Introduction
Blood stasis syndrome (BSS) is an interesting
research area in studies of traditional Asian and Western medicine
(1) focused on vascular disease.
In Korean Traditional Medicine (KTM), BSS is considered to be
caused by blood circulation and Qi circulatory disturbances, which
are the result of accidents, surgery and stress (2). Clinically, BSS is characterized by
pain, bleeding and coagulation, in the vasculature and the muscles.
It is diagnosed based on increased viscosity of the blood, red
blood cell (RBC) deformability, and the acceleration of RBC
maturation, platelet aggregation and microcirculatory dysfunction
(3). Recently, various studies
have reported that BSS is important in metabolic diseases (MDs)
(4), including obesity,
atherosclerosis and cardiovascular disease (5). Previous studies have demonstrated
that MDs are closely associated with inflammation in vascular
diseases (6,7).
Sobokchukeo-Tang (ST) is a well-known formula that
is used for treating primary dysmenorrhea caused by BSS in Korea
and China. ST is used to treat BSS, including uterine myoma,
primary dysmenorrhea and chronic pelvic inflammation (8). Other reports have described the
efficacy of ST for treating vascular disorders and pain (9), endometriosis (10), cancer (11) and menstrual irregularities in
vivo (12).
It is established that the levels of tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) are increased in MD
patients (13). Lipid diseases,
including obesity and atherosclerosis, are associated with the
elevated concentration of inflammatory markers, including
C-reactive protein and proinflammatory cytokines, including
interleukin-1β (IL-1β), IL-6 and TNF-α (14–16).
Peroxisome proliferator-activated receptors (PPARs)
are ligand-dependent transcription factors that regulate lipid and
glucose metabolism (17). PPARs
predominantly include three subtypes; δ, β and γ. The major role of
PPAR-γ in adipocytes is the regulation of adipogenesis and lipid
homeostasis, whereas PPAR-δ is expressed in hepatocytes,
enterocytes and the renal proximal tubule cells of the kidney.
Despite research into PPAR-α and PPAR-γ, the molecular function of
PPAR-β remains unclear. However, PPAR-β is expressed in many
regions of tissues and cells, with relatively high levels present
in the brain, adipose tissue and skin (18). The CCAAT/enhancer-binding protein
(C/EBP) family also serves an important role in modulating
adipocytes (19). These adipogenic
transcriptional factors modulate lipid production in the immune
system.
The present study observed the anti-inflammatory
effects of ST extracts on macrophage cell lines. The
anti-adipogenesis efficacy of ST on mouse fibroblast cell lines was
also investigated. The results demonstrated that ST modulated
adipokine expression under inflammatory conditions.
Materials and methods
Preparation of the herbal formula
Each of the 10 herbal components of ST were mixed as
listed in Table I. All herbal
components were purchased from Omniherb (Deagu, Korea) in 2012. The
origins of each herb were confirmed by Dr Jun-Kyung Lee of Hyemin
Dispensary of Oriental Medicine (Jeonju, Korea). A voucher specimen
(BS-6) was deposited at the KM fundamental Research Division, Korea
Institute of Oriental Medicine (Daejeon, Korea). The extracts were
prepared in our laboratory from a mixture of chopped crude herbs.
Extraction was performed using distilled water at 100°C for 3 h by
reflux extraction, using the extractor COSMOS-660 (Kyungseo Machine
Co., Incheon, Korea). The solution was filtered through filter
paper. The extract was freeze-dried to create a powder (extraction
yield, 13.04%). The prepared powder was stored at −70°C.
 | Table I.Composition of Sobokchukeo-Tang. |
Table I.
Composition of Sobokchukeo-Tang.
| Name of herbs | Binomial name | Amount (g) |
|---|
| Foeniculi
Fructus | Foeniculum vulgare
Mill. | 4.0 |
| Zingiberis
Rhizoma | Zingiber
officinale Roscoe | 0.8 |
| Carthami Flos | Corydalis ternata
Nakai | 4.0 |
| Myrrha | Commiphora molmol
Engler | 4.0 |
| Angelicae Sinens
Radix | Angelica gigas
N. | 12.0 |
| Cnidii Rhizoma | Cnidium officinale
Makino | 4.0 |
| Cinnammomi
Cortex | Cinnamomum
loureirii Nees | 4.0 |
| Paeoniae Rubra
Radix | Paeonia obovata
Maxim. | 8.0 |
| Typhae Pollen | Typha angustifolia
L. | 12.0 |
| Trogopterori
Faeces | Trogopterus
xanthipes | 8.0 |
High performance liquid chromatography
(HPLC) analysis
The lyophilized extract (10 mg) was dissolved in 70%
methanol (5 ml) and then filtered through a 0.2 µm membrane filter
(Woongki Science Co., Ltd., Seoul, Korea) before being injected
into HPLC for component analysis. The purity of the ten standard
compounds was ≥98.0% using HPLC analysis. The HPLC-grade solvents,
methanol, acetonitrile and water were obtained from J.T. Baker
(Phillipsburg, NJ, USA). Trifluoroacetic acid (analytical reagent
grade) and the standards were procured from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany).
The HPLC system consisted of a Waters Alliance 2695
system coupled with a 2998 photodiode array detector (Waters
Corporation, Mitford, MA, USA). Data processing was performed with
Empower software, version 3 (Waters Corporation). The 10 components
in ST were separated using a Luna 5 µm C18 100A column (4.6×250 mm,
5 µm particle size, no. 00G-4252-E0; Phenomenex, Inc., Torrance,
CA, USA). The monitoring was performed at 230 nm for three
compounds (1, albiflorin; 2, paeoniflorin; and 3, benzoic acid),
280 nm for five compounds (4, gallic acid; 5, coumarin; 6, cinnamic
acid; 7, cinnamic aldehyde; and 8,6-gingerol) and 320 nm for two
compounds [9, nodakenin; and 10, ferulic acid (10)]. The mobile phases consisted of
water with 0.1% (v/v) trifluoroacetic acid (solvent A) and
acetonitrile (solvent B) at a flow rate of 1.0 ml/min. The gradient
conditions changed as presented in Table II. The injection volume was 10
µl.
 | Table II.Composition of mobile phase for
chromatographic separation. |
Table II.
Composition of mobile phase for
chromatographic separation.
| Time (min) | Solvent A
(%)a | Solvent B
(%)b |
|---|
| 0 | 95 |
5 |
| 30 | 40 | 60 |
| 40 | 0 | 100 |
| 45 | 0 | 100 |
| 50 | 95 |
5 |
| 60 | 95 |
5 |
Cell culture and cytotoxicity
The RAW 264.7 murine macrophage cell line, the
3T3-L1 mouse embryonic fibroblast cell line and the THP-1 human
acute monocytic leukemia cell line were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). The RAW 264.7
[5.5% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 1% penicillin/streptomycin (P/S)] and
3T3-L1 (10% calf serum and 1% P/S) cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.). The THP-1 cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.; 10% FBS, 1%
P&S). The culture flask was maintained at 37°C in a humidified
atmosphere consisting of 5% CO2 and 95% air.
The cell cytotoxicity was detected by using Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Briefly, the RAW 264.7, 3T3-L1 and THP-1 cells
were seeded at 3×103, 8×102 and
1×104 cells/well in 96-well plates. After incubation
overnight, the cells were treated with 0–1,000 µg/ml ST for 24 h.
CCK-8 solution (10 µl) was added to each well. After 4 h, the
absorbance was measured at 450 nm using a Benchmark Plus microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the
percentages of the control (without ST) were calculated.
Anti-inflammatory activity
To confirm the levels of cytokines in the RAW 264.7
cells, the cells were cultured with 5×104 cells per well
in 48-well plates with lipopolysaccharide (LPS; Escherichia
coli 0111:B4; Sigma-Aldrich, Merck Millipore; 1 µg/ml) for 24 h
to induce inflammation. The cells were treated with ST extract
(62.5–500 µg/ml). The IL-6 (cat. no. DY406) and TNF-α (cat. no.
DY410) concentration in the supernatant was analyzed using ELISA
(R&D Systems, Inc., Minneapolis, MN, USA).
THP-1 cells were cultured at 1×106
cells/well in 6-well plates in the presence of phorbol 12-myristate
13-acetate (20 ng/ml; Sigma-Aldrich; Merck Millipore) for 24 h to
induce differentiation into macrophage-like cells. Differentiated
cells were then incubated with serum-free medium for 1 day at 37°C
and 5% CO2. Cells were treated with LPS (1 µg/ml) in
RPMI medium (10% FBS and 1% P/S) in the presence or absence of ST
extracts (62.5–500 µg/ml). The cells were incubated for 24 h, then
the supernatant was taken to measure the concentration of
proinflammatory cytokines [IL-1β (cat. no. KHC0014), IL-6 (cat. no.
KHC0061C) and TNF-α (cat. no. KHC3014C); Invitrogen; Thermo Fisher
Scientific, Inc.].
3T3-L1 cell culture and
differentiation
To induce adipocyte differentiation, the 3T3-L1
cells were cultures in 6-well plates at 3×105 cells/well
to confluence. After 2 days, the cells were treated with a
differentiation mixture containing 1 µM dexamethasone, 5 mM
3-isobutyl-1-methylxanthine and 1 µg/ml insulin (Sigma-Aldrich;
Merck Millipore) in DMEM with 10% FBS (MDI) to induce the
preadipocytes to differentiate. After 2 days, the medium was
replaced with DMEM with 10% FBS and 1 µM insulin. Cultures were
incubated for 2 days, after which the culture medium was replaced
again with DMEM (10% FBS) and repeated at 2 day intervals until day
7. SB203580, a p38 mitogen-activated protein kinase (MAPK)
inhibitor (Cell Signaling Technologies, Inc., Danvers, MA, USA) was
used as the positive control. The triglyceride (TG; BioAssay
Systems, Hayward, CA, USA; cat. no. ETGA-200) was detected by
colorimetric method in the cell lysates at 570 nm using a
microplate reader (Benchmark Plus; Bio-Rad Laboratories, Inc.). The
leptin (R&D Systems, Inc.; cat. no. MOB00) concentrations were
measured by ELISA in supernatant at 450 nm using a microplate
reader.
Oil Red O (ORO) staining
MDI-induced differentiated 3T3-L1 cells were treated
with ST at concentrations of 62.5, 125, 250 and 500 µg/ml. The fat
droplets were visualized using ORO staining. The cells were fixed
with 10% formalin for 1 h, washed with 60% isopropanol, and dried.
Then, the cells were stained with 0.5% ORO solution in 60%
isopropanol for 10 min and then washed four times with distilled
water. The images of the stained cells were acquired using an
inverted contrast phase microscope (Olympus Corporation, Tokyo,
Japan). To detect the absorbance of the sample, the stained cells
were dissolved in DMSO and absorbance was measured at 570 nm by a
microplate reader.
Anti-adipogenesis activity
The TG and leptin production was measured on day 7
after differentiation. The cells were washed three times with PBS
and scraped in 500 µl 5% Triton X-100. The cell lysate was
centrifuge at 848 × g for 3 min at 4°C to remove the fat
layers. The supernatant was assayed for TG production, according to
the manufacturer's protocol (BioAssay Systems; cat. no. ETGA-200).
The supernatant in the differentiated 3T3-L1 cells treated with ST
were used to determine the leptin concentration according to the
manufacturer's protocol (R&D Systems, Inc.; cat. no.
MOB00).
Protein expression
Cells were treated with ST (62.5, 125, 250 and 500
µg/ml) for 5 days and then washed twice with ice-cold PBS. The cell
lysates were prepared with radioimmunoprecipitation cell lysis
buffer (GenDEPOT, Barker, TX, USA). The lysates were centrifuged at
15,928 × g for 15 min at 4°C. The concentration of protein
was measured using the Bicinchoninic Acid Protein Assay kit (Thermo
Fisher Scientific, Inc.). A total of 30 µg of each protein was
separated by electrophoresis using 4–20% Criterion™ TGX™ precast
gels (Bio-Rad Laboratories, Inc.) and transferred onto
polyvinylidene fluoride membranes (GE Healthcare Life Sciences,
Chalfont, UK). The membranes were blocked with 5% skim milk and
incubated with primary antibodies (1:1,000 dilutions; β-actin (cat.
no. sc-81178), PPAR-γ (cat. no. sc-7273), C/EBPα (cat. no. sc-61);
Santa Cruz Biotechnology, Inc., Dallas, Texas. USA) overnight at
4°C. The next day, the membranes were incubated with goat
anti-rabbit secondary antibodies (1:5,000; cat. no. 170-6515;
Bio-Rad Laboratories, Inc.) for 1 h at room temperature, and
immunoreactive proteins were detected using an enhanced
chemiluminescence assay kit (Thermo Fisher Scientific, Inc.). Bands
were detected using a ChemiDoc™ XRS + image analyzer (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error and
were analyzed by analysis of variance and the Bonferroni multiple
comparison method using Systat 13.0 (Systat Software Inc., San
Jose, CA USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
HPLC analysis
Satisfactory results were obtained using mobile
phases consisting of 1.0% (v/v) trifluoroacetic acid (solvent A)
and acetonitrile with 1.0% (v/v) trifluoroacetic acid (solvent B).
Quantitation was achieved using photodiode array detection in the
region 200–400 nm based on the retention times and UV spectra
compared with the standards. The UV absorbance was recorded at 230
nm for three compounds, 280 nm for five compounds and 320 nm for
two compounds. The retention times of compounds 1–10 were 12.80,
13.44, 19.32, 6.60, 21.60, 23.41, 24.64, 30.30, 15.17 and 16.44
min, respectively (Fig. 1A).
Fig. 1B presents the chromatograms
of the ST extract solutions.
 | Figure 1.High performance liquid chromatography
chromatogram of (A) standard mixture and (B) Sobokchukeo-Tang. I,
230 nm; II, 280 nm; and III, 330 nm. (1) Alboflorin, (2) peoniflorin, (3) benzoic acid, (4) gallic acid, (5) coumarin, (6) cinnamic acid, (7) cinnamic aldehyde, (8) 6-gingerol, (9) nodakenin (10) and ferulic acid. AU, absorbance
unit. |
Anti-inflammatory activity
There was no cytotoxicity up to 1,000 µg/ml ST (data
not shown). The cytokine concentrations were detected in the
supernatants of LPS-treated RAW 264.7 cells and THP-1 cells. The
inflammatory efficacy was examined using mouse and human cell
lines.
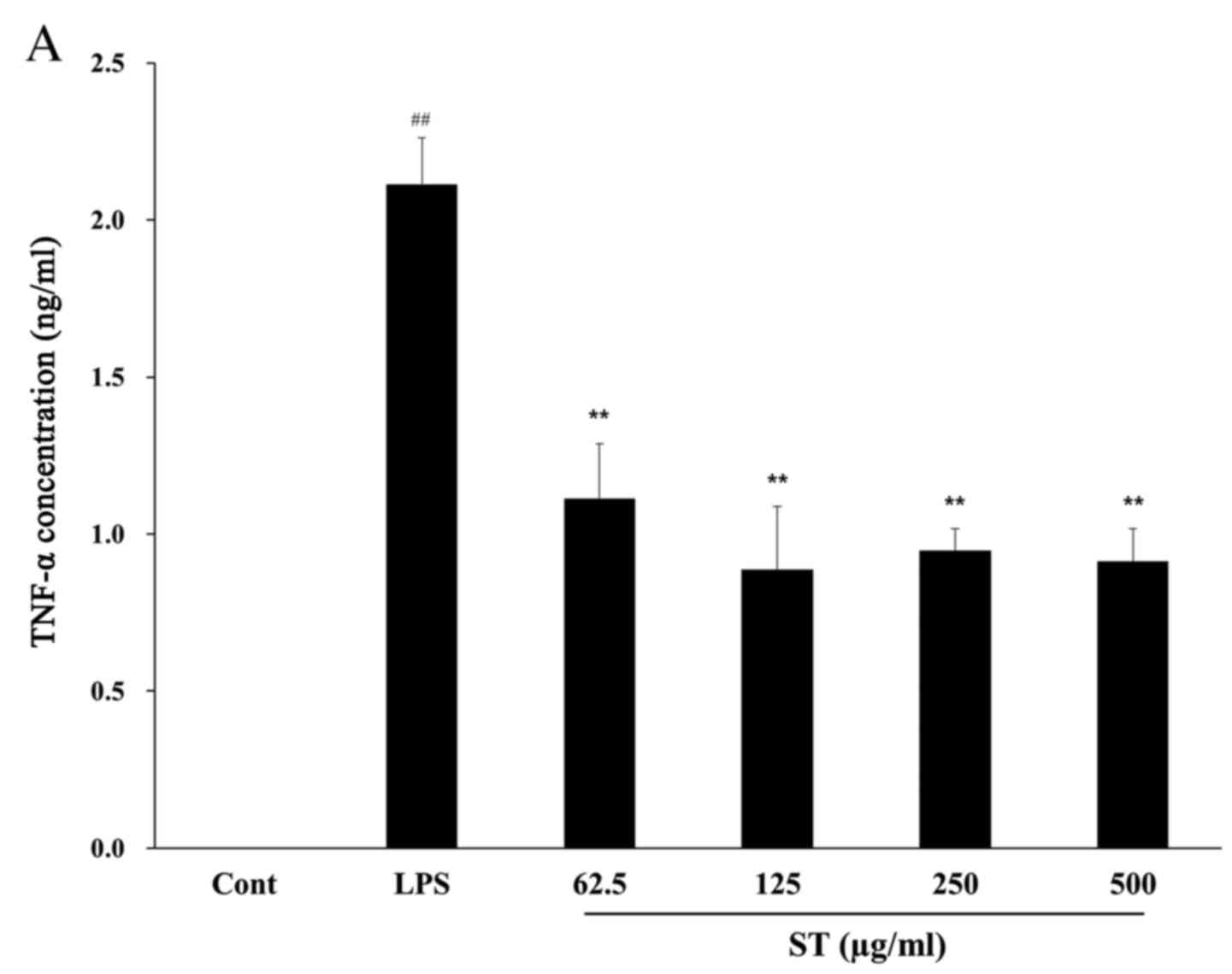
ST significantly inhibited the production of TNF-α
by up to 57% in LPS-treated RAW 264.7 mouse cells compared with
cells treated with LPS only (P<0.01; Fig. 2A). The IL-6 concentration in the
LPS-treated group (11.87±1.95 ng/ml) exhibited a significant
increase of ~72-fold compared with the control group (0.16±0.076
ng/ml; P<0.0001) and IL-6 was significantly reduced by ST (500
µg/ml) by 59–65% compared with LPS treatment (P=0.008; Fig. 2B).
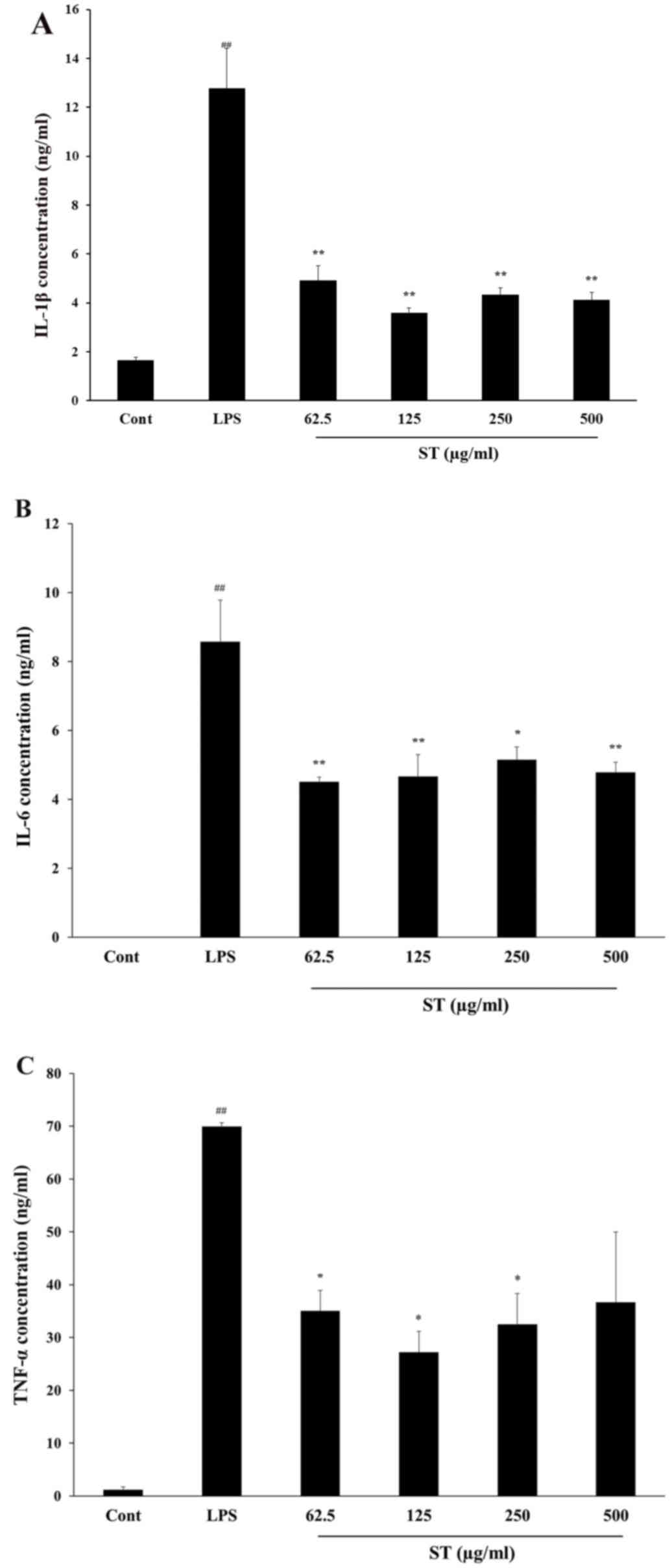
Cytokine levels were also measured in human THP-1
cells. The LPS-treated group exhibited significantly increased
concentrations of IL-1β (12.79±1.61 ng/ml; P<0.0001), IL-6
(8.58±1.21 ng/ml; P<0.0001) and TNF-α (69.95±0.75 ng/ml;
P<0.0001) compared with the control. The ST (500 µg/ml)
treatment reduced the IL-1β (4.11±0.32 ng/ml), IL-6 (4.79±0.30
ng/ml) and TNF-α (36.70±13.31 ng/ml) released concentrations
compared with LPS-only treatment (P<0.01; Fig. 3). Thus, the data confirmed that ST
suppressed IL-6 and TNF-α, anti-inflammatory cytokines, in mouse
and human cell lines.
Lipid accumulation in adipocytes
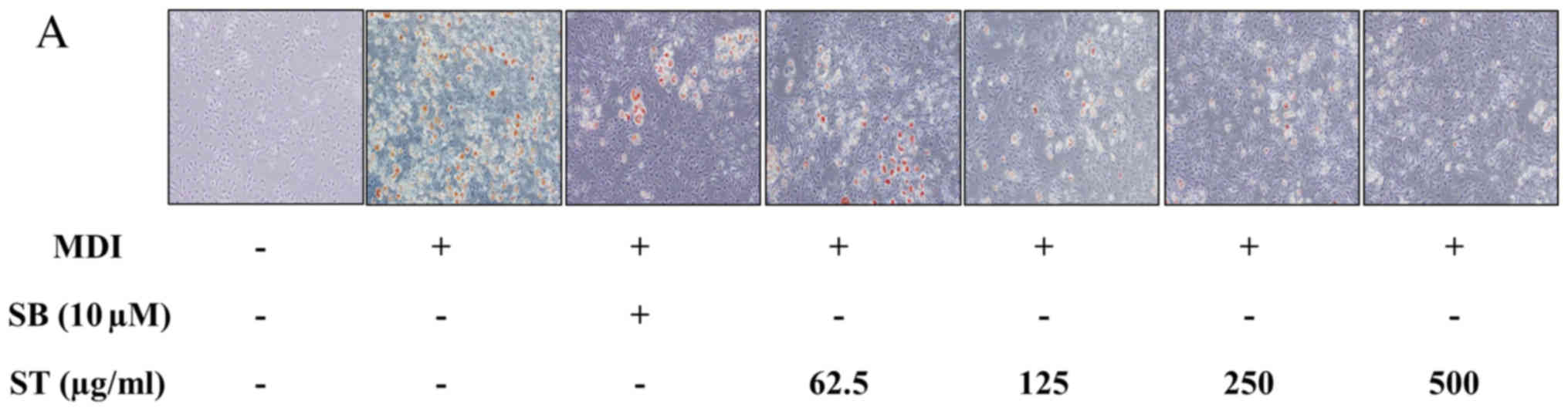
3T3-L1 cells were incubated in MDI-differentiation
medium in the presence or absence of ST extracts. SB203580
treatment was the positive control, and basal growth medium
treatment was the negative control. Lipid accumulation was observed
by ORO staining on day 7. The retained dye by the fat droplets was
dissolved with DMSO and measured at a wavelength of 570 nm by
microplate reader. The fat droplets were increased by
MDI-differentiation medium. When SB203580 was administered in the
MDI-induced well as a positive control, the fat droplets were
inhibited by up to 72%. In the ST treatment group, it was observed
that the fat droplets were reduced by ST in a dose-dependent manner
(Fig. 4A and B). This suggested
that the ST treatment inhibited lipid accumulation and adipogenesis
in a dose dependent manner.
Intracellular lipid regulation
The intracellular TG and leptin content was
quantified at 7 days post-differentiation of the preadipocytes. The
TG content was significantly increased in the cells cultured with
MDI by 4-fold (142.74±4.14 µM) compared with the control group
(33.18±0.89 µM; P<0.0001). The positive control, SB203580,
significantly inhibited TG production in MDI-induced cells compared
with MDI treatment alone (P=0.003). The ST-treated group suppressed
the TG production by up to 50% compared with MDI treatment only
(Fig. 5A).
When 3T3-L1 cells were treated with MDI, the leptin
content increased to 156.64±9.50 pg/ml. In the positive control,
the leptin content was significantly inhibited by ~78% (34.42±1.93
pg/ml; P<0.01; Fig. 5B), and in
the ST-treated group, the leptin concentration was significantly
inhibited by up to 95% (P<0.01). Thus, ST suppressed the release
of TG and leptin from adipocytes.
Protein expression in
adipogenesis
The cell protein levels of PPAR-γ and C/EBPα were
determined. Differentiated cells were treated with various
concentrations of ST for 5 days, and the protein levels of PPAR-γ
and C/EBPα were determined by western blotting. As demonstrated in
Fig. 5C, SB203580 suppressed
PPAR-γ and C/EBPα expression compared with differentiated cells
treated with MDI only. The ST-treated groups exhibited reduced
PPAR-γ and C/EBPα protein levels in a dose-dependent manner. These
results indicated that ST inhibits 3T3-L1 pre-adipocyte
differentiation partially through PPAR-γ and C/EBPα via p38 MAPK
signaling.
Discussion
BSS is a blood circulation disorder. Various
diseases, including hyperviscosity, ischemic brain injury,
microvascular accidents, atherosclerosis, hypertension and pain,
are caused by BSS, which can be explained by the inflammatory
vascular pathology (20). The
traditional herbal formula, ST, is used for the treatment of BSS,
pain, cancer and menstrual irregularities. The current study
confirmed the efficacy of the anti-inflammatory activity of ST by
using adipocytes and macrophages.
Obesity-associated inflammation is suspected to
contribute to various diseases, including cancer, cardiovascular
disease and diabetes. The proinflammatory cytokines/chemokines,
including IL-1β, IL-6, TNF-α, adipokines and leptin, are important
for the initiation and development of MDs (21). The present study investigated
whether ST modulates the proinflammatory cytokines, IL-6 and TNF-α,
and confirmed the cytokine releasing levels in mouse and human
macrophage cell lines. ST inhibited the release of proinflammatory
cytokines compared with the levels in LPS-treated cells. ST may
improve pain and cancer by inhibiting pro-inflammatory
activity.
Obesity causes chronic inflammatory reactions
associated with pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α)
and adipokines. Numerous studies have indicated that adipocytes
produce considerable concentrations of IL-1β, IL-6 and TNF-α
(22–25). There is a tendency for
differentiated adipocytes to undergo apoptosis, followed by
macrophage infiltration of the developed adipocytes. Adipose tissue
from obese subjects has a higher concentration of secreted
cytokines, including IL-1β, IL-6, IL-8 and TNF-α (26). Adipose tissue significantly
contributes to the production of cytokines. TNF-α is a positively
associated with adipocyte size and plasma adipokine levels
(27). The current study
demonstrated that IL-6 and TNF-α release is inhibited by ST in a
concentration-dependent manner in LPS-treated RAW 264.7 cells and
differentiated THP-1 cells, thus, providing another association
between fat tissue and inflammation in obesity. Additionally, ST
inhibited the production of TG and leptin in 3T3-L1 cells.
Adipocytes are a storage tissue for overnutrition,
and it is recognized that adipocytes release numerous factors that
regulate inflammation and metabolism. The mechanisms resulting in
macrophage development to adipose tissue are currently under
investigation. Increased concentrations and release of cytokines
and chemokines has been implicated in this process (28). We hypothesize that the reduction in
TG accumulation and leptin release following ST treatment is
partially mediated by reduced fatty acid synthesis. Leptin serves a
crucial role in the endocrine system regarding obesity; it
stimulates appetite suppression and regulates energy consumption
(29). It is well known that the
blood concentration of leptin is closely associated with the TG
concentration. The amount of adipose tissue is dependent on
circumstances and hormones, including insulin and gonadotropins
(30). In the current study, ST
inhibited the TG and leptin levels in MDI-induced differentiated
3T3-L1 cells. In addition, ST inhibited adipocyte differentiation
and lipid droplet formation.
The expression of the adipogenic markers, PPAR-γ and
C/EBPα, were inhibited by ST treatment during adipogenesis of
MDI-induced. SB203580 (p38 MAPK inhibitor) was used as a positive
control. The role of p38MAPK in adipocyte differentiation remains a
controversial topic. p38 activation is altered by
MDI-differentiation of 3T3-L1 (31) and suppression of p38 early in
MDI-differentiation of 3T3-L1 cells was demonstrated to decrease
adipocyte development (32). In
the current study, treatment with ST inhibited the PPAR-γ and
C/EBPα expression, which suggested that ST may suppress adipocyte
differentiation partially by inhibiting p38 MAPK. It was also
observed that SB203580 inhibited PPAR-γ and C/EBPα expression.
In conclusion, the results of the current study
demonstrated that ST has anti-inflammatory efficacy in LPS-treated
macrophages and inhibits adipogenesis in MDI-induced 3T3-L1
adipocytes, as indicated by the significant reduction in TG and
leptin accumulation without any cytotoxicity. Furthermore, the
suppressive effects of ST are potentially mediated by the
downregulated expression of adipogenesis-associated genes. Thus, ST
may act as a therapeutic agent to prevent lipid-associated
diseases, including obesity and atherosclerosis.
Acknowledgements
This research was supported by grants from Korea
Institute of Oriental Medicine (grant no. K14280).
References
|
1
|
Liu Y, Yin HJ, Shi DZ and Chen KJ: Chinese
herb and formulas for promoting blood circulation and removing
blood stasis and antiplatelet therapies. Evid Based Complement
Alternat Med. 2012:1845032012.PubMed/NCBI
|
|
2
|
Jeon BH, Woo WH and Jeong WY: The review
of blood stasis concept in oriental medicine. Korean J Oriental
Pathology. 4:93–102. 1989.
|
|
3
|
Wang T, Jia C, Chen Y, Li X and Cheng J:
Analysis on establishment and affecting factors of qi stagnation
and blood stasis rat model. Zhongguo Zhong Yao Za Zhi.
37:1629–1633. 2012.(In Chinese). PubMed/NCBI
|
|
4
|
Lee TC, Lo LC and Wu FC: Traditional
Chinese medicine for metabolic syndrome via TCM pattern
differentiation: Tongue diagnosis for predictor. Evid Based
Complement Alternat Med. 2016:19712952016.PubMed/NCBI
|
|
5
|
Liu X, Guo C, Ma X, Tian R, Zhang Y and
Yin H: Relationship between serum estrogen levels and blood stasis
syndrome in postmenopausal women with coronary heart disease. Pak J
Med Sci. 31:25–30. 2015.PubMed/NCBI
|
|
6
|
Navarro JF and Mora C: Role of
inflammation in diabetic complications. Nephrology Dialysis
Transplantation. 20:2601–2604. 2005. View Article : Google Scholar
|
|
7
|
Jeffcoate WJ, Game F and Cavanagh PR: The
role of proinflammatory cytokines in the cause of neuropathic
osteoarthropathy (acute Charcot foot) in diabetes. Lancet.
366:2058–2061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gunter RN: Blood Stasis: China's classical
concept in modern medicine. Elsvier; China: 2006
|
|
9
|
Zhuo QH, Jiang XF and Zhang YM: Clinical
observation of Shaofu zhuyu decoction treating for dysmenorrhea
with adolescent functional of 126 cases. Clinical Journal of
Experiment Traditional Medicine Formulae. 8:58–59. 2008.
|
|
10
|
Yang SJ and Jin CS: A study on the effects
of sobokchukeo-tang on the isolated uterine muscle of rats. J
Orient Obestet Gynecol. 18:72–84. 2005.
|
|
11
|
Shin WW, Choi JS, Khil JH and Kim SH:
Study on antitumor activity of sobokchukeotang and
kamisocokchukeotang. J Korean Orient Med. 22:22–30. 2001.
|
|
12
|
Yun YH, Lee DN, Seo IB and Kim HJ: Effects
of sobokchukeo-tang on the development of experimentally induced
endometriosis in rats. J Orient Obestet Gynecol. 19:141–161.
2006.
|
|
13
|
Cheng YX, Zhou LL, Yan YM, Chen KX and Hou
FF: Diabetic nephropathy-related active cyclic peptides from the
roots of Brachystemma calycinum. Bioorg Med Chem Lett.
21:7434–7439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kern PA, Ranganathan S, Li C, Wood L and
Ranganathan G: Adipose tissue tumor necrosis factor and
interleukin-6 expression in human obesity and insulin resistance.
Am J Physiol Endocrinol Metab. 280:E745–E751. 2001.PubMed/NCBI
|
|
15
|
Maachi M, Pieroni L, Bruckert E, Jardel C,
Fellahi S, Hainque B, Capeau J and Bastard JP: Systemic low-grade
inflammation is related to both circulating and adipose tissue
TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat
Metab Disord. 28:993–997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skurk T, Alberti-Huber C, Herder C and
Hauner H: Relationship between adipocyte size and adipokine
expression and secretion. J Clin Endocrinol Metab. 92:1023–1033.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yaw HP, Ton SH, Chin HF, Karim MK,
Fernando HA and Kadir KA: Modulation of lipid metabolism in
glycyrrhizic acid-treated rats fed on a high-calorie diet and
exposed to short or long-term stress. Int J Physiol Pathophysiol
Pharmacol. 7:61–75. 2015.PubMed/NCBI
|
|
18
|
Kota B, Huang TH and Roufogalis BD: An
overview on biological mechanisms of PPARs. Pharmacol Res.
51:85–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He Q, Huang C, Zhao L, Feng J, Shi Q, Wang
D and Wang S: α-Naphthoflavone inhibits 3T3-L1 pre-adipocytes
differentiation via modulating p38MAPK signaling. Int J Clin Exp
Pathol. 6:168–178. 2013.PubMed/NCBI
|
|
20
|
Park B, You S, Jung J, Lee JA, Yun KJ and
Lee MS: Korean studies on blood stasis: An overview. Evid Based
Complement Alternat Med. 3:20152015.
|
|
21
|
Gokulakrishnan K, Amutha A, Ranjani H,
Bibin SY, Balakumar M, Pandey GK, Anjana RM, Ali MK, Narayan KM and
Mohan V: Relationship of adipokines and proinflammatory cytokines
among asian indian with obesity and youth onset type 2 diabetes.
Endocr Pract. 21:1143–1151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CJ, Jian DY, Lin MW, Zhao JZ, Ho LT
and Juan CC: Evidence in Obese Children: Contribution of
hyperlipidemia, obesity-inflammation and insulin sensitivity. PLoS
One. 10:e01259352015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akram Z, Abduljabbar T, Abu Hassan MI,
Javed F and Vohra F: Cytokine profile in chronic periodontitis
patients with and without obesity: A systematic review and
meta-analysis. Dis Markers. 2016:48014182016.PubMed/NCBI
|
|
24
|
Basinska K, Marycz K, Śieszek A and Nicpoń
J: The production and distribution of IL-6 and TNF-a in
subcutaneous adipose tissue and their correlation with serum
concentrations in Welsh ponies with equine metabolic syndrome. J
Vet Sci. 16:113–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eichelmann F, Schwingshackl L, Fedirko V
and Aleksandrova K: Effect of plant-based diets on obesity-related
inflammatory profiles: A systematic review and meta-analysis of
intervention trials. Obes Rev. 17:1067–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hotamisligil GS, Arner P, Caro JF,
Atkinson RL and Spiegelman BM: Increased adipose tissue expression
of tumor necrosis factor-alpha in human obesity and insulin
resistance. J Clin Invest. 95:2409–2415. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Winkler G, Kiss S, Keszthelyi L, Sápi Z,
Ory I, Salamon F, Kovács M, Vargha P, Szekeres O, Speer G, et al:
Expression of tumor necrosis factor (TNF)-alpha protein in the
subcutaneous and visceral adipose tissue in correlation with
adipocyte cell volume, serum TNF-alpha, soluble serum
TNF-receptor-2 concentrations and C-peptide level. Eur J
Endocrinol. 149:129–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goossens GH and Blaak EE: Adipose tissue
dysfunction and impaired metabolic health in human obesity: A
matter of oxygen? Front Endocrinol (Lausanne). 6:552015.PubMed/NCBI
|
|
29
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pósa A, Szabó R, Kupai K, Csonka A, Szalai
Z, Veszelka M, Török S, Daruka L and Varga C: Exercise training and
calorie restriction influence the metabolic parameters in
ovariectomized female rats. Oxid Med Cell Longev.
2015:7870632015.PubMed/NCBI
|
|
31
|
Takenouchi T, Takayama Y and Takezawa T:
Co-treatment with dexamethasone and octanoate induces adipogenesis
in 3T3-L1 cells. Cell Biol Int. 28:209–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Engelman JA, Lisanti MP and Scherer PE:
Specific inhibitors of p38 mitogen-activated protein kinase block
3T3-L1 adipogenesis. J Biol Chem. 273:32111–32120. 1988.
|