balasubramanian K: Effect of Bisphenol-A
on insulin signal transduction and glucose oxidation in liver of
adult male albino rat. Environ Toxicol Pharmacol. 35:300–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Cruz SC, Jubendradass R, Jayakanthan M,
Rani SJ and Mathur PP: Bisphenol A impairs insulin signaling and
glucose homeostasis and decreases steroidogenesis in rat testis: An
in vivo and in silico study. Food Chem Toxicol. 50:1124–1133. 2012.
View Article : Google Scholar
|
|
27
|
Copps KD and White MF: Regulation of
insulin sensitivity by serine/threonine phosphorylation of insulin
receptor substrate proteins IRS1 and IRS2. Diabetologia.
55:2565–2582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo S: Insulin signaling, resistance and
the metabolic syndrome: Insights from mouse models into disease
mechanisms. J Endocrinol. 220:T1–T23. 2014. View Article : Google Scholar
|
|
29
|
Schenk S, Saberi M and Olefsky JM: Insulin
sensitivity: Modulation by nutrients and inflammation. J Clin
Invest. 118:2992–3002. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alonso-Magdalena P, Vieira E, Soriano S,
Menes L, Burks D, Quesada I and Nadal A: Bisphenol A exposure
during pregnancy disrupts glucose homeostasis in mothers and adult
male offspring. Environ Health Perspect. 118:1243–1250. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lebrun P and Van Obberghen E: SOCS
proteins causing trouble in insulin action. Acta Physiol (Oxf).
192:29–36. 2008. View Article : Google Scholar
|
|
32
|
Zheng YY, Wang LF, Fan XH, Wu CH, Huo N,
Lu HY, Xu XY and Wei L: Association of suppressor of cytokine
signalling 3 polymorphisms with insulin resistance in patients with
chronic hepatitis C. J Viral Hepat. 20:273–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suchy D, Łabuzek K, Machnik G, Kozłowski M
and Okopień B: SOCS and diabetes-ups and downs of a turbulent
relationship. Cell Biochem Funct. 31:181–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zolotnik IA, Figueroa TY and Yaspelkis BB
III: Insulin receptor and IRS-1 co-immunoprecipitation with SOCS-3
and IKKα/β phosphorylation are increased in obese Zucker rat
skeletal muscle. Life Sci. 91:816–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sachithanandan N, Fam BC, Fynch S, Dzamko
N, Watt MJ, Wormald S, Honeyman J, Galic S, Proietto J,
Andrikopoulos S, et al: Liver-specific suppressor of cytokine
signaling-3 deletion in mice enhances hepatic insulin sensitivity
and lipogenesis resulting in fatty liver and obesity. Hepatology.
52:1632–1642. 2010. View Article : Google Scholar : PubMed/NCBI
|