Introduction
Acne is a common skin condition occurring at the
transition between puberty and adult age, which is
quasi-physiological and usually self-limited. Currently, there are
different clinical forms of acne. Acne vulgaris is the most common
form, but there are also some difficulties in diagnosing forms such
as fulminans acne (1), whose
diagnosis often requires skin biopsy. It is crucial to distinguish
the border between the acne specific to age and that of
pathological condition because, under certain circumstances, acne
can be a cutaneous expression of hormonal disorders (2).
Women have three major sources of androgens
(3): i) the ovaries, stimulated by
the pituitary hormones follicle-stimulating hormone (FSH) and
luteinizing hormone (LH), which produce small quantities of
androgens [dehydroepiandrosterone sulfate (DHEA-S) and
testosterone] that can be released per se in the circulation
or converted into estrogen by the enzyme aromatase, which is
present in the ovarian follicle cells. At this level, disorders of
androgen excess are represented by functional ovarian
hyperandrogenism, whereas androgen-secreting tumors occur rarely.
ⅱ) The adrenal gland produces DHEA-S which can be metabolized in
more potent androgens such as androstenedione and testosterone; and
ⅲ) the skin, which has all the enzymes required for converting the
weak androgens into strong androgens such as testosterone and in
the synthesis of androgens.
In sebaceous glands, the increased activity of these
enzymes sustains the major role of androgens in inducing skin
lesions. Thus persistent acne can be explained in adult women with
high levels of testosterone and DHEA-S, which are practically the
most important hormones for the diagnosis of endocrine acne
(2,3).
According to the Global Acne Grading System (GAGS),
each type of acneiform lesion has a gravity score: no lesions, 0;
comedones, 1; papules, 2; pustules, 3; and nodules, 4. The local
score was calculated using the formula: Factor × grade 0–4.
Depending on the location of acne, the factor had the following
values: forehead, 2; right cheek, 2; left cheek, 2; chin, 1; thorax
and upper torso, 1. The sum of the local scores was the global
score which settled acne severity. A global score of 1–18 signified
mild acne; 19–30, moderate acne; 31–38, severe acne; and a global
score >39, very severe acne (4).
The persistence of acne in adulthood or its late
onset (in women >25 years) suggests an endocrine cause due to
hyperandrogenism (5). Although the
most common cause of hyperandrogenism is represented by PCOS, the
differential diagnoses with Cushing's syndrome, ovarian or adrenal
androgen-secreting tumors, acromegaly or with non-endocrine
disorders, Apert syndrome, Behçet's syndrome and SAHA syndrome
(seborrhoea, acne, hirsutism and alopecia) are of importance
(6).
The diagnosis of PCOS should be suspected in the
presence of hyperandrogenism and the following clinical
manifestations: severe acne that reoccurs after isotretinoin
therapy associated with hirsutism, oligomenorrhea or amenorrhea
(defined as the presence of <8 menstrual cycles per year),
androgenic alopecia, seborrhea and acanthosis nigricans on the
backhead, digits, inguinal or periocular - an insulin resistance
marker. Those clinical signs must also be correlated with
laboratory tests for hyperandrogenism and with transvaginal and
pelvic ultrasound (7).
The aim of the present study was to assess the
prevalence of hormonal profile disturbances according to age in
women with papulopustular and nodulocystic acne resistant to
conventional therapy (retinoid therapy, topical benzoyl peroxide
and azelaic acid, local and/or systemic antibiotherapy or
isotretinoin).
Materials and methods
Patient data
This observational cross-sectional study included 72
patients, aged 15–36 years, who were tested between May and October
2014 in the Department of Dermatology, Emergency Regional Hospital
(Craiova, Romania). The patients suffered from moderate and severe
forms of papulopustular and nodulocystic acne and were unresponsive
to classical dermatological treatment or had clinical manifestation
of hyperandrogenism. The patients were divided into two age groups:
the first one (I) included 40 patients, aged 15–22 years, and the
second one (II) included 32 patients, aged 23–36 years.
Informed consent was obtained from each patient
>18 years of age and parental informed consent for those <18
years was obtained. The study was conducted in accordance with the
World Medical Association Declaration of Helsinki and approved by
the Institutional Ethics Committee of the Emergency Regional
Hospital.
Inclusion criteria for the sudy were: acne resistant
to conventional dermatological therapy (retinoid therapy, topical
benzoyl peroxide and azelaic acid, local and/or systemic
antibiotherapy or isotretinoin); acne accompanied by a
hyperandrogenic status: hirsutism, intense facial seborrhea,
irregular menses, androgenic alopecia, voice changes; refractive
acne with polycystic ovaries evidenced on endovaginal ultrasound;
sudden onset of acne in women aged >23 years, unresponsiveness
to local and/or systemic antibiotherapy or isotretinoin therapy,
and a body mass index (BMI) of >29 kg/m2.
Exclusion criteria for the study were: pregnant
women, nursing mothers, cosmetic acne, rosacea, seborrheic
dermatitis, patients with functional hyperprolactinemia due to
estrogen therapy (oral contraceptives in the last 3 months),
patients under treatment with drugs that cause acne (progesterone,
glucocorticoids, lithium, selective reuptake serotonin inhibitors,
isoniazid, phenytoin as well as vitamins B2, B6 and B12) and other
medication including: dopamine antagonists (phenothiazines,
haloperidol, metoclopramide, reserpine, methyldopa), cimetidine,
verapamil, and monoamine oxidase inhibitors.
Endocrine evaluation
A hormonal profile was performed on each patient,
including FSH, LH, estradiol, prolactin, total testosterone, DHEA-S
and plasma cortisol using ELISA (ELx808IU device; BioTek, Winooski,
VT, USA).
Testosterone normal levels for women were considered
as ≤0.9 ng/ml. Excluding adrenal disorders, an increased serum
level of testosterone indicated an ovarian source. Moderate
increases of 1.5 ng/ml were suggestive for PCOS, whereas values
>20 ng/ml strongly suggested androgen-secreting tumors. DHEA-S
(normal levels ≤1 mg/24 h) and serum testosterone were used as a
screening test for acne associated with hirsutism. Approximately
84% of women with hirsutism have high levels of androgens. High
levels of DHEA-S are typical for PCOS (9).
Normal levels of FSH (follicular phase) were settled
as ≤15 mIU/ml, while LH was ≤25 mIU/ml. An LH/FSH ratio >2 was
considered abnormal and was associated with PCOS albeit it is not
characteristic of PCOS. Additionally, women with PCOS with a normal
LH/FSH ratio were reported to be overweight and have insulin
resistance (10).
The particular role of androgen excess in the
etiology of refractive acne is well known, while the role of
estrogen is incompletely elucidated. Estrogens increase sex
hormone- binding globulin (SHBG) acting through liver (11). SHBG has a high binding affinity for
testosterone, and an outstanding estrogen affinity. Since
testosterone and dihydrotestosterone are the primary androgens
involved in the etiology of refractory acne, decreased levels of
SHBG lead to poor response to treatment (12).
On the other hand, the role of estrogens in
modulating the sebum production is little understood. To reduce the
sebum production, an increased quantity of estrogens is necessary,
greater than the one necessary for the inhibition of ovulation,
administered for a long time. In the present study, we evaluated
estradiol levels during the follicular phase of the menstrual cycle
(normal range, 46–607 pmol/l).
Prolactin levels (normal range, 132–498 mIU/l) were
also measured considering a number of studies that reported high
levels of prolactin in women with PCOS and refractive acne
(13). PCOS occurs as a
consequence of multiple factors including the abnormal release of
gonadotropins and ovarian dysfunction. Alteration in dopamine
turnover can also perturb the release of gonadotropins, being a
common cause for both PCOS and hyperprolactinemia (14). However, hyperprolactinemia is most
often transitory, as a very small percentage of patients with PCOS
and hyperprolactinemia have high levels of prolactin. Other studies
reported no relationship between PCOS and hyperprolactinemia, while
high levels of estrogens stimulate moderate prolactin secretion
(14).
Serum cortisol measurement was performed at 8:00
a.m. and normal levels were settled at 5–23 µg/dl. A moderate
increase was indicated when double levels occurred in PCOS, and
high levels were most frequently associated with Cushing's syndrome
(15). In these cases a
differential diagnosis with Cushing's and pseudo-Cushing's
syndromes using a dexamethazone suppression test was performed. In
addition, hypercortisolism was predisposed to excessive cutaneous
sebum secretion and through immunosuppression allowed the bacteria
Propionibacterium acnesto to multiply, causing acne. It is
well known that the plasmatic cortisol is increased during stress,
explaining the exacerbation of acne in stressful periods (16).
Statistical analysis
Data entry and statistical processing were performed
using Stata 13 software (StataCorp LP, College Station, TX, USA).
The correlations between continuous variables were assessed using
the Pearson's correlation coefficient r and were graphically
represented through scatter p groups with superimposed linear fit
curves. P<0.05 was considered or all the statistical tests.
Results
Hormonal disturbances
In the first group of 40 patients aged 15–22 years
we observed the following hormonal disturbances: increased total
testosterone in 6 patients (15%), increased DHEA-S in 4 patients
(10%) that presented oligomenorrhea associated with an intense
seborrheic secretion, increased estradiol in 5 patients (12.5%),
decreased FSH in 2 patients (5%) and increased LH in 4 patients
(10%) (Fig. 1). Serum cortisol and
prolactin levels were within normal range.
In the second group of 32 patients aged 23–36 years,
6 patients had refractory acne (persistent acne), 10 patients had
moderate acne (and sudden onset after age of 23) and 16 patients
had refractory acne and at least one sign of hyperandrogenism
(irregular menstrual cycles in 10 patients, intense seborrheic
status associated with deepening of the voice, and androgenic
alopecia or hirsutism in 12 patients). In this group, we found the
following hormonal disturbances: increased total testosterone in 18
patients (56.25%), increased DHEA-S in 18 patients (56.25%). From
the last 18 patients, 11 (61.11%) had refractory acne associated
with the clinical manifestation of hyperandrogenism and 7 of them
(38.8%) had refractory late-onset (after 23 years) acne associated
without clinical manifestation of hyperandrogenism. Eight patients
who experienced clinical hyperandrogenism showed normal values of
total testosterone and DHEA-S, probably through an increase of the
cutaneous androgen receptor number or through an increased receptor
sensitivity. Estradiol levels were increased in 11 patients
(34.3%), LH levels were increased in 8 patients (25%),
hyperprolactinemia occurred in 2 patients (6.25%) and
hypercortisolism in 4 patients (12.5%). FSH levels were decreased
in 6 patients (18.75%) (Fig.
1).
Clinical features of patients and
hormone level distribution
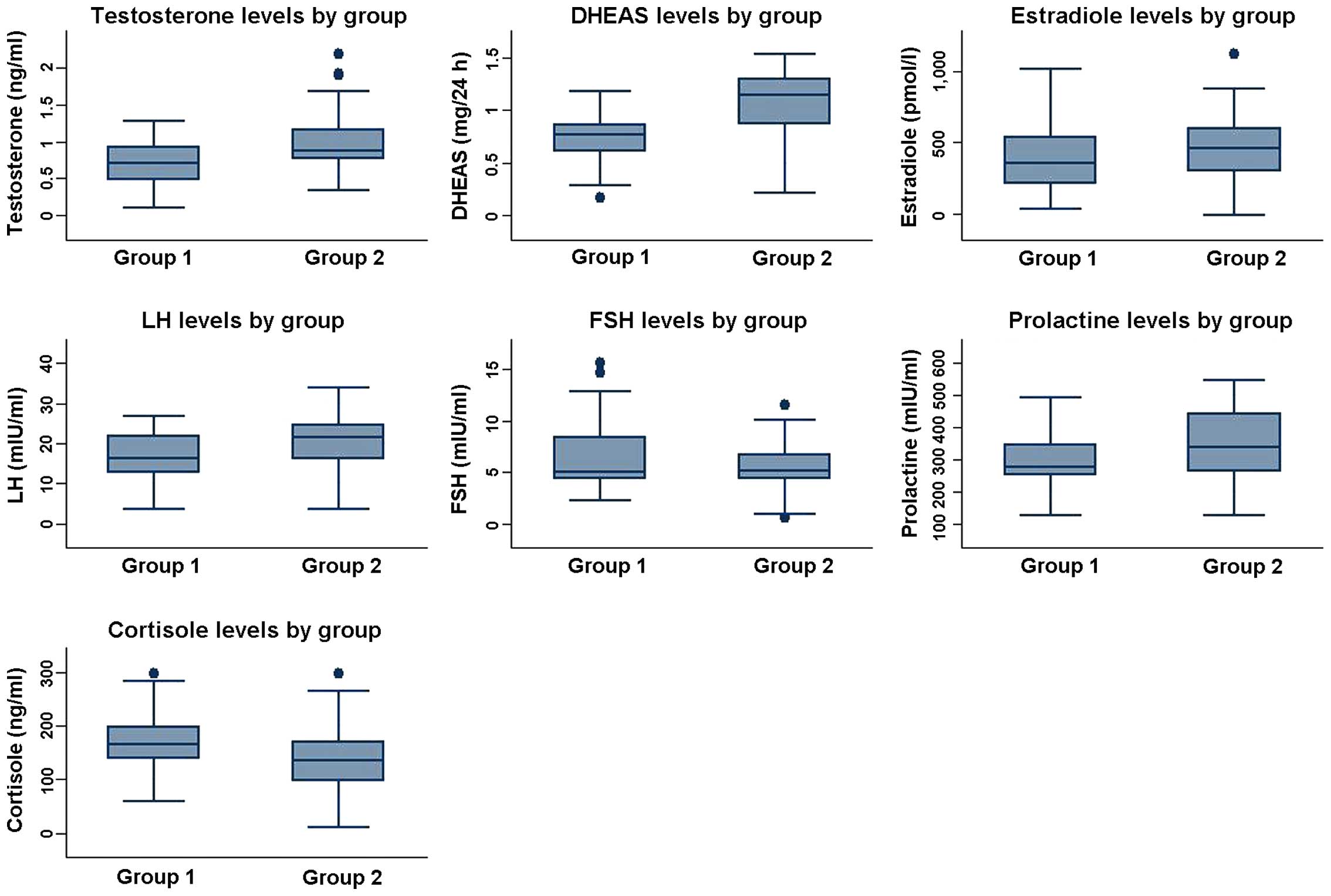
Demographic and biochemical data analysis are shown
in Table I and Fig. 2.
 | Table I.Clinical and laboratory features of
patients with refractory acne. |
Table I.
Clinical and laboratory features of
patients with refractory acne.
| Parameter | Mean ± SD (group I:
40 patients) | Mean ± SD (group II:
32 patients) | P-value |
|---|
| Age (years) | 18.30±2.311 | 31.25±4.683 | <0.001 |
| BMI
(kg/m2) | 20.321±6.782 | 25.789±7.751 | 0.021 |
| Testosterone
(ng/ml) | 0.642±0.352 | 0.971±0.505 | <0.001 |
| DHEA-S (mg/24 h) | 0.854±0.279 | 1.056±0.331 | <0.001 |
| Estradiol
(pmol/l) | 380.625±223.535 | 410.531±237.838 | 0.588 |
| FSH (mIU/ml) | 6.31±3.579 | 4.545±3.566 | 0.041 |
| LH (mIU/ml) | 14.875±6.711 | 20.156±8.215 | 0.004 |
| Prolactin
(mIU/ml) | 281.85±91.113 | 353.969±102.841 | 0.002 |
| Serum cortisol
(µg/dl) | 155.45±46.568 | 137.625±61.901 | 0.182 |
Testosterone levels were lower in group I
(0.642±0.352 ng/ml) vs. group II (0.972±0.505 ng/ml), and the
differences were highly significant (p<0.0001). DHEA-S was also
decreased in group I (0.854±0.279 mg/24 h) vs. group II
(1.056±0.331 mg/24 h), and the differences were highly significant
(p=0.001).
Estradiol was at high levels in 5 patients belonging
to group I (380.625±223.535 pmol/l) and 11 patients from group II
(410.531±237.838 pmol/l). No statistical significant differences
were found (p=0.588). After performing endovaginal ultrasound to
these patients, we found increased ovarian volume with microcysts
at the periphery of the ovary. Furthermore, in 4 patients in whom
severe nodulocystic and conglobate acne was evident, the
endovaginal ultrasound also showed micropolycystic ovaries, but
without associated hormonal disturbances.
In group I, no abnormal levels of prolactin and
serum cortisol which could impact the development of acne were
documented. By contrast, 2 patients (6.25%) from group II had
hyperprolactinemia (137.625±61.901), and 4 patients (12.5%) had
hypercortisolism (353.969±102.841). The increase in prolactin
levels was significant in group II (p=0.002), while the increase of
cortisol was non-significant (p=0.182).
An LH/FSH ratio >2 commonly suggests PCOS and
according to this, we registered low levels of FSH in 2 patients
(5%) belonging to group I and LH was increased in 4 patients (10%)
lacking its pulsatile secretion profile. One patient presented
PCOS. The hormone levels were 6.31±3.579 mIU/ml for FSH and
14.875±6.711 mIU/ml for LH. In group II FSH was decreased in 6
patients (18.75%) and LH was increased in 8 patients (25%). The FSH
levels were 4.545±3.566 mIU/ml and the LH levels were 20.156±8.215
mIU/ml. These hormonal disturbances suggest that in general the
hyperandrogeny from endocrine acne progresses in the context of
ovarian functional hyperandrogeny. The FSH decrease was
statistically significant in patients from group II (p=0.041) and
the LH increase was highly significant in patients from group II
(p=0.004).
After an assessment of age-related hormonal
variations, we observed a direct correlation between age and
increase in DHEA-S and testosterone levels in patients with
refractory acne (Fig. 3).
No significant statistical correlation between
abnormal hormonal profiles and the severity of acne was
identified.
Discussion
Acne is a disorder of the pilosebaceous unit that
was considered common among adolescents. However, it has been shown
that acne may be extended to adulthood, even at the ages of 36–44
years (16). The current treatment
of acne vulgaris uses local, laser or intense pulsed light (IPL)
therapy (17).
In most of the cases, the poor response to the
treatment of acne in teenager females can be related to an
unhealthy eating behavior (rich in dairy products or with high
glycemic index), an overuse of cosmetic products in order to mask
the skin lesions or a decreased compliance to treatment (18).
A high resistance to local of systemic
antibiotherapy and a high recurrence rate after use of isotretinoin
have been reported (19). These
aspects indicate a hormonal etiology of acne and specific treatment
should be initiated (20).
Hormonal therapy includes: androgen receptor blockers
(spironolactone, flutamide, cyproterone acetate) or oral
contraceptives, which inhibit ovarian production of androgens and
thus reduce sebum secretion (21).
Therapeutic decision should be taken considering the
hormonal profile, ultrasound aspect of the ovaries and the
localization and severity of acne. In our study, the findings
regarding hormonal abnormalities reported in patients from group I
cannot be correlated with the presence of refractory acne as only 6
patients presented high testosterone levels and 4 patients had high
DHEA-S. The remaining patients did not require hormonal therapy but
conventional treatment.
In group II, the hormonal disturbances were much
more frequent, as 18 patients were found to have high levels of
testosterone and DHEA-S. Patients presented with PCOS, hirsutism,
androgenic alopecia and refractory acne and the best option of
treatment was represented by blocking agents of the androgenic
receptors. We did not find any correlation regarding the hormonal
levels and severity of acne. However there was a positive
relationship between the clinical manifestation of hyperandrogenism
and the clinical form of acne: papulopustular acne was associated
with virilisation signs compared with comedonian acne. According to
this, 10 of the 16 patients with acne and associated signs of
hyperandrogenism had nodular and papulopustular acne, while the
other 6 patients had predominantly comedonic lesions.
We also found a positive relationship between the
number of acne localizations and the presence of hyperandrogenism
signs. To exemplify, at least two locations of acne (most commonly:
thorax, shoulders, jaw, neck and cheeks) were associated with
irregular menstrual cycles and intense seborrhea of the face and
scalp. Thus, the presence of acne on those sites, accompanied by
intense facial seborrhea, hirsutism and irregular menses could
reveal endocrine disorders that should be thoroughly investigated
(22,23).
In our study, the most common localizations of acne
were: neck and jaw, 18 patients; perioral, 14 patients; lateral
region of the cheeks, 12 patients; anterior and posterior thorax, 8
patients; while 28 patients (87.5%) presented at least two regions
affected by acne. On the other hand, the absence of the clinical
manifestation of hyperandrogenism cannot exclude the coexistence of
an endocrine disorder thus, the acne of the adult woman can be
considered a hyperandrogenism sign. In our study group, 6 patients
without any sign of hyperandrogenism registered high levels of
androgens, and 8 patients with obvious clinical signs of
hyperandrogenism showed a normal hormonal profile. The results
suggest that the increase in androgenic hormones cannot by itself
explain refractory acne.
Patients with adrenal tumors have virilization
signs, caused by high testosterone levels, but not always including
acne. Many studies sustain the importance of raised testosterone
and DHEA-S levels in acne development in women (23). However, in most of the cases, there
are not any hormonal dysregulations, suggesting a peripheral
malfunction, in the pilosebaceous unit, rather than in the
hormone-producing glands. It has been reported that the areas of
skin affected by acne are capable of converting testosterone to
dihydrotestosterone up to 20-fold more than unaffected skin,
commonly the result of idiopathic functional peripheral
hyperandrogenism at the sebocyte level. There are some differences
in androgen metabolism by sebaceous glands depending on the body
region, thus, facial skin has greater hormonal conversion ability
than posterior thorax, which explains the distribution of acne in
this region. Thus, sebum production is a sensitive marker of
androgenic activity (23).
The present study has several limitations related to
the methodology used, including a cross-sectional observational
approach, lack of data under therapy and response to androgen
receptor blockage or oral contraceptives, as well as heterogeneity
of etiology of acne in the study groups.
In conclusion, refractory acne in post-adolescent
women is a therapeutic challenge and requires a multidisciplinary
approach. Acne can be the sign of PCOS, but the diagnosis needs to
be certified through corroboration of both clinical signs and
laboratory results. Isotretinoin is an important therapeutic
option, albeit hormonal treatment such as androgen receptor
blockers and/or oral contraceptives can be recommended even when
serum androgen levels are normal, when conventional dermatological
treatment alone does not lead to any improvement.
Acknowledgements
This study received financial support through the
project entitled ‘Innovative algorithms for diagnosis and treatment
of acne’ from the operational program POS CCE 2007–2013, grant no.
456/3.04.2013, ID no. 1433/SMIS 41582.
References
|
1
|
Brănişteanu DE, Cotrutz CE, Luca MC,
Molodoi DA, Stoica LE, Ianoşi SL, Cianga CM and Brănişteanu DC:
Morphopathological stigmata in acne fulminans. Rom J Morphol
Embryol. 56:1185–1190. 2015.PubMed/NCBI
|
|
2
|
Kamangar F and Shinkai K: Acne in the
adult female patient: A practical approach. Int J Dermatol.
51:1162–1174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danby FW: New, relevant information and
innovative interventions in the management of acne. G Ital Dermatol
Venereol. 146:197–210. 2011.PubMed/NCBI
|
|
4
|
Adityan B, Kumari R and Thappa DM: Scoring
systems in acne vulgaris. Indian J Dermatol Venereol Leprol.
75:323–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Surcel M and Stamatian F: Folliculogenesis
disturbances within the polycystic ovarian syndrome and possible
consequences on oocyte quality. Acta Endo (Buc). 8:267–287. 2012.
View Article : Google Scholar
|
|
6
|
Georgescu CE: Polycystic ovary syndrome
endocrine and cardio-metabolic abnormalities: How to manage? Acta
Endo (Buc). 11:77–84. 2015. View Article : Google Scholar
|
|
7
|
Köşüş N, Köşüş A and Turhan NÖ:
Relationship of ovarian volume with mean platelet volume and lipid
profile in patients with polycystic ovary syndrome. Exp Ther Med.
2:1141–1144. 2011.PubMed/NCBI
|
|
8
|
Xia Y, Wang W, Wang L, Shen S, Cao Y, Yi
L, Gao Q and Wang Y: hOGG1 gene polymorphisms and susceptibility to
polycystic ovary syndrome. Biomed Rep. 4:421–426. 2016.PubMed/NCBI
|
|
9
|
Ozdemir S, Ozdemir M, Görkemli H, Kiyici A
and Bodur S: Specific dermatologic features of the polycystic ovary
syndrome and its association with biochemical markers of the
metabolic syndrome and hyperandrogenism. Acta Obstet Gynecol Scand.
89:199–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gudovan E, Diaconescu C, Oros S and Neamtu
C: Autoimmune thyroiditis associated with polycystic ovary
syndrome: Comments about 25 cases. Acta Endo (Buc). 4:173–180.
2008. View Article : Google Scholar
|
|
11
|
Baldani DP, Skrgatic L, Cerne JZ, Oguic
SK, Gersak BM and Gersak K: Association between serum levels and
pentanucleotide polymorphism in the sex hormone binding globulin
gene and cardiovascular risk factors in females with polycystic
ovary syndrome. Mol Med Rep. 11:3941–3947. 2015.PubMed/NCBI
|
|
12
|
Rich P: Hormonal contraceptives for acne
management. Cutis 81 (Suppl). 13–18. 2008.
|
|
13
|
O'Connell K and Westhoff C: Pharmacology
of hormonal contraceptives and acne. Cutis 81 (Suppl). 8–12.
2008.
|
|
14
|
Bracero N and Zacur HA: Polycystic ovary
syndrome and hyperprolactinemia. Obstet Gynecol Clin North Am.
28:77–84. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kovacs P: Hyperprolactinemia and
polycystic ovary syndrome. Medscape: Accessed. Apr
10–2003.http://www.medscape.com/viewarticle/451707
|
|
16
|
Yanovski JA, Cutler GB Jr, Chrousos GP and
Nieman LK: The dexamethasone-suppressed corticotropin-releasing
hormone stimulation test differentiates mild Cushing's disease from
normal physiology. J Clin Endocrinol Metab. 83:348–352. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ianosi S, Neagoe D, Calbureanu M and
Ianosi G: Investigator-blind, placebo-controlled, randomized
comparative study on combined vacuum and intense pulsed light
versus intense pulsed light devices in both comedonal and
papulopustular acne. J Cosmet Laser Ther. 15:248–254. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Collier CN, Harper JC, Cafardi JA,
Cantrell WC, Wang W, Foster KW and Elewski BE: The prevalence of
acne in adults 20 years and older. J Am Acad Dermatol. 58:56–59.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reynolds RC, Lee S, Choi JY, Atkinson FS,
Stockmann KS, Petocz P and Brand-Miller JC: Effect of the glycemic
index of carbohydrates on acne vulgaris. Nutrients. 2:1060–1072.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu JT, Xuan M, Zhang YN, Liu HW, Cai JH,
Wu YH, Xiang XF, Shan GQ and Cheng B: The efficacy of autologous
platelet-rich plasma combined with erbium fractional laser therapy
for facial acne scars or acne. Mol Med Rep. 8:233–237.
2013.PubMed/NCBI
|
|
21
|
Lowenstein EJ: Diagnosis and management of
the dermatologic manifestations of the polycystic ovary syndrome.
Dermatol Ther (Heidelb). 19:210–223. 2006. View Article : Google Scholar
|
|
22
|
Thiboutot D: Acne: Hormonal concepts and
therapy. Clin Dermatol. 22:419–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jensterle M, Goricar K and Janez A:
Metformin as an initial adjunct to low-dose liraglutide enhances
the weight-decreasing potential of liraglutide in obese polycystic
ovary syndrome: Randomized control study. Exp Ther Med.
11:1194–1200. 2016.PubMed/NCBI
|