Introduction
Numerous studies have confirmed that there is a
positive association between tumorigenesis and diabetes. The risk
of malignancy in patients with diabetes is ~2 times higher compared
with non-diabetic individuals (1,2). The
incidence of breast cancer has increased rapidly; according to
GLOBOCAN 2008 statistics, there were ~1.38 million new cases of
breast cancer annually and ~30% of patients succumbed to tumor
metastases (3). A US cohort study,
after a 20-year follow up, reported that women with type 2 diabetes
mellitus (T2DM) had a 17% increased risk of breast cancer compared
with non-diabetic individuals (4).
The choice of hypoglycemic therapy for patients with T2DM that
develop tumors has an important influence on the development of the
tumor. This is an important issue that has received increased
interest in T2DM research.
Liraglutide, a glucagon-like peptide (GLP)-1
analogue, has been widely employed as a novel antidiabetic drug as
it increases insulin secretion and improves glycemic control.
Studies have demonstrated that GLP-1 exhibits various
extra-pancreatic effects, including the promotion of glycogen
synthesis and fat production in the liver, skeletal muscle and
adipose tissue, and roles in the activation of the feeding center
and cardiovascular protection (5–7). The
association between GLP-1 and tumors remains unclear at present.
Ligumsky et al (8)
demonstrated that the selective GLP-1 receptor exists on the
surface of MDA-MB-231 breast cancer cells, and a GLP-1 receptor
agonist acted on the GLP-1 receptor to inhibit the proliferation
and promote the apoptosis of MDA-MB-231 cells. However, it has also
been reported that GLP-1 receptor agonists have the potential to
increase the risk of pancreatic and thyroid cancer (9,10).
MicroRNAs (miRNAs/miRs) exist widely in organisms
and are involved in the regulation of numerous physiological and
pathological processes. An increasing number of studies have
demonstrated that miRNAs may be involved in tumor formation by
regulating the expression of tumor-associated genes (11–13).
In breast cancer, miRNAs that are closely associated with
metastasis are termed ‘metastamiRs’ (14). These miRNAs regulate the metastasis
of breast cancer by modulating the signaling pathways associated
with epithelial-mesenchymal transition and tumor metastasis
(15). miR-27a is highly expressed
in breast cancer, gastric cancer, pancreatic cancer and colon
cancer as an oncogenic miRNA (16,17).
It functions by regulating the apoptosis, cell cycle and
differentiation of breast cancer cells (18,19).
Our previous study demonstrated that metformin may
activate AMP-activated protein kinase (AMPK) in MCF-7 cells and
downregulate the expression of miR-27a. AMPK is a key molecule in
the regulation of biological energy metabolism (20). AMPK activation strongly inhibits
the proliferation of various types of tumor cells and is therefore
a promising antitumor target. AMPK consists of two subunits, α1 and
α2. In breast cancer tissues and adjacent tissues, the expression
of the AMPKα1 subunit is abundant, while the expression of AMPKα2
in breast cancer tissues is significantly lower compared with in
adjacent tissues (21).
Furthermore, breast epithelial carcinoma exhibits a marked
reduction in AMPKα2 expression (22).
The existing literature has reported that
liraglutide activates AMPK in muscle, liver and islet β-cells,
exerting various biological effects (23–25).
However, to the best of the authors' knowledge, whether liraglutide
downregulates the expression of miR-27a and activates AMPKα2 to
affect the proliferation and apoptosis of breast cancer cells is
not currently clear. Therefore, the present study selected MCF-7
human breast cancer cells and aimed to perform a preliminary
investigation of the effects of liraglutide on the proliferation
and apoptosis of MCF-7 cells, and investigate the potential
underlying mechanism.
Materials and methods
Cell culture
MCF-7 cell lines were obtained from the Cell Bank of
the Type Culture Collection of Chinese Academy of Sciences
(Beijing, China). Cells were cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin in humidified air at 37°C with 5% CO2. The
media was replaced every 1–2 days.
Cell transfection
Briefly, 20 nM mimic (5′-UUCACAGUGGCUAAGUUCCGC-3′)
or inhibitor (5′-GCGGAACUUAGCCACUGUGAA-3′) of miR-27a (Shanghai
GenePharma Co., Ltd., Shanghai, China) were transfected into 6-well
plates at a cell density of 1×106 cells per well using
the transfection reagent Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) to activate or
inactivate miR-27a activity, respectively. Negative controls for
mimics (5′-UUGUACUACACAAAAGUACUG-3′) and inhibitors
(5′-CAGUACUUUUGUGUAGUACAA-3′) were employed. A mock group, which
consisted of cells treated with Lipofectamine 2000 only, was also
included. At 6 h after transfection, the transfection solution was
replaced with RPMI-1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. For RNA extraction and
protein isolation, cells were treated for 48 h and then harvested.
miRNA transfection efficiencies were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Detection of cell proliferation by
cell counting Kit (CCK)-8 assay
MCF-7 cells were seeded in 96-well plates at 1,000
cells per well in 100 µl cell culture medium and incubated at 37°C
for 24 h. All cells were divided into the following groups: Blank
wells containing medium only, untreated control cells and test
cells treated with different concentrations of liraglutide (10,
100, 1,000 or 10,000 nM). Cells were either treated with 10, 100,
1,000 and 10,000 nM liraglutide for 24 h or 1,000 nM liraglutide
for 24, 48 and 72 h in an 5% CO2, humidified atmosphere
at 37°C. Each group was established three holes. Following
treatment with liraglutide or transfection with miR-27a
mimics/inhibitors, 10 µl CCK-8 solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added into each well and
incubated for 2 h in an 5% CO2, humidified atmosphere at
37°C, and the absorbance was detected at 450 nm. The CCK-8 assay
was performed three times.
Colony formation assay
Cells were seeded into 6-well plates (500
cells/well) and incubated at 37°C in an environment with 5%
CO2 for 2 days. Subsequently, cells were treated with
1,000 nM liraglutide for 48 h. The medium was refreshed with medium
containing 10% FBS once every three days, and the colony formation
was observed after 14 days. The number of colonies of more than 50
cells was counted using the inverted fluorescent microscope
(IX51-A12PH; Olympus Corporation, Tokyo, Japan). Image
magnification, ×10. To identify colonies, 0.1% crystal violet
staining was used for 20 min at 37°C (Beyotime Institute of
Biotechnology, Haimen, China) and representative photographs were
captured. Each experiment was performed in triplicate.
Cell apoptosis detection by flow
cytometry
Cells were digested with 0.25% trypsin for 2 min and
a single-cell suspension was prepared. Cells were subsequently
seeded into 12-well plates (5×104 cells/well) and
incubated at 37°C in an environment with 5% CO2
overnight. Following treatment with 1,000 nM liraglutide 48 h or
transfection with miR-27a mimic/inhibitor 24 h, the medium was
transferred to a centrifuge tube (containing apoptotic or necrotic
cells). PBS was used to wash the adherent cells, followed by
digestion with 0.25% trypsin to prepare a single-cell suspension.
Subsequently, the suspension was transferred to a centrifuge tube
and centrifuged at 800 × g for 5 min at 4°C. Subsequently, cell
suspension (100 µl) was transferred to a 1.5 ml Eppendorf tube, and
5 µl annexin V and 5 µl propidium iodide was added, according to
the protocol of FITC Annexin V Apoptosis Detection kit I (BD
Biosciences, Franklin Lakes, NJ, USA). After 15 min incubation at
room temperature in the dark, flow cytometry was performed to
analyze the changes in cell apoptosis using FACS Aria I (BD
Biosciences, Franklin Lakes, NJ, USA).
AMPK small interfering (si)RNA
transfection
AMPKα2 siRNA (5′-GAGAAGCAGAAGCACGACGTT-3′) and
scrambled control (5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from
Cell Signaling Technology, Inc., (Danvers, MA, USA). Cells were
incubated at 37°C and 5% CO2 until they reached a
confluency of 70%. Subsequently, all transient transfections were
performed with 100 nM AMPKα2 siRNA or scrambled control using
Lipofectamine 2000, according to the manufacturer's protocol. After
6 h, the transfection mixtures were replaced with RPMI-1640 with
10% FBS. Cells were harvested at 48 h post-transfection.
RT-qPCR
Following treatment with 0, 10, 100 and 1,000 nM
liraglutide for 48 h or transfection with miR-27a
mimics/inhibitors, total miRNA from MCF-7 cells was extracted with
an PureLink™ miRNA Isolation kit (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The expression of miR-27a was measured using a mirVana™
qRT-PCR miRNA Detection kit (Invitrogen; Thermo Fisher Scientific,
Inc.). The stemloop-RT primer of miR-27a is
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGTGGA-3′. The
thermocycling parameters of reverse transcriptase are 25°C for 10
min, 42°C for 1 h and 85°C for 5 min. U6 small nuclear RNA was used
for the normalization of relative abundance of miR-27a. The primer
of miR-27a is 5′-TTCACAGTGGCTAAGTTCCGC-3′ and universal primer R is
5′-CCAGTGCAGGGTCCGAGGT-3′. The primer of U6 is
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and 5′-ACGCTTCACGAATTTGCGTGTC-3′. A
total of 100 ng cDNA product amplified in the reverse transcription
step above was added to a 20 µl reaction volume using the following
amplification program: 95°C for 5 min, followed by 40 amplification
cycles of denaturation at 95°C for 10 sec, annealing at 62°C for 20
sec, and elongation at 72°C for 10 sec. For AMPKα2 mRNA expression
analysis, which was performed following treatment with 0, 10, 100
and 1,000 nM liraglutide for 48 h, transfection with miR-27a
mimics/inhibitors or transfection with or without miR-27a mimics
followed by treatment with 1,000 nM liraglutide for 48 h. Total RNA
was extracted using TRIzol™ LS Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), and the reverse-transcription
reactions were performed using a TransScript First-Strand cDNA
Synthesis SuperMix (Transgene Bio, Inc., Peking, China). The
thermocycling parameters of reverse transcriptase are 25°C for 10
min, 42°C for 1 h and 85°C for 5 min. qPCR amplification was
performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.,
Dalian, China). A total of 100 ng of the cDNA product amplified in
the reverse transcription step above was added to a 20 µl reaction
volume using the following thermocycling parameters: 95°C for 5
min, followed by 40 amplification cycles of denaturation at 95°C
for 10 sec, annealing at 58°C for 20 sec, and elongation at 72°C
for 10 sec. The primers of AMPKα2 are forwards,
5′-GCCAAGAAGCAAATGAGAATG-3′, and reverse,
5′-GACACAACGCAAACTCCTGA-3′. The primers of GAPDH are forwards,
5′-ACCACAGTCCATGCCATCAC-3′, and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′. The expression levels of the target
genes were normalized to GAPDH. Standard PCR samples were analyzed
with a Bio-Rad iQ™ 5 thermal cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Melting curves were
generated for each RT-qPCR to verify the specific amplification
products and primer dimers of each PCR reaction. All qPCR reactions
were performed in triplicate. miR-27a and AMPKα2 expression levels
were normalized using the comparative Cq method (26), and relative fold changes were
calculated by the 2−ΔΔCq equation.
Western blot analysis
Following treatment with 0, 10, 100 and 1,000 nM
liraglutide for 48 h, transfection with miR-27a mimics/inhibitors,
transfection with or without miR-27a mimics followed by treatment
with 1,000 nM liraglutide for 48 h, or transfection with AMPKα2
siRNA followed by treatment with 1,000 nM liraglutide for 48 h,
cells were lysed with Radioimmunoprecipitation assay Lysis and
Extraction Buffer (Thermo Fisher Scientific, Inc.). The cytosolic
extracts were prepared by centrifuging the lysates twice at 10,800
× g for 10 min at 4°C. The protein concentration in each lysate was
measured by a bicinchoninic acid assay. Equal amounts of protein
(10 µl, 500 ng/ml) were separated by 10% SDS-PAGE minigels.
Following electrophoresis, proteins were transferred onto a
nitrocellulose membrane and blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) in 0.1% TBS-Tween (TBST) at room
temperature for 2 h. Subsequently, the membranes were incubated
with a rabbit anti-AMPKα2 monoclonal antibody (1:1,000; cat. no.
2757; Cell Signaling Technology, Inc.), a rabbit anti-proliferating
cell nuclear antigen (PCNA) polyclonal antibody (1:1,000; cat. no.
13110; Cell Signaling Technology, Inc.), a rabbit
anti-cleaved-caspase-3 polyclonal antibody (1:1,000; cat. no. 9664;
Cell Signaling Technology, Inc.), or GAPDH antibody (1:2,000; cat.
no. 10494-1-AP; Protein Tech Group, Inc., Chicago, USA) overnight
at 4°C. After thorough rinsing with TBST, membranes were incubated
with the second antibody goat anti-rabbit immunoglobulin G
(H+L)-horseradish peroxidase (1:10,000; LK2001; Sungene Biotech,
Co., Ltd., Tianjin, China) for 1 h. After rinsing, chemiluminescent
detection was performed using a Pierce™ ECL Western
Blotting Substrate (Invitrogen; Thermo Fisher Scientific, Inc.),
followed by exposing and developing X-ray film. Western blotting
results were analyzed using an Image Lab 4.0 software system
(Bio-Rad Laboratories, Inc.). Western blotting was performed at
least three times.
Statistical analysis
Each experiment included at least three replicates.
Data are presented as the mean ± standard deviation. A one-way
analysis of variance was used followed by Tukey's or Dunnett's
post-hoc test to analyze the differences between multiplegroups.
Statistical analyses were performed using SPSS 21.0 software (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference. Graphs were produced using
GraphPad Prism v5.0 software (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
Liraglutide inhibits the proliferation
and promotes the apoptosis of MCF-7 breast cancer cells
In order to investigate the effect of liraglutide on
the proliferation of MCF-7 human breast cancer cells, cells were
treated with 10, 100, 1,000 and 10,000 nM liraglutide for 48 h and
a CCK-8 assay was performed to detect the optical density at 450
nm. Optical density was decreased in cells treated with liraglutide
in a dose-dependent manner compared with the control group
(Fig. 1A and B). The cell survival
rate values were calculated and were 90.13, 62.80, 41.44 and
11.05%, respectively, in 10, 100, 1,000 and 10,000 nM treatment
groups (Fig. 1B). These results
indicated that liraglutide exhibited a marked inhibitory effect on
the proliferation of MCF-7 cells in a dose-dependent manner.
Further CCK-8 assays were performed to investigate whether
liraglutide inhibits the proliferation of MCF-7 cells in a
time-dependent manner. The results demonstrated that there was no
significant difference in the proliferation between the control and
liraglutide groups at 24 h, but significant differences were
observed between control and liraglutide groups at 48 and 72 h
time-points (Fig. 1C). The
calculated cell survival rates were 86.88, 54.69 and 43.41% for
liraglutide-treated cells at 24, 48 and 72 h (Fig. 1D). There was no significant
difference between the control and liraglutide groups at 24 h;
however, compared with the control group, the cell survival rates
following liraglutide treatment were significantly decreased at 48
and 72 h time-points (Fig. 1D).
These results indicated that liraglutide exhibited a marked
inhibitory effect on the proliferation of MCF-7 cells in a
time-dependent manner.
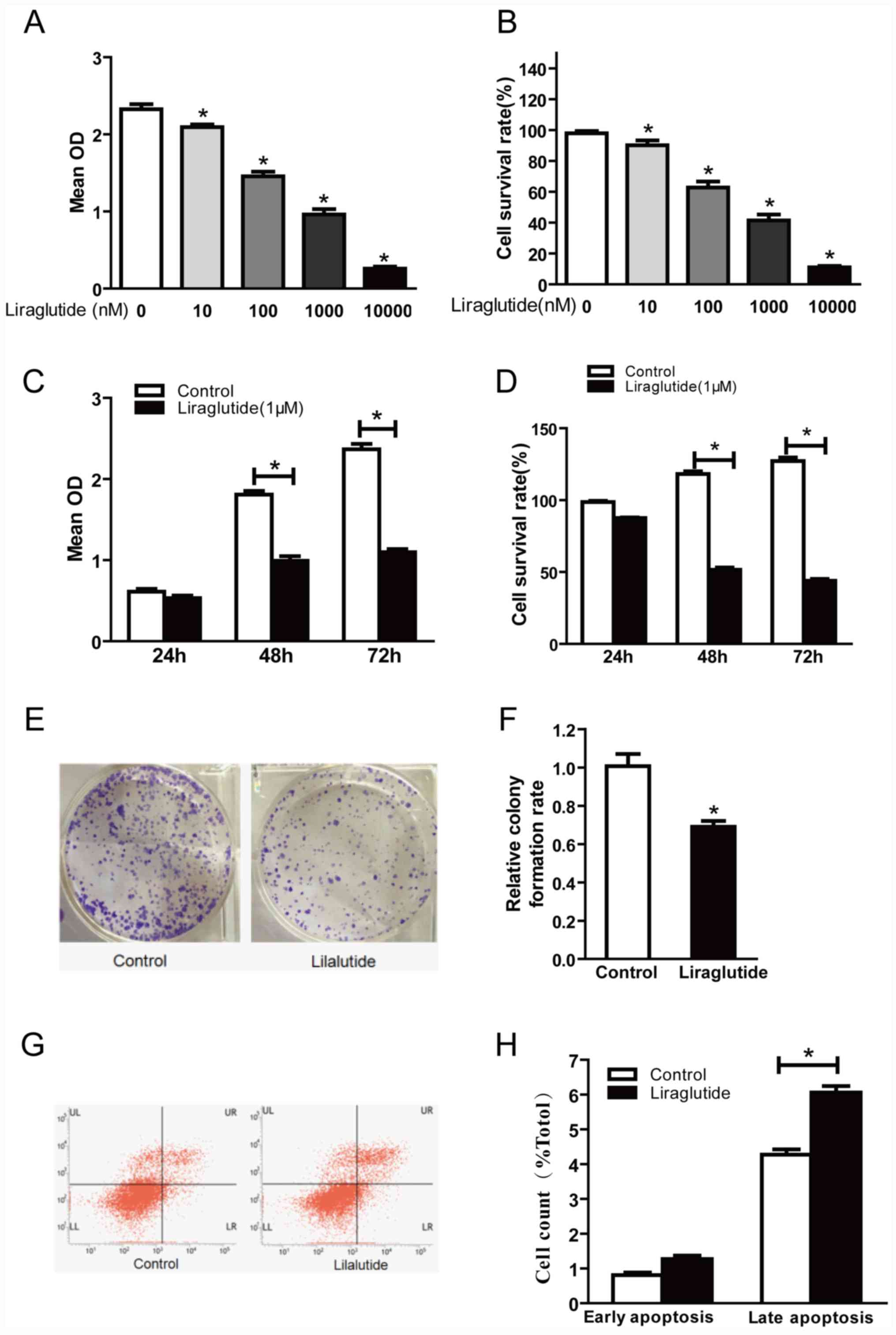 | Figure 1.Liraglutide inhibits the
proliferation and promotes apoptosis in the MCF-7 breast cancer
cell line. (A) CCK-8 assay results demonstrated that with
increasing liraglutide concentration, the mean OD values were
reduced. (B) Cell survival rates in each group were calculated
following CCK-8 assays. (C) When 1,000 nM liraglutide was employed
for 48 and 72 h, cell proliferation was markedly inhibited. (D)
Cell survival rates in each group were calculated following CCK-8
assays. (E) Representative images of cell colony formation assays.
(F) Quantification of cell colony formation assay results confirmed
that the colony formation capacity decreased significantly
following treatment with 1,000 nM liraglutide. (G) Representative
flow cytometry plots in control cells or cells treated with 1,000
nM liraglutide. LR quadrant represents early apoptotic cells, and
UR quadrant represents late apoptotic cells. (H) Quantified flow
cytometry results demonstrated that the percentage of late
apoptotic cells in the liraglutide treatment group increased
significantly compared with the control group. Data are presented
as the mean ± standard deviation. For parts A and B, *P<0.05 vs.
0 nM liraglutide group; for parts C, D, F and H, *P<0.05, as
indicated by brackets. CCK-8, Cell Counting Kit-8; OD, optical
density. |
The cell colony formation assay is another
laboratory technique used to determine alterations in cell
proliferation. Following treatment of cells with 1,000 nM
liraglutide for 48 h, colony formation assay results demonstrated
that significantly fewer colonies were formed compared with the
control group (69.37 vs. 100% in liraglutide and control groups,
respectively; P<0.05; Fig. 1E and
F). These results further confirmed that liraglutide exhibits a
marked inhibitory effect on the proliferation of MCF-7 cells.
The effects of liraglutide on the apoptosis of MCF-7
cells were assessed by flow cytometry. The results demonstrated
that treatment with 1,000 nM liraglutide for 48 h led to an
increase in the percentage of apoptotic cells, compared with the
control group. The percentages of early apoptotic and late
apoptotic cells in the liraglutide treatment group were 1.26±0.18
and 6.06±0.32%, respectively, and the early apoptosis and late
apoptosis percentages in the control group were 0.81±0.13 and
4.27±0.26%, respectively. The difference in the percentage of late
apoptotic cells between the control and liraglutide treatment
groups was statistically significant (P<0.05; Fig. 1G and H), while the difference in
the percentage of early apoptotic cells was not significant
(Fig. 1G and H). These results
demonstrated that liraglutide may promote the apoptosis of MCF-7
cells.
Liraglutide inhibits miR-27a
expression and upregulates AMPKα2
The results of RT-qPCR analysis demonstrated that
treatment with 100 and 1,000 nM liraglutide significantly inhibited
the expression of miR-27a and significantly increased the mRNA
expression of AMPKα2, compared with untreated control cells
(P<0.05; Fig. 2A and B). The
expression levels of miR-27a were reduced by 15.5, 44.5 and 61.8%,
respectively, in the 10, 100 and 1,000 nM treatment groups,
compared with the control group (Fig.
2A). Compared with the control group, the mRNA expression of
AMPKα2 was increased by 1.61, 6.63 and 9.25 times in 10, 100 and
1,000 nM treatment groups, respectively (Fig. 2B). In addition, liraglutide
treatment (100 and 1,000 nM) significantly increased the expression
of AMPKα2 at the protein level, compared with the control group, as
determined by western blotting (P<0.05; Fig. 2C). The protein expression of AMPKα2
was 1.5, 4.0 and 6.8 times higher in the 10, 100 and 1,000 nM
treatment groups, respectively, compared with the control group
(Fig. 2C). These results indicated
that miR-27a may promote the proliferation, and inhibit the
apoptosis, of MCF-7 human breast cancer cells, and liraglutide may
mediate its effects by downregulating miR-27a expression.
Therefore, miR-27a may be a potential target for the prevention and
treatment of breast cancer. Subsequently, experiments were
performed using miR-27a mimics/inhibitors to investigate whether
liraglutide may inhibit the proliferation and promote the apoptosis
of MCF-7 cells by targeting miR-27a and influencing expression of
AMPKα2, which has previously been validated as a target gene of
miR-27a (27).
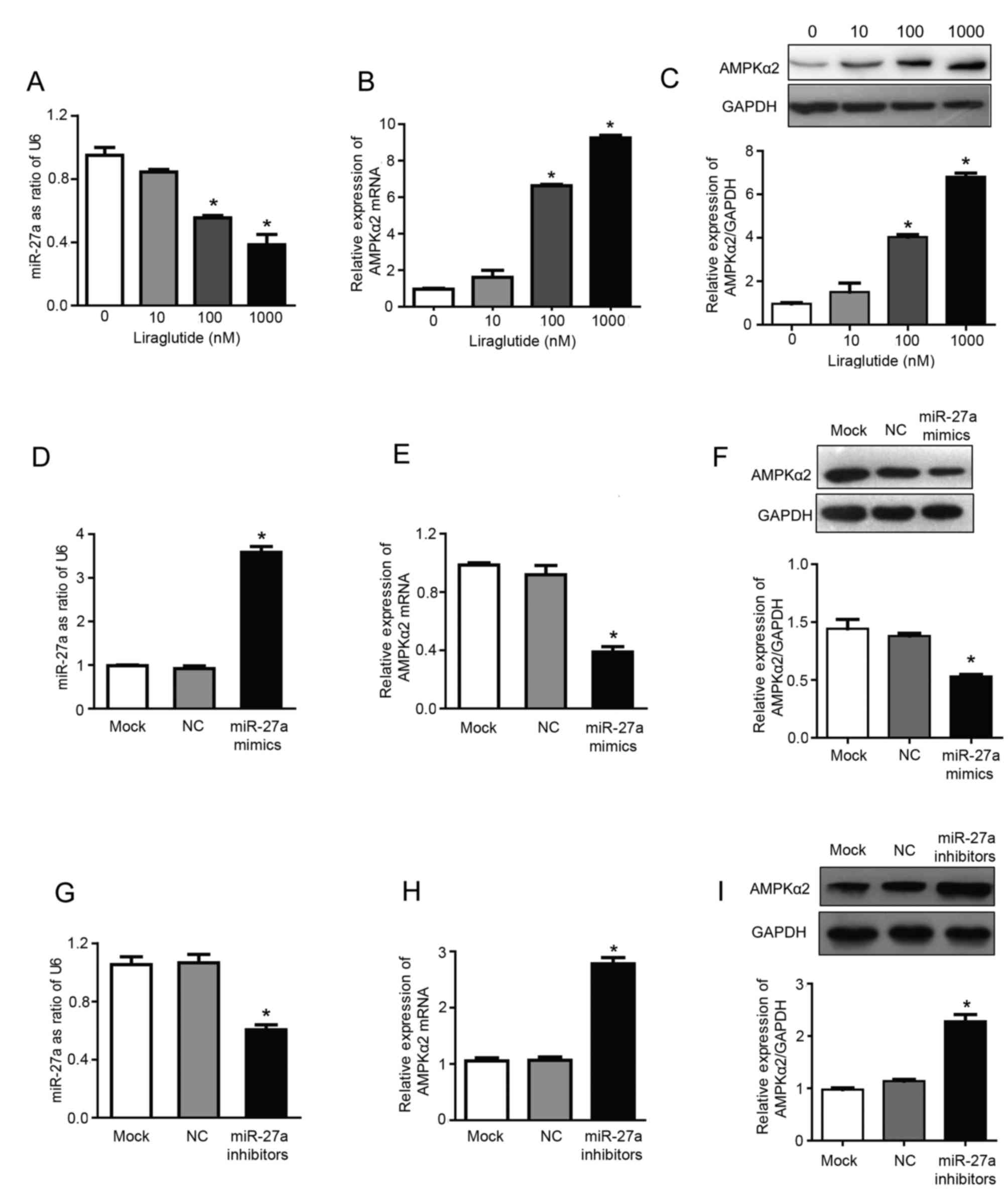 | Figure 2.Liraglutide inhibits miR-27a
expression and subsequently upregulates AMPKα2. Following treatment
with 10, 100 and 1,000 nM liraglutide for 48 h, RT-qPCR was
performed to determine the mRNA levels of (A) miR-27a and (B)
AMPKα2. (C) Western blotting was performed to measure the protein
levels of AMPKα2. (D) RT-qPCR confirmed that transfection with
miR-27a mimics led to the successful overexpression of miR-27a in
MCF-7 cells. (E) RT-qPCR demonstrated that the mRNA levels of
AMPKα2 were downregulated following transfection with miR-27a
mimics. (F) Western blotting demonstrated that AMPKα2 protein
expression was downregulated following transfection with miR-27a
mimics. (G) RT-qPCR confirmed that transfection with miR-27a
inhibitors led to the successful reduction of miR-27 levels in
MCF-7 cells. (H) RT-qPCR demonstrated that AMPKα2 mRNA expression
was increased following transfection with miR-27a inhibitors. (I)
Western blotting demonstrated that AMPKα2 protein expression was
increased following transfection with miR-27a inhibitors. Data are
presented as the mean ± standard deviation. For parts A-C,
*P<0.05 vs. 0 nM liraglutide; for parts D-I, *P<0.05 vs. NC
group. miR, microRNA; AMPKα2, AMP-activated protein kinase
catalytic subunit α2; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; NC, negative control; U6, U6 small
nuclear RNA. |
The present study confirmed that miR-27a exhibits a
regulatory effect on the expression of AMPKα2. miR-27a mimics and
inhibitors were transfected into MCF-7 cells. RT-qPCR results
confirmed that transfection with miR-27a mimics was successful
(Fig. 2D), and the results also
demonstrated that overexpression of miR-27a led to downregulation
of AMPKα2 mRNA expression by 57.77%, compared with the NC group
(P<0.05; Fig. 2E). In addition,
following transfection with miR-27a mimics, AMPKα2 protein
expression was also downregulated by 52.55%, compared with the NC
group (P<0.05; Fig. 2F).
Successful transfection of miR-27a inhibitors was confirmed by
RT-qPCR (Fig. 2G). Furthermore,
following transfection with miR-27a inhibitors, AMPKα2 mRNA
expression was increased ~2.61 times (P<0.05; Fig. 2H) and AMPKα2 protein expression was
increased ~2.27 times (P<0.05; Fig.
2I) compared with the NC group. These results indicated that
miR-27a may negatively regulate AMPKα2.
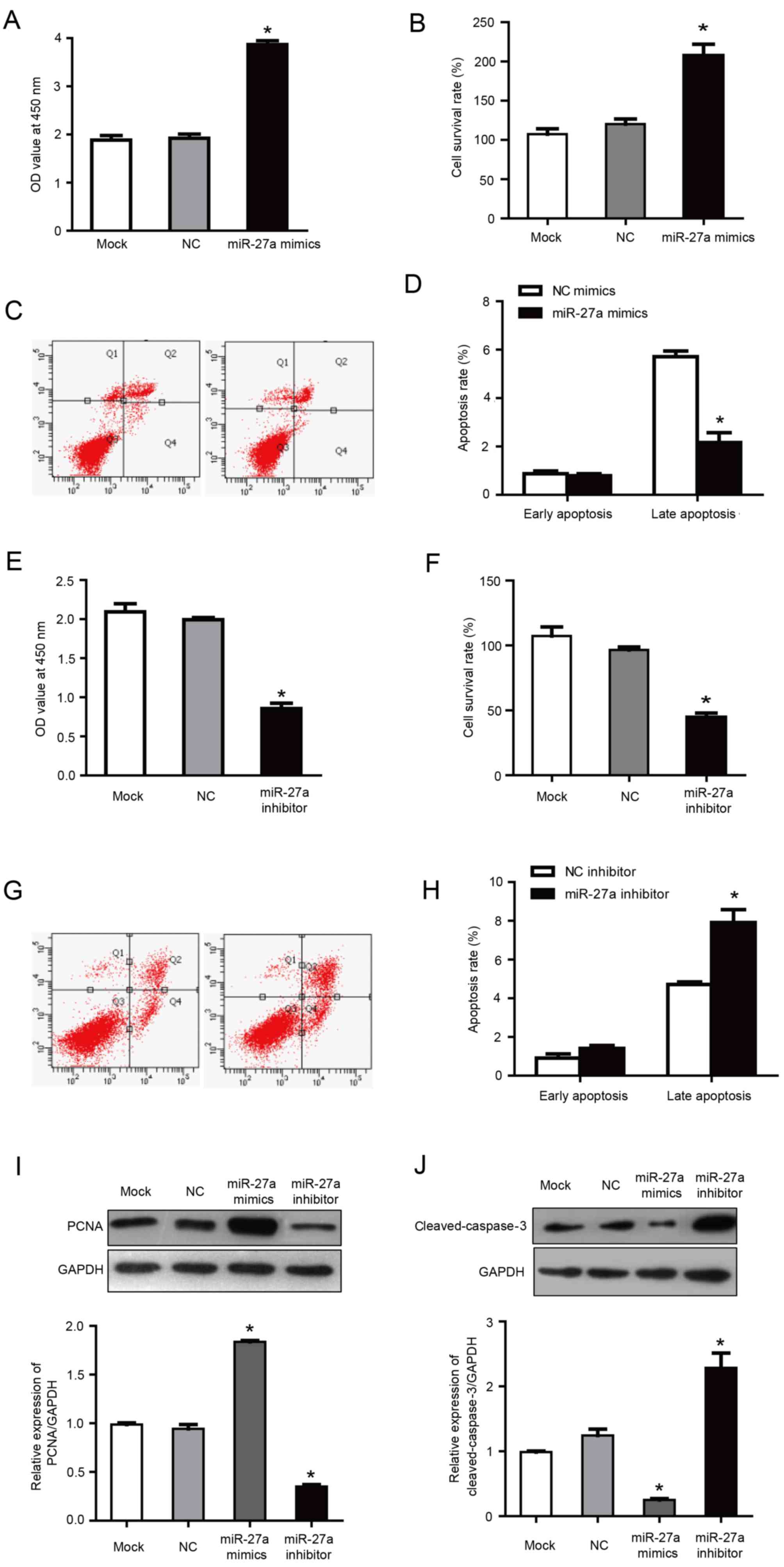
miR-27a promotes the proliferation of
MCF-7 human breast cancer cells and inhibits apoptosis
It was previously reported that miR-27a, as a
carcinogenic miRNA, regulates key target genes to control the cell
cycle check point to affect the growth of breast cancer cells
(16). In order to investigate the
role of miR-27a in the proliferation and apoptosis of MCF-7 cells,
MCF-7 cells were transfected with miR-27a mimics and inhibitors.
Following transfection with miR-27a mimics, the proliferation and
cell survival rate was increased significantly compared with the
negative control group (P<0.05; Fig. 3A and B). Furthermore, flow
cytometry demonstrated that, compared with the negative control
group, the percentage of late apoptotic cells was significantly
reduced in the cells transfected with miR-27a mimics (2.16±0.41 vs.
5.71±0.23% in miR-27a mimics and negative control groups,
respectively; P<0.05; Fig. 3C and
D). Correspondingly, in cells transfected with miR-27a
inhibitor, the proliferation and cell survival were significantly
lower compared with the negative control group (P<0.05; Fig. 3E and F), and the late apoptosis
percentage was increased significantly compared with the negative
control group (6.91±0.27 vs. 4.71±0.23% in miR-27a inhibitor and
negative control groups, respectively; P<0.05; Fig. 3G and H). There was no statistical
difference between the NC group and the mock group. These results
indicated that miR-27a promoted the proliferation and inhibited the
apoptosis of MCF-7 cells.
PCNA is only expressed in normal proliferating cells
and tumor cells. It has an important role in the initiation of cell
proliferation and is a good indicator of the cell proliferation. In
a pre-test, no significant differences were observed between
negative control mimics and negative control inhibitor groups and
the mock group (data not shown). Therefore, although separate
mimics and inhibitor negative controls were used for all
aforementioned transfection results, negative control mimics were
selected as a common control for the western blotting results
presented in Fig. 3I and J.
Previous studies have also included the use of a common control for
mimics and inhibitor experiments (28,29).
Western blotting demonstrated that the expression of
PCNA was increased in cells transfected with miR-27a mimics, and
was increased by ~2 times compared with the NC group (P<0.05;
Fig. 3I). Furthermore, PCNA
expression was reduced in the group transfected with miR-27a
inhibitors, with an expression of ~43.22% of the NC group
(P<0.05; Fig. 3I). These
results further demonstrated that miR-27a may inhibit the
proliferation of MCF-7 human breast cancer cells.
Caspase-3 is a key apoptotic factor in the caspase
family. Western blotting demonstrated that the expression of
cleaved-caspase-3 was decreased in cells transfected with miR-27a
mimics, with an expression of ~28.56% of the NC group (P<0.05;
Fig. 3J). Correspondingly,
following transfection with miR-27a inhibitor, the
cleaved-caspase-3 expression was ~2.1 times higher compared with
the NC group (P<0.05; Fig. 3J).
These results further indicated that miR-27a may promote the
apoptosis of MCF-7 cells.
Upregulation of miR-27a reverses the
activation effects of liraglutide on AMPKα2
The previously discussed results indicated that the
promotion of apoptosis by liraglutide may be associated with the
activation of the AMPKα2 pathway, and miR-27a negatively regulates
AMPKα2. Therefore, we hypothesized that the effect of liraglutide
on apoptosis may be mediated via reduced miR-27a expression and
activation of the AMPKα2 pathway.
The present study further investigated the effect of
liraglutide in MCF-7 cells following transfection with miR-27a
mimics. The results demonstrated that AMPKα2 expression was
increased by liraglutide in MCF-7 cells, compared with the control
group, while its expression in the liraglutide + miR-27a mimics
group was decreased compared with the liraglutide + negative
control mimics group (Fig. 4A and
B). These results indicated that the overexpression of miR-27a
potentially impaired the effect of liraglutide on apoptosis in
MCF-7 cells.
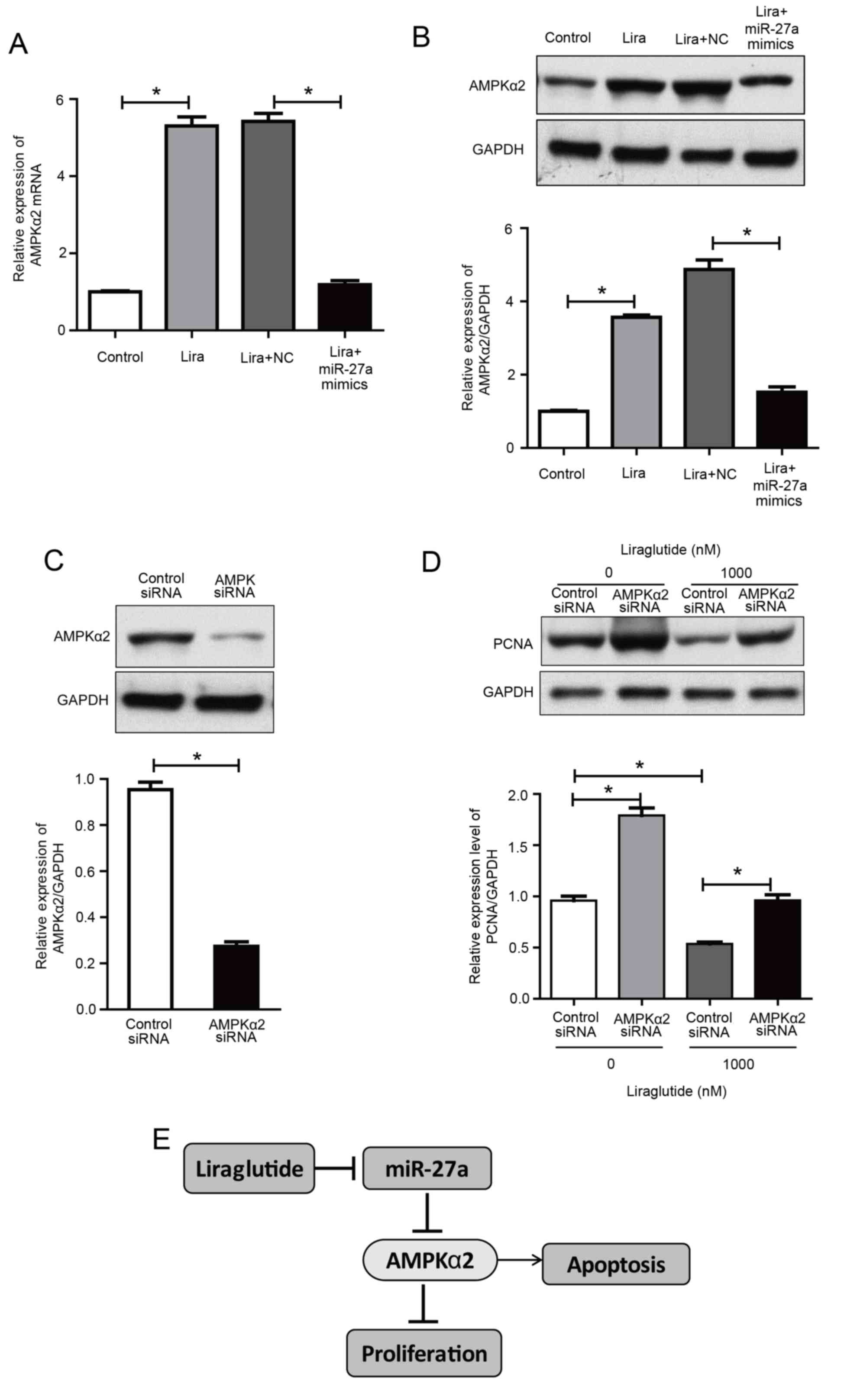 | Figure 4.Upregulation of miR-27a reverses the
liraglutide-induced activation of AMPKα2 and AMPKα2 siRNA inhibits
liraglutide-induced inhibition of proliferation in MCF-7 cells. (A)
AMPKα2 mRNA levels were measured by reverse
transcription-quantitative polymerase chain reaction in MCF-7 cells
transfected with or without miR-27a mimics and subsequently treated
with liraglutide for 48 h. (B) AMPKα2 protein levels were measured
by western blotting in MCF-7 cells transfected with or without
miR-27a mimics and subsequently treated with liraglutide for 48 h.
(C) MCF-7 cells were transfected with control siRNA or AMPKα2 siRNA
for 48 h and western blotting was performed to measure AMPKα2
levels and confirm successful transfection. (D) MCF-7 cells were
transfected with AMPKα2 siRNA and cultured with or without 1,000 nM
liraglutide for 48 h. The expression of PCNA was determined by
western blot analysis. GAPDH served as the loading control. (E)
Summary diagram of the regulatory pathway in the present study. In
breast cancer cells, liraglutide inhibits miR-27a expression and
subsequently activates the AMPK pathway, thereby leading to the
induction of cell apoptosis. Data are presented as the mean ±
standard deviation. *P<0.05, as indicated by brackets. miR,
microRNA; AMPKα2, AMP-activated protein kinase catalytic subunit
α2; siRNA, small interfering RNA; PCNA, proliferating cell nuclear
antigen; NC, negative control; Lira, liraglutide. |
AMPKα2 knockdown reduces the
inhibitory effect of liraglutide on MCF-7 cells
To determine whether AMPK is responsible for the
inhibition of proliferation induced by liraglutide, MCF-7 cells
were transfected with AMPKα2 siRNA. As demonstrated in Fig. 4C, cells exhibited a marked decrease
in AMPKα2 protein expression, compared with the control siRNA
group. With or without liraglutide treatment, knockdown of AMPKα2
increased the protein expression of PCNA, compared with cells
transfected with control siRNA, indicating that proliferation was
increased following AMPKα2 knockdown (Fig. 4D). These results indicate that AMPK
activation may be essential for the inhibitory effect of
liraglutide on the proliferation of MCF-7 cells. These results
demonstrated that liraglutide-induced inhibition of proliferation
may be associated with the AMPK pathway. Taken together, the
results of the current study indicate that liraglutide, potentially
through the miR-27a/AMPKα2 pathway, may promote apoptosis in MCF-7
cells (Fig. 4E).
Discussion
Breast cancer is among the most common types of
malignant tumors that affect women, according to statistics from
developed countries in North America and Europe (30). Several studies have reported that
breast cancer incidence and mortality risk are closely associated
with metabolic disease (31–33).
The present study reported that liraglutide, which is widely
employed for hypoglycemic treatment, also exhibited an inhibitory
effect on the proliferation of MCF-7 cells and promoted apoptosis.
These effects may be associated with the inhibition of miR-27a
expression by liraglutide, and miR-27a negatively regulates AMPKα2
expression.
GLP-1 exerts its function primarily through binding
to its receptor. GLP-1 receptors are not exclusive to the pancreas,
and are also present in the gastrointestinal tract, brain, heart,
aorta, kidney, lung, peripheral nervous system and other tissues
and organs (34). In addition to a
role in regulating blood glucose, GLP-1 also exhibits numerous
extra-hypoglycemic effects. Liraglutide, a GLP-1 analogue, has 97%
homology with GLP-1 in the human body and regulates blood glucose
through various mechanisms. It has been reported that sustained
GLP-1 receptor activation may increase the incidence of colorectal
cancer in patients with T2DM that exhibit hyperinsulinemia
(35). However, a meta-analysis
demonstrated that the use of liraglutide did not increase the
incidence of acute pancreatitis and pancreatic cancer in patients
with T2DM (36). Ligumsky et
al (8) reported that
exenatide, a GLP-1 receptor agonist, led to sustained activation of
the GLP-1 receptor, which subsequently led to the inhibition of
proliferation and promotion of apoptosis in MDA-MB-231 cells
through the cyclic AMP pathway.
MCF-7 is the most commonly employed breast cancer
cell line in research, and is estrogen receptor positive with a
high proliferation rate. The results of the current study
demonstrated that liraglutide effectively inhibited the
proliferation of MCF-7 cells in a concentration- and time-dependent
manner. Furthermore, colony formation assay and flow cytometry
results also confirmed that liraglutide inhibited the proliferation
of MCF-7 cells and promoted cell apoptosis.
AMPK is an important kinase that regulates energy
homeostasis and also a key protein involved in various cellular
signaling pathways, including those involved in the regulation of
the cell cycle and apoptosis. A recent study reported that
liraglutide activated AMPK in the muscle cells of mice, thereby
regulating muscle cell translocation of glucose transporter 4
(37). Furthermore, Miao et
al (24) demonstrated that
liraglutide promoted the proliferation of insulin-producing β cells
through the AMPK/mechanistic target of rapamycin kinase (mTOR)
signaling pathway, while Ben-Shlomo et al (38) reported that liraglutide inhibited
the formation of fatty liver via the AMPK pathway. The present
study also indicated that liraglutide activates AMPK. Therefore,
liraglutide-induced inhibition of proliferation and apoptosis
promotion in MCF-7 cells may be associated with the activation of
AMPK.
miRNAs exhibit their biological roles through
specific recognition of the 3′untranslated regions within target
genes and through complementary base-pairing to inhibit target gene
expression. miR-27, which includes miR-27a and miR-27b, has
important biological functions. As an oncogenic miRNA, it has been
reported to be highly expressed in breast, gastric, pancreatic and
colon cancer. It has been reported that miR-27a regulates cell
growth and differentiation, and is implicated in drug resistance,
dose-dependently. Furthermore, miR-27a may promote the metastasis
of cancer cells through induction of epithelial-mesenchymal
transition. miR-27a was also reported to be involved in cell
apoptosis, cell cycle checkpoints and metabolism (39). The results of the present study
also confirmed that miR-27a exhibited an important role in
inhibiting proliferation and promoting apoptosis in MCF-7 cells.
Therefore, miR-27a may be employed as an effective target for the
prevention and treatment of breast cancer. However, miR-27a
inhibitor is not yet suitable for clinical application. The present
study employed liraglutide, which is widely used in the clinic, to
interfere with MCF-7 human breast cancer cells, and the results
demonstrated that liraglutide inhibited miR-27a expression, which
may provide a novel treatment option for the prevention and control
of breast cancer by targeting miR-27a.
Our previous study verified that AMPKα2 was a target
gene of miR-27a. AMPKα2 is one of the catalytic subunits of AMPK
and its expression in MCF-7 human breast cancer cells is
suppressed, as it functions as a tumor suppressor (27). Fox et al (21) investigated AMPKα2 expression among
tumor samples, non-tumorous adjacent (ADJ) breast epithelial tissue
and normal epithelial tissue samples, and the results demonstrated
that AMPKα2 protein expression was reduced by 27% in tumor samples
compared with the patient-matched ADJ samples and by 37% compared
with normal epithelial tissue samples. Further experiments
indicated that AMPKα2 arrested the cell cycle through cyclin D1 and
reduced protein biosynthesis through the mTOR pathway. Furthermore,
the same study reported that AMPKα2 may act directly on P53,
resulting in MCF-7 cell apoptosis. The results of the present study
demonstrated that liraglutide inhibited the proliferation and
promoted the apoptosis of MCF-7 cells, which may occur via
inhibition of miR-27a expression and subsequent upregulation of the
miR-27a target gene, AMPKα2.
In conclusion, the present study demonstrated that
the hypoglycemic drug liraglutide inhibited the proliferation and
promoted the apoptosis of MCF-7 cells, exhibiting potential
anti-breast cancer effects. In addition, the results demonstrated
that liraglutide may function as an miR-27a inhibitor, thereby
potentially providing a novel method for the clinical prevention
and treatment of breast cancer. It has been previously reported
that certain antidiabetic drugs, including insulin and
sulfonylureas antidiabetic drugs, may affect the levels of insulin,
inflammatory factors and insulin-like growth factor-1, thus
increasing tumor risk in patients with T2DM (40,41).
The current study may provide a basis for the selection of
hypoglycemic agents in patients with T2DM, particularly in patients
with breast cancer. However, various issues are associated with the
present study. For example, the results demonstrated that 100 nM
liraglutide inhibited miR-27a expression in MCF-7 cells, while 10
nM liraglutide did not lead to a statistically significant decline.
In addition, further investigation is required to determine whether
100 nM liraglutide may lead to any side effects prior to clinical
application. In conclusion, the current study may provide a novel
direction for investigating the roles of GLP-1 besides its
glucose-lowering effects.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant no. 81400784) and the Natural
Science Foundation of Tianjin (grant no. 16JCYBJC26800).
References
|
1
|
Noto H, Osame K, Sasazuki T and Noda M:
Substantially increased risk of cancer in patients with diabetes
mellitus: A systematic review and meta-analysis of epidemiologic
evidence in Japan. J Diabetes Complications. 24:345–353. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buysschaert M and Sadikot S: Diabetes and
cancer: A 2013 synopsis. Diabetes Metab Syndr. 7:247–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michels KB, Solomon CG, Hu FB, Rosner BA,
Hankinson SE, Colditz GA and Manson JE: Nurses' Health Study: Type
2 diabetes and subsequent incidence of breast cancer in the Nurses'
Health Study. Diabetes Care. 26:1752–1758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dardevet D, Moore MC, Neal D, DiCostanzo
CA, Snead W and Cherrington AD: Insulin-independent effects of
GLP-1 on canine liver glucose metabolism: Duration of infusion and
involvement of hepatoportal region. Am J Physiol Endocrinol Metab.
287:E75–E81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sancho V, Trigo MV, González N, Valverde
I, Malaisse WJ and Villanueva-Peñacarrillo ML: Effects of
glucagon-like peptide-1 and exendins on kinase activity, glucose
transport and lipid metabolism in adipocytes from normal and type-2
diabetic rats. J Mol Endocrinol. 35:27–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pannacciulli N, Le DS, Salbe AD, Chen K,
Reiman EM, Tataranni PA and Krakoff J: Postprandial glucagon-like
peptide-1 (GLP-1) response is positively associated with changes in
neuronal activity of brain areas implicated in satiety and food
intake regulation in humans. Neuroimage. 35:511–517. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ligumsky H, Wolf I, Israeli S, Haimsohn M,
Ferber S, Karasik A, Kaufman B and Rubinek T: The peptide-hormone
glucagon-like peptide-1 activates cAMP and inhibits growth of
breast cancer cells. Breast Cancer Res Treat. 132:449–461. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quoyer J, Longuet C, Broca C, Linck N,
Costes S, Varin E, Bockaert J, Bertrand G and Dalle S: GLP-1
mediates antiapoptotic effect by phosphorylating Bad through a
beta-arrestin 1-mediated ERK1/2 activation in pancreatic
beta-cells. J Biol Chem. 285:1989–2002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elashoff M, Matveyenko AV, Gier B,
Elashoff R and Butler PC: Pancreatitis, pancreatic, and thyroid
cancer with glucagon-like peptide-1-based therapies.
Gastroenterology. 141:150–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Chen YJ, Xu K, Xu H, Shen XZ and
Tu RQ: Circulating microRNAs as a fingerprint for endometrial
endometrioid adenocarcinoma. PLoS One. 9:e1107672014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samantarrai D, Dash S, Chhetri B and
Mallick B: Genomic and epigenomic cross-talks in the regulatory
landscape of miRNAs in breast cancer. Mol Cancer Res. 11:315–328.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun L and Fang J: Epigenetic regulation of
epithelial-mesenchymal transition. Cell Mol Life Sci. 73:4493–4515.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Mertens-Talcott SU, Zhang S, Kim K,
Ball J and Safe S: MicroRNA-27a indirectly regulates estrogen
receptor {alpha} expression and hormone responsiveness in MCF-7
breast cancer cells. Endocrinology. 151:2462–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang W, Yu F, Yao H, Cui X, Jiao Y, Lin L,
Chen J, Yin D, Song E and Liu Q: miR-27a regulates endothelial
differentiation of breast cancer stem like cells. Oncogene.
33:2629–2638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hardie DG: AMP-activated protein kinase:
An energy sensor that regulates all aspects of cell function. Genes
Dev. 25:1895–1908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fox MM, Phoenix KN, Kopsiaftis SG and
Claffey KP: AMP-activated protein kinase alpha 2 isoform
suppression in primary breast cancer alters AMPK growth control and
apoptotic signaling. Genes Cancer. 4:3–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hadad SM, Baker L, Quinlan PR, Robertson
KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG,
et al: Histological evaluation of AMPK signalling in primary breast
cancer. BMC Cancer. 9:3072009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Ni CL, Yao Z, Chen LM and Niu WY:
Liraglutide enhances glucose transporter 4 translocation via
regulation of AMP-activated protein kinase signaling pathways in
mouse skeletal muscle cells. Metabolism. 63:1022–1030. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong
YP, Shu H, Liu Y and Li CL: The human glucagon-like peptide-1
analogue liraglutide regulates pancreatic beta-cell proliferation
and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides.
39:71–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ben-Shlomo S, Zvibel I, Shnell M, Shlomai
A, Chepurko E, Halpern Z, Barzilai N, Oren R and Fishman S:
Glucagon-like peptide-1 reduces hepatic lipogenesis via activation
of AMP-activated protein kinase. J Hepatol. 54:1214–1223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao W, Zhang X, Liu J, Sun B, Tang H and
Zhang H: miR-27a-mediated antiproliferative effects of metformin on
the breast cancer cell line MCF-7. Oncol Rep. 36:3691–3699. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei W, Zhang Q, Wang Z, Yan B, Feng Y and
Li P: miR-219-5p inhibits proliferation and clonogenicity in
chordoma cells and is associated with tumor recurrence. Oncol Lett.
12:4568–4576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Azumi J, Tsubota T, Sakabe T and Shiota G:
miR-181a induces sorafenib resistance of hepatocellular carinoma
cells through downregulation of RASSF1 expression. Cancer Sci.
107:1256–1262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prado A, Andrades P and Parada F: Recent
developments in the ability to predict and modify breast cancer
risk. J Plast Reconstr Aesthet Surg. 63:1581–1587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schrauder MG, Fasching PA, Haberle L, Lux
MP, Rauh C, Hein A, Bayer CM, Heusinger K, Hartmann A, Strehl JD,
et al: Diabetes and prognosis in a breast cancer cohort. J Cancer
Res Clin Oncol. 137:975–983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Juanjuan L, Wen W, Zhongfen L, Chuang C,
Jing C, Yiping G, Changhua W, Dehua Y and Shengrong S: Clinical
pathological characteristics of breast cancer patients with
secondary diabetes after systemic therapy: A retrospective
multicenter study. Tumour Biol. 36:6939–6947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fidan-Yaylalı G, Dodurga Y, Seçme M and
Elmas L: Antidiabetic exendin-4 activates apoptotic pathway and
inhibits growth of breast cancer cells. Tumour Biol. 37:2647–2653.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baggio LL and Drucker DJ: Biology of
incretins: GLP-1 and GIP. Gastroenterology. 132:2131–2157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simonsen L, Pilgaard S, Orskov C,
Rosenkilde MM, Hartmann B, Holst JJ and Deacon CF: Exendin-4, but
not dipeptidyl peptidase IV inhibition, increases small intestinal
mass in GK rats. Am J Physiol Gastrointest Liver Physiol.
293:G288–G295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alves C, Batel-Marques F and Macedo AF: A
meta-analysis of serious adverse events reported with exenatide and
liraglutide: Acute pancreatitis and cancer. Diabetes Res Clin
Pract. 98:271–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Ni CL, Yao Z, Chen LM and Niu WY:
Liraglutide enhances glucose transporter 4 translocation via
regulation of AMP-activated protein kinase signaling pathways in
mouse skeletal muscle cells. Metabolism. 63:1022–1030. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ben-Shlomo S, Zvibel I, Shnell M, Shlomai
A, Chepurko E, Halpern Z, Barzilai N, Oren R and Fishman S:
Glucagon-like peptide-1 reduces hepatic lipogenesis via activation
of AMP-activated protein kinase. J Hepatol. 54:1214–1223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F and
Yu F: MiR-27 as a prognostic marker for breast cancer progression
and patient survival. PLoS One. 7:e517022012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith U and Gale EA: Does diabetes therapy
influence the risk of cancer? Diabetologia. 52:1699–1708. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer: A consensus report. CA Cancer J Clin.
60:207–221. 2010. View Article : Google Scholar : PubMed/NCBI
|