Introduction
Acute lymphoblastic leukemia (ALL) predominantly
occurs in children between the ages of 2 and 5 years, but may also
occur in adults (1–4). Over the past decade, under different
treatments, the survival rate of childhood ALL has approached ~90%
(1,5); however, the treatment of adult ALL
still needs improvement (2,6). The
activation or inhibition of various genes, including lysine
methyltransferase 2A, IKARS family zinc-finger 1, AT-rich
interaction domain 5B, CCAAT/enhancer-binding protein and cyclin
dependent kinase inhibitor 2A, may lead to the development of ALL
(7,8).
The GATA-binding protein family of zinc-finger
transcription factors comprises six members, including GATA1,
GATA2, GATA3, GATA4, GATA5 and GATA6 (9), which bind to GATA sequences in the
DNA with the consensus 5′-WGATAR-3′, where W is either T or A
nucleotides and R is either G or A (10,11).
GATA4 was originally revealed to serve an important role in cardiac
development (12,13); subsequent studies have reported
that GATA4 mediated apoptosis (14,15)
and regulated the mRNA expression of B cell lymphoma 2
(BCL2) (16) and
BCL-XL (17),
and serves as a survival factor in carcinoma. A number of GATA
family proteins have been revealed to serve inhibitory roles in
leukemia, including GATA1, GATA2 and GATA3 (18–21).
However, the expression and function of GATA4 in ALL remains
unknown.
The present study demonstrated that GATA4 expression
was upregulated in ALL and binds to the promoter region of
BCL2 and mouse double minute 2 homolog (Mdm2),
activating their transcription. MDM2 protein expression was
revealed to negatively regulate the expression of p53, thereby
inhibiting apoptosis and promoting cell cycle. In conclusion, the
present results suggested that GATA4 may be a marker for ALL
diagnosis and a target of molecular therapy.
Materials and methods
Cell culture and transfection
MOLT-4 adult ALL, Jurkat childhood ALL cell lines
and H9 normal human T lymphocyte cell lines were purchased from
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) that contained 1% penicillin-streptomycin and 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2. Then ~4×105 cells were
transfected with pCMV-Tag 2B empty vector (Youbio, Hunan, China)
FLAG-GATA4 (pCMV-Tag 2B-GATA4) or small interfering RNA (siRNA)
against scrambled (SCR), GATA4 siRNA (siGATA4) or siGATA4 and sip21
simultaneously using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according the manufacturer's protocol.
Following 48 h transfection, cells were collected by 800 × g
centrifugation at 4°C for 5 min and used for the further
experiments. siRNA sequences were as follows: siGATA4#1,
AACCGGCCGCTCATCAAGCCT; siGATA4#2, AATCTCGTAGATATGTTTGAC; sip21,
AACTTCGACTTTGTCACCGAG.
Blood samples
A total of 42 blood samples from patients with ALL
and 42 samples from healthy controls were collected from patients
at the First Hospital of Jilin University (Changchun, China)
between 2010 and 2016. All patients had been informed about the
nature of the experiments and provided written informed consent.
The study was approved by the Ethics Committee of the First
Hospital of Jilin University.
Western blot analysis
Cells (~4×106) were lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing a protease inhibitor
cocktail (Beijing Transgen Biotech Co., Ltd., Beijing, China) at
4°C for 30 min. Protein concentrations were measured using the
Bradford Reagent (Beyotime Institute of Biotechnology). A total of
40 µg protein was loaded in each well and resolved by 8% SDS-PAGE.
Proteins were transferred to polyvinylidene fluoride membranes,
which were subsequently blocked with 5% non-fat milk at 37°C for 1
h. The membranes were incubated with the following primary
antibodies at 4°C overnight: anti-GATA4 (ab124265; 1:600) anti
GADD45 (ab105060; 1:3,000), anti-p21 (ab109520; 1:2,000),
anti-14-3-3σ (ab14123; 1:2,000) and anti-β-actin (1:1,000) from
Abcam (Cambridge, MA, USA); and anti-FLAG (F1804 1:5,000),
anti-MDM2 (SAB4300601; 1:2,000), anti-BCL2 (SAB4300340; 1:500) and
anti-p53 (P9249; 1:1,000) from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Membranes were washed three times with TBS +
1% Tween-20 and incubated with horseradish peroxidase-conjugated
secondary goat anti-mouse immunoglobulin (Ig)G (ab6728; 1:5,000;
Abcam) or goat anti-rabbit IgG (ab6721; 1:5,000; Abcam) at room
temperature for 1 h. Immunoreactive protein bands were visualized
using the Enhanced Chemiluminescence kit (Beyotime Institute of
Biotechnology). Each experiment was performed at least 3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 3×106 cells
using the RNAprep Pure kit (Tiangen Biotech Co., Ltd., Beijing,
China), following the manufacturer's protocol. Total RNA (2 µg) was
reverse transcribed into cDNA using the FastKing RT kit (Tiangen
Biotech Co., Ltd.), according to the manufacturer's protocol. qPCR
was performed with the RealMaster mix SYBR-Green (Tiangen Biotech
Co., Ltd.) and an ABI PRISM 7700 Real Time Thermal Cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The RT-qPCR conditions
were as follows: 5 min at 95°C, denaturation at 95°C for 30 sec,
annealing at 57°C for 30 sec and extension at 72°C for 60 sec,
performed for 34 cycles. The primers were as follows: GATA4,
5′-CCCAATCTCGTAGATATGTTTGAC-3′ and 5′-CCGTTCATCTTGTGGTAGAG-3′;
BCL2, 5′-GCTATAACTGGAGAGTGCTG-3′ and 5′-ACTTGATTCTGGTGTTTCCC-3′;
MDM2, 5′-TCAAACTCCTGACCTCAAGTG-3′ and
5′-TTACCATCATAAGCCTACAGACC-3′; p53, 5′-GACCTATGGAAACTACTTCCTG-3′
and 5′-CATTCTGGGAGCTTCATCTG-3′; p21, GCATGACAGATTTCTACCAC-3′ and
5′-GACTAAGGCAGAAGATGTAGAG; GADD45, 5′-CGAGAACGACATCAACATCC-3′ and
5′-ATGAATGTGGATTCGTCACC-3′; 14–3-3σ, 5′-AGACAACCTAACACTTTGGAC-3′
and 5′-AGGAAAGAAGGATGACACCC-3′; GAPDH, 5′-CATTTCCTGGTATGACAACGA-3′
and 5′-TACATGGCAACTGTGAGGAG-3′. Relative mRNA expression levels of
indicated genes were calculated by the 2−ΔΔCq method
(22). GAPDH was used as internal
control and for normalization. Each experiment was performed at
least 3 times.
Cell Counting Kit-8 (CCK-8)
proliferation assay
Following 48 h transfection, cells (4×103
cells/well in 100 µl RPMI-1640 medium) were cultured in 96-well
plates at 37°C. CCK-8 solution (10 µl; Beyotime Institute of
Biotechnology) was added to each well and incubated at 37°C for 3
h. A microplate reader was used to measure the absorbance at 450
nm; the absorbance indicated cells proliferation. Each experiment
was performed at least 3 times.
Apoptosis assay
Adriamycin was purchased from Selleck Chemicals
(Houston, TX, USA) and dissolved in phosphate-buffered saline
(PBS). Following 48 h transfection, Adriamycin (5 µg/ml) was added
to the culture medium and incubated for 24 h at 37°C to induce
apoptosis. Subsequently, apoptotic rates were examined with the
Annexin V/fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(Abcam), according to the manufacturer's protocol. Briefly,
approximately 4×105 cells were collected by 800 × g
centrifugation at 4°C for 5 min, washed with PBS and stained with
Annexin V and propidium iodide. Attune™ NxT Software (version 1.0;
Thermo Fisher Scientific, Inc.) was used to analyze apoptotic cells
using a flow cytometer. Each experiment was performed at least 3
times.
Transwell migration and invasion
assay
Costar Transwell chambers (Corning Inc., Corning,
NY, USA) were used to detect the cell migration and invasion
ability. Uncoated Transwell inserts were used to assess cell
migration ability, whereas Transwell inserts precoated with
Matrigel (BD Biosciences, San Jose, CA, USA) were used to determine
cell invasion ability. Jurkat cells were transfected for 48 h,
resuspended in RPMI-1640 without FBS, and plated (2×104
cells) into the upper chamber; the lower chamber was filled with
500 µl RPMI-1640 supplemented with 10% FBS. Cells were incubated at
37°C with 5% CO2 in a humidified air atmosphere for 24
h. Following incubation, cells in the upper chamber were removed
with cotton swabs, and cells in the lower chamber were fixed with
4% paraformaldehyde and stained with 0.5% crystal violet. Stained
cells were counted (6 fields were counted for each membrane) under
a light microscope. Each experiment was performed at least 3
times.
Chromatin immunoprecipitation
(ChIP)
ChIP analysis was performed with the Chromatin
Immunoprecipitation Assay kit (Beyotime Institute of
Biotechnology), according to manufacturer's protocol. Briefly,
cells were grown to 85–95% confluence, washed cells three times
with PBS and chemically cross-linked with 1% formaldehyde for 20–30
min at 37°C. Subsequently, cells were lysed with lysis buffer at
4°C for 30 min and sonicated 3 cycles at 4°C, each cycle of 15
times. Following lysis, 3 µg GATA4 antibody was added to the lysis
solution and incubated at 4°C overnight. Anti-rabbit IgG was used
as control. Protein A beads were used to isolate
antibody-interacted DNA fragments. The binding chromatin was
purified by Qiagen PCR Purification kit (Qiagen China Co., Ltd.,
Shanghai, China). Chromatin fragments was analyzed by PCR and
detected by gel electrophoresis. The PCR assay was performed using
2XEasyTaq PCR SuperMix (Transgene, Beijing, China) according to
manuscript's protocol. The PCR conditions were as follows: 5 min at
95°C, denaturation at 95°C for 30 sec, annealing at 56°C for 30 sec
and extension at 72°C for 30 sec, performed for 32 cycles. The
primers were as follows: BCL2, 5-GGACTTCTGCGAATACCG-3′ and
5-GTCCCTGAGGGCTTCATT-3′; MDM2, 5-CGGGTTCACAGGATTGTC-3′ and
5-GATGCTGGTTACCGTTGG-3′. Each experiment was performed at least 3
times.
Wound-healing assay
Cells (3×105) were transfected with
vector, GATA4 or SCR, siGATA4, when the density of cells was almost
90–100%, cells were scratched with a 20 µl pipette and photographed
at 0 h or 48 h under a light microscope. The distance of cells
migration indicated the migration ability of cells. Each experiment
was performed at least 3 times.
Dual-luciferase reporter assay
The promoter sequence of MDM2 or BCL2 was cloned
into a pGL3 firefly luciferase reporter plasmid vector. Jurkat
cells (3×106) were co-transfected with 0.2, 0.5, 1 and 2
µg FLAG-GATA4, Renilla and pGL3-MDM2-promoter or pGL3-BCL2-promoter
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). The Renilla luciferase plasmid vector was used as an
internal control. Following 24 h transfection at 37°C, firefly and
Renilla luciferase activities were measured through the
Dual-Luciferase Reporter Assay system (Promega Corporation,
Madison, WI, USA). Firefly luciferase activity was normalized to
Renilla luciferase activity. Each experiment was performed at least
3 times.
Statistical analysis
All data were expressed as the mean ± standard
deviation; and each experiment was performed at least three times.
The data were analyzed by SSPS 19.0 (IBM Corp., Armonk, NY, USA).
Comparisons between groups were made by one-way analysis of
variance followed by Tukey's test, and comparisons between two
groups were made with Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
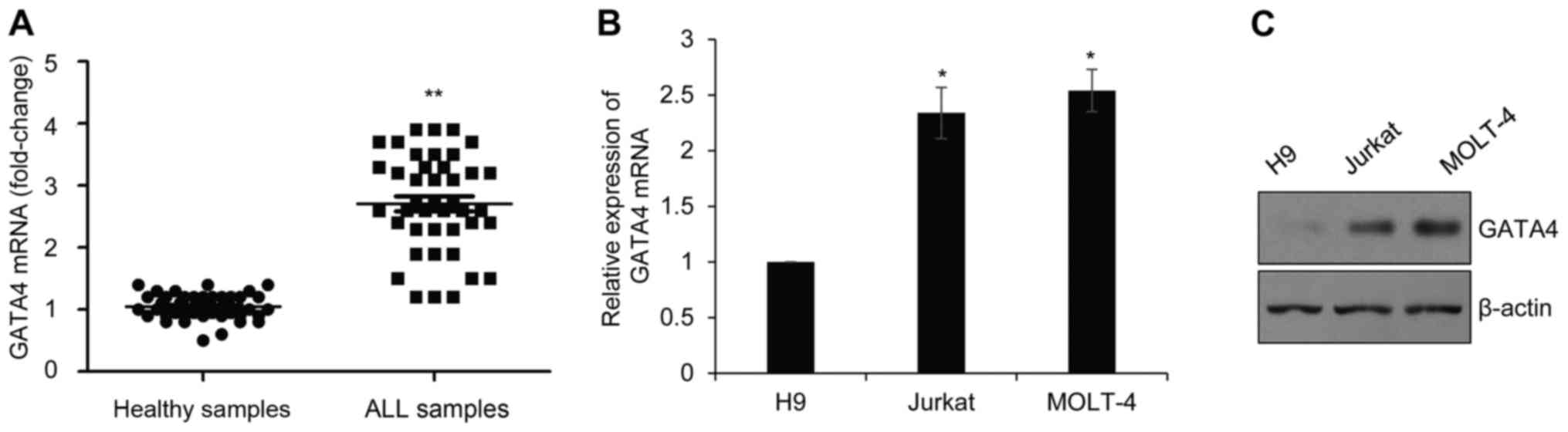
GATA4 is highly expressed in childhood
ALL
To determine the expression of GATA4 in childhood
ALL, blood samples from 42 patients with ALL and 42 healthy
controls were collected. RT-qPCR was used to assess GATA4 mRNA
expression (Fig. 1A). Compared
with healthy control group, the expression of GATA4 mRNA was
significantly higher in the childhood ALL samples. In addition,
GATA4 mRNA and protein expression was examined in Jurkat and MOLT-4
ALL cell lines, using the H9 normal human T lymphocyte cell lines
as a control. The results indicated that the mRNA and protein
levels of GATA4 expression were upregulated in ALL cell lines
compared with H9 (Fig. 1B and C,
respectively).
GATA4 promotes cell proliferation by
inhibiting apoptosis and facilitating G1/S phase
transition
To further analyze the function of GATA4 in ALL,
GATA4 was either overexpressed or silenced in Jurkat and MOLT-4
cell lines, and subsequent mRNA and protein expression levels were
determined by RT-qPCR and western blotting, respectively. The
results indicated that the expression of GATA4 was upregulated in
FLAG-GATA4-transfected cells, but downregulated in
siGATA4-transfected cells (Fig. 2A and
B); transfection with siGATA4#2 appeared to be more efficient
compared with siGATA4#1, therefore siGATA4#2 was used for the
further experiments.
 | Figure 2.GATA4 promotes cell proliferation by
inhibiting apoptosis and facilitating G1/S phase
transition. Jurkat and MOLT-4 ALL cells were transfected with
Vector control, FLAG-GATA4, SCR, siGATA4#1 or siGATA4#2 for 48 h.
(A) GATA4 mRNA expression levels were determined by reverse
transcription-quantitative polymerase chain reaction. *P<0.05;
GATA4 vs. vector, siGATA4 vs. SCR. (B) GATA4 protein expression was
examined by western blotting. (C) Transfected cells proliferation
was examined by Cell Counting Kit-8 assay. *P<0.05 GATA4 vs.
vector, siGATA4 vs. SCR. (D) Apoptosis rates and (E) cell cycle
analysis were detected by flow cytometry. *P<0.05 GATA4 vs.
vector, siGATA4 vs. SCR. GATA4, GATA-binding factor 4; OD, optical
density; SCR, scramble; si, small interfering RNA. |
To elucidate the function of GATA4 on the
progression of ALL, CCK-8 cell viability analysis was conducted. As
shown in Fig. 2C, the
proliferation of Jurkat cells that ectopically expressed GATA4 was
significantly increased compared with the Vector control group.
Conversely, siGATA4 knockdown of GATA4 expression significantly
suppressed cell proliferation compared with expression in the SCR
control (Fig. 2C); similar results
were observed in MOLT-4 cells (Fig.
2C). To determine the mechanism of GATA4 in proliferation, the
role of GATA4 in apoptosis was examined using flow cytometry.
Compared with the control groups, the rate of apoptosis was
decreased in the cells that overexpressed GATA4; however, the
apoptotic rate was increased in the cells transfected with siGATA4
(Fig. 2D). In addition, cell cycle
analysis revealed that overexpression of GATA4 promoted
G1/S phase transition; however, inhibition of GATA4
arrested cell cycle at G0/G1 phase (Fig. 2E). These results suggested that
GATA4 may facilitate cell proliferation by inhibiting cell
apoptosis and promoting cell G1/S phase transition.
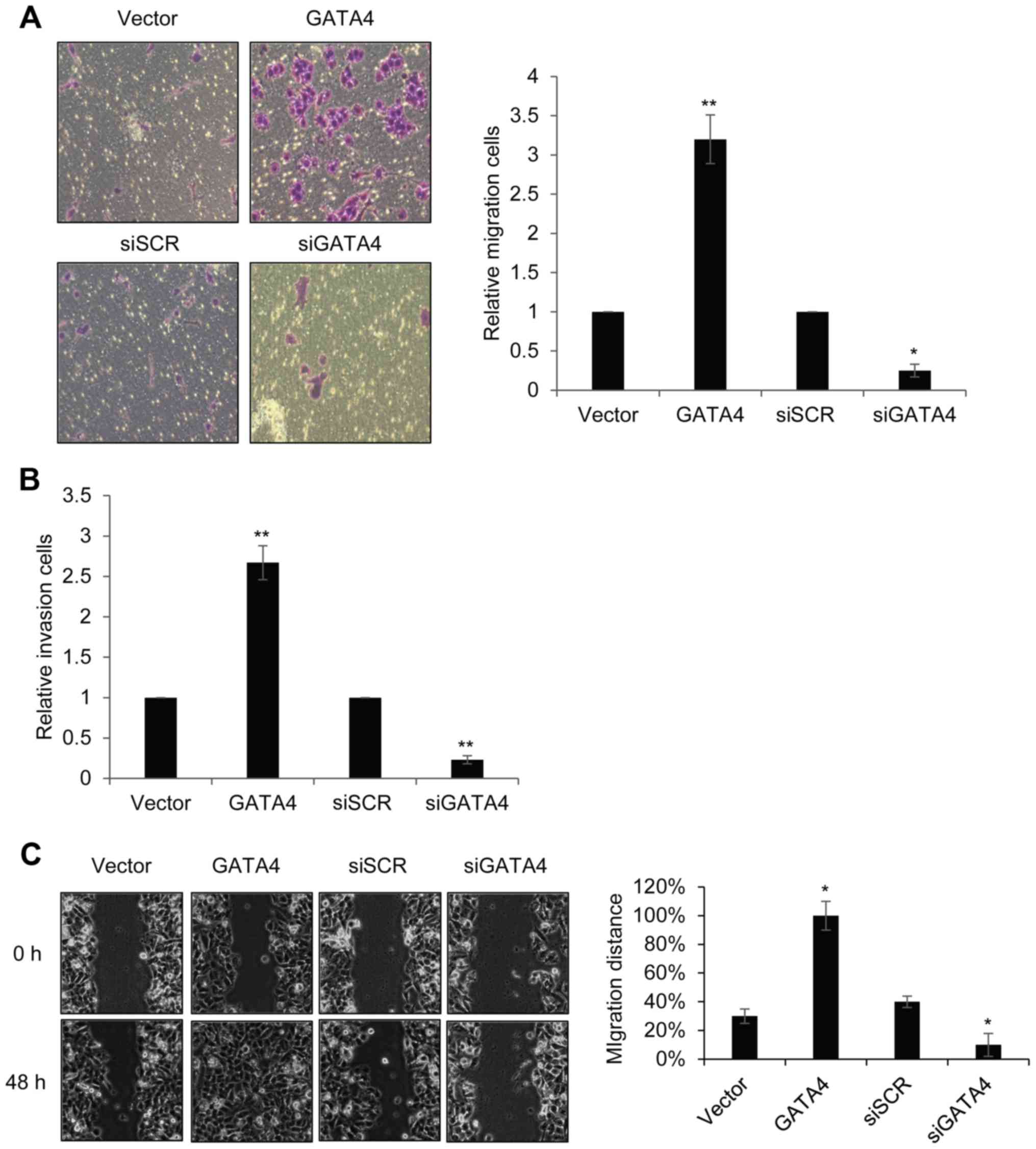
GATA4 facilitates migration and
invasion in ALL cells
To further investigate the roles of GATA4 on ALL
progression, Transwell analysis was performed to explore the
migratory and invasive abilities of ALL cells under different
experimental conditions. The number of migrating cells in the GATA
overexpression group was as significantly higher compared with the
Vector control group (Fig. 3A). By
contrast the number of migrating cells in the siGATA4 was
significantly decreased compared with the SCR control group
(Fig. 3A). Invasion analysis
results revealed similar results (Fig.
3B). Results from the wound-healing assay confirmed that GATA4
expression promoted cell migration: Overexpression of GATA4
significantly increased cell migration distance following 48 h
culture; however, knockdown of GATA4 expression lead to a decreased
in cell migration distance (Fig.
3C). These data suggested that GATA4 may serve a role in
promoting cell migration and invasion.
GATA4 inhibits apoptosis by regulating
the transcriptional activity BCL2 and MDM2 in ALL
GATA4 has been previously reported to regulate the
apoptotic process by regulating the transcription of BCL2 in
embryonic stem cells or ovarian follicles (14,15,23,24).
However, whether GATA4 mediated apoptosis in this manner in ALL was
unknown. To explore the mechanism of GATA4 on apoptosis in ALL, the
expression of BCL2 protein and mRNA in cells in which GATA4 is
overexpressed or silenced was examined. The results revealed that
both the protein and mRNA levels of expression of BCL2 were notably
increased in GATA4-overexpression cells (Fig. 4A and B, respectively). Cell
apoptosis was also previously demonstrated to be regulated by the
p53-MDM2 pathway (25); therefore,
the expression of p53 and MDM2 was examined in GATA4-overexpressing
Jurkat cells. The expression level of MDM2 protein was increased,
whereas the expression of p53 protein was decreased in cells that
ectopically expressed GATA4 (Fig.
4A). Similarly, the mRNA expression level of MDM2 was
significantly increased and the mRNA expression level of p53 was
slightly decreased in cells overexpressing GATA4 (Fig. 4 and B). The results suggested that
GATA4 may regulate apoptosis in ALL through BCL2 and the p53-MDM2
pathway.
As GATA4 is a zinc-finger-containing transcription
factor, the present study hypothesized that GATA4 activated the
transcription of BCL2 and MDM2. To verify this
hypothesis, ChIP analysis was performed with anti-GATA4 in Jurkat
cells (Fig. 4C). The results
suggested that GATA4 interacted with the promoter region of BCL2
and MDM2 in Jurkat cells. In addition, results from the
dual-luciferase reporter assay demonstrated that GATA4 activated
the transcription of BCL2 and MDM2 (Fig. 4D). These results demonstrated that
GATA4 may target the promoter of BCL2 and MDM2 to
change the expression levels of BCL2 and MDM2.
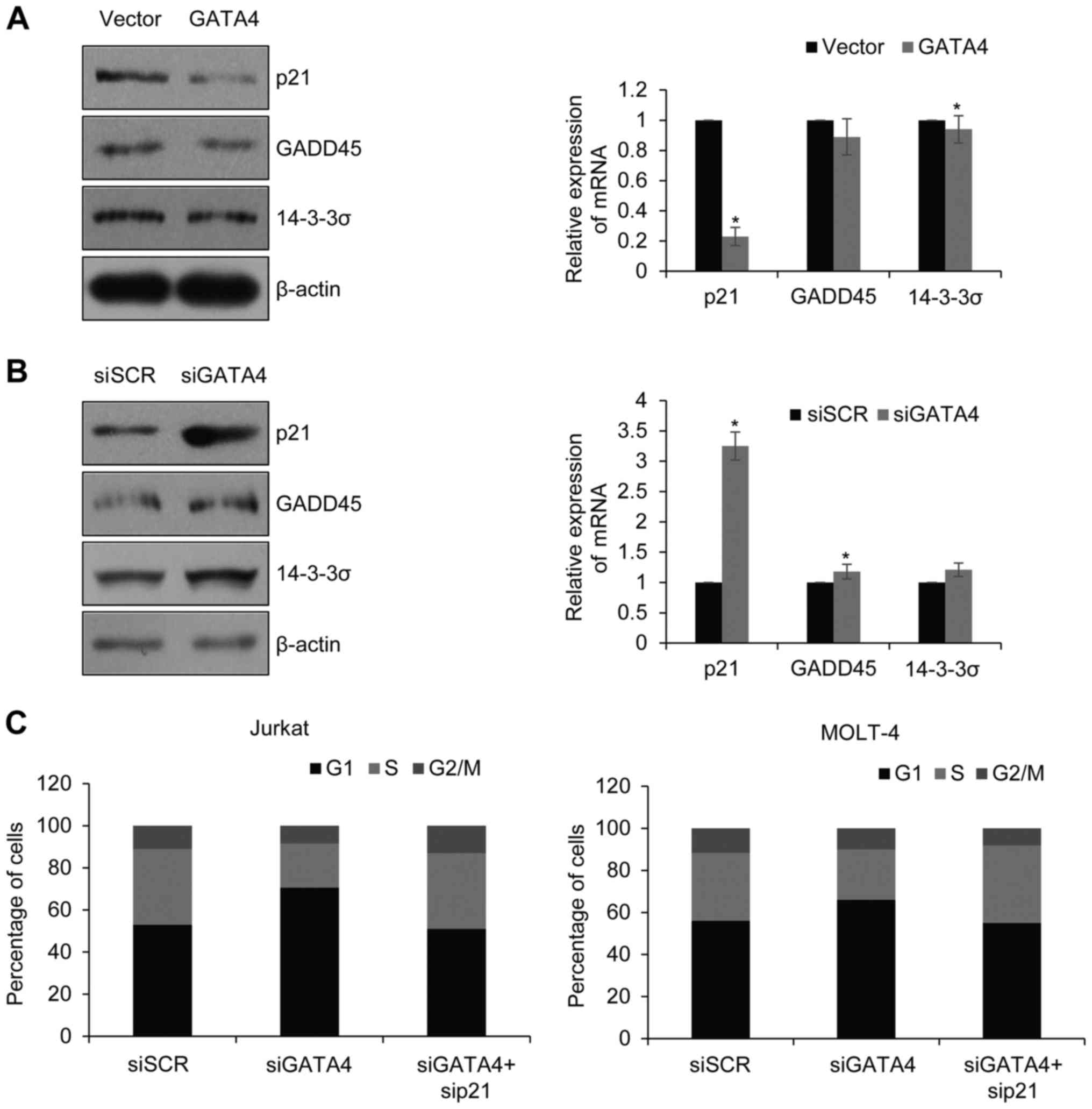
GATA4 promotes cell cycle through
inhibition of p53-regulated p21 in ALL
It was found that GATA4 negatively regulated p53
through transcriptional activating MDM2. The p53 pathway has been
found to regulate cell cycle through transcriptional activating of
p21, GADD45 and 14–3-3σ. p21 interacted with Cyclin-CDK complex and
inhibited it phosphorylating Rb and resulted in cells arresting at
G1 phase. To investigate the mechanism of GATA4
facilitation of G1/S phase transition, the expression of
p21, GADD45 and 14-3-3σ were examined in Jurkat cells in which
GATA4 was overexpressed or silenced (Fig. 5A and B, respectively). Consistent
with our hypothesis, the expression of p21 was decreased in
GATA4-overexpressing cells, whereas no notable changes in the
protein expression levels of GADD45 and 14-3-3σ were observed
(Fig. 5A). However, knockdown of
GATA4 increased the expression of p21 (Fig. 5B). Next, GATA4 and p21 were knocked
down together, and the cell cycle determined. It was found that
when GATA4 and p21 were silenced together, the percentage of cells
at S was increased compared with the group which only silenced
GATA4; similar results were observed in MOLT-4 cells (Fig. 5C). Together, GATA4 facilitated
G1/S transition through p53-p21 pathway.
Discussion
ALL is a malignant hematologic cancer that arises
from the hematopoietic precursors of lymphoid blood cell lineage.
ALL predominantly occurs in children. T-ALL represents 25% of adult
ALL cases and 10–15% of pediatric cases (26), resulting from the malignant
transformation of T cell progenitors. Although there are several
treatments for ALL, patients still have poor prognosis (4,27,28).
The GATA family of transcription factors serve
various roles in the process of cell proliferation and
differentiation (29,30). The expression of GATA4 has been
extensively studied in a number of carcinomas (31–34).
Previous studies have reported that the expression of GATA4 was low
in several cancers, including colorectal cancer, adrenocortical
tumors, epithelial ovarian cancer and hepatocellular carcinoma,
which suggested that GATA4 may serve as a tumor suppressor
(35). By contrast, high
expression levels of GATA4 were previously demonstrated in the Huh6
pediatric liver tumor cell line (36). In addition, it was reported that
GATA family members, including GATA1, GATA2 and GATA3, served
inhibiting functions in leukemia (18–21),
although the function of GATA4 in ALL remained unknown. The present
study demonstrated that GATA4 mRNA expression was significantly
upregulated in child patients with ALL compared with healthy
children. CCK-8 analysis revealed that high expression of GATA4
promoted proliferation in ALL cells. Cell cycle analysis
demonstrated that the inhibition of GATA4 arrested cells at
G0/G1 phase, whereas ectopic expression of
GATA4 promoted G1/S phase transition. Previous studies
have demonstrated that GATA1 regulated cell cycle arrest through
activation of p21 and GATA3 regulated hematopoietic stem cell
maintenance and cell-cycle entry (37–39).
The above evidence indicated that the GATA family serve different
functions in the cell cycle and the reason why they do so requires
further investigation. Results from the present study also
indicated that GATA4 suppressed apoptosis in ALL cells. In
addition, the Transwell assays revealed that GATA4 may regulate ALL
cell invasive ability. Cell apoptosis is an important mechanism
that prevents cell overgrowth and DNA damage. Inhibition cell
apoptosis promotes cancer cell proliferation. GATA4 was also
demonstrated to activate the transcription of BCL2, thereby
suppressing cell apoptosis, and GATA4 may also regulate apoptosis
through regulation of the p53-MDM2 pathway.
A recent report suggested that GATA4 may facilitate
hepatoblastoma cell proliferation by regulating the expression of
DKK3 and microRNA (miRNA) miR125b (40). A number of other studies have also
demonstrated that miRNAs and transcription factors are closely
correlated in gene regulatory networks (41), and bioinformatics analyses
suggested that the promoter sequences of most mammalian miRNA genes
included at least one GATA box, which indicated that GATA4 may
transcriptionally regulate certain miRNAs. A detailed molecular
mechanism of GATA4 remains to be investigated, and miRNAs may be an
important factor.
In conclusion, the present study revealed GATA4
served a key function in ALL, and high expression of GATA4 may
serve an important function in the development of ALL. GATA4 was
demonstrated to regulate the cell cycle and apoptosis through the
regulation of the transcriptional activity MDM2 and
BCL2. GATA4 may be a potential target of ALL molecular
therapy.
References
|
1
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bassan R and Hoelzer D: Modern therapy of
acute lymphoblastic leukemia. J Clin Oncol. 29:532–543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stanulla M and Schrappe M: Treatment of
childhood acute lymphoblastic leukemia. Semin Hematol. 46:52–63.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pui CH, Campana D, Pei D, Bowman WP,
Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu
M, et al: Treating childhood acute lymphoblastic leukemia without
cranial irradiation. N Engl J Med. 360:2730–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pieters R, Schrappe M, De Lorenzo P, Hann
I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster
A, et al: A treatment protocol for infants younger than 1 year with
acute lymphoblastic leukaemia (Interfant-99): An observational
study and a multicentre randomised trial. Lancet. 370:240–250.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papaemmanuil E, Hosking FJ, Vijayakrishnan
J, Price A, Olver B, Sheridan E, Kinsey SE, Lightfoot T, Roman E,
Irving JA, et al: Loci on 7p12.2, 10q21.2 and 14q11.2 are
associated with risk of childhood acute lymphoblastic leukemia. Nat
Genet. 41:1006–1010. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trevino LR, Yang W, French D, Hunger SP,
Carroll WL, Devidas M, Willman C, Neale G, Downing J, Raimondi SC,
et al: Germline genomic variants associated with childhood acute
lymphoblastic leukemia. Nat Genet. 41:1001–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molkentin JD: The zinc finger-containing
transcription factors GATA-4, −5 and −6. Ubiquitously expressed
regulators of tissue-specific gene expression. J Biol Chem.
275:38949–38952. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evans T, Reitman M and Felsenfeld G: An
erythrocyte-specific DNA-binding factor recognizes a regulatory
sequence common to all chicken globin genes. Proc Natl Acad Sci
USA. 85:5976–5980. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orkin SH: GATA-binding transcription
factors in hematopoietic cells. Blood. 80:575–581. 1992.PubMed/NCBI
|
|
12
|
Arceci RJ, King AA, Simon MC, Orkin SH and
Wilson DB: Mouse GATA-4: A retinoic acid-inducible GATA-binding
transcription factor expressed in endodermally derived tissues and
heart. Mol Cell Biol. 13:2235–2246. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelley C, Blumberg H, Zon LI and Evans T:
GATA-4 is a novel transcription factor expressed in endocardium of
the developing heart. Development. 118:817–827. 1993.PubMed/NCBI
|
|
14
|
Heikinheimo M, Ermolaeva M, Bielinska M,
Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS and Wilson DB:
Expression and hormonal regulation of transcription factors GATA-4
and GATA-6 in the mouse ovary. Endocrinology. 138:3505–3514. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaskivuo TE, Anttonen M, Herva R, Billig
H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M and Tapanainen
JS: Survival of human ovarian follicles from fetal to adult life:
apoptosis, apoptosis-related proteins and transcription factor
GATA-4. J Clin Endocrinol Metab. 86:3421–3429. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyrönlahti A, Rämö M, Tamminen M,
Unkila-Kallio L, Butzow R, Leminen A, Nemer M, Rahman N, Huhtaniemi
I, Heikinheimo M and Anttonen M: GATA-4 regulates Bcl-2 expression
in ovarian granulosa cell tumors. Endocrinology. 149:5635–5642.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jääskeläinen M, Nieminen A, Pökkylä RM,
Kauppinen M, Liakka A, Heikinheimo M, Vaskivuo TE, Klefström J and
Tapanainen JS: Regulation of cell death in human fetal and adult
ovaries-role of Bok and Bcl-X (L). Mol Cell Endocrinol. 330:17–24.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bergman M and Ringertz N: Gene expression
pattern of chicken erythrocyte nuclei in heterokaryons. J Cell Sci.
97:167–175. 1990.PubMed/NCBI
|
|
19
|
Shimamoto T, Ohyashiki K, Ohyashiki JH,
Kawakubo K, Fujimura T, Iwama H, Nakazawa S and Toyama K: The
expression pattern of erythrocyte/megakaryocyte-related
transcription factors GATA-1 and the stem cell leukemia gene
correlates with hematopoietic differentiation and is associated
with outcome of acute myeloid leukemia. Blood. 86:3173–3180.
1995.PubMed/NCBI
|
|
20
|
Ono Y, Fukuhara N and Yoshie O: TAL1 and
LIM-only proteins synergistically induce retinaldehyde
dehydrogenase 2 expression in T-cell acute lymphoblastic leukemia
by acting as cofactors for GATA3. Mol Cell Biol. 18:6939–6950.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Dong L, Liu G, Lin Y, Zhang J, Jia
S, Lu S, Pian H, Yao B and Chen H: GATA-2 gene expression in
leukemia patients and its significance. Zhonghua Xue Ye Xue Za Zhi.
22:27–29. 2001.(In Chinese). PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi S, Lackey T, Huang Y, Bisping E,
Pu WT, Boxer LM and Liang Q: Transcription factor gata4 regulates
cardiac BCL2 gene expression in vitro and in vivo. FASEB J.
20:800–802. 2006.PubMed/NCBI
|
|
24
|
Zhang SJ, Shi JY and Li JY: GATA-2 L359 V
mutation is exclusively associated with CML progression but not
other hematological malignancies and GATA-2 P250A is a novel single
nucleotide polymorphism. Leuk Res. 33:1141–1143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haupt Y, Barak Y and Oren M: Cell
type-specific inhibition of p53-mediated apoptosis by mdm2. EMBO J.
15:1596–1606. 1996.PubMed/NCBI
|
|
26
|
Ferrando AA, Neuberg DS, Staunton J, Loh
ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland
DG, et al: Gene expression signatures define novel oncogenic
pathways in T cell acute lymphoblastic leukemia. Cancer cell.
1:75–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldberg JM, Silverman LB, Levy DE, Dalton
VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE and Asselin BL:
Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber
Cancer Institute acute lymphoblastic leukemia consortium
experience. J Clin Oncol. 21:3616–3622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oudot C, Auclerc MF, Levy V, Porcher R,
Piguet C, Perel Y, Gandemer V, Debre M, Vermylen C, Pautard B, et
al: Prognostic factors for leukemic induction failure in children
with acute lymphoblastic leukemia and outcome after salvage
therapy: The FRALLE 93 study. J Clin Oncol. 26:1496–1503. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Kang J, Deng X, Guo B, Wu B and
Fan Y: Knockdown of GATAD2A suppresses cell proliferation in
thyroid cancer in vitro. Oncol Rep. 37:2147–2152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walker EM, Thompson CA and Battle MA:
GATA4 and GATA6 regulate intestinal epithelial cytodifferentiation
during development. Dev Biol. 392:283–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castro IC, Breiling A, Luetkenhaus K,
Ceteci F, Hausmann S, Kress S, Lyko F, Rudel T and Rapp UR:
MYC-induced epigenetic activation of GATA4 in lung adenocarcinoma.
Mol Cancer Res. 11:161–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anttonen M, Pihlajoki M, Andersson N,
Georges A, L'hôte D, Vattulainen S, Färkkilä A, Unkila-Kallio L,
Veitia RA and Heikinheimo M: FOXL2, GATA4 and SMAD3 co-operatively
modulate gene expression, cell viability and apoptosis in ovarian
granulosa cell tumor cells. PLoS One. 9:e855452014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Färkkilä A, Andersson N, Bützow R, Leminen
A, Heikinheimo M, Anttonen M and Unkila-Kallio L: HER2 and GATA4
are new prognostic factors for early-stage ovarian granulosa cell
tumor-a long-term follow-up study. Cancer Med. 3:526–536. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mehta G, Kumarasamy S, Wu J, Walsh A, Liu
L, Williams K, Joe B and de la Serna IL: MITF interacts with the
SWI/SNF subunit, BRG1, to promote GATA4 expression in cardiac
hypertrophy. J Mol Cell Cardiol. 88:101–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Agnihotri S, Wolf A, Munoz DM, Smith CJ,
Gajadhar A, Restrepo A, Clarke ID, Fuller GN, Kesari S and Dirks
PB: A GATA4-regulated tumor suppressor network represses formation
of malignant human astrocytomas. J Exp Med. 208:689–702. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soini T, Haveri H, Elo JM, Kauppinen M,
Kyrönlahti A, Salo MK, Lohi J, Andersson LC, Wilson DB and
Heikinheimo M: Transcription factor GATA-4 is abundantly expressed
in childhood but not in adult liver tumors. J Pediatr Gastroenterol
Nutr. 54:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goldfarb AN: Coordinating red cell
differentiation with cell cycle arrest: GATA-1 activation of p21.
Cell Cycle. 9:20612010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Papetti M, Wontakal SN, Stopka T and
Skoultchi AI: GATA-1 directly regulates p21 gene expression during
erythroid differentiation. Cell Cycle. 9:1972–1980. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ku CJ, Hosoya T, Maillard I and Engel JD:
GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle
entry. Blood. 119:2242–2251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pei Y, Yao Q, Yuan S, Xie B, Liu Y, Ye C
and Zhuo H: GATA4 promotes hepatoblastoma cell proliferation by
altering expression of miR125b and DKK3. Oncotarget. 7:77890–77901.
2016.PubMed/NCBI
|
|
41
|
Safe S: MicroRNA-specificity protein (sp)
transcription factor interactions and significance in
carcinogenesis. Curr Pharmacol Rep. 1:73–78. 2015. View Article : Google Scholar : PubMed/NCBI
|