Introduction
Osteoporosis is a systemic skeletal disease leading
to fragility fractures and has become a major health problem in the
world (1). Osteoporosis primarily
affects women, and it has been reported that ~40% of postmenopausal
women in the USA suffer from osteoporosis (2,3).
With an ageing population, the medical and socioeconomic burden of
osteoporosis is expected to gradually increase globally in the near
future (4–6). Although mechanisms underlying the
pathogenesis of osteoporosis remain to be elucidated, an number of
studies suggested that the reduction of bone mineral density (BMD)
and deterioration of bone microarchitecture are responsible for the
development and progression of osteoporosis. Therefore, a novel
treatment for osteoporosis should aim to prevent excessive bone
resorption and to enhance bone formation (1).
Coenzyme Q10 (CoQ10), also known as ubiquinone,
ubidecarenone or coenzyme Q, is a lipid-soluble vitamin-like
substance. It serves a role in the electron transport chain
involved in the generation and regulation of cellular bioenergy
(7,8). CoQ10 demonstrates bioenergetic and
antioxidant properties (9).
Therefore, it is commonly used for the treatment of a variety of
diseases, including heart, metabolic, nervous system and
reproductive system diseases, and cancer (10). CoQ10 prevents the onset of bone
disease and regulates osteoblast and osteoclast differentiation
(11,12). However, little is known about the
efficacy of CoQ10 for the treatment of osteoporosis.
Therefore, the aim of the present study was to
investigate the effects of CoQ10 on osteoblastic cell proliferation
and differentiation in vitro, and on therapeutic efficacy of
the treatment of osteoporosis in a rat model of the disease in
vivo, as well as the underlying mechanism. Proliferation and
differentiation of rat bone marrow stromal cells (BMSCs), and
levels of osteoblastogenic markers, were determined following
treatment with different concentrations of CoQ10. Serum levels of
bone metabolism markers were determined in ovariectomy
(OVX)-induced rats with osteoporosis treated or untreated with
CoQ10. Effects of CoQ10 on morphological bone changes and cellular
signaling pathways were investigated. The present study provides
novel insights into potential treatment strategies of
osteoporosis.
Materials and methods
Animals and experimental design
Female Sprague-Dawley (SD) rats (8-months old,
250–350 g, n=48) were obtained from the Experimental Animal Center
of Dalian Medical University (Dalian, China). All rats were
acclimatized for at least one week prior to the experiments.
Animals were housed individually in standard laboratory cages with
controlled temperature and humidity (22–25°C; 50–65%) under a 12-h
light/dark cycle. Rats had access to food and water ad
libitum. A total of 8 rats were randomly selected and used in
the in vitro experiment, and BMSCs were obtained from them.
Remaining rats (n=40) were used to conduct in vivo
experiments. All experiments were approved by the Ethics Committee
on Animals at Dalian University (Dalian, China) and performed in
accordance with the standards set by the Guide for the Care and Use
of Laboratory Animals published by the National Institutes of
Health (Bethesda, MD, USA).
Primary culture of BMSCs and treatment
with CoQ10
BMSCs were isolated as previously described
(13,14). SD rats were subjected to euthanasia
by inhalation of 70% CO2 and femurs and tibias were
harvested. Bone marrow was removed, filtered through a 70-µm nylon
mesh and centrifuged at 1,200 × g for 8 min at 4°C. Collected cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin (all, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2
for 3 days. Subsequently, adherent cells were washed three times
with PBS and cultured in DMEM until 90% confluence was attained.
Following 3 or 4 passages, BMSCs were treated with vehicle (soya
bean oil) or various concentrations of CoQ10 (10, 20 or 100 µM,
Sigma-Aldrich; Thermo Fisher Scientific, Inc.). CoQ10 was dissolved
in soya bean oil at 40°C.
Cell proliferation
Viability of BMSCs was assessed by using
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells
(2×104 cells/ml) were seeded in 96-well plates and
treated with a vehicle or various concentrations of CoQ10 (10, 20
or 100 µM) for 24 h. On days 1, 3, 5 and 7 prior to the treatment,
cells were incubated with an MTT solution (20 µl, 0.5 mg/ml) for 4
h at 37°C, followed by an addition of dimethylsulfoxide
(Sigma-Aldrich; Merck KGaA). The absorbance was measured at a
wavelength of 590 nm with a SpectraMax 340 microplate reader
(Molecular Devices LLC, Sunnyvale, CA, USA).
Alkaline phosphatase (ALP) activity
assay
The activity of ALP was determined as previously
described (12). BMSCs
(5×103 cells/ml) were seeded in 96-well plates and
incubated with vehicle or 10, 20 or 100 µM CoQ10 for 24 h at room
temperature. Following pre-treatment with vehicle or CoQ10, 20 µl
p-nitrophenyl phosphate (pNPP; 1 mg/ml; Sigma-Aldrich; Merck KGaA)
was added to the RIPA buffer solution for 30 min at 37°C on days 1,
3, 5, and 7 of the experiment, according to the manufacturer's
protocol. Enzymatic activity was quantified by ALP ELISA kit (cat.
no. 687242; Antibody Research Corp., St. Peters, MO, USA) and
absorbance measured at a wavelength of 405 nm with a SpectraMax
Plus384 reader (Molecular Devices LLC).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Expression of osteogenic markers, runt-associated
transcription factor 2 (RUNX-2) and osteocalcin (OCN), was assessed
by RT-qPCR. Total RNA was isolated from BMSCs using the RNeasy Mini
kit (Qiagen Inc., Valencia, CA, USA) according to the
manufacturer's protocol. First-strand complementary DNA was
synthesized using Superscript III Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription
was performed at 55°C for 60 min. Expression levels were determined
using SYBR green-based qPCR (SYBR Green PCR Master Mix; Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR was undertaken
according to the following parameters: 1 predenaturation cycle of 1
min at 94°C, 40 cycles of 95°C for 30 sec, 60°C for 30 sec, and
72°C for 2 min, and a final extension at 72°C for 5 min. All
primers were supplied by Genepharma Co., Ltd. (Shanghai, China) and
are listed in Table I. Expression
was normalized to GAPDH using the comparative 2−ΔΔCq
method (15).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Target | Forward primer
5′→3′ | Reverse primer
5′→3′ |
|---|
| RUNX-2 |
TAAGAGGGTGGGGGCAGTCA |
CAGACCAGACAACACCTTTGACG |
| OCN |
TCTCTCTGCTCACTCTGCTG |
ATTTTGGAGCAGCTGTGCCG |
| GAPDH |
AAGAAGGTGGTGAAGCAGGC |
TCCACCACCCTGTTGCTGTA |
Western blot analysis
Total protein was extracted from BMSCs or rat femurs
using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China). Protein concentration
was assessed using a Bicinchoninic Acid protein assay kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Proteins were separated by 10–12% SDS-PAGE and
transferred to nitrocellulose membranes (EMD Millipore, Billerica,
MA, USA) and blocked with 5% dried skimmed milk in Tris buffered
saline with Tween-20. Membranes were then probed with: Anti
phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K, 1:1,000, cat.
no. mAb4249), phosphorylated (p)-PI3K (1:1,000, cat. no. 4228),
RAC-alpha serine/threonine-protein kinase (AKT, 1:1,000, cat. no
4691), p-AKT (1:1,000, cat. no 4060, all Cell Signaling Technology,
Inc., Danvers, MA, USA) or phosphatidylinositol 3,4,5-trisphosphate
3-phosphatase and dual-specificity protein phosphatase PTEN (PTEN;
cat. no. ab32199; Abcam, Cambridge, USA) primary antibodies
overnight at 4°C. Anti-GAPDH (1:2,000, cat. no. sc-25778) Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was used as a loading
control and was incubated overnight at 4°C. Following washing with
PBS, membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:5,000, cat. no.
sc-2372, Santa Cruz Biotechnology, Inc.). Immunoreactive protein
bands were visualized by enhanced chemiluminescence Western
blotting substrate (Roche Applied Science, Penzberg, Germany).
In vivo experiment
The rats were randomly divided into 5 groups (n=8
per group): i) the sham group, where rats were subjected to a sham
surgery that involved exposing but not removal of ovaries, and
received normal feedstuff for 3 months; ii) the OVX group, where
rats were subjected to OVX operation and received normal feedstuff
for 3 months; iii) the OVX+1 mg/kg CoQ10 group, where rats were
subjected to OVX and treated with 1 mg/kg CoQ10 by intraperitoneal
injection every 5 days for 3 months; iv) the OVX+10 mg/kg CoQ10
group where, rats were subjected to OVX and treated with 10 mg/kg
CoQ10 by intraperitoneal injection every 5 days for 3 months; and
v) the OVX+20 mg/kg CoQ10 group, where rats were subjected to OVX
and treated with 20 mg/kg CoQ10 by intraperitoneal injection every
5 days for 3 months.
OVX and sample collection
Rats were subjected to OVX as previously described
(16). Animals were anesthetized
by an intraperitoneal injection of 8% chloral hydrate solution
(Sigma-Aldrich; Merck KGaA). Following anesthesia, rats were
subjected to bilateral OVX by the dorsal approach. Six hours after
the last dose of CoQ10, all animals were sacrificed. Blood samples
(4 ml) were collected from hearts and stored at −20°C. Blood
samples were then centrifuged at 1,200 × g for 10 min at room
temperature. Whole femurs were dissected and fixed in 4%
paraformaldehyde for a subsequent micro-computed tomography (CT)
analysis.
Biochemical analysis of serum
Levels of serum estrogen (E2) (cat. no. IB79329,
IBL-America Inc., Spring Lake, MN, USA) and serum bone metabolism
markers, including calcium, parathyroid hormone (PTH) (cat. no.
60–2500, Immutopics Inc., San Clemente, CA, USA) and
osteoprotegerin (OPG) (cat. no. BE69058; IBL-America Inc.), were
measured by ELISA. Serum calcium levels were determined using an
Accucare Calcium Arsenazo III kit (Serotec Co., Ltd., Hokkaido,
Japan).
Micro-CT analysis of femur bones
High-resolution micro CT scans (SCANCO viva; version
4.0; Scanco Medical AG, Brüttisellen, Switzerland) were performed
on the sham- and CoQ10-treated joints. Changes in BMD and bone
volume/total volume (BV/TV) ratio were evaluated using the
Image-Pro software (version 7.0; Media Cybernetics Inc., Rockville,
MD, USA) and SCANCO microCT software packages (version 6.0). The
average trabecular number (Tb.N), average trabecular thickness
(Tb.Th) and average trabecular separation (Tb.Sp) were assessed by
the plate model 16 set-up: Tb.N=0.5 bone surface (BS)/TV; Tb.Th=2
BV/BS; Tb.Sp=2 (TV-BV)/BS (17).
Statistical analysis
All experiments were repeated at least three times.
The data are expressed as the mean ± standard error. Student's
t-test was used to determine differences between the two groups,
and one-way analysis of variance was performed to compare
differences among three groups, followed by the
Student-Newman-Keuls post-hoc test. P<0.05 was considered to
indicate statistically significant differences. Results were
analyzed using SPSS software (version 18.0; SPSS, Chicago, IL,
USA).
Results
Effect of CoQ10 on the viability of
BMSCs
The viability of BMSCs was analyzed by MTT following
1, 3, 5 and 7 days of treatment with various concentrations of
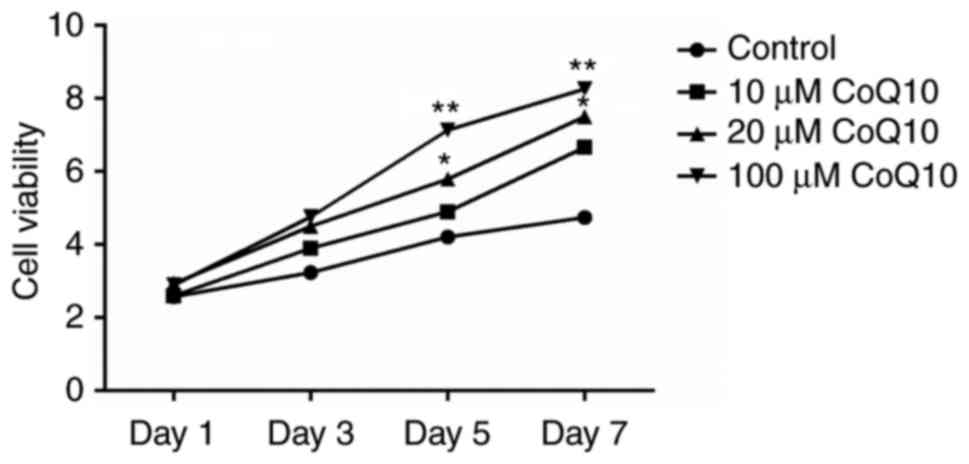
CoQ10 (10, 20 or 100 µM). As presented in Fig. 1, 10 µM CoQ10 increased cell
viability but the difference was not statistically significant.
Cell viability was not significantly altered on days 1 and 3 at any
dose. CoQ10 administrated at a dose of 20 or 100 µM exhibited
significant stimulatory effects on cell viability on days 5 and 7
(P<0.05 or P<0.01) in a dose-dependent pattern. The data
suggests that CoQ10 promotes viability of BMSCs.
Effect of CoQ10 on the expression of
osteogenic markers in BMSCs
RUNX-2, OCN and ALP are osteogenic markers.
Following treatment with different concentrations of CoQ10 (10, 20
or 100 µM), the expression levels of RUNX-2, OCN and ALP in BMSCs
on days 1, 3, 5, and 7 were measured by RT-qPCR and ELISA. As
indicated in Fig. 2, the
expression of RUNX-2, OCN and ALP was significantly increased by
the treatment with 20 or 100 µM CoQ10 on days 3, 5, and 7
(P<0.05, P<0.01, or P<0.001), in a dose-dependent manner.
No significant differences were observed on day 1 and following
treatment with 10 µM CoQ10 on any day. The results suggest that 20
and 100 µM CoQ10 can promote osteogenic differentiation of
BMSCs.
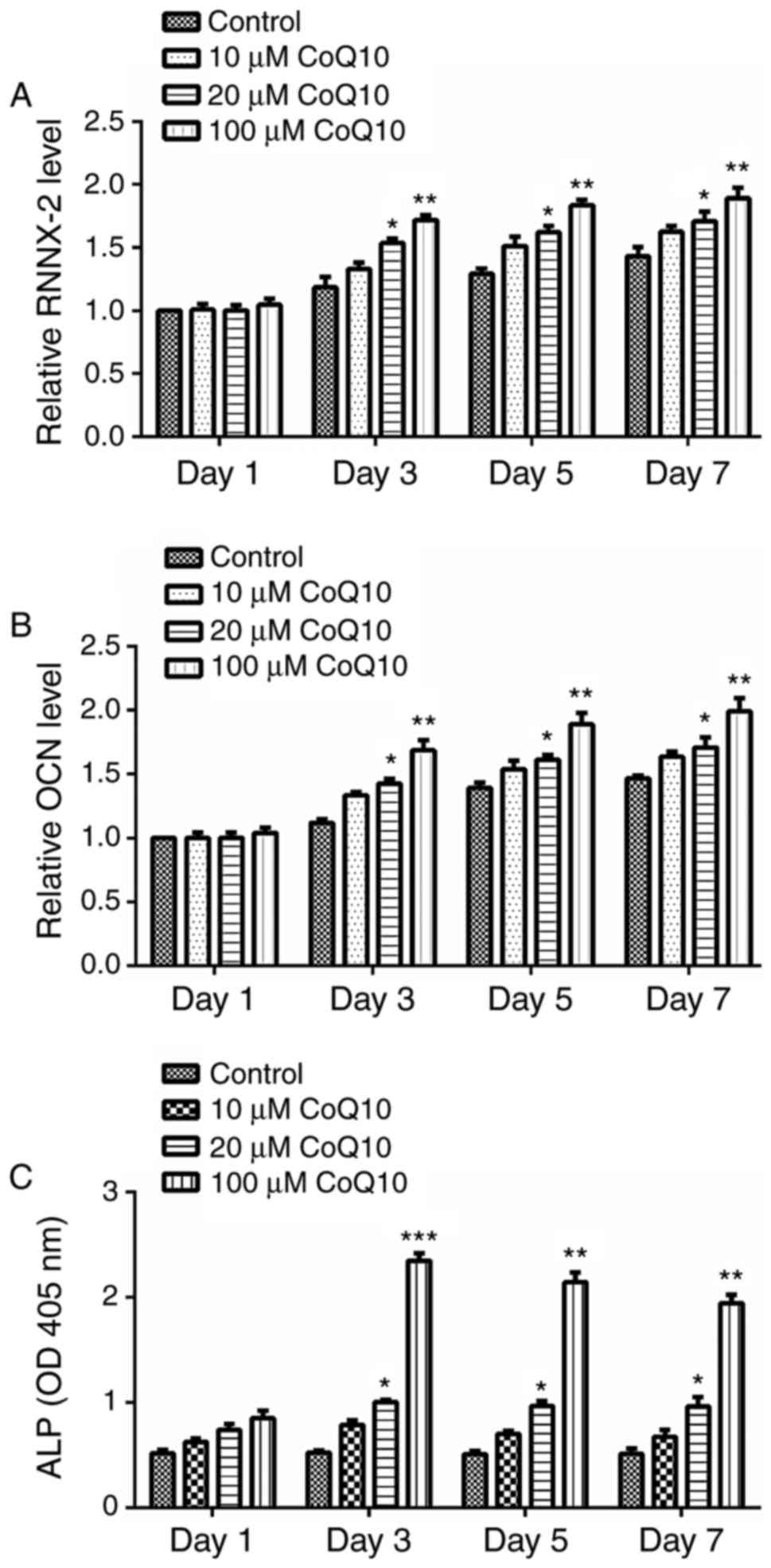 | Figure 2.Effect of CoQ10 on expression of
osteogenic markers in BMSCs. A total of 1, 3, 5 and 7 days
following treatment with 10, 20 or 100 µM CoQ10, the expression
levels of (A) RUNX-2, (B) OCN, and (C) ALP were measured in BMSCs.
The results revealed that the levels of RUNX-2, OCN and ALP was all
significantly increased on days 3, 5, and 7 by treatment with 20 or
100 µM CoQ10. Data are presented as the mean ± standard error.
*P<0.05, **P<0.01 or ***P<0.001 vs. the respective control
group. CoQ10, coenzyme Q10; BMSCs, bone marrow stromal cells;
RUNX-2, runt-associated transcription factor 2; OCN, osteocalcin;
ALP, alkaline phosphatase. |
Effect of CoQ10 on serum E2 levels and
serum bone metabolism markers
To investigate the effects of CoQ10 on osteoporosis,
a rat model of osteoporosis was established by OVX followed by the
administration of CoQ10. Serum levels of E2 and bone metabolism
markers (calcium, OPG and PTH) were determined following
administrating different concentrations of CoQ10. As presented in
Fig. 3, the levels of E2, calcium
and OPG was significantly decreased in the OVX group compared with
the sham group (all P<0.05), while PTH was significantly
increased in the OVX group, compared with the sham group
(P<0.05). Administration of 20 mg/kg CoQ10 significantly
increased the levels of calcium and OPG and decreased the levels of
PTH (all P<0.05) but exhibited no significant effect on the
levels of E2 compared with the OVX group. Furthermore, no
significant differences were observed in the abundance of E2,
calcium, OPG and PTH between the OVX group and OXY+1 or 10 mg/kg
treatment groups. The results demonstrated that 20 mg/kg CoQ10 can
increase serum calcium and OPG and decrease PTH levels, which may
be beneficial for patients with osteoporosis.
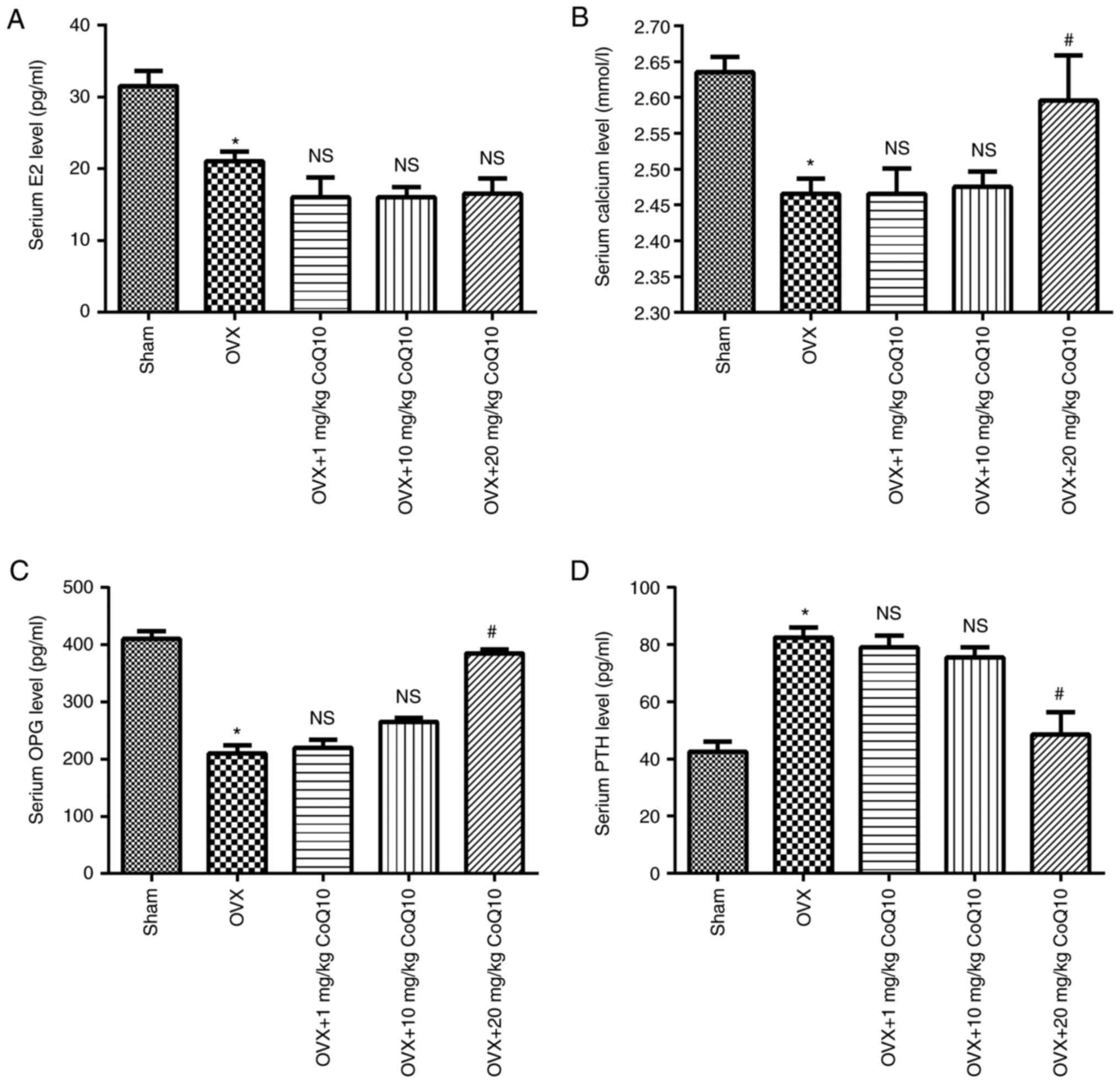 | Figure 3.Effect of CoQ10 on serum E2 levels
and serum bone metabolism markers. OVX-induced rats with
osteoporosis were treated with 1, 10 and 20 mg/kg CoQ10. The serum
levels of E2 and bone metabolism markers (calcium, OPG and PTH),
were determined. The results demonstrated that administration of 20
mg/kg CoQ10 exhibited no significant effect on (A) E2 levels but
significantly increased the levels of (B) calcium, (C) OPG and (D)
PTH, compared with the OVX group. Data are presented as the mean ±
standard error. *P<0.05 vs. the sham group;
#P<0.05 vs. the OVX group. CoQ10, coenzyme Q10; OVX,
ovariectomy; E2, estrogen; OPG, osteoprotegerin; PTH, parathyroid
hormone; NS, no significance. |
Effect of CoQ10 on bone morphology
parameters
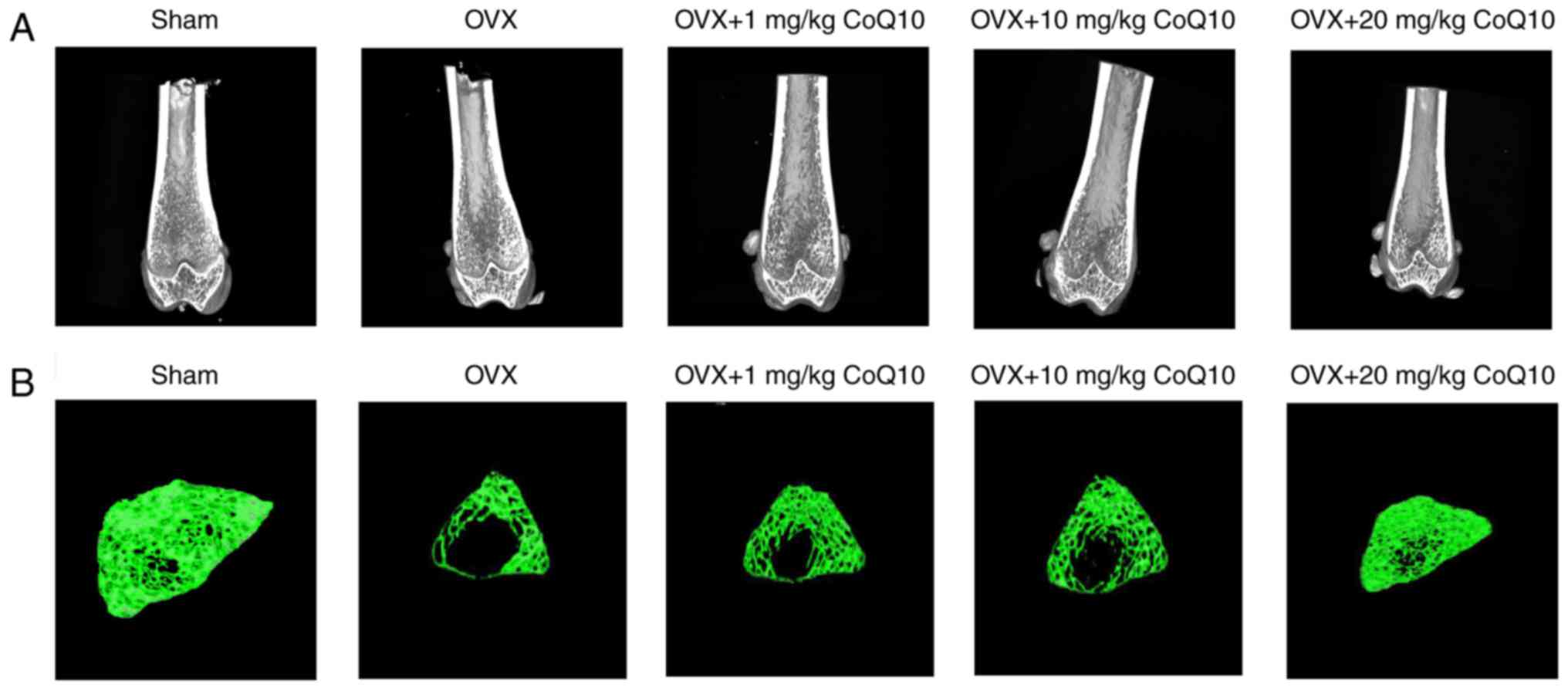
Micro-CT analysis was performed to evaluate bone
morphology parameters. The results are presented in Table II. BMD, BV/TV, Tb.N. and Tb.Th.
were significantly decreased in the OVX group compared with the
sham group, while Tb.Sp. significantly increased (all P<0.001).
Administration of CoQ10 (1, 10, or 20 mg/kg) significantly
increased BMD and Tb.N. and reduced Tb.Sp, compared with the OVX
group (all P<0.001). Only administration of 20 mg/kg CoQ10
exhibited significant stimulatory effects on BV/TV and Tb.Th,
compared with the OVX group (all P<0.001). Fig. 4A presents micrographs of the
vertical section of distal femur in different groups. The analysis
revealed poor trabecular structure in the OVX group compared with
the sham group. CoQ10 treatment improved the trabecular
microstructure (Fig. 4A) and
trabecular thickness (Fig. 4B) in
a dose-dependent manner compared with OVX group. The results
demonstrated that CoQ10 may protect against osteoporosis by
improving bone formation.
 | Table II.Effect of CoQ10 on bone morphology
parameters. |
Table II.
Effect of CoQ10 on bone morphology
parameters.
| Group | BMD
(mg/cm−2) | BV/TV (%) | Tb.N. (/mm) | Tb.Th.
(mm−1) | Tb.Sp.
(mm−1) |
|---|
| Sham |
785.04±30.05 |
66.31±2.52 |
6.12±0.28 |
1.05±0.10 |
0.58±0.06 |
| OVX |
378.66±26.06a |
27.01±4.74a |
3.38±0.55a |
0.71±0.03a |
2.01±0.19a |
| OVX+ 1 mg/kg
CoQ10 |
498.77±18.91b |
29.09±2.92 |
4.37±0.35b |
0.64±0.01 |
1.57±0.19b |
| OVX+ 10 mg/kg
CoQ10 |
499.24±13.41b |
29.79±3.66 |
4.42±0.31b |
0.68±0.07 |
1.59±0.18b |
| OVX+ 20 mg/kg
CoQ10 |
665.27±30.57b |
54.33±2.67b |
5.70±0.44b |
0.85±0.03b |
0.75±0.07b |
Effect of CoQ10 on the PTEN/PI3K/AKT
pathway
It has been previously demonstrated that activation
of the PI3K/AKT pathway protects against osteoporosis and controls
multiple cell processes, including cell proliferation,
differentiation and cell apoptosis. PTEN is a known negative
regulator of PI3K/AKT. Therefore, mRNA and protein expression
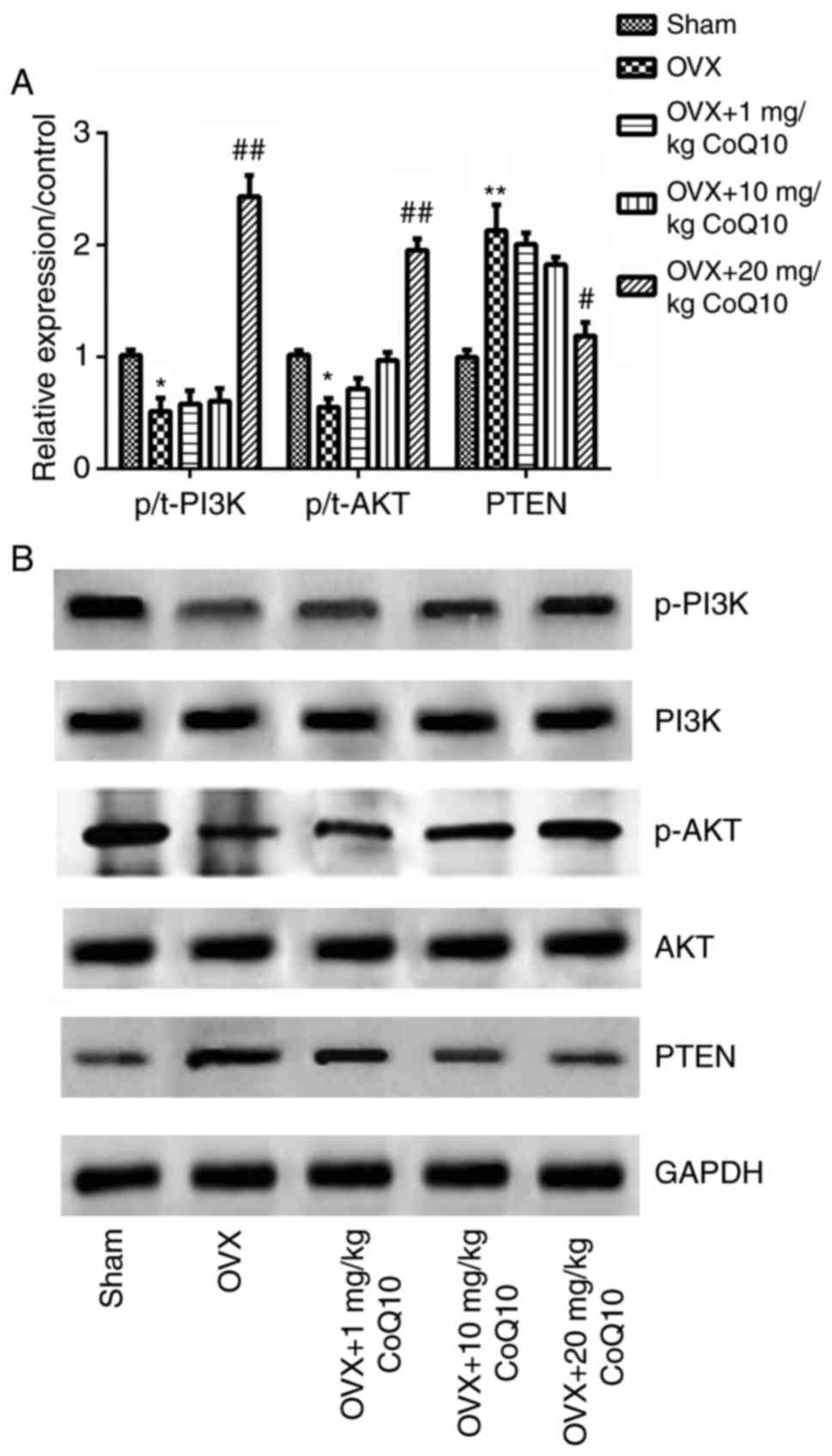
levels of PTEN/PI3K/AKT key factors, were analyzed. The results
revealed that OVX significantly decreased the levels of
phosphorylated to total ratio (p/t)-PI3K and p/t-AKT but
significantly increased the levels of PTEN compared with the sham
group (P<0.05 or P<0.01). There were no significant
differences in the levels of p/t-PI3K, p/t-AKT and PTEN following
administration of 1 or 10 mg/kg CoQ10 compared with the OVX group.
However, significant differences were observed in the relative
protein expression of p/t-PI3K, p/t-AKT and PTEN following
administration of 20 mg/kg CoQ10, compared with the OVX group
(P<0.05 or P<0.01; Fig. 5A and
B). The above results indicate that CoQ10 activates the
PTEN/PI3K/AKT signaling pathway.
Discussion
In the present study, potential therapeutic effects
of CoQ10 on osteoporosis were investigated in vivo and in
vitro. The results revealed that CoQ10 significantly increased
the proliferation and osteogenic differentiation of BMSCs in a
dose-dependent manner. In addition, Therefore, CoQ10 significantly
decreased bone resorption and enhanced bone formation. Furthermore,
the results demonstrated that CoQ10 activated the PTEN/PI3K/AKT
signaling pathway. CoQ10 presents protective effects on
osteoporosis and may be a potential candidate for the treatment of
osteoporosis.
In previous studies, several different types of
drugs have been proposed for bone treatment, including
bisphosphonates, estrogen and raloxifene (18–21).
However, in spite of their effectiveness, certain drugs present
side effects, including thromboembolism and oesophageal irritation
(1,22–24).
Therefore, novel drugs that aim to improve bone therapy are
required. CoQ10 demonstrates membrane stabilizing activity and is
an antioxidant with free radical scavenging activity and
cell-protective effects. Effects of CoQ10 on human diseases have
been widely studied, revealing the protective role of CoQ10 in
heart failure, cancer, muscular dystrophy and periodontal disease
(10,25–27).
A previous study (11)
demonstrated that CoQ10 acts as an inhibitor of osteoclastogenesis
by downregulating the production of reactive oxygen species (ROS),
and suggested that CoQ10 presents potential therapeutic
implications in the treatment of osteoporosis and other bone
diseases. However, both reports only focused on cell experiments,
and the mechanism underlying effects of CoQ10 were not completely
elucidated.
In the present study, experiments were performed on
cell culture and rat models of osteoporosis. For the in
vitro study, BMSCs were extracted from experimental rats. BMSCs
are multipotent progenitor cells demonstrating a capacity to
differentiate into multiple lineages, incluging osteoblasts,
chondrocytes, adipocytes and myoblasts (28–30).
The results of the present study confirmed that CoQ10 enhances the
proliferation of BMSCs and promotes the osteogenic differentiation
in a dose-dependent manner; these observations are similar to the
results of a previous study (12).
The above results were further verified by the observation that
CoQ10 can promote the expression of osteoblastogenic markers,
including RUNX-2, OCN and ALP, suggesting induction of a correct
function of differentiated osteoblast cells (31). Treatment with 20 and 100 µM CoQ10
exhibited the highest impact on the differentiation of BMSCs. The
levels of RUNX-2, OCN and ALP increased significantly 7 days
following treatment, suggesting that the effects of CoQ10 are
dose-dependent.
A previous study investigated the absorption,
metabolism and pharmacokinetics of CoQ10 (32). Absorption of CoQ10 is slow and
limited due to its hydrophobic properties and large molecular
weight. It has been demonstrated that solubilized CoQ10
formulations exhibit increased bioavailability (32). Therefore, there is an association
between the plasma CoQ10 levels and the dose of CoQ10 ingested at a
given time point. To verify whether CoQ10 acts in a dose-dependent
manner, therapeutic effects of CoQ10 on the OVX-induced model of
osteoporosis were assessed using different doses (1, 10 and 20
mg/kg) of CoQ10. Following three months of treatment, serum levels
of E2 and bone metabolism markers including calcium, OPG and PTH,
were verified. CoQ10 supplementation exhibited no significant
impact on the levels of E2, indicating that the effects of CoQ10 on
osteoporosis are likely to be independent of E2. Significant
changes in the levels of calcium, OPG and PTH were induced by 20
mg/kg CoQ10. Low serum calcium is an indicator of osteoporosis for
postmenopausal women and OPG, a tumor necrosis factor receptor
family member, is a key regulator of bone resorption (33). PTH is regarded a modulator of the
development of osteoporosis, and excess PTH leads to osteoporosis
(34). The results of the present
study indicated that CoQ10 protects against osteoporosis and may
regulate bone metabolism. Furthermore, the results of the present
study demonstrated that CoQ10 supplementation effectively reversed
osteoporotic changes and maintained bone structure by increasing
BMD, BV, Tb.N and Tb.Th, and decreasing Tb.Sp. These results
indicate a potential for safe therapeutic use of CoQ10 in the
treatment of human diseases.
It has been previously demonstrated that
PTEN/PI3K/AKT is a signaling pathway regulating several biological
processes, including cell proliferation and metabolism (35). PTEN is a dual lipid and protein
phosphatase, which mainly targets lipid phosphatidylinositol-3,
4,5-triphosphate and negatively regulates activation of PI3K and
AKT (35,36). In vivo and in vitro
studies suggested that the PI3K/AKT signaling pathway is
responsible for the inhibition of osteoporosis by enhancing
osteoblast proliferation and differentiation, and bone formation
(37–39). Therefore, the present study
hypothesized that the effects of CoQ10 on OVX-induced osteoporosis
may be involved in the regulation of the PTEN/PI3K/AKT signaling
pathway. The results of the present study demonstrated that OVX
significantly increased the expression of PTEN but decreased the
expression of p-PI3K and p-AKT, indicating that OVX inactivated the
PI3K/AKT pathway. CoQ10 supplementation reserved these results, and
the expression levels of p-PI3K and p-AKT were significantly
increased in a dose-dependent manner. The present study obtained
similar results to a previous study that revealed that CoQ10 can
protect against amyloid beta-induced neuronal cell death by
activating the P13K pathway (40).
In conclusion, the present study demonstrated that
CoQ10 supplementation promotes proliferation and differentiation of
BMSCs, and effectively suppresses OVX-induced bone loss by
reversing osteoporotic changes and maintaining bone structure.
These effects may be mediated by activation of the PTEN/PI3K/AKT
signaling pathway. However, further clinical studies should be
performed to verify these results.
References
|
1
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burge R, Dawson-Hughes B, Solomon DH, Wong
JB, King A and Tosteson A: Incidence and economic burden of
osteoporosis-related fractures in the United States, 2005–2025. J
Bone Miner Res. 22:465–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ray NF, Chan JK, Thamer M and Melton LJ
III: Medical expenditures for the treatment of osteoporotic
fractures in the United States in 1995: Report from the National
Osteoporosis Foundation. J Bone Miner Res. 12:24–35. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qu B, Ma Y, Yan M, Wu HH, Fan L, Liao DF,
Pan XM and Hong Z: The economic burden of fracture patients with
osteoporosis in western China. Osteoporos Int. 25:1853–1860. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European Union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the International Osteoporosis Foundation (IOF)
and the European Federation of Pharmaceutical Industry Associations
(EFPIA). Arch Osteoporos. 8:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becker DJ, Kilgore ML and Morrisey MA: The
societal burden of osteoporosis. Curr Rheumatol Rep. 12:186–191.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groneberg DA, Kindermann B, Althammer M,
Klapper M, Vormann J, Littarru GP and Döring F: Coenzyme Q10
affects expression of genes involved in cell signalling, metabolism
and transport in human CaCo-2 cells. Int J Biochem Cell Biol.
37:1208–1218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linnane AW, Kios M and Vitetta L: Coenzyme
Q(10)-its role as a prooxidant in the formation of superoxide
anion/hydrogen peroxide and the regulation of the metabolome.
Mitochondrion. 7 Suppl:S51–S61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Littarru GP and Tiano L: Bioenergetic and
antioxidant properties of coenzyme Q10: Recent developments. Mol
Biotechnol. 37:31–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jolliet P, Simon N, Barré J, Pons JY,
Boukef M, Paniel BJ and Tillement JP: Plasma coenzyme Q10
concentrations in breast cancer: Prognosis and therapeutic
consequences. Int J Clin Pharmacol Ther. 36:506–509.
1998.PubMed/NCBI
|
|
11
|
Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS,
Park HK and Kwon IK: Antioxidants, like coenzyme Q10, selenite, and
curcumin, inhibited osteoclast differentiation by suppressing
reactive oxygen species generation. Biochem Biophys Res Commun.
418:247–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moon HJ, Ko WK, Jung MS, Kim JH, Lee WJ,
Park KS, Heo JK, Bang JB and Kwon IK: Coenzyme q10 regulates
osteoclast and osteoblast differentiation. J Food Sci.
78:H785–H891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panepucci RA, Siufi JL, Silva WA Jr,
Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT and Zago
MA: Comparison of gene expression of umbilical cord vein and bone
marrow-derived mesenchymal stem cells. Stem Cells. 22:1263–1278.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu H, VandeVord PJ, Mao L, Matthew HW,
Wooley PH and Yang SY: Improved tissue-engineered bone regeneration
by endothelial cell mediated vascularization. Biomaterials.
30:508–517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke B, Xu Z, Ling Y, Qiu W, Xu Y, Higa T
and Aruoma OI: Modulation of experimental osteoporosis in rats by
the antioxidant beverage effective microorganism-X (EM-X). Biomed
Pharmacother. 63:114–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laib A and Rüegsegger P: Calibration of
trabecular bone structure measurements of in vivo three-dimensional
peripheral quantitative computed tomography with
28-microm-resolution microcomputed tomography. Bone. 24:35–39.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eriksen EF, Díez-Pérez A and Boonen S:
Update on long-term treatment with bisphosphonates for
postmenopausal osteoporosis: A systematic review. Bone. 58:126–135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan JZ, Yang L, Meng GL, Lin YS, Wei BY,
Fan J, Hu HM, Liu YW, Chen S, Zhang JK, et al: Estrogen improves
the proliferation and differentiation of hBMSCs derived from
postmenopausal osteoporosis through notch signaling pathway. Mol
Cell Biochem. 392:85–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewiecki EM, Miller PD, Harris ST, Bauer
DC, Davison KS, Dian L, Hanly DA, McClung MR, Yuen CK and Kendler
DL: Understanding and communicating the benefits and risks of
denosumab, raloxifene, and teriparatide for the treatment of
osteoporosis. J Clin Densitom. 17:490–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramalho-Ferreira G, Faverani L, Prado F,
Garcia I and Okamoto R: Raloxifene enhances peri-implant bone
healing in osteoporotic rats. Int J Oral Maxillofac Surg.
44:798–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lloyd M: Treatment of postmenopausal
osteoporosis. N Engl J Med. 339:2021998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kendler D: Osteoporosis: Therapies now and
in the future. Climacteric. 14:604–605. 2011.PubMed/NCBI
|
|
25
|
Caso G, Kelly P, McNurlan MA and Lawson
WE: Effect of coenzyme q10 on myopathic symptoms in patients
treated with statins. Am J Cardiol. 99:1409–1412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lister RE: Coenzyme Q10 and periodontal
disease. Br Dent J. 179:200–201. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morisco C, Trimarco B and Condorelli M:
Effect of coenzyme Q10 therapy in patients with congestive heart
failure: A long-term multicenter randomized study. Clin Investig.
71 8 Suppl:S134–S136. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tuli R, Tuli S, Nandi S, Wang ML,
Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG
and Tuan RS: Characterization of multipotential mesenchymal
progenitor cells derived from human trabecular bone. Stem Cells.
21:681–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Blanc K and Pittenger M: Mesenchymal
stem cells: Progress toward promise. Cytotherapy. 7:36–45. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neve A, Corrado A and Cantatore FP:
Osteoblast physiology in normal and pathological conditions. Cell
Tissue Res. 343:289–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhagavan HN and Chopra RK: Coenzyme Q10:
Absorption, tissue uptake, metabolism and pharmacokinetics. Free
Radic Res. 40:445–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bekker PJ, Holloway D, Nakanishi A,
Arrighi M, Leese PT and Dunstan CR: The effect of a single dose of
osteoprotegerin in postmenopausal women. J Bone Miner Res.
16:348–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Orimo H, Fujita T and Yoshikawa M:
Increased sensitivity of bone to parathyroid hormone in
ovariectomized rats. Endocrinology. 90:760–763. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maehama T and Dixon JE: The tumor
suppressor, PTEN/MMAC1, dephosphorylates the lipid second
messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem.
273:13375–13378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xi JC, Zang HY, Guo LX, Xue HB, Liu XD,
Bai YB and Ma YZ: The PI3K/AKT cell signaling pathway is involved
in regulation of osteoporosis. J Recept Signal Transduct Res.
35:640–645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Zeng X, Zhang L and Zheng X:
Stimulatory effect of puerarin on bone formation through activation
of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Med.
73:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ
and Lim DS: Fibroblast growth factor-2 and −4 promote the
proliferation of bone marrow mesenchymal stem cells by the
activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem
Cells Dev. 17:725–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi H, Park HH, Koh SH, Choi NY, Yu HJ,
Park J, Lee YJ and Lee KY: Coenzyme Q10 protects against amyloid
beta-induced neuronal cell death by inhibiting oxidative stress and
activating the P13K pathway. Neurotoxicology. 33:85–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|