Introduction
Atherosclerosis is a complex pathology involving
several processes, including subendothelial retention of
atherogenic lipoproteins, oxidative stress, inflammation and
cellular proliferation (1).
Vascular smooth muscle cells (VSMCs) may contribute to the
development of atherosclerosis through the production of
inflammatory cytokines, such as monocyte chemoattractant protein-1,
and the synthesis of matrix proteins (2).
Reactive oxygen species (ROS), for example
superoxide anion (O2−) and hydrogen peroxide
(H2O2), are physiological and
pathophysiological signaling molecules that participate in the
development of atherosclerosis (3,4).
Excessive production of ROS, partially through upregulation of DNA
damage pathways, is a central mechanism that mediates pathological
activation of VSMCs. In addition, ROS activate multiple
pro-inflammatory transcription factors, including nuclear factor
erythroid 2-related factor 2, nuclear factor-κB (NF-κB), and
activator protein 1, which regulate the expression of adhesion
molecules and chemokines in VSMCs (5). Therefore, targeting ROS is an
important therapeutic strategy for atherosclerosis.
Tormentic acid (TA) is a triterpene isolated from
the stem bark of the plant Vochysia divergens. Previous
studies have demonstrated that TA has anticancer, anti-oxidant,
anti-inflammatory and hypoglycemic properties (6–9). TA
was demonstrated to suppress high-fat diet-induced diabetes and
hyperlipidemia via glucose transporter 4 and adenosine
monophosphate-activated protein kinase phosphorylation (10). Fogo et al (11) previously reported that TA
significantly reduced VSMC proliferation and survival. In addition,
TA inhibited lipopolysaccharide-induced inducible nitric oxide
synthase (iNOS), cyclooxygenase-2, and tumor necrosis factor-α
(TNF-α) expression in RAW264.7 cells (12). However, the impact of TA on
H2O2-induced oxidative stress and
inflammation in rat VSMCs (RVSMCs) remains unclear. Therefore, the
aim of the present study was to investigate whether TA suppressed
H2O2-induced oxidative stress and
inflammation in RVSMCs, and to determine the molecular
mechanisms.
Materials and methods
Animal and RVSMC preparation
Female Sprague Dawley (SD) rats (age, 6 weeks;
weight, 180–200 g) were obtained from the Animal Breeding Center of
the People's Hospital of Tianjin City (Tianjin, China). They were
housed in barrier facilities with 12 h light/dark cycles at 22±2°C,
and had access to laboratory chow (Jiangsu Xietong Medicine
Biological Engineering Co., Ltd., Jiangsu, China) and tap water
ad libitum. After 1 week of feeding, the animals were
anesthetized by subcutaneous injection of sodium pentobarbital (40
mg/kg body weight). All experiments were performed in accordance
with the institutional guidelines for animal care. This study was
approved by the ethics committee of the People's Hospital of
Tianjin City (Tianjin, China).
RVSMCs were enzymatically isolated from the aortas
of female SD rats according to the methods described in a previous
study (13), and were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany),
100 U/ml penicillin, 100 µg/ml streptomycin and 200 mM
L-glutamine in a humidified 5% CO2 atmosphere at 37°C.
For all experiments, RVSMCs (used at passages 5–8) were cultured to
70–80% confluence and serum-starved in DMEM without FBS for 24
h.
H2O2-induced
oxidant stress
Cells were pretreated with various concentrations of
TA (12.5, 25 and 50 µM; Shaanxi Institute for Food and Drug
Control, Shaanxi, China) for 2 h, followed by the addition of
H2O2 (100 µM final concentration) for
a further 24 h. Controls performed were 2 h TA pretreatment without
H2O2 stimulation, and 24 h
H2O2 treatment without 2 h TA
pretreatment.
Cell viability assay
Cell viability was assessed by Cell Counting Kit-8
(CCK-8) assay (Beyotime Institute of Biotechnology, Shanghai,
China). In brief, RVSMCs were seeded into 96-well plates at
1×104 cells per well and cultured for 24 h to adhere.
Following the described H2O2 and TA
treatments, 10 µl CCK-8 reagent was added to each well and
the cells incubated for a further 2 h. Finally, the absorbance was
read at 570 nm (A570) using a Bio-Rad enzyme-linked
immunosorbent assay (ELISA) microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The viability of cells was
calculated as (A570 of treated groups/A570 of
control group) ×100%.
Measurement of cellular ROS levels
RVSMCs were stained with 5 µM dihydroethidium
(DHE; Molecular Probes; Thermo Fisher Scientific, Inc.) for 30 min
at 37°C. Fluorescence of DHE was measured with a fluorescence
microscope (excitation wavelength 488 nm and emission wavelength
585 nm), quantified using ImageJ software (version, 1.46; National
Institutes of Health, Bethesda, MD, USA).
ELISA assay
Following RVSMC incubation with TA (12.5, 25 and 50
µM) for 24 h, the cells were exposed to
H2O2 for a further 2 h. Samples of the
supernatant were collected from each well to measure TNF-α (cat.
no. RAB0479; Sigma-Aldrich), interleukin (IL)-6 (cat. no. 10406;
Sigma-Aldrich) and IL-1β (cat. no. RAB0278; Sigma-Aldrich) levels
by ELISA.
Western blot analysis
Cell lysate was prepared from RVSMCs using lysis
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). Equal
amounts of protein samples (30 µg total protein per lane)
were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 10% gels and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). After
blocking with 2% non-fat dry milk in Tris-buffered saline (TBS) for
1 h at room temperature, the membranes were probed with the
following primary antibodies in TBS plus 0.1% Tween-20 (TBST)
containing 5% BSA (Sigma-Aldrich; Merck Millipore) at 4°C
overnight: Mouse anti-rabbit nitric oxide synthase (NOS; cat. no.
N7782; dilution, 1:1,000; Sigma-Aldrich; Merck Millipore); mouse
anti-rabbit nicotinamide adenine dinucleotide phosphate (NADPH)
-oxidase 1 (NOX1; cat. no. SAB2108601; dilution, 1:2,000;
Sigma-Aldrich; Merck Millipore); mouse anti-rabbit neutrophil
cytosolic factor 1 (p47phox; cat. no. sc-14015; dilution, 1:1,500;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA); and mouse
anti-rabbit glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat.
no. sc-25778; dilution, 1:1,500; Santa Cruz Biotechnology, Inc.).
Membranes were then washed three times in TBST for 5 min per wash
before they were incubated with goat anti-rabbit horseradish
peroxidase-conjugated anti-IgG secondary antibody (cat. no.
sc-2054; dilution, 1:3,000; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Immune complexes were visualized using
enhanced chemiluminescence reagent (Gibco; Thermo Fisher
Scientific, Inc.). Developed films were scanned, and the optical
densities were analyzed using ImageJ software (version, 1.37;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical differences were analyzed using one-way analysis of
variance, followed by Dunnett's multiple comparison post-hoc test.
Differences in the cumulative clinical score were analyzed using
the non-parametric Mann-Whitney test. P<0.05 was considered to
indicate a statistically significant difference.
Results
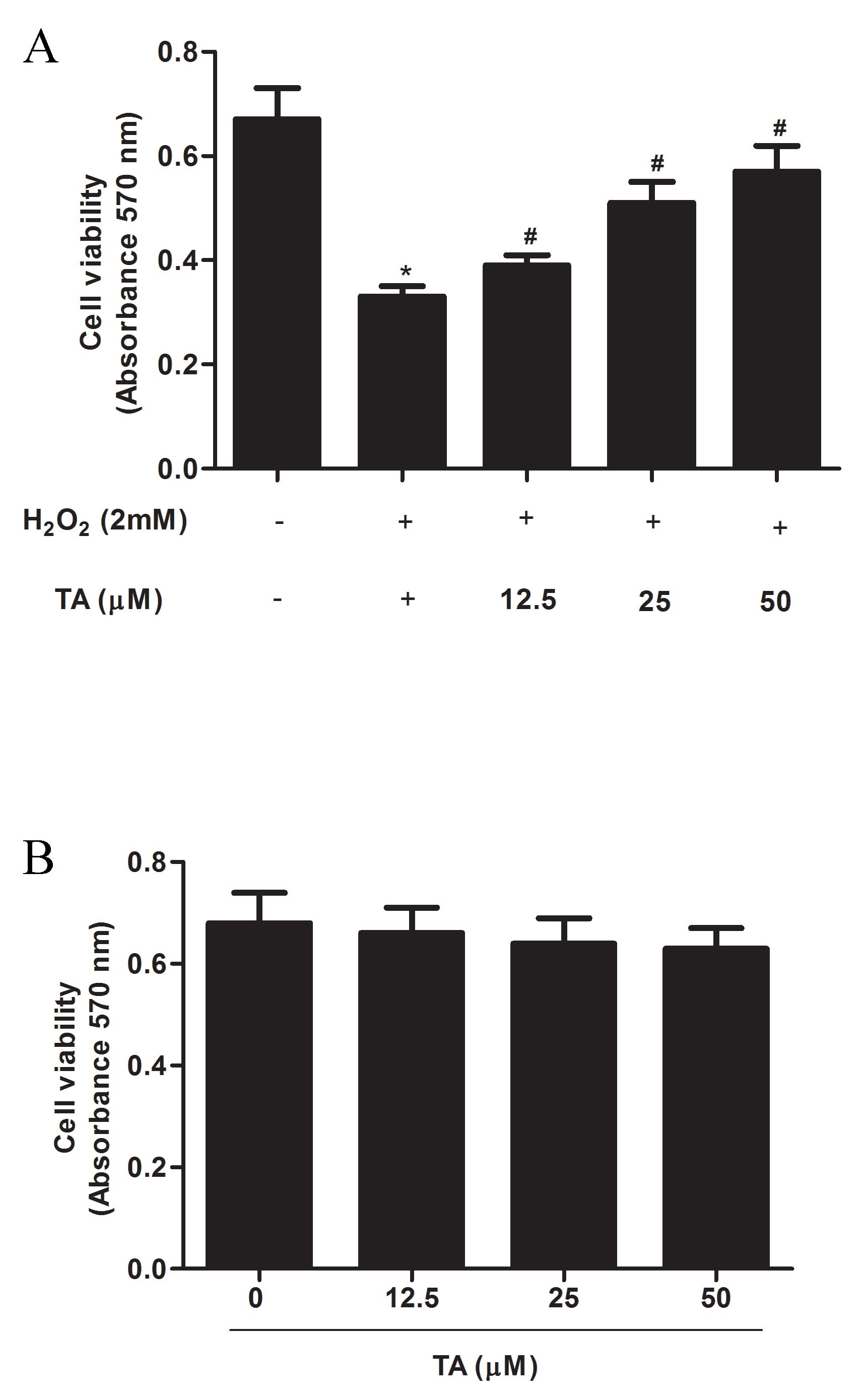
Effects of TA on RVSMC viability
The effect of TA on cell viability in
H2O2-induced RVSMCs was examined by CCK-8
assay. The significant decrease in cell viability resulting from
H2O2 treatment (P=0.017) compared with
untreated control; Fig. 1A), was
significantly attenuated by TA i n a dose- dependent manner
(P<0.05; Fig. 1A). To exclude
any proliferative effect of TA from analysis, cell viability was
assessed following TA treatment alone, and was demonstrated to be
unaffected by treatment with TA at any of the concentrations tested
(12.5, 25, and 50 µM) compared with untreated control
(Fig. 1B). Thus, the
concentrations 12.5, 25 and 50 µM were used in subsequent
experiments.
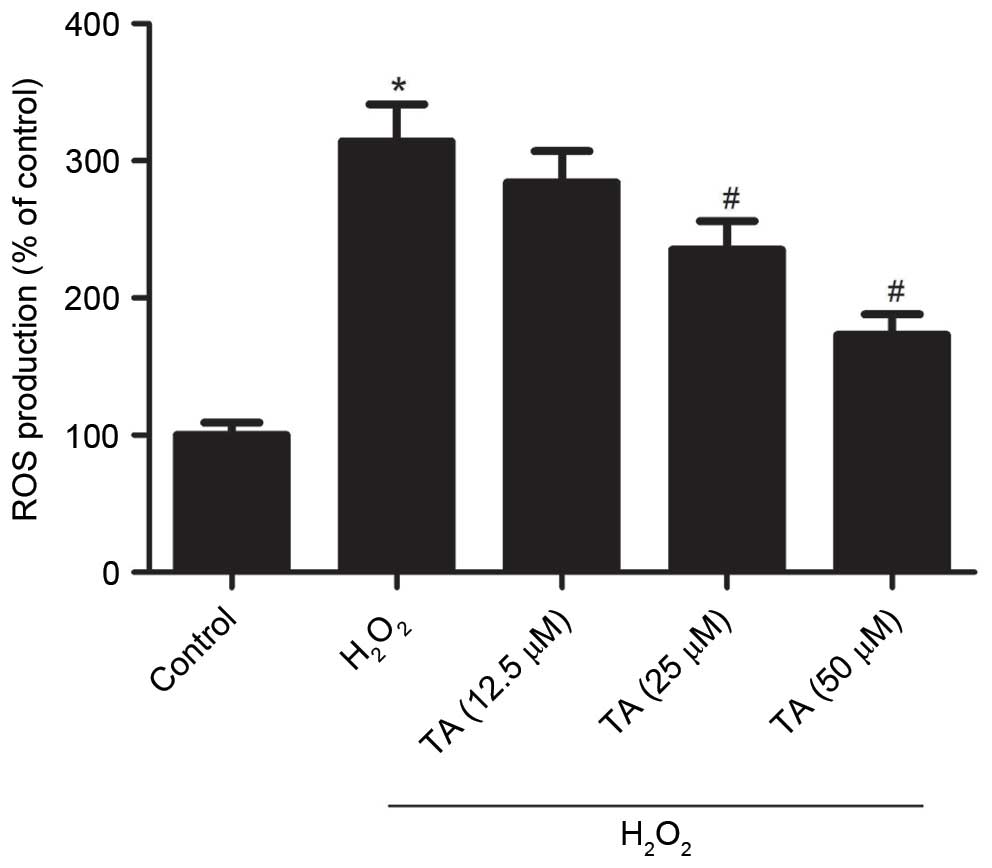
Effect of TA on ROS generation in RVSMCs
exposed to H2O2
As increased ROS levels, resulting in oxidative
stress, are considered to be important in the pathogenesis of
atherosclerosis, the effect of TA on ROS generation in RVSMCs
exposed to H2O2 was investigated. Treatment
with H2O2 for 2 h significantly increased the
production of ROS compared with untreated control cells (P=0.019;
Fig. 2). However, pretreatment
with 25 µM (P=0.041) and 50 µM TA (P=0.024)
significantly inhibited ROS generation compared with cells treated
with H2O2 only (Fig. 2).
Effect of TA on iNOS, NOX1 and p47phox
protein expression in RVSMCs exposed to
H2O2
The effect of TA on iNOS, NOX1 and p47phox protein
expression levels were evaluated by western blot analysis in
RVSMCsexposed to H2O2. Treatment with
H2O2 for 2 h significantly increased the
protein expression levels of iNOS (P=0.017), NOX1 (P=0.026) and
p47phox (P=0.031) compared with untreated control cells (F ig. 3A
and B). However, pretreatment with TA significantly inhibited the
H2O2-induced expression of all three proteins
in RVSMCs in a dose-dependent manner, compared with cells treated
with H2O2 only (P<0.05; Fig. 3A and B).
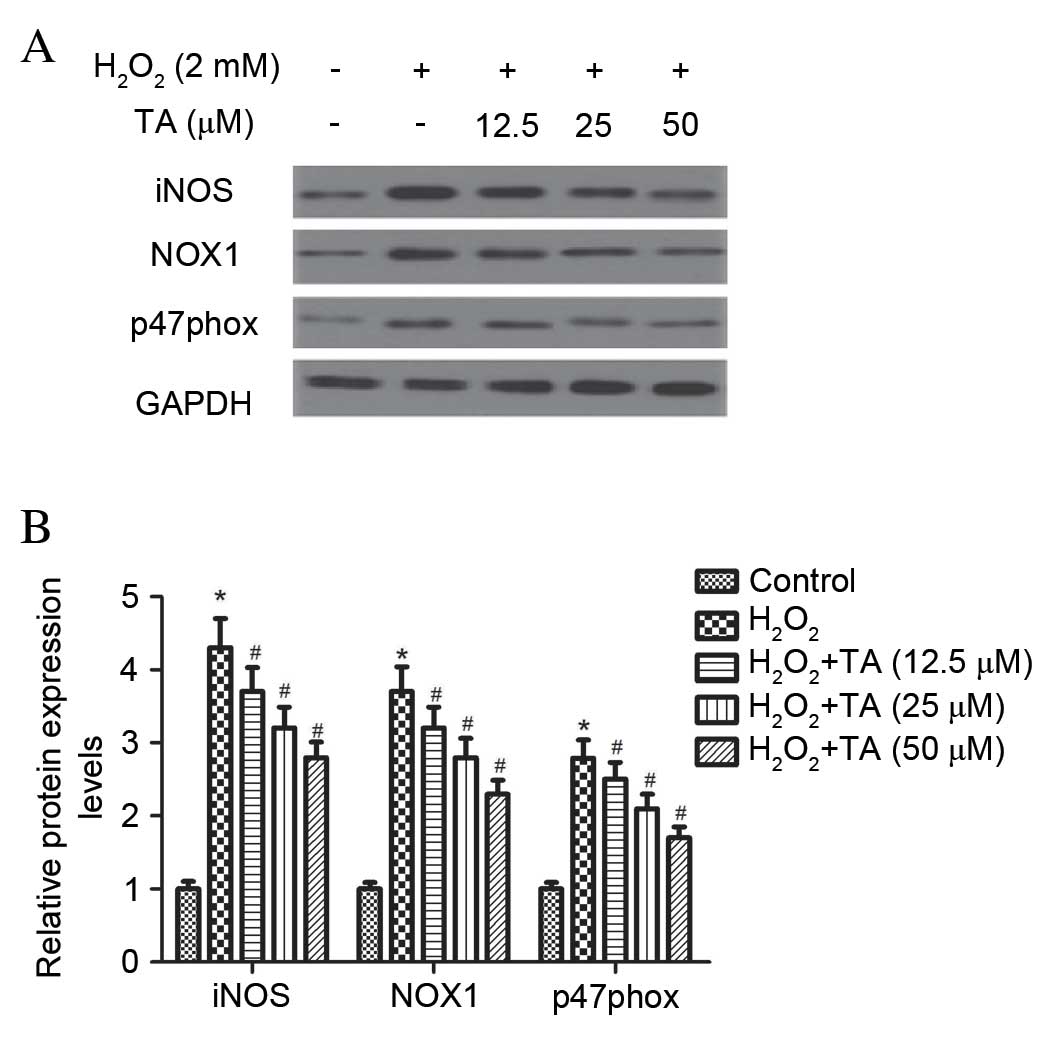 | Figure 3Effect of TA on iNOS and NADPH
oxidase expression in RVSMCs exposed to H2O2.
RVSMCs were pretreated with various concentrations of TA (0, 12.5,
25 and 50 µM) for 2 h, followed by the addition of
H2O2 (100 µM as final concentration)
for another 24 h. (A) Proteins were subjected to western blot
analysis and (B) the relative protein levels of iNOS, p47phox and
NADPH oxidase 1 were analyzed, using GAPDH as the loading control.
Data are presented as the mean ± standard deviation obtained from
five individual experiments performed in triplicate.
*P<0.05 vs. untreated control; #P<0.05
vs. H2O2-treated control. RVSMC, rat vascular
smooth muscle cell; TA, tormentic acid; iNOS, inducible nitric
oxide synthase; NADPH, nicotinamide adenine dinucleotide phosphate;
H2O2, hydrogen peroxide; NOX1, NADPH oxidase
1; p47phox, neutrophil cytosolic factor 1; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Effect of TA on TNF-α, IL-6 and IL-1β
production in RVSMCs induced with H2O2
To investigate the anti-inflammatory effects of TA
on RVSMCs, TNF-α, IL-6 and IL-1β production was evaluated using
ELISA, revealing that H2O2 significantly
increased the production of TNF-α (P= 0.021; Fig. 4A), IL-6 (P=0.016; Fig. 4B) and IL-1β (P=0.031; Fig. 4C) in the RVSMCs, compared with
untreated cells. Compared with cells treated with
H2O2 only, TA significantly decreased the
production of TNF-α (P=0.028; Fig.
4A), IL-6 (P=0.023; Fig. 4B)
and IL-1β (P= 0.016; Fig. 4C) in a
dose-dependent manner.
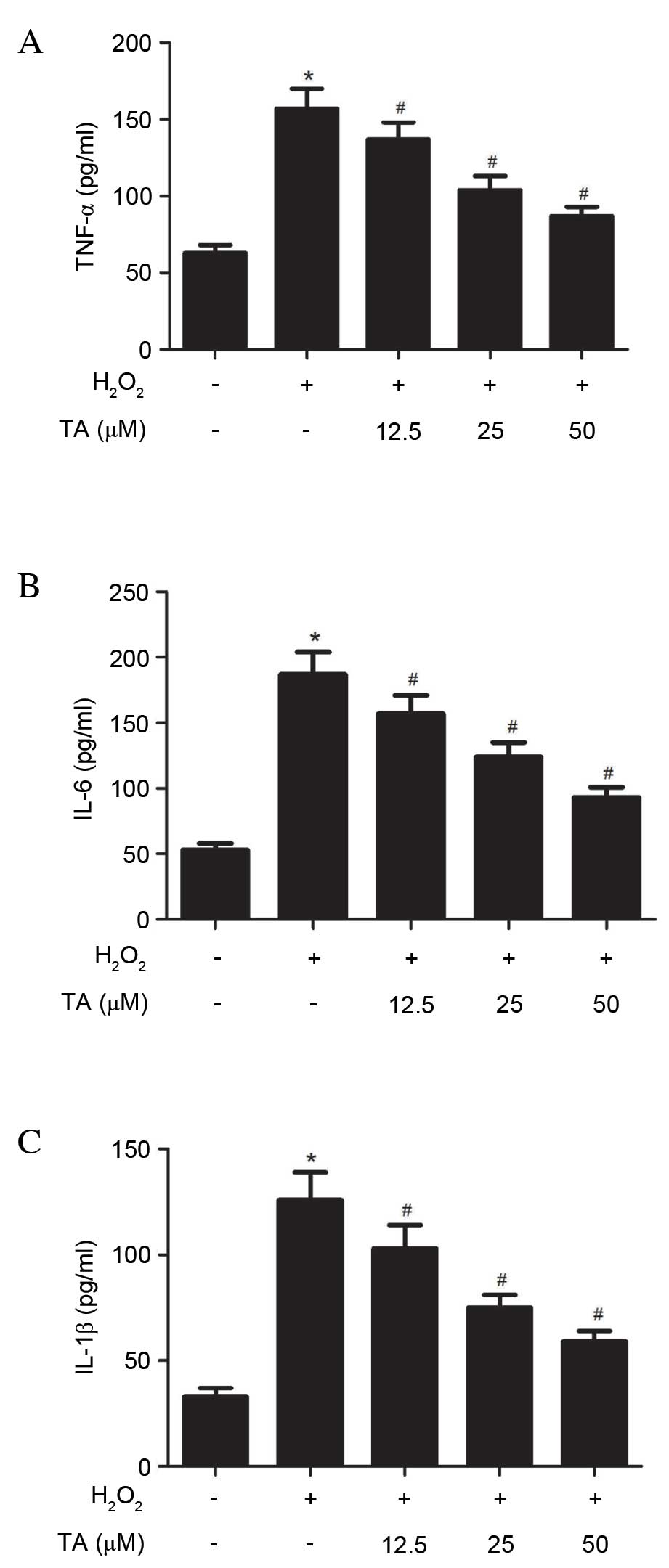 | Figure 4Effects of TA on TNF-α, IL-6 and
IL-1β production in RVSMCs induced with H2O2.
RVSMCs were pretreated with various concentrations of TA (0, 12.5,
25 and 50 µM) for 2 h, followed by treatment with
H2O2 (final concentration 100 µM) for
a further 24 h. Enzyme-linked immunosorbent assay was performed to
quantify (A) TNF-α, (B) IL-6 and (C) IL-1β levels. Data are
presented as the mean ± standard deviation obtained from five
individual experiments performed in triplicate.
*P<0.05 vs. untreated control; #P<0.05
vs. H2O2-treated control. RVSMC, rat vascular
smooth muscle cell; TNF-α, tumor necrosis factor-α;
H2O2, hydrogen peroxide; TA, tormentic acid;
IL, interleukin. |
Effects of TA on NF-ĸB signaling pathway
in H2O2-induced RVSMCs
As NF-ĸB has been previously reported to be
important in the regulation of cytokine production, the effects of
TA on H2O2-induced NF-ĸB activation were
investigated. As demonstrated in Fig.
5, H2O2 significantly increased NF-ĸB p65
phosphorylation (P=0.018; Fig. 5A and
B) and IĸBα degradation (P=0.027; Fig. 5A and C) compared with untreated
cells. However, TA pretreatment prevented NF-ĸB p65 phosphorylation
(P=0.013; Fig. 5A and B) and IĸBα
degradation (P=0.019; Fig. 5A and
C) induced by H2O2 in RVSMCs in a
dose-dependent manner, compared with cells treated with
H2O2 only. No differences in the levels of
NF-κB p65 were observed in H2O2-induced RVSMC
(Fig. 5A; quantitative data not
shown).
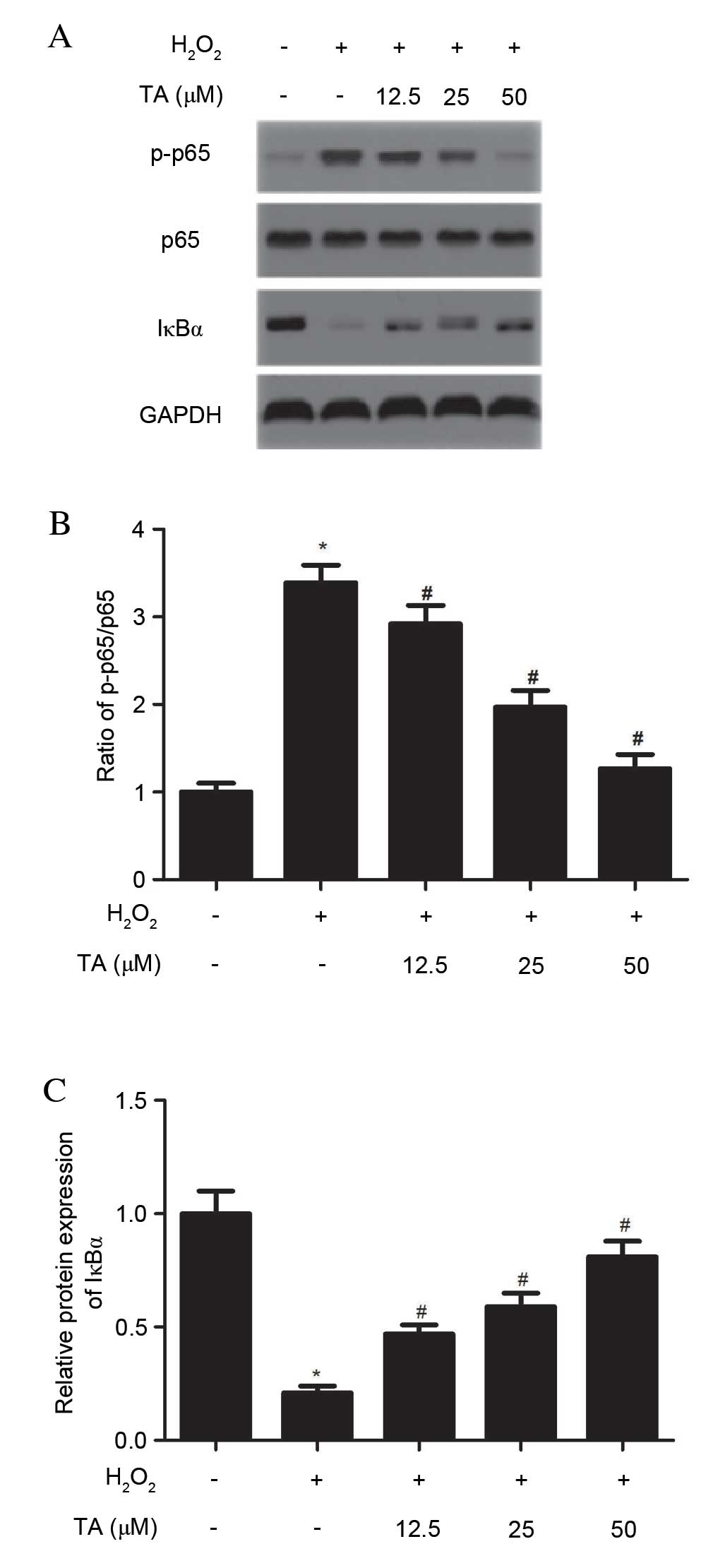 | Figure 5Effects of TA on the NF-ĸB signaling
pathway in H2O2-induced RVSMCs. RVSMCs were
pretreated with various concentrations of TA (0, 12.5, 25 and 50
µM) for 2 h, followed by the addition of
H2O2 (final concentration 100 µM) for
a further 24 h. (A) Proteins were subjected to western blot
analysis to detect relative protein levels of NF-ĸB p65, p-NF-ĸB
p65 and IĸBα, using GAPDH as the loading control. For each
treatment, (B) the ratio of phosphorylated to non-phosphorylated
NF-ĸB p65, and (C) the relative protein levels of IĸBα were
analyzed. Data are presented as the mean ± standard deviation
obtained from five individual experiments performed in triplicate.
*P<0.05 vs. untreated control; #P<0.05 vs.
H2O2-treated control. RVSMC, rat vascular
smooth muscle cell; NF-ĸB, nuclear factor-ĸB;
H2O2, hydrogen peroxide; TA, tormentic acid;
p-, phosphorylated; IĸBα, NF-κB inhibitor-α. |
Discussion
The present study demonstrated that TA inhibits
H2O2-induced ROS generation in RVSMCs, and
H2O2-induced expression of iNOS and NOX1 in
RVSMCs. In addition, TA was demonstrated to significantly decrease
the production of TNF-α, IL-6 and IL-1β. Furthermore, TA
pretreatment reduced NF-ĸB p65 phosphorylation and IĸBα degradation
induced by H2O2 in RVSMCs.
Oxidative stress is frequently involved in
cardiovascular disease and is a common feature of early
stage-atherosclerosis as a response to vascular injury (14,15).
H2O2 has previously been demonstrated to
activate signaling pathways to stimulate ROS production in vascular
cells (16–18). The present study demonstrated that
treatment with TA significantly inhibits the generation of ROS
induced by H2O2 i n RVSMCs.
Previous studies have reported that iNOS may
exacerbate atherosclerosis, as ApoE−/− mice lacking the
iNOS gene exhibit decreased atherosclerotic lesion formation
compared with ApoE−/− mice (19). NOXs are transmembrane enzymes that
transport electrons from cytoplasmic NADPH to molecular oxygen,
leading to superoxide generation, and are therefore an important
source of vascular ROS (20).
Several studies have demonstrated that NOX1 expression is increased
in atherosclerosis (21,22), and that p47phox is an essential
component of NOX (23). In smooth
muscle cells, H2O2 activates NOX, resulting
in the production of O2−, and, consequently,
oxidant-induced injury (24).
Similarly, the present study observed that
H2O2 significantly increases the expression
of iNOS, NOX1 and p47phox in RVSMCs, whereas pretreatment with TA
significantly abrogates this effect.
Inflammatory cytokines are involved in the early
stages of atherosclerosis (25).
TNF-α is the earliest and primary endogenous mediator in the
process of inflammation, and is involved in promoting inflammatory
cell infiltration, injuring vascular endothelial cells and
stimulating the generation of ROS (26). IL-1β is one of the most potent
pro-inflammatory cytokines and endogenous pyrogens, and stimulates
the acute phase response (27). In
response to oxidative stimuli, VSMCs undergo a phenotypic change to
a 'proliferative, migrating and synthetic' state, characterized by
excess extracellular matrix and inflammatory cytokine production
(28). In accordance with these
results, the present study demonstrated that
H2O2 significantly increases the production
of TNF-α, IL-6 and IL-1β in RVSMCs. However, TA significantly
attenuated H2O2-induced production of TNF-α,
IL-6 and IL-1β i n RVSMCs.
NF-ĸB represents a family of transcription factors,
including p50 and p65 that are important in the regulation of
inflammatory responses (29). In
response to oxidative stress, Activated IκB kinase phosphorylates
the NF-κB inhibitor, IκB, resulting in its polyubiquitination and
proteasomal degradation (30). IκB
degradation leads to the translocation of NF-κB p50 and p65 to the
nucleus, which results in the transcription of a variety of genes
participating in diverse cellular processes, including
inflammation, proliferation, apoptosis, and cellular senescence
(31). Pierce et al
(32) observed that the NF-κB
inhibitor, salsalate, increases IκB expression levels, and
decreases the levels of NF-κB and p47phox NADPH oxidase subunit in
endothelial cells. The activation of NF-κB by ROS has also
previously been demonstrated to induce TNF-α, IL-6 and IL-1β
release in VSMCs (33). The
present study demonstrated that pretreatment of RVSMCs with TA
prevents H2O2-induced NF-ĸB p65
phosphorylation and IĸBα degradation in a dose-dependent manner.
This suggests that TA may reduce H2O2-induced
ROS generation through the action of NOX, and reduces TNF-α, IL-6
and IL-1β protein expression levels and induction of iNOS through
inhibition of NF-ĸB signaling activation.
In conclusion, the present study demonstrated that
TA inhibits H2O2-induced oxidative stress and
inflammation in RVSMCs via inhibition of the NF-ĸB signaling
pathway. TA may, therefore, have potential as a pharmacological
agent in the prevention or treatment of atherosclerosis.
References
|
1
|
Insull W Jr: The pathology of
atherosclerosis: Plaque development and plaque responses to medical
treatment. Am J Med. 122(Suppl1): S3–S14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
3
|
Al Ghouleh I, Khoo NK, Knaus UG,
Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM,
Kelley EE, Bauer PM, et al: Oxidases and peroxidases in
cardiovascular and lung disease: New concepts in reactive oxygen
species signaling. Free Radic Biol Med. 51:1271–1288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Csányi G, Taylor WR and Pagano PJ: NOX and
inflammation in the vascular adventitia. Free Radic Biol Med.
47:1254–1266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clempus RE and Griendling KK: Reactive
oxygen species signaling in vascular smooth muscle cells.
Cardiovasc Res. 71:216–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banno N, Akihisa T, Tokuda H, Yasukawa K,
Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J and
Nishino H: Triterpene acids from the leaves of Perilla frutescens
and their anti-inflammatory and antitumor-promoting effects. Biosci
Biotechnol Biochem. 68:85–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park GH, Lee JY, Kim DH, Cho YJ and An BJ:
Anti-oxidant and antiinflammatory effects of Rosa multiform root. J
Life Sci. 21:1120–1126. 2011. View Article : Google Scholar
|
|
8
|
Bortalanza LB, Ferreira J, Hess SC, Delle
Monache F, Yunes RA and Calixto JB: Anti-allodynic action of the
tormentic acid, a triterpene isolated from plant, against
neuropathic and inflammatory persistent pain in mice. Eur J
Pharmacol. 453:203–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivorra M, Paya M and Villar A:
Hypoglycemic and insulin release effects of tormentic acid: A new
hypoglycemic natural product. Planta Med. 54:282–285. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu JB, Kuo YH, Lin CH, Ho HY and Shih CC:
Tormentic acid, a major component of suspension cells of Eriobotrya
japonica, suppresses high-fat diet-induced diabetes and
hyperlipidemia by glucose transporter 4 and AMP-activated protein
kinase phosphorylation. J Agric Food Chem. 62:10717–10726. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fogo AS, Antonioli E, Calixto JB and
Campos AH: Tormentic acid reduces vascular smooth muscle cell
proliferation and survival. Eur J Pharmacol. 615:50–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
An HJ, Kim IT, Park HJ, Kim HM, Choi JH
and Lee KT: Tormentic acid, a triterpenoid saponin, isolated from
Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α
expression through inactivation of the nuclear factor-κb pathway in
RAW 264.7 macrophages. Int Immunopharmacol. 11:504–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chamley-Campbell J, Campbell GR and Ross
R: The smooth muscle cell in culture. Physiol Rev. 59:1–61.
1979.PubMed/NCBI
|
|
14
|
Parthasarathy S, Khan-Merchant N,
Penumetcha M and Santanam N: Oxidative stress in cardiovascular
disease. J Nucl Cardiol. 8:379–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harrison D, Griendling KK, Landmesser U,
Hornig B and Drexler H: Role of oxidative stress in
atherosclerosis. Am J Cardiol. 91:7A–11A. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Torres M and Forman HJ: Redox signaling
and the MAP kinase pathways. Biofactors. 17:287–296. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griendling KK, Sorescu D, Lassègue B and
Ushio-Fukai M: Modulation of protein kinase activity and gene
expression by reactive oxygen species and their role in vascular
physiology and pathophysiology. Arterioscler Thromb Vasc Biol.
20:2175–2183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taniyama Y and Griendling KK: Reactive
oxygen species in the vasculature: Molecular and cellular
mechanisms. Hypertension. 42:1075–1081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Detmers PA, Hernandez M, Mudgett J,
Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock
J, et al: Deficiency in inducible nitric oxide synthase results in
reduced atherosclerosis in apolipoprotein E-deficient mice. J
Immunol. 165:3430–3435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bedard K and Krause KH: The NOX family of
ROS-generating NAPDH oxidase: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheehan AL, Carrell S, Johnson B, Stanic
B, Banfi B and Miller FJ Jr: Role for Nox1 NADPH oxidase in
atherosclerosis. Atherosclerosis. 216:321–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dikalova AE, Góngora MC, Harrison DG,
Lambeth JD, Dikalov S and Griendling KK: Upregulation of Nox1 in
vascular smooth muscle leads to impaired endothelium-dependent
relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol.
299:H673–H679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barry-Lane PA, Patterson C, van der Merwe
M, Hu Z, Holland SM, Yeh ET and Runge MS: p47phox is required for
atherosclerotic lesion progression in ApoE(−/−) mice. J Clin
Invest. 108:1513–1522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li WG, Miller FJ Jr, Zhang HJ, Spitz DR,
Oberley LW and Weintraub NL: H(2)O(2)-induced O(2) production by a
non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem.
276:29251–29256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Fu Y, Zhang W, Su G, Liu B, Guo M,
Li F, Liang D, Liu Z, Zhang X, et al: Salidroside attenuates
inflammatory responses by suppressing nuclear factor-κB and mitogen
activated protein kinases activation in lipopolysaccharide-induced
mastitis in mice. Inflamm Res. 62:9–15. 2013. View Article : Google Scholar
|
|
27
|
Dinarello C: The IL-1 family and
inflammatory diseases. Clin Exp Rheumatol. 20(5 Suppl 27): S1–S13.
2002.
|
|
28
|
O'Brien KD, McDonald TO, Chait A, Allen MD
and Alpers CE: Neovascular expression of E-selectin, intercellular
adhesion molecule-1 and vascular cell adhesion molecule-1 in human
atherosclerosis and their relation to intimal leukocyte content.
Circulation. 93:672–682. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory disease. N Engl J Med. 336:1066–1071. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tumur Z, Shimizu H, Enomoto A, Miyazaki H
and Niwa T: Indoxyl sulfate upregulates expression of ICAM-1 and
MCP-1 by oxidative stress-induced NF-kappaB activation. Am J
Nephrol. 31:435–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pierce GL, Lesniewski LA, Lawson BR, Beske
SD and Seals DR: Nuclear factor-{kappa}B activation contributes to
vascular endothelial dysfunction via oxidative stress in
overweight/obese middle-aged and older humans. Circulation.
119:1284–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|