Introduction
Intervertebral disc degeneration disease is an
important health problem worldwide, resulting in a large economic
burden (1). During degeneration,
the intervertebral discs exhibit extensive histomorphological
alterations, including apoptosis of nucleus pulposus (NP) cells,
lamellae disorganization of the annulus fibrosis, as well as
thinning and calcification of the cartilaginous endplates (2,3).
Furthermore, the supply of nutrients, particularly oxygen and
glucose, is significantly decreased and in some cases even
non-existent. The increased rate of NP cell death during
degeneration coincides with a decrease in nutrients supplied to the
disc, indicating that a reduction in the supply of nutrients may be
an important factor involved in NP cell death in human discs
(4).
B-cell lymphoma 2 (Bcl-2)/adenovirus E1B
19-kDa-interacting protein 3 (BNIP3) is a member of a unique family
of death-inducing mitochondrial proteins, which possesses a single
Bcl-2 homology 3 (BH3) domain that acts as a pro-death factor
(5). Under normal conditions,
BNIP3 is expressed at low levels in various cell types, where it is
primarily localized to the cytosol (5,6).
However, BNIP3 expression is increased under hypoxic conditions and
is translocated from the cytosol to the mitochondria, where it
causes mitochondrial dysfunction and subsequently induces cell
death. Furthermore, apoptosis-inducing factor (AIF) is a key factor
in caspase-independent cell death (7), which is anchored to the outer face of
the inner mitochondrial membrane. In response to apoptotic stimuli,
AIF is released into the cytosol and translocates to the nucleus,
where it induces chromatin condensation and large-scale DNA
fragmentation (~50 kb) in a caspase 3-independent manner (8,9). Our
previous study reported that BNIP3 expression was upregulated in NP
cells in response to nutrient deprivation (ND) (10). However, the exact role of BNIP3 in
ND-induced NP cell death, as well as the underlying mechanism,
remains to be elucidated. Accordingly, the present study
hypothesized that during disc degeneration, the nutrient supply
induces alterations in the microenvironment of NP cells, which may
increase BNIP3 expression and subsequently cause mitochondrial
dysfunction, thereby resulting in the release and translocation of
AIF to the nucleus, ultimately inducing apoptosis of NP cells. To
confirm this hypothesis, the role of BNIP3 in ND-induced cell death
and the potential involvement of AIF in this process were
investigated. The present study is the first, to the best of our
knowledge, to demonstrate that ND induced cell death of NP cells
partially via activation of the BNIP3/AIF signalling pathway,
thereby providing a novel insight into the potential mechanism
underlying ND-induced NP cell death during disc degeneration.
Materials and methods
Isolation and culture of NP cells from
caudal discs
All animal experiments conducted in the present
study were approved by the Animal Care and Use Committee of the
Third Military Medical University (Chongqing, China; approval no.
SYXC-2015-00012) and were conducted in strict accordance with the
recommendations outlined in the Guide for the Care and Use of
Laboratory Animals adopted by the National Institutes of Health
(11). Thirty male Sprague-Dawley
rats (150–200 g; age, 8–10 weeks) were provided by the Laboratory
Animal Centre of the Third Military Medical University (Chongqing,
China). The animals were given free access to food and water, and
were housed 3 mice/cage in a room maintained at a constant
temperature (23±1°C) and humidity (60±1%) with a 12-h light-dark
cycle. All efforts were made to lessen suffering and to minimize
the number of animals used. NP cells from Sprague-Dawley rats were
isolated from caudal discs as previously described (10). The NP cells were removed from both
halves of each disc using blunt forceps and pooled. Pooled NP cells
were digested with 0.2% (w/v) collagenase II for 4 h at 37°C. The
digested tissue and solution were then filtered through a Falcon
40-µm filter to remove non-digested fragments and were washed three
times by centrifugation (5 min at 1,118 × g). Typically, cell
cultures were maintained for 3 days either under normal control
conditions [Dulbecco's modified Eagle's medium (DMEM) with 5 mM
glucose and 10% fetal bovine serum (FBS; all from Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA); 37°C, 21% O2]
or varying conditions for the ND groups (DMEM without FBS or
glucose; 37°C, 21% O2).
Cell viability and apoptosis
assays
Cell viability was detected using the trypan blue
exclusion method, as described previously (12). Apoptosis was detected using an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
Apoptosis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The stained cells
were analysed by flow cytometry; fluorescence emission was detected
at 530 and 575 nm, and excitation was detected at 488 nm.
Separation of cytosolic, mitochondrial
and nuclear fractions
The cytosolic, mitochondrial and nuclear fractions
were isolated as described previously, with some modifications
(13). Briefly, 2×107
cells were harvested by trypsinization following treatment and were
washed once with PBS. Subsequently, the cells were
digitonin-permeablilized for 5 min on ice at a density of
4×107/ml in cytosolic extraction buffer (75 mM NaCl, 1
mM NaH2PO4, 8 mM
Na2HPO4, 250 mM sucrose, 1 mM
phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin, 5 µg/ml aprotinin
and 0.05% digitonin). Cells were then transferred to a pre-cooled
tissue homogenizer and homogenized 30 times using a tight pestle.
The homogenized cells were centrifuged at 800 × g for 5 min at 4°C
and the supernatant was separated from the pellet comprising
mitochondria and cellular debris. The supernatant containing
cytoplasmic protein was further purified by centrifugation at
13,000 × g at 4°C for 10 min. The pellet, containing the
mitochondrial fraction was resuspended in 50 µl homogenization
buffer (10 mM Tris HCl, pH 6.7, 0.15 mM MgCl2, 0.25 M
sucrose, 1 mM PMSF and 1 mM DTT). Mitochondrial fractions were
stored at −80°C until further use. For cytosolic and nuclear
extraction, the K266-100 Nuclear/Cytosol Fractionation kit
Biovision, Inc. (Milpitas, CA, USA) was used. Protein contents were
determined using the Bradford assay with bovine serum albumin
(Hyclone; GE Healthcare Life Sciences) as standard.
Cytofluorimetric analysis of
mitochondrial transmembrane potential (Δψm)
Δψm was measured using the lipophilic cationic probe
JC-1. After treatment, the cells were subjected to flow cytometry
as described previously (14). The
excitation wavelength was 490/525 nm and emission was monitored at
530/590 nm for JC-1 single probes and aggregates, respectively.
RNA interference
Small interfering RNA (siRNA) targeting BNIP3 (cat.
no. sc-37452) and AIF (cat. no. sc-29194) along with control siRNA
(cat. no. sc-44230) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). NP cells (2×105) were
transfected in serum-free DMEM with Lipofectamine 2000 transfection
reagent (4 µg/well; Thermo Fisher Scientific, Inc.) containing 100
nM siRNA for 5–7 h at 37°C according to the manufacturer's
protocol. Subsequently, the cells were cultured in DMEM for an
additional 24 h at 37°C. As previously indicated, cells were then
subjected to ND for up to 72 h, harvested, and underwent further
analysis.
Plasmids and transfection
The pEGFP-N1-BNIP3 plasmid was designed by
Invitrogen (Thermo Fisher Scientific, Inc.) as previously described
(15). Cells (2×105)
were cultured in antibiotic-free DMEM for 24 h at 37°C and were
then transfected with BNIP3 and control plasmids (3 µg/well) using
Opti-MEM®I reduced serum media (Hyclone; GE Healthcare
Life Sciences) and Lipofectamine 2000 transfection reagent (4
µg/well; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 24 h post-transfection, the
cells were washed and subjected to ND for the indicated
durations.
Western blot analysis
Protein samples (40–60 µg; extracted as described in
the Separation of cytosolic, mitochondrial and nuclear
fractions subsection) were separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes, which were
incubated at 4°C for 12 h with the following primary antibodies:
Polyclonal anti-BNIP3 (cat. no. ab10433; dilution, 1:300; Abcam,
Cambridge, MA, USA), monoclonal anti-AIF (cat. no. ab32516; 1:500;
Abcam), anti-cytochrome c oxidase IV (cat. no. ab14744; Cox
IV; 1:10,000; Abcam), anti-histone H1 (cat. no. ab203337; 1:500;
Abcam), and anti-β-actin (cat. no. A1978; 1:2,000; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Subsequently, the membranes were
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (cat. no. ab150028; 1:10,000;
Abcam) at 37°C for 2 h. Immunoblotting was detected using an
enhanced chemiluminescence kit (Beyotime Institute of
Biotechnology, Haimen, China) and images were captured using a
FluorChem 8900 imager (Beyotime Institute of Biotechnology).
Western blots were semi-quantified using ImageJ v4.02 software
(National Institutes of Health, Bethesda, MD, USA).
Caspase 3 activity analysis
The generation of p-nitroaniline (pNA) from the
caspase tetrapeptide substrate Ac-DEVD-pNA (Beyotime Institute of
Biotechnology) was used as an index of caspase 3 activity, as
described previously (16).
Optical density of free pNA was measured at an absorption
wavelength of 405 nm using a microplate spectrophotometer (Dynex
Technologies, Inc., Chantilly, VA, USA).
Statistical analysis
All values are presented as the mean ± standard
deviation. Differences between samples were assessed by one-way
analysis of variance with a Bonferroni post hoc test for multiple
comparisons using SPSS v22.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
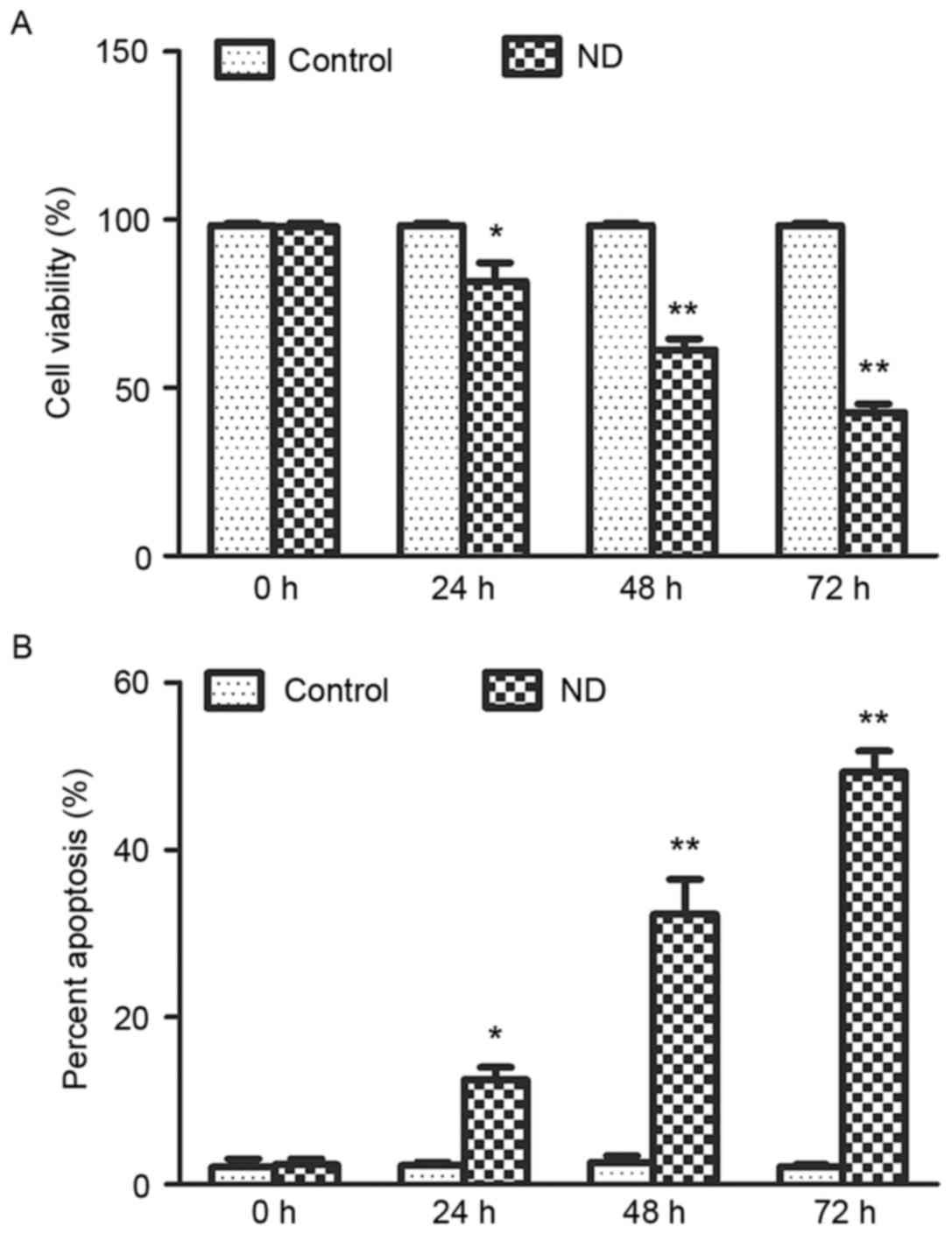
ND induces NP cell death
The effects of ND on the viability of NP cells were
determined using a trypan blue exclusion assay. The results
detected a time-dependent decrease in viability of NP cells
subjected to ND for up to 72 h (Fig.
1A). As determined using the Annexin V-FITC/PI apoptosis assay,
the mode of cell death was predominantly apoptosis (Fig. 1B). Together, these data suggested
that ND induced cell death, particularly apoptosis, of NP cells,
which was consistent with the findings of our previous study
(10).
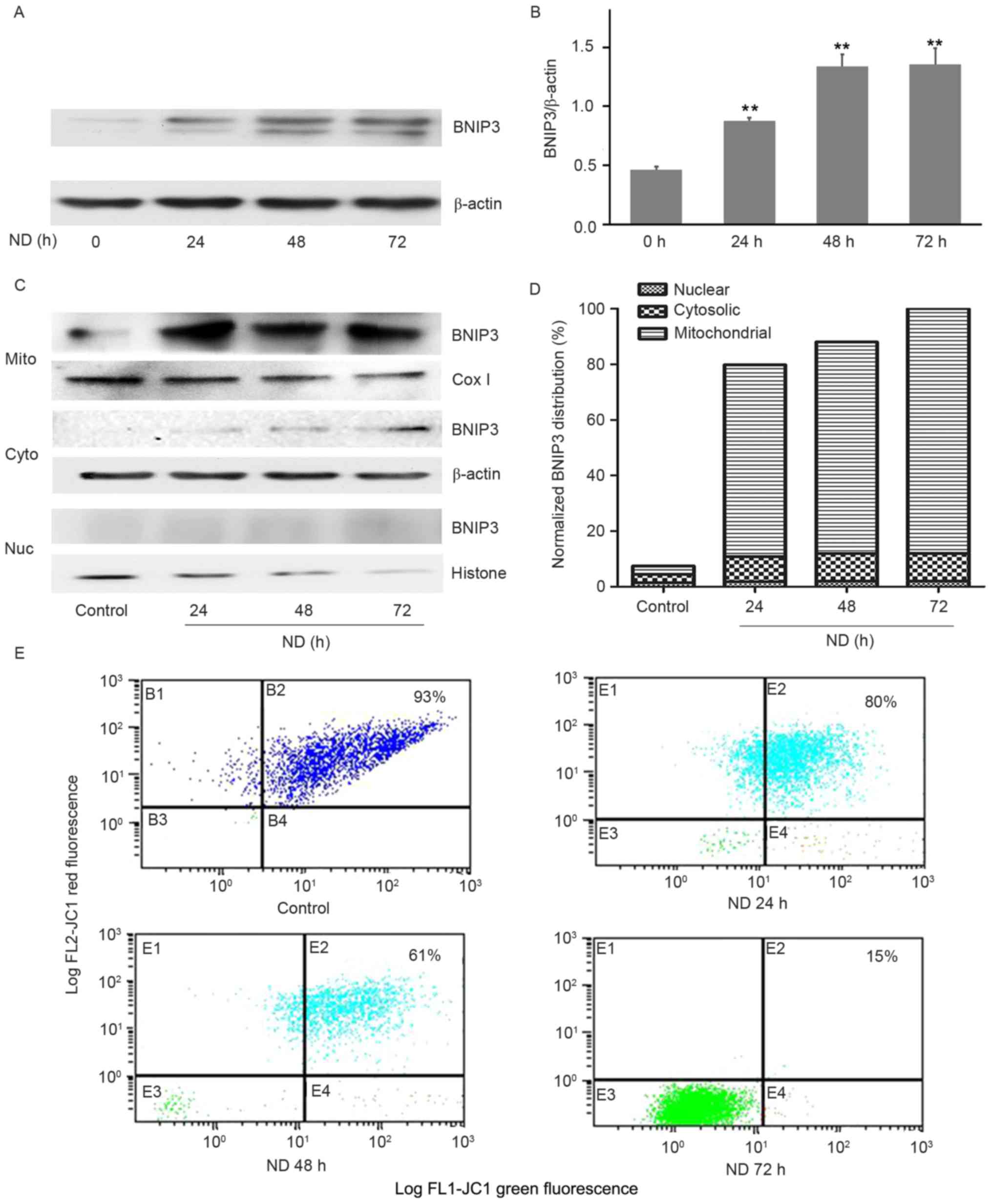
ND-induced cell death is associated
with increased BNIP3 expression and mitochondrial translocation in
NP cells
To determine whether ND was able to alter BNIP3
expression, cells were subjected to ND for up to 72 h and protein
expression was detected by western blot analysis. BNIP3 was
expressed at a low level in the control cells, whereas ND
significantly increased BNIP3 expression over the 72-h observation
period (Fig. 2A and B); this
exhibited a similar trend to the cell death response.
To examine how BNIP3 may induce NP cell death, the
subcellular localization of ND-induced BNIP3 was detected.
Differential centrifugation was used to prepare mitochondrial,
cytosolic and nuclear fractions, which were probed with a BNIP3
antibody. As loading controls, the cytosolic fraction was probed
with a β-actin antibody, the mitochondrial fraction with a Cox IV
antibody and the nuclear fraction with a histone H1 antibody. As
presented in Fig. 2C and D, ND
increased the mitochondrial translocation of BNIP3. A small amount
of BNIP3 was present in the cytosolic fraction, whereas none was
observed in the nuclear fraction. In addition, the Δψm of cells
cultured under normal and ND conditions was detected using JC-1. As
shown in Fig. 2E, ~93% of the
control cells exhibited double green-red fluorescence, whereas
there was a time-dependent decrease in the percentage of ND-treated
cells exhibiting double green-red fluorescence (~80% at 24 h, ~61%
at 48 h and ~15% at 72 h), confirming a significant alteration in
Δψm. These data suggested that ND may upregulate the expression of
BNIP3 thereby inducing its translocation to mitochondria and
causing mitochondrial dysfunction.
ND induces NP cell death in a
BNIP3-dependent manner
In order to further determine the role of BNIP3 in
ND-induced cell death, BNIP3 was overexpressed or silenced in NP
cells using a transient transfection method. As shown in Fig. 3A and B, BNIP3 protein expression
was significantly upregulated in NP cells transfected with
pEGFP-N1-BNIP3. Conversely, BNIP3 expression in BNIP3
siRNA-transfected cells was markedly lower than that of the control
cells (Fig. 3C and D).
Furthermore, ND-induced death of NP cells was significantly
increased by BNIP3 overexpression (Fig. 3E and F). However, as expected,
ND-induced NP cell death was markedly inhibited by BNIP3 knockdown
(Fig. 3G and H). Together, these
results suggested that ND stimulated NP cell death via BNIP3
regulation.
AIF serves a key role in
BNIP3-activated cell death in ND-treated NP cells
The involvement of AIF in ND-induced NP cell death
was investigated. As presented in Fig.
4A and B, the total amount of AIF did not vary with exposure
time to ND. However, AIF expression in the mitochondrial fraction
was decreased with exposure time to ND; expression began to
markedly decrease after ND for 48 h and expression had disappeared
from the mitochondria after 72 h of ND. Furthermore, AIF expression
was detected in the nuclear fraction after ND for 48 h and
accumulated in a time-dependent manner, thus suggesting that AIF
released from the mitochondria was translocated to the nucleus. In
addition, BNIP3 overexpression increased the protein expression
levels of AIF in NP cells exposed to ND, whereas BNIP3 knockdown
inhibited ND-induced AIF expression (Fig. 4C and D).
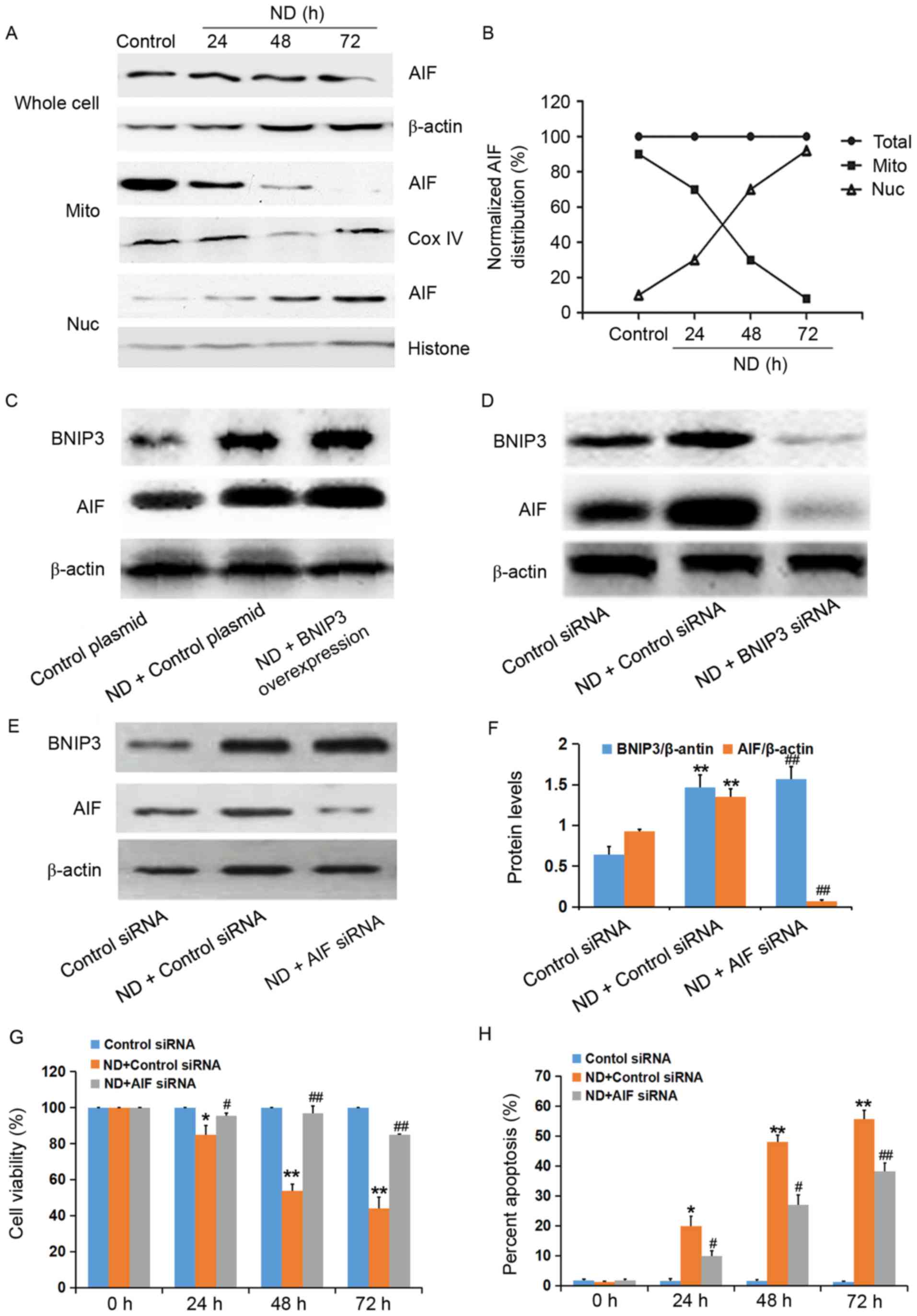 | Figure 4.AIF serves a key role in BNIP3-induced
death of nucleus pulposus cells exposed to ND. Cells were subjected
to ND for up to 72 h. (A) Extracts of cell fractions from control
and treated cells were subjected to western blot analysis with an
anti-AIF antibody. (B) Levels of AIF distribution were normalized
as the percentage of the total amount of AIF at each time. NP cells
were transfected with either a plasmid expressing BNIP3 or with
BNIP3 siRNA. Following 24 h, the cells were subjected to ND for a
further 72 h. BNIP3 expression was detected in the (C) plasmid and
(D) siRNA groups by western blot analysis. (E) AIF was knocked down
by AIF siRNA. At 24 h post-transfection, the cells were subjected
to ND for a further 72 h. Cells were collected and lysed, and
western blot analysis was performed. (F) Protein expression was
semi-quantified for the siRNA groups. (G) Cell viability was
determined using the trypan blue exclusion method. (H) Percentage
of apoptotic cells was determined using the Annexin V-fluorescein
isothiocyanate/propidium iodide assay. Data are presented as the
mean ± standard deviation of three experiments. *P<0.05,
**P<0.01 vs. the control group; #P<0.05,
##P<0.01 vs. the ND+control siRNA-treated group. AIF,
apoptosis-inducing factor; BNIP3, B-cell lymphoma 2/adenovirus E1B
19 kDa-interacting protein; Cox IV, cytochrome c oxidase IV; Mito,
mitochondrial; ND, nutrient deprivation; Nuc, nuclear; siRNA, small
interfering RNA. |
In order to further determine the involvement of AIF
in BNIP3-induced death of NP cells exposed to ND, AIF siRNA was
used to knockdown AIF expression. As shown in Fig. 4E and F, ND-induced BNIP3 expression
was not affected by AIF siRNA. However, ND-induced NP cell death
was abolished following transfection with AIF siRNA (Fig. 4G and H). These results indicated
that BNIP3-induced death of NP cells exposed to ND was dependent on
AIF.
BNIP3-induced death of NP cells
exposed to ND is independent of caspase 3
To determine whether the death of NP cells was
caspase-dependent, caspase 3 activity was measured using a
microplate spectrophotometer following ND. According to the optical
density values (Table I), during
the 72-h culture period, caspase 3 activity was not significantly
altered compared with in the control cells. These findings
suggested that BNIP3-indcued NP cell death may be independent of
caspase 3.
 | Table I.Results of caspase 3 activity
assay. |
Table I.
Results of caspase 3 activity
assay.
| Group | OD value |
|---|
| Control | 0.036±0.005 |
| ND 24 h | 0.028±0.002 |
| ND 48 h | 0.027±0.004 |
| ND 72 h | 0.032±0.008 |
Discussion
Previous studies have reported that the initial
characteristic of intervertebral disc degeneration appears to be
associated with loss of NP cells, which indicates that NP cells
serve an important role in the maintenance of disc health (17,18).
Understanding the mechanisms underlying NP cell death will help to
further elucidate the pathomechanism of disc degeneration. It has
previously been reported that a decrease in nutrient supply is the
key factor leading to the death of NP cells during disc maturation
and degeneration (19). However,
the exact underlying mechanisms remain to be elucidated. Therefore,
in the present study, a cell culture model under ND conditions was
created to investigate the roles of BNIP3 (a nutrient-sensitive
protein) and AIF (a mitochondrial pro-death protein) in the death
pathway of NP cells.
Mitochondria serve an important role in cell death
responses through the release of mitochondrial pro-death proteins,
including AIF, endonuclease G and cytochrome c (20). Members of the Bcl-2 family control
the release of these proteins. Bcl-2 is an integral membrane
protein that prevents apoptosis through inhibition of protein
efflux, where BNIP3, Bcl-2-associated X protein and BH3
interacting-domain death agonist are translocated from the
cytoplasm to the outer mitochondrial membrane and trigger apoptosis
upon release (21). As reported
previously, the mitochondrial translocation of BNIP3 is involved in
the ND-induced death of neurons (22). The results of the present study
demonstrated that ND stimulated NP cell death via the regulation of
BNIP3 expression and mitochondrial translocation. A previous study
demonstrated that autophagy attenuates the catabolic effect of NP
cells under inflammatory conditions, which is sustained by nuclear
factor (NF)-κB and inhibition of c-Jun N-terminal kinase (19). In addition, the stromal
cell-derived factor-1/C-X-C motif chemokine receptor 4 axis
promotes cell apoptosis in human degenerative NP cells via the
NF-κB pathway (20). To the best
of our knowledge, the findings of the present study are the first
to provide direct evidence of ND-induced NP cell death in a
BNIP3-dependent manner, which is an important complement to
previous studies.
Although caspase-dependent apoptosis is the main
pathway of cell death, considerable evidence has suggested the
importance of caspase-independent pathways (23–25).
The mitochondrial apoptogenic factor AIF may induce apoptosis
without cytochrome c-mediated caspase activation. In
response to apoptotic stimuli, AIF is released into the cytosol and
translocated to the nucleus where it induces chromatin condensation
and large-scale DNA fragmentation (7). Notably, the results of the present
study demonstrated that AIF was released from the mitochondria and
translocated to the nucleus. Previous studies have indicated the
need for a caspase 3-dependent step in the release of AIF (26,27).
However, the results of the present study reported that caspase 3
expression was not altered during ND-induced NP cell death. These
results suggested that ND was capable of inducing the translocation
of AIF into the nucleus during the progression of cell death in a
caspase-independent manner.
It has previously been reported that BNIP3 is able
to rapidly induce mitochondrial dysfunction by directly interacting
with voltage-dependent anion channel/porin and adenine nucleotide
translocase to stimulate opening of the mitochondrial permeability
transition pore, thereby dissipating Δψm (28). The rapid loss of Δψm can lead to
depletion of adenosine triphosphate (ATP), calcium ion imbalance,
and eventual loss of plasma membrane integrity prior to subsequent
execution of cell death (29).
Another study demonstrated that AIF can also damage mitochondrial
function, resulting in the loss of Δψm (30). It has also been indicated that the
rapid and progressive depletion of intracellular ATP content
triggers the disruption of Δψm in cells and induces
caspase-independent apoptotic death (31). Therefore, the present study
hypothesized that, in addition to the release of AIF, a
BNIP3-mediated mitochondrial energetic failure may be associated
with ND-induced NP cell death.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that ND-induced NP cell
death may partially occur via activation of the BNIP3/AIF
signalling pathway. These results provide additional experimental
evidence to elucidate the pathomechanism of intervertebral disc
degeneration.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272028 and
81472131).
Glossary
Abbreviations
Abbreviations:
|
AIF
|
apoptosis-inducing factor
|
|
ATP
|
adenosine triphosphate
|
|
BNIP3
|
B-cell lymphoma 2/adenovirus E1B 19
kDa-interacting protein
|
|
ND
|
nutrient deprivation
|
|
NP
|
nucleus pulposus
|
|
pNA
|
p-nitroaniline
|
References
|
1
|
Dagenais S, Caro J and Haldeman S: A
systematic review of low back pain cost of illness studies in the
United States and internationally. Spine J. 8:8–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishii T, Tsuji H, Sano A, Katoh Y, Matsui
H and Terahata N: Histochemical and ultrastructural observations on
brown degeneration of human intervertebral disc. J Orthop Res.
9:78–90. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thompson JP, Pearce RH, Schechter MT,
Adams ME, Tsang IK and Bishop PB: Preliminary evaluation of a
scheme for grading the gross morphology of the human intervertebral
disc. Spine (Phila Pa 1976). 15:411–415. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ward E, Evans SE and Stern CD: The role of
the somites and notochord in vertebral column development. Int J
Exp Pathol. 96:A112015.
|
|
5
|
Velde C Vande, Cizeau J, Dubik D, Alimonti
J, Brown T, Israels S, Hakem R and Greenberg AH: BNIP3 and genetic
control of necrosis-like cell death through the mitochondrial
permeability transition pore. Mol Cell Biol. 20:5454–5468. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee H and Paik SG: Regulation of BNIP3 in
normal and cancer cells. Mol Cells. 21:1–6. 2006.PubMed/NCBI
|
|
7
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye H, Cande C, Stephanou NC, Jiang S,
Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G and Wu H:
DNA binding is required for the apoptogenic action of apoptosis
inducing factor. Nat Struct Biol. 9:680–684. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung EC, Joza N, Steenaart NA, McClellan
KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS,
Shore GC, et al: Dissociating the dual roles of apoptosis-inducing
factor in maintaining mitochondrial structure and apoptosis. EMBO
J. 25:4061–4073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Wang J and Zhou Y: Upregulation of
BNIP3 and translocation to mitochondria in nutrition deprivation
induced apoptosis in nucleus pulposus cells. Joint Bone Spine.
79:186–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goodman JR: The Association For Assessment
And Accreditation Of Laboratory Animal Care International fails to
meaningfully address concerns regarding its accreditation program.
J Appl Anim Welf Sci. 18:314–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 111:A3.B.1–3. 2015. View Article : Google Scholar
|
|
13
|
Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi
L and Mi MT: Resveratrol regulates mitochondrial reactive oxygen
species homeostasis through Sirt3 signaling pathway in human
vascular endothelial cells. Cell Death Dis. 5:e15762014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santos JH, Hunakova L, Chen Y, Bortner C
and Van Houten B: Cell sorting experiments link persistent
mitochondrial DNA damage with loss of mitochondrial membrane
potential and apoptotic cell death. J Biol Chem. 278:1728–1734.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahn BH, Kim HS, Song S, Lee IH, Liu J,
Vassilopoulos A, Deng CX and Finkel T: A role for the mitochondrial
deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad
Sci USA. 105:14447–14452. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carvour M, Song C, Kaul S, Anantharam V
and Kanthasamy A and Kanthasamy A: Chronic low-dose oxidative
stress induces caspase-3-dependent pkcdelta proteolytic activation
and apoptosis in a cell culture model of dopaminergic
neurodegeneration. Ann N Y Acad Sci. 1139:197–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oegema TR Jr: The role of disc cell
heterogeneity in determining disc biochemistry: A speculation.
Biochem Soc Trans. 30:839–844. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hunter CJ, Matyas JR and Duncan NA: The
notochordal cell in the nucleus pulposus: A review in the context
of tissue engineering. Tissue Eng. 9:667–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pozdniakov AL and Khvylia SI: Role of the
alimentary factor in the development of morphological changes in
the blood vessels in an experiment. Vestn Akad Med Nauk SSSR.
65–70. 1986.(In Russian). PubMed/NCBI
|
|
20
|
Zanna C, Ghelli A, Porcelli AM, Martinuzzi
A, Carelli V and Rugolo M: Caspase-independent death of Leber's
hereditary optic neuropathy cybrids is driven by energetic failure
and mediated by AIF and Endonuclease G. Apoptosis. 10:997–1007.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Yang X, Zhang S, Ma X and Kong J:
BNIP3 upregulation and EndoG translocation in delayed neuronal
death in stroke and in hypoxia. Stroke. 38:1606–1613. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang TM, Qi SN, Zhao N, Yang YJ, Yuan HQ,
Zhang B and Jin S: Induction of apoptosis through
caspase-independent or caspase-9-dependent pathway in mouse and
human osteosarcoma cells by a new nitroxyl spin-labeled derivative
of podophyllotoxin. Apoptosis. 18:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oishi S, Tsuji M, Hasegawa H and Oguchi K:
High glucose induce apoptosis in a human lymphoma (U937) cells by a
caspase-independent pathway. J Pharmacol Sci. 112:258p2010.
|
|
25
|
Madeo F, Carmona-Gutierrez D, Ring J,
Büttner S, Eisenberg T and Kroemer G: Caspase-dependent and
caspase-independent cell death pathways in yeast. Biochem Biophys
Res Commun. 382:227–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arnoult D, Gaume B, Karbowski M, Sharpe
JC, Cecconi F and Youle RJ: Mitochondrial release of AIF and EndoG
requires caspase activation downstream of Bax/Bak-mediated
permeabilization. EMBO J. 22:4385–4399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arnoult D, Karbowski M and Youle RJ:
Caspase inhibition prevents the mitochondrial release of
apoptosis-inducing factor. Cell Death Differ. 10:845–849. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HM, Cheung P, Yanagawa B, McManus BM
and Yang DC: BNips: A group of pro-apoptotic proteins in the Bcl-2
family. Apoptosis. 8:229–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prabhakaran K, Li L, Mills EM, Borowitz JL
and Isom GE: Up-regulation of uncoupling protein 2 by cyanide is
linked with cytotoxicity in mesencephalic cells. J Pharmacol Exp
Ther. 314:1338–1345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang YH, Yi MJ, Kim MJ, Park MT, Bae S,
Kang CM, Cho CK, Park IC, Park MJ, Rhee CH, et al:
Caspase-independent cell death by arsenic trioxide in human
cervical cancer cells: Reactive oxygen species-mediated poly
(ADP-ribose) polymerise-1 activation signals apoptosis-inducing
factor release from mitochondria. Cancer Res. 64:8960–8967. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ballif BA and Blenis J: Molecular
mechanisms mediating mammalian mitogen-activated protein kinase
(MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ.
12:397–408. 2001.PubMed/NCBI
|