Introduction
Hepatocellular carcinoma (HCC) is the most common
malignancy of the liver and the third leading cause of
cancer-related death worldwide. Despite the development of various
therapies, the outcome for HCC patients remains poor. Generally,
HCC patients have a 1-year survival rate of <50% and a 5-year
survival rate of 10% (1).
Annually, ~0.11 million people die from liver cancer in China, and
the major reason for frequent HCC relapses are intrahepatic and
distant metastases that develop after the curative surgical
resection or transplantation (2).
Moreover, HCC is insensitive to chemotherapy and radiotherapy
(3). Therefore, novel HCC
treatments such as gene therapy and molecular targeted therapy
should be investigated (4). As a
multitarget anticancer drug, sorafenib has been approved for the
treatment of HCC, but showed a very low success rate (5). Gene therapy represents an alternative
form of cancer treatment and was shown to have high efficiency,
specificity, and few serious side effects (4).
Melanoma differentiation-associated
gene7/interleukin-24 (MDA7/IL24), a member of the IL10 cytokine
family, was originally identified as a gene associated with the
terminal differentiation and irreversible growth suppression of
metastatic human melanoma cells (6). MDA7/IL24 is considered to be secreted
by the immune system and melanocytes alone (7,8).
Several reports demonstrated the loss of MDA7/IL24
expression during the progression of melanoma, and a significant
inverse correlation between the loss of this gene and tumor
invasion, suggesting that MDA7/IL24 may have anticancer effects
(6,7,9,10).
Additionally, our previous studies demonstrated that MDA7/IL24 has
multiple anticancer functions, selectively inducing cancer cell
apoptosis, but also showing immunomodulatory and antiangiogenic
properties and strong antitumor bystander effects, which makes this
molecule an ideal candidate for cancer gene therapy (9–13).
We constructed MDA7/IL24-expressing lentiviral
particles, and evaluated the effects of lentivirus-mediated
MDA7/IL24 expression on HCC cell proliferation and colony-forming
ability. Moreover, we explored the mechanisms underlying
MDA7/IL24-mediated HCC regression (14).
Materials and methods
Cell lines and culture conditions
HCC cell line SMMC-7721 was obtained from Cell Bank
of Chinese Academy of Sciences (Shanghai, China), and maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) and 100 U/ml of penicillin-streptomycin.
The cells were incubated at 37°C in a humidified atmosphere with 5%
CO2. In addition, the cell line is not contaminated or
mis-identified according to the Database of Cross-Contaminated or
Misidentified Cell Lines.
Recombinant lentiviral particle
construction and infection
We constructed MDA7/IL24 gene expression
plasmid, while an empty plasmid was used as a negative control.
Following this, MDA7/IL24-expressing plasmid or the negative
control plasmid, together with pHelper 1.0 and pHelper 2.0 (pVSVG-I
and pCMVΔR 8.92 plasmids, respectively), were added to 293T cells
with Lipofectamine 2000 (Invitrogen, Shanghai, China), according to
the manufacturer's instructions. After 48 h of transfection,
supernatants containing viral particles were collected and
centrifuged (1,006 g, 20 min) to get rid of cell debris, and
filtered through 0.45-µm polyvinylidene fluoride (PVDF) membranes.
HCC cells were infected with MDA7/IL24-expressing lentiviral
particles or the controls at the multiplicity of infection (MOI) of
20. The infected cells expressing green fluorescent protein (GFP)
were observed under a fluorescence microscope (Micro Publisher
3.3RTV; Olympus, Tokyo, Japan). The cells were collected and total
RNA was extracted to determine the efficiency of knockdown.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted and purified from
SMMC-7721-infected cells, using Trizol reagent (Invitrogen)
following the manufacturer's instructions. RT was performed to
generate cDNA molecules, using M-MLV reverse transcriptase
(Promega, Madison, WI, USA) and oligo(dT) primers (Sangon,
Shanghai, China), following the manufacturers' instructions. The
expression of MDA7/IL24 was determined by quantitative
real-time (qRT-) PCR, using a PCR assay kit (TransGen Biotech,
Beijing, China). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) cDNA was amplified as an internal reference.
MDA7/IL24 primer set: Forward, 5′-TTGCCTGGGTTTTACCCTGC-3′ and
reverse, 5′-AAGGCTTCCCACAGTTTCTGG-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. PCR conditions included initial
denaturation (95°C for 10 sec) and then 40 cycles of amplification
(95°C for 15 sec and 55°C for 15 sec). MDA7/IL24 relative
expression was normalized to GAPDH levels by the
2−ΔΔCt method (15).
MTT assay
To investigate the effects of MDA7/IL24
overexpression on cell viability, MTT assay was performed three
times. SMMC-772 cells in the logarithmic growth phase were cultured
for 24 h in 96-well plates (1×105 cells per well). After
the infection, cells were incubated for additional 72 h.
Mitochondrial function was evaluated by MTT colorimetric assay.
Briefly, the medium was removed and a fresh medium containing 0.5
mg/ml MTT was added to each well. The cells were incubated at 37°C
for 4 h. Following this, the supernatants were removed, 50 µl
dimethylsulfoxide (DMSO) was added to each well, and samples were
incubated for 30 min at 37°C with gentle shaking. Finally,
absorbance was determined using a microplate reader at 490 nm. Cell
viability was calculated as the ratio of the absorbance determined
in the samples infected with the MDA7/IL24 overexpression
plasmid to that of the control group (untreated cells).
Colony formation assay
Infected and untreated SMMC-7721 cells were plated
in six-well plates (200 cells/well) and cultured in a 5%
CO2 incubator at 37°C for 14 days. The cells were washed
twice with PBS and fixed in 4% paraformaldehyde for 30 min. Cell
colonies were stained with Giemsa dye (Chemicon, Temecula, CA, USA)
for 20 min, and washed with double distilled water several times.
Colony numbers were counted under a fluorescence microscope.
Cell cycle
Cells were cultured in 12-cell plates. After 5 days,
the cells were collected and fixed with cold 70% ethanol overnight
at −20°C, and then washed with cold PBS for one time. The fixed
cells were treated with RNase and stained with propidium iodide
(Sigma, St. Louis, MO, USA). The stained cells were analyzed by
flow cytometer and ModFit LT software (Verity Software House,
Topsham, ME, USA).
Cell apoptosis
Cell apoptosis was performed using Annexin V PE and
7-AAD apoptosis detection kit (BD Bioscience, San Diego, CA, USA)
according to the manufacturer's instructions. Cells were collected
after cultured 5 days, washed and resuspended with 1xbinding
buffer. Then 5 µl Annexin V was added into 200 µl of the above cell
suspension and incubated at room temperature in the dark for 15
min. After incubation, 5 µl 7-AAD was added the cell apoptosis was
detected using the flow cytometer.
Microarray processing and
analysis
Total RNA isolated from SMMC-7721 cells infected
with either lentiviral vector expressing (LV-)MDA7/IL24 (n=3) or
negative control lentivirus (n=3) was subjected to microarray
analysis, to determine the global transcriptomic profile of each
cell group, using Affymetrix human GeneChip according to the
manufacturer's instructions. Microarray hybridization, washing, and
staining were performed using the GeneChip Hybridization Wash and
Stain kit (Affymetrix, Santa Clara, CA, USA). Arrays were then
scanned using the GeneChip Scanner 3,000 to obtain raw data
(Affymetrix). Significant differences in the expression of the
analyzed genes between SMMC-7721 cells infected with either
LV-MDA7/IL24 or negative control lentivirus were obtained based on
P<0.05.
Western blot analysis
Seventy-two h after the infection of SMMC-7721 cells
with either LV-MDA7/IL24 or negative control lentivirus, the cells
were collected and washed with PBS twice, and the lysis buffer was
added to extract the cellular proteins. Afterwards, the lysates
were centrifuged at 14,000 rpm at 4°C for 10 min and the
supernatants were collected. BCA method was applied to determine
the protein concentration. Twenty micrograms of protein sample
obtained from the infected cells was separated by 10% SDS-PAGE and
then transferred to PVDF membrane. PVDF membranes were blocked at
4°C overnight with 5% bovine serum albumin and incubated with
monoclonal antibodies against B cell lymphoma protein-2 (BCL2;
1:500; ab692; Abcam, Cambridge, UK), Cyclin E (1:1,000; cat. no.
4132), p-ERK1/2 (1:800; cat. no. 4370), p-AKT (1:2,000; cat. no.
4060) and caspase-3 (1:1,000; cat. no. 9662; all CST Biological
Reagents Co., Ltd., Shanghai, China). Thereafter, the horseradish
peroxidase-conjugated secondary antibody we added for each
corresponding primary antibody. The obtained blots were analyzed
using enhanced chemiluminescence. GAPDH was detected on the same
membrane as a loading control.
Statistical analysis
Student's t-test was performed for data analysis.
Statistical analysis was performed using the SPSS version 22.0
software (SPSS, Chicago, IL, USA). All data were presented as mean
± standard deviation (SD) of the results obtained in three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
LV-MDA7/IL24 infection induces
MDA7/IL24 overexpression in SMMC-7721 cells
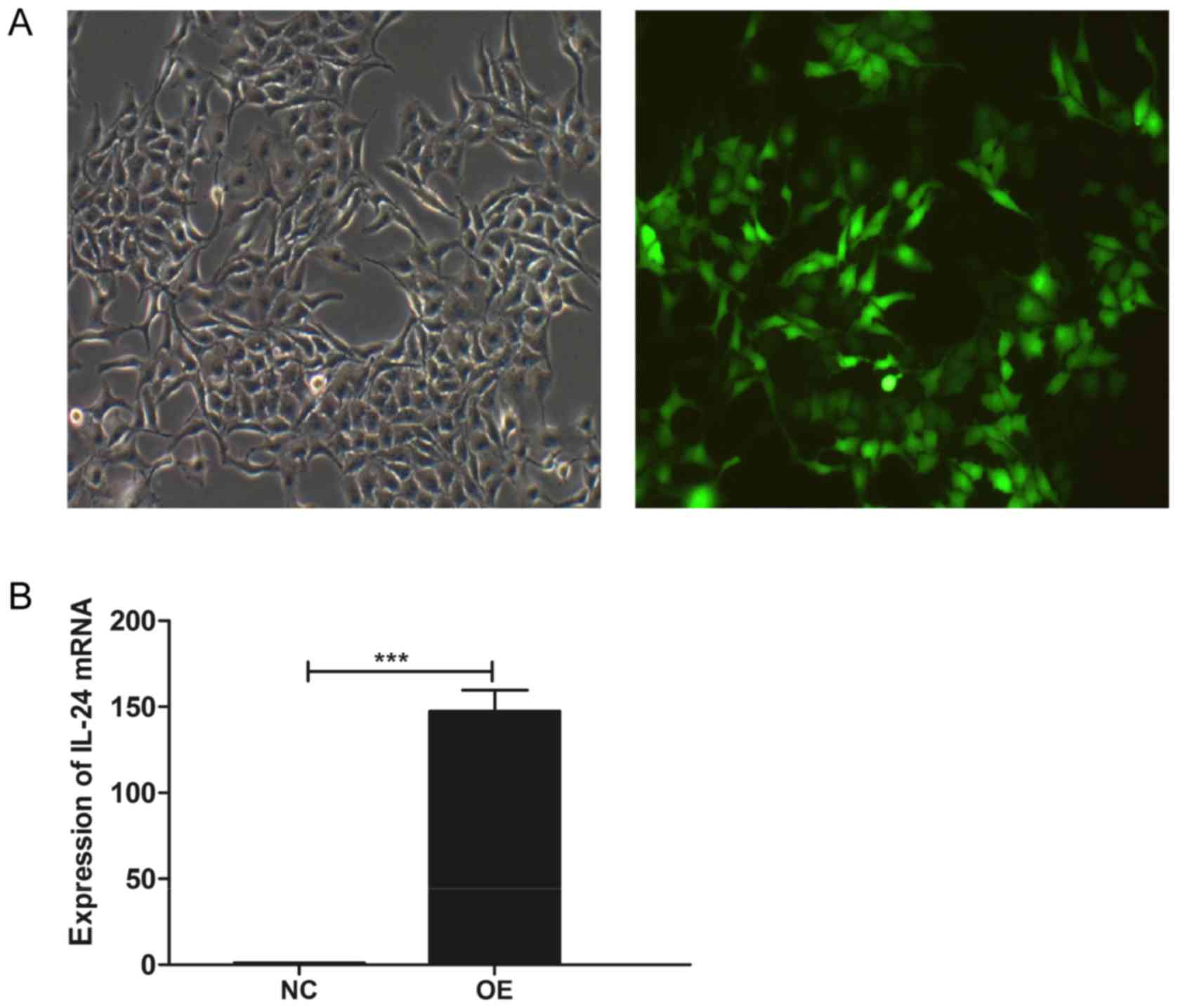
As presented in Fig.
1A, SMMC-7721 cells were shown to be GFP-positive following the
infection with the lentiviral particles, indicating a high
efficiency of the infection. Further analysis demonstrated a
significant upregulation of MDA7/IL24 expression in these
cells (P<0.001), compared with that in the cells infected with
the negative control lentiviruses (Figs. 1B and 2).
MDA7/IL24 overexpression inhibits
SMMC-7721 cell proliferation and colony-forming ability
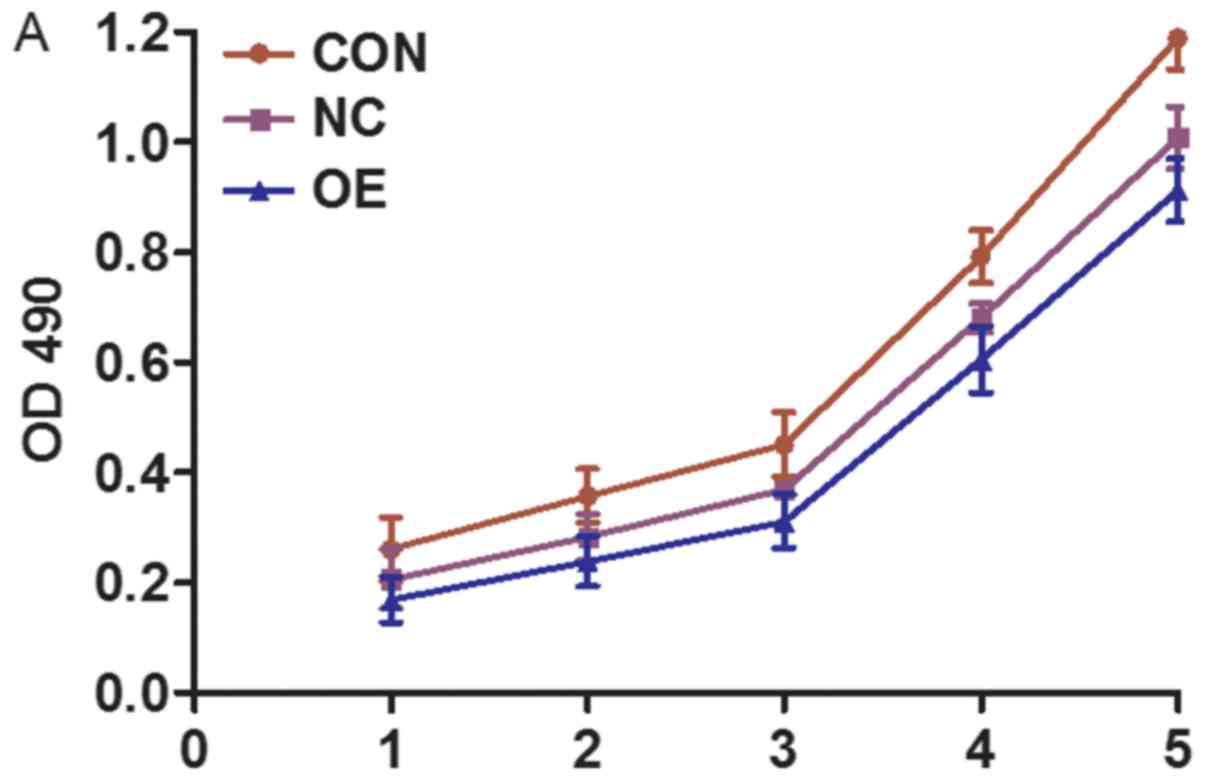
LV-MDA7/IL24-infected SMMC-7721 cells were shown to
have a decreased growth rate, in comparison with the untreated and
negative control-treated cells (P<0.05). At day 5, we determined
that the optical density at 490 nm (OD490) of LV-MDA7/IL24-infected
cells was 0.921±0.013, while those of the negative control-infected
and untreated cells were 0.988±0.007 and 1.094±0.007, respectively
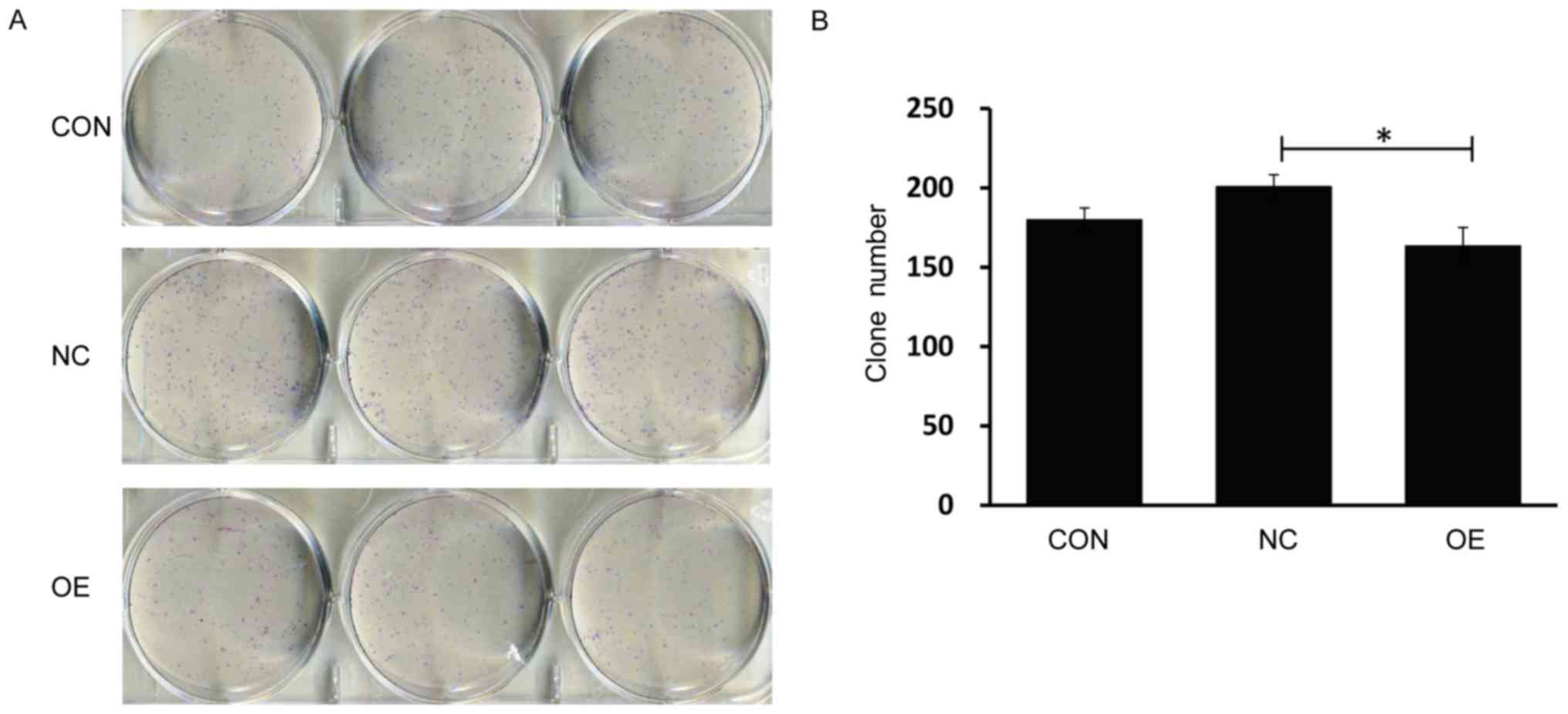
(Fig. 3). Colony-forming ability
of SMMC-7721 cells was analyzed by crystal violet staining, and the
number of cells per colony significantly decreased after
LV-MDA7/IL24 infection (P<0.05). Furthermore, we determined the
number of colonies, and this number was found to decrease to 163±12
in samples overexpressing MDA7/IL24, compared with those in
the untreated and negative control-treated cells (180±8 and 201±8,
respectively) (Fig. 4).
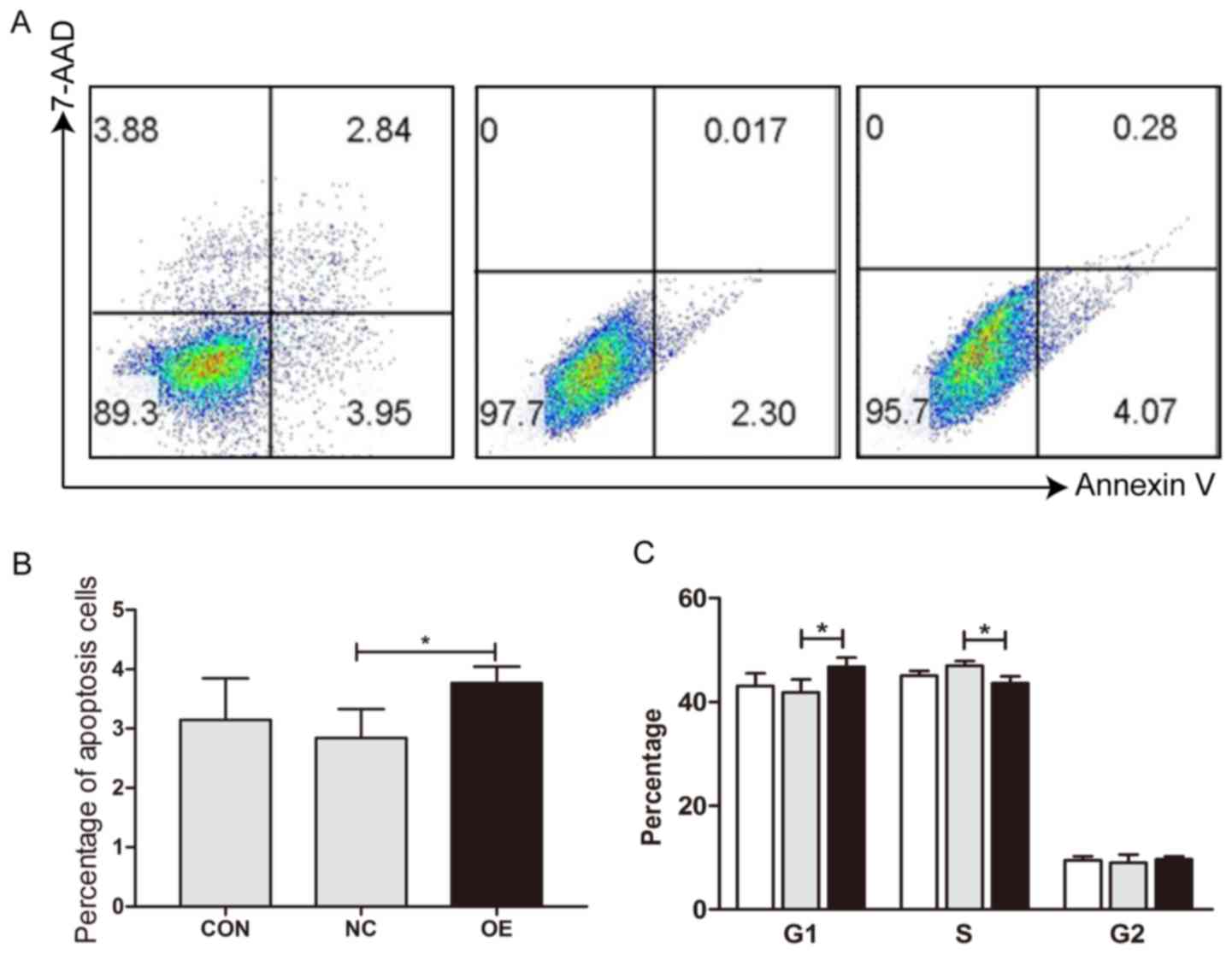
MDA7/IL24 overexpression induces cell
arrest and apoptosis
To demonstrate the reasons why MDA7/IL24
overexpression results in reduced cell viability, apoptosis and
cell cycle of MDA7/IL24 positive and negative HCC cells were
examined by flow cytometry. The results showed that MDA7/IL24
overexpression induced cell apoptosis (Fig. 5A and B) and elevated the percentage
of G1 phage cells in SMMC-7721 cells (Fig. 5C).
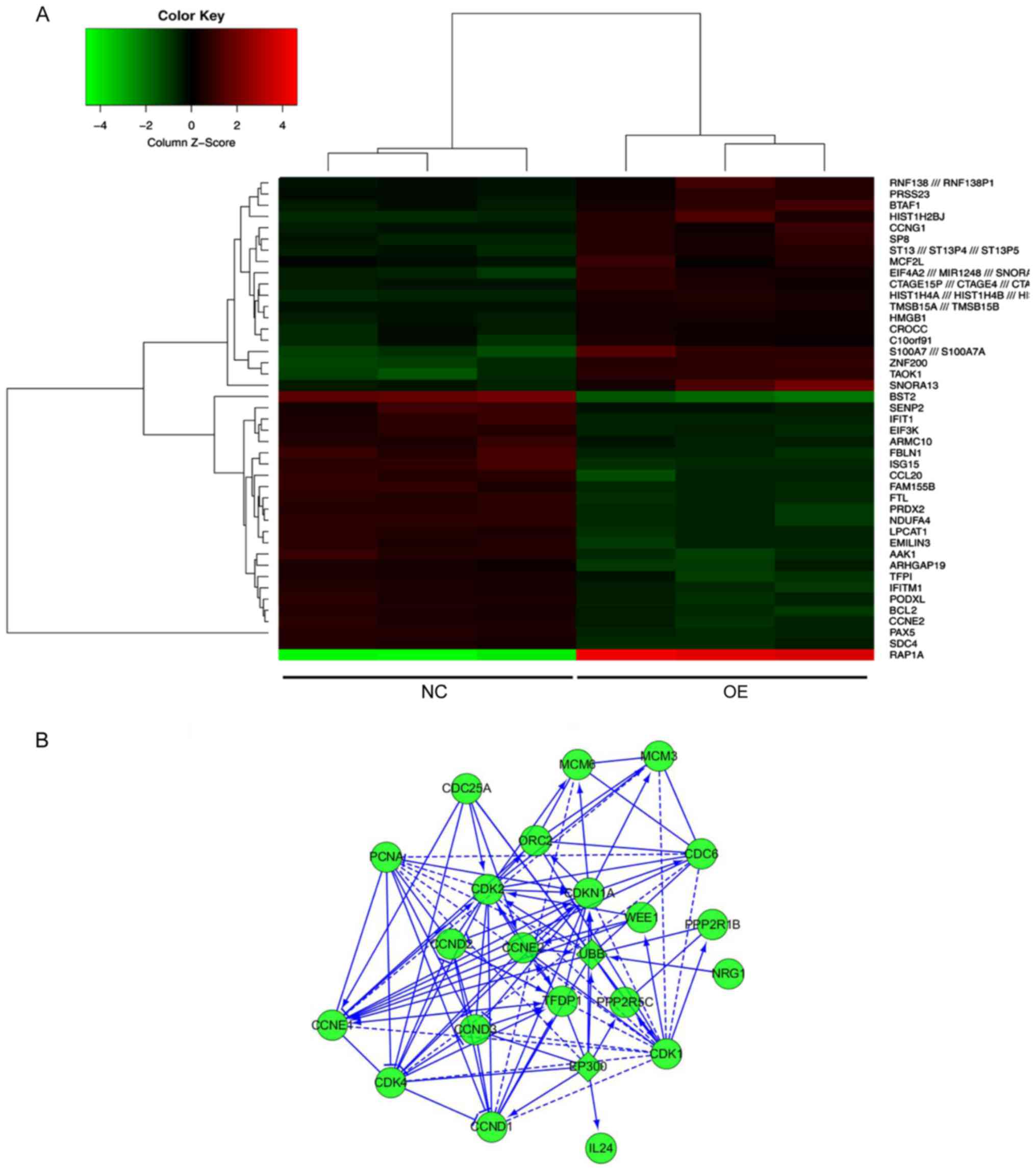
MDA7/IL24 overexpression affects
multiple cancer development pathways
To explore the molecular mechanisms underlying the
anticancer effects of MDA7/IL24, we performed microarray analysis
and gene expression profiling in cells infected with either
LV-MDA7/IL24 or the negative control. A significant difference in
the expression levels between these cells was observed for 43
genes, 20 with upregulated and 23 with downregulated expression
(Fig. 6A). KEGG pathway analysis
demonstrated that these genes were significantly enriched in three
pathways, including cell cycle regulation, DNA synthesis and
transcriptional and apoptosis (Fig.
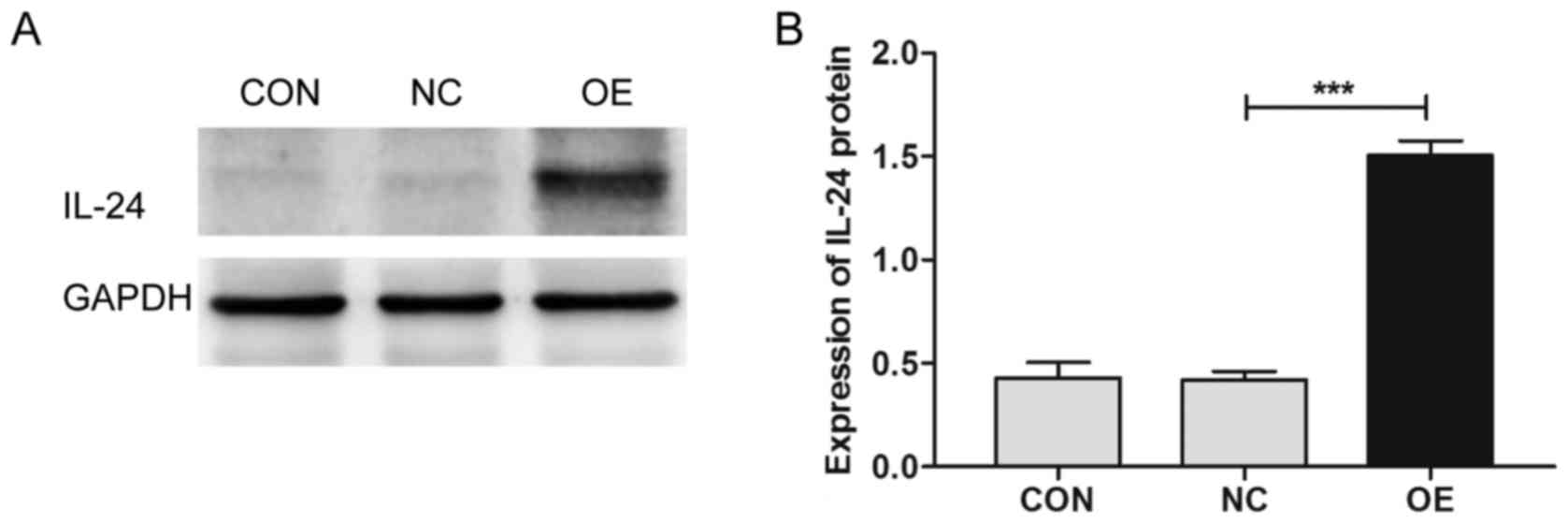
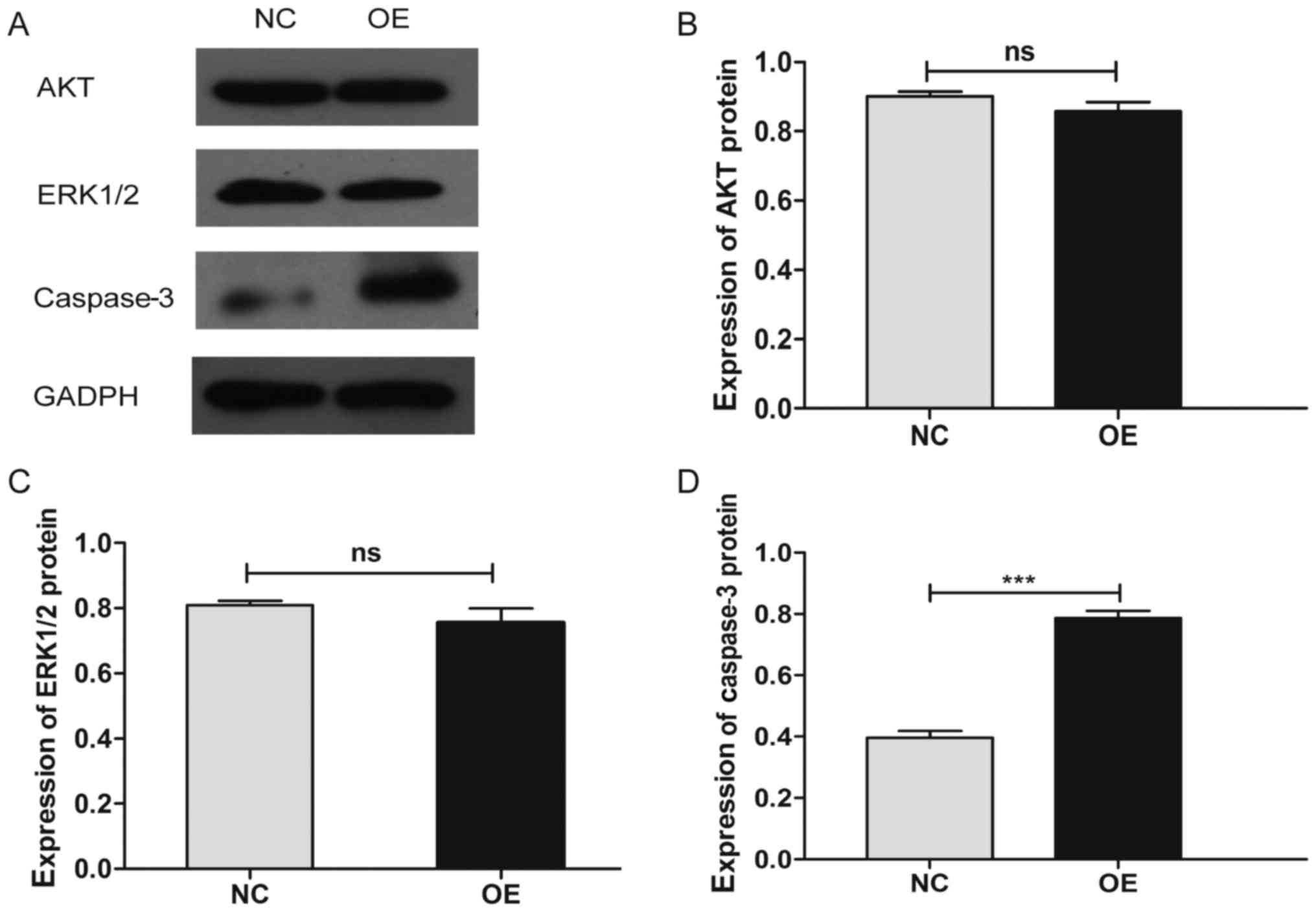
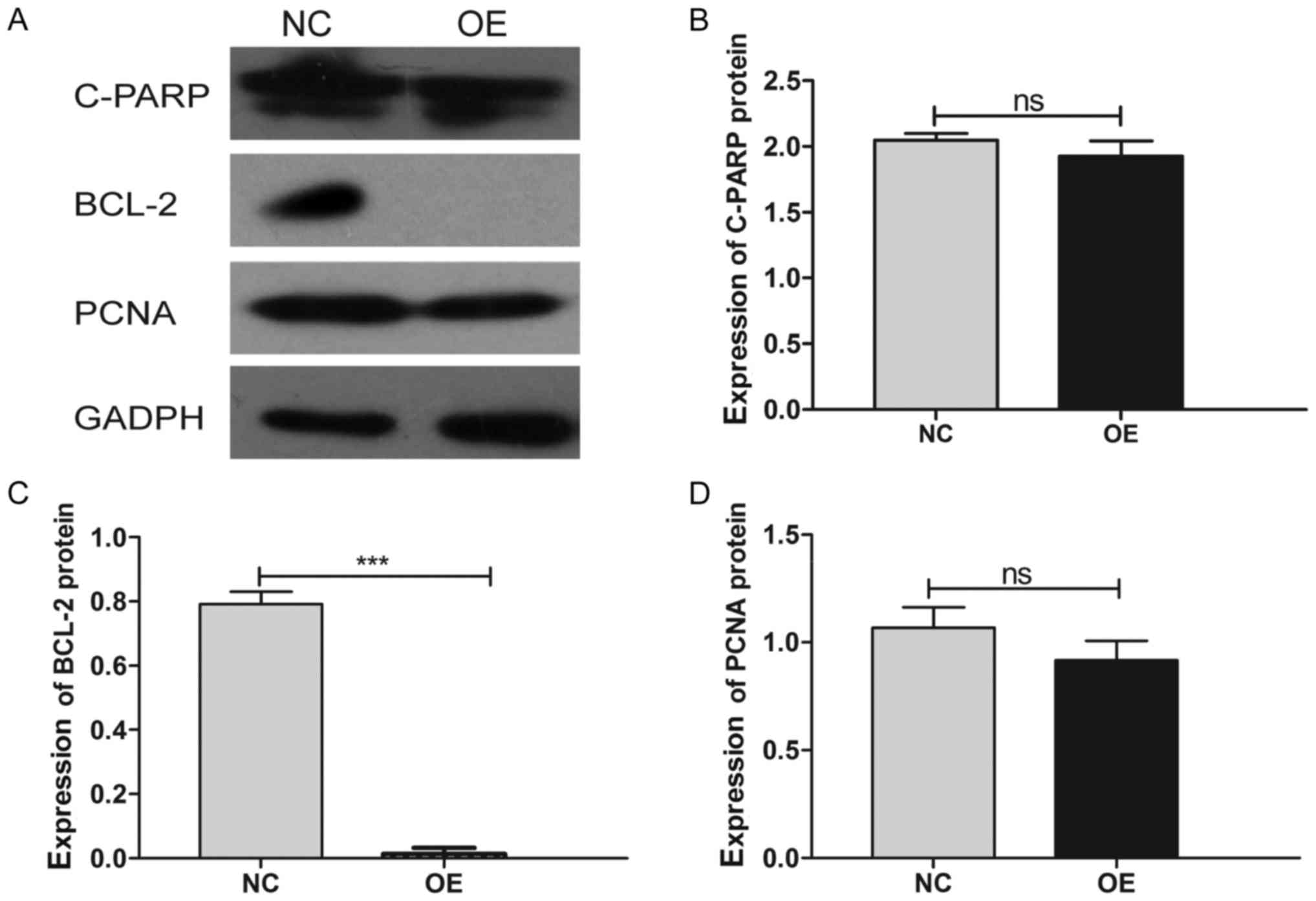
6B). Additionally, we verified the differential expression of
several key molecules involved in these two pathways by western
blotting, which showed that the upregulation of MDA7/IL24
induces the expression of caspase-3 and downregulates the
expression of p-AKT, p-ERK1/2, CCNE2, and BCL2 (Figs. 7–10).
Discussion
Gene therapy has become a focus of current
investigations aimed at improving HCC treatment (16). MDA7/IL24 is a member of the IL10
cytokine family, and was shown to induce apoptosis in different
cancers specifically, including HCC (17), lung cancer (18), melanoma (19), breast cancer (20), pancreatic cancer (21), cervical cancer (22), and prostate cancer (23), but it does not affect normal cells
(24). Therefore, this gene may
represent an ideal gene therapy target (25). Additionally, MDA7/IL24 can induce
the activation of immune system response aimed against cancer
cells, and effectively inhibit neoplastic angiogenesis. It was
demonstrated that the overexpression of this molecule and
chemotherapy have synergistic effects (26). However, MDA7/IL24
overexpression mediated by lentiviruses has not been investigated
previously.
Here, we constructed a LV-MDA7/IL24, and
confirmed that the expression levels of this gene increase in
SMMC-7721 cells infected with lentiviral particles carrying this
vector. This demonstrated that the recombinant lentiviruses
effectively promote the expression of MDA7/IL24 in HCC
cells. MTT and colony formation assays demonstrated that
lentivirus-mediated MDA7/IL24 expression markedly inhibits
HCC cell proliferation. The number of cells and cell growth rate of
LV-MDA7/IL24-infected cells were shown to be significantly
decreased. In accordance with the results obtained in a previous
study (27), we demonstrated the
anticancer effects of MDA7/IL24.
However, the molecular mechanisms underlying the
effects of MDA7/IL24 on HCC cells remain unclear. We performed gene
expression profiling of SMMC-7721 cells infected with LV-MDA7/IL24,
demonstrating that many genes show significantly different
expression between MDA7/IL24-overexpressing cells and the
control cells. We further performed functional pathway analysis,
and several pathways involving the differentially expressed genes
were found to be involved in cancer development (14), for example, G1/S checkpoint and
G2/M DNA damage signaling pathway. These pathways are crucial for
cell cycle regulation, DNA synthesis, and transcription.
Previously, it was reported that MDA7/IL24 induces IL20/IL22
receptor-independent apoptosis by modulating multiple apoptotic
signaling pathways such as mitochondrial pathway, MAPK, PKR, GADD
pathways, and others (28–30), and is involved in the accumulation
of BAX and BCL2 (31). The results
of our western blot analysis showed that MDA7/IL24 induces the
expression of caspase-3 and inhibits the expression of p-AKT,
p-ERK1/2, CCNE2, and BCL2.
CCNE2 and p-ERK1/2 are molecules involved in cell
cycle regulation, which can induce tumor progression by regulating
cell cycle transition. Cyclin E2 is a member of cyclin E family,
which forms cyclin E-CDK2 complex with CDK2 (6). This complex promotes cell cycle
progression by regulating G1/S phase transition, and the
dysregulation of cyclin E2-CDK2 activity was shown to be involved
in tumor development (32).
Moreover, the overexpression of cyclin E2 was shown to be
associated with poor survival of breast cancer patients (33). P-ERK1/2 is the activated form of
ERK1/2, which plays an important role in cell proliferation by
regulating cell cycle progression (34). P-ERK1/2 was demonstrated to be a
HCC prognostic marker, since increased p-ERK1/2 levels correlate
with a decrease in the overall survival (35). MDA7/IL24 overexpression induces
CCNE2 and p-ERK1/2 downregulation, indicating that this molecule
suppresses tumor progression by regulating cell cycle transition.
Just as demonstrated by the experimental results, IL-24
overexpression induced G1 arrest in human HCC cells.
BCL2 is a 26-kDa oncoprotein, and its carcinogenic
property is closely associated with the anti-apoptotic activity
(36). As a regulator of
apoptosis, BCL2 may promote tumor cell survival and inhibit
apoptosis through the regulation of mitochondrial membrane
permeability and the induction of tumor angiogenesis (37). BCL2 overexpression is related to
tumor progression (38). P-AKT, an
active form of AKT, plays an important role in the inhibition of
tumor cell apoptosis, promoting tumor cell proliferation and
angiogenesis (39). Many studies
demonstrated that p-AKT is highly activated in many tumors, and its
abnormal expression was shown to be closely related to tumor
development and progression (40).
Members of caspase family are key elements in the process of
apoptosis, and their activation and abnormal expression can induce
apoptosis through the interaction with other factors (41). Caspase-3 is the most important
member of the caspase family involved in the process of apoptosis,
mediating signaling triggered by many other molecules (42). In the present study, the expression
levels of p-AKT, BCL2, and caspase-3 were shown to differ between
the cells overexpressing MDA7/IL24 and the controls,
indicating that MDA7/IL24 can inhibit tumor progression by inducing
apoptosis, which is consistent with the results of cell functional
experiments.
Taken together, our results demonstrate that
MDA7/IL24 can inhibit the proliferation and suppress tumorigenicity
of HCC cells in vitro. Furthermore, the MDA7/IL24 exerts its
effects through the regulation of cell cycle transition and the
induction of apoptosis. Therefore, we demonstrated that MDA7/IL24
has anticancer functions, its overexpression inhibits HCC
progression, and it may represent a novel therapeutic target for
cancer treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China Youth Fund (81201673). The
manuscript was edited and proofread by Editage.
References
|
1
|
Wang CJ, Xiao CW, You TG, Zheng YX, Gao W,
Zhou ZQ, Chen J, Xue XB, Fan J and Zhang H: Interferon-α enhances
antitumor activities of oncolytic adenovirus-mediated IL-24
expression in hepatocellular carcinoma. Mol Cancer. 11:312012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han B, Liu SH, Guo WD, Zhang B, Wang JP,
Cao YK and Liu J: Notch1 downregulation combined with
interleukin-24 inhibits invasion and migration of hepatocellular
carcinoma cells. World J Gastroenterol. 21:9727–9735. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Shi L, Zhang X, Kang X, Wen Y, Qian
H, Zhou Y, Xu W, Zhang Y, Wu M and Yin Z: Recombinant adenovirus
IL-24-Bax promotes apoptosis of hepatocellular carcinoma cells in
vitro and in vivo. Cancer Gene Ther. 17:771–779. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao CW, Xue XB, Zhang H, Gao W, Yu Y,
Chen K, Zheng JW and Wang CJ: Oncolytic adenovirus-mediated
MDA-7/IL-24 overexpression enhances antitumor activity in
hepatocellular carcinoma cell lines. Hepatobiliary Pancreat Dis
Int. 9:615–621. 2010.PubMed/NCBI
|
|
6
|
Huo W, Li ZM, Zhu XM, Bao YM and An LJ:
MDA-7/IL-24 suppresses tumor adhesion and invasive potential in
hepatocellular carcinoma cell lines. Oncol Rep. 30:986–992. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
8
|
Huang EY, Madireddi MT, Gopalkrishnan RV,
Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ,
Alexandre D, et al: Genomic structure, chromosomal localization and
expression profile of a novel melanoma differentiation associated
(mda-7) gene with cancer specific growth suppression and apoptosis
inducing properties. Oncogene. 20:7051–7063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellerhorst JA, Prieto VG, Ekmekcioglu S,
Broemeling L, Yekell S, Chada S and Grimm EA: Loss of MDA7
expression with progression of melanoma. J Clin Oncol.
20:1069–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarkar D, Su ZZ, Vozhilla N, Park ES,
Gupta P and Fisher PB: Dual cancer specific targeting strategy
cures primary and distant breast carcinomas in nude mice. Proc Natl
Acad Sci USA. 102:pp. 14034–14039. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caudell EG, Mumm JB, Poindexter N,
Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S
and Grimm EA: The protein product of the tumor suppressor gene,
melanoma differentiation-associated gene 7, exhibits
immunostimulatory activity and is designated IL-24. J Immunol.
168:6041–6046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher PB, Gopalkrishnan RV, Chada S,
Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT and Dent P: mda7/IL-24,
a novel cancer selective apoptosis inducing cytokine gene: From the
laboratory into the clinic. Cancer Biol Ther. 2 4 Suppl 1:S23–S37.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tong AW, Nemunaitis J, Su D, Zhang Y,
Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, et
al: Intratumoral injection of INGN 241, a nonreolicating
adenovector expressing the melanoma-differentiation associated
gene-7 (mda7/IL-24): Biologic outcome in advanced cancer patients.
Mol Ther. 11:160–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lebedeva IV, Sauane M, Gopalkrishnan RV,
Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A,
Dent P and Fisher PB: Mda7/IL-24: Exploiting cancer's Achilles'
heel. Mol Ther. 11:4–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Z, Lin J, Sun Z, Ni J and Sha Y:
RNAi-mediated downregulation of CDKL1 inhibits growth and
colony-formation ability, promotesapoptosis of human melanoma
cells. J Dermatol Sci. 79:57–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diao J, Wu C, Zhang J, Liu J, Zhang X, Hao
P, Zhao S and Zhang Z: Loss of diacylglycerol kinase-Ζ inhibits
cell proliferation and survival in human gliomas. Mol Neurobiol.
53:5425–5435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue XB, Xiao CW, Zhang H, Lu AG, Gao W,
Zhou ZQ, Guo XL, Zhong MA, Yang Y and Wang CJ: Oncolytic adenovirus
SG600-IL24 selectively kills hepatocellular carcinoma cell lines.
World J Gastroenterol. 16:4677–4684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Ye Z, Zhong J, Xiang J and Yang J:
Adenovirus-mediated Il-24 expression suppresses hepatocellular
carcinoma growth via induction of cell apoptosis and cycling arrest
and reduction of angiogenesis. Cancer Biother Radiopharm. 22:56–63.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emdad L, Lebedeva IV, Su ZZ, Gupta P,
Sarkar D, Settleman J and Fisher PB: Combinatorial treatment of
non-small cell lung cancers with gefitinib and Ad.mda-7 enhances
apoptosis-induction and reverses resistance to a single therapy. J
Cell Physiol. 210:549–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarkar D, Su ZZ, Lebedeva IV, Sauane M,
Gopalkrishnan RV, Valerie K, Dent P and Fisher PB: mda-7 (IL-24)
mediates selective apoptosis in human melanoma cells by inducing
the coordinated overexpression of the GADD family of genes by means
of p38 MAPK. Proc Natl Acad Sci USA. 99:pp. 10054–10059. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McKenzie T, Liu Y, Fanale M, Swisher SG,
Chada S and Hunt KK: Combination therapy of Ad-mda7 and trastuzumab
increases cell death in Her-2/neu-overexpressing breast cancer
cells. Surgery. 136:437–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lebedeva IV, Su ZZ, Sarkar D,
Gopalkrishnan RV, Waxman S, Yacoub A, Dent P and Fisher PB:
Induction of reactive oxygen species renders mutant and wild-type
K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced
apoptosis. Oncogene. 24:585–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi H, Wei LL, Yuan CF, Yang JX, Yi FP, Ma
YP and Song FZ: Melanoma differentiation-associated
gene-7/interleukin 24 inhibits invasion and migration of human
cervical cancer cells in vitro. Saudi Med J. 11:1671–1675.
2007.
|
|
24
|
Menezes ME, Shen XN, Das SK, Emdad L, Guo
C, Yuan F, Li YJ, Archer MC, Zacksenhaus E, Windle JJ, et al:
MDA-7/IL-24 functions as a tumor suppressor gene in vivo in
transgenic mouse models of breast cancer. Oncotarget.
6:36928–36942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito Y, Miyahara R, Gopalan B, Litvak A,
Inoue S, Shanker M, Branch CD, Mhashilkar AM, Roth JA, Chada S and
Ramesh R: Selective induction of cell cycle arrest and apoptosis in
human prostate cancer cells through adenoviral transfer of the
melanoma differentiation associated-7 (mda-7)/interleukin-24
(IL-24) gene. Cancer Gene Ther. 12:238–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawabe S, Nishikawa T, Munshi A, Roth JA,
Chada S and Meyn RE: Adenovirus-mediated mda-7 gene expression
radiosensitizes non-small cell lung cancer cells via
TP53-independent mechanisms. Mol Ther. 6:637–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sauane M, Gopalkrishnan RV, Sarkar D, Su
ZZ, Lebedeva IV, Dent P, Pestka S and Fisher PB: MDA-7/IL-24: Novel
cancer growth suppressing and apoptosis inducing cytokine. Cytokine
Growth Factor Rev. 14:35–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sauane M, Gopalkrishnan RV, Lebedeva I,
Mei MX, Sarkar D, Su ZZ, Kang DC, Dent P, Pestka S and Fisher PB:
Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through
JAK/STAT-independent pathways. J Cell Physiol. 196:334–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su ZZ, Lebedeva IV, Sarkar D,
Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P
and Fisher PB: Melanoma differentiation associated gene-7,
mda-7/IL-24, selectively induces growth suppression, apoptosis and
radiosensitization in malignant gliomas in a p53-independent
manner. Oncogene. 22:1164–1180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su ZZ, Lebedeva IV, Sarkar D, Emdad L,
Gupta P, Kitada S, Dent P, Reed JC and Fisher PB: Ionizing
radiation enhances therapeutic activity of mda-7/IL-24: Overcoming
radiation- and mda-7/IL-24-resistance in prostate cancer cells
overexpressing the antiapoptotic proteins bcl-xL or bcl-2.
Oncogene. 25:2339–2348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tahara I, Miyake K, Hanawa H, Kurai T,
Hirai Y, Ishizaki M, Uchida E, Tajiri T and Shimada T: Systemic
cancer gene therapy using adeno-associated virus type 1 vector
expressing MDA-7/IL24. Mol Ther. 15:1805–1811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Z, Liu J, Wang C, Wang Y, Jiang Y and
Guo M: MicroRNA-25 regulates small cell lung cancer cell
development and cell cycle through cyclin E2. Int J Clin Exp
Pathol. 7:7726–7734. 2014.PubMed/NCBI
|
|
33
|
Ye L, Guo L, He Z, Wang X, Lin C, Zhang X,
Wu S, Bao Y, Yang Q, Song L and Lin H: Upregulation of E2F8
promotes cell proliferation and tumorigenicity in breast cancer by
modulating G1/S phase transition. Oncotarget. 17:23767–23771.
2016.
|
|
34
|
Hwang HC and Clurman BE: Cyclin E in
normal and neoplastic cell cycles. Oncogene. 24:2776–2786. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao S, Qiu ZX, Zhang L and Li WM:
Prognostic values of ERK1/2 and p-ERK1/2 expressions for poor
survival in non-small cell lung cancer. Tumor Biol. 36:4143–4150.
2015. View Article : Google Scholar
|
|
36
|
Li Q and Yang Z: Expression of
phospho-ERK1/2 and PI3-K in benign and malignant gallbladder
lesions and its clinical and pathological correlations. J Exp Clin
Cancer Res. 28:652009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sabokrouh A, Vaisi-Raygani A, Goodarzi MT,
Khatami S, Taghizadeh-Jahed M, Shahabadi N, Lakpour N and Shakiba
Y: Comparison between platinum-azidothymidine and azidothymidine
effects on Bcl-2 and telomerase gene expression in rats with
hepatocellular carcinoma. Avicenna J Med Biotech. 7:50–56.
2015.
|
|
38
|
Zhao N, Sun BC, Zhao XL, Wang Y, Meng J,
Che N, Dong XY and Gu Q: Role of Bcl-2 and its associated miRNAs in
vasculogenic mimicry of hepatocellular carcinoma. Int J Clin Exp
Pathol. 8:15759–15768. 2015.PubMed/NCBI
|
|
39
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: Implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu LF, Ni JY, Sun HL, Chen YT and Wu YD:
Effects of hypoxia-inducible factor-1α silencing on the
proliferation of CBRH-7919 hepatoma cells. World J Gastroenterol.
19:1749–1759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mackenzie RW and Elliott BT: Akt/PKB
activation and insulin signaling: A novel insulin signaling pathway
in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes.
7:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui R, Kim T, Fassan M, Meng W, Sun HL,
Jeon YJ, Vicentini C, Tili E, Peng Y, Scarpa A, et al: MicroRNA-224
is implicated in lung cancer pathogenesis through targeting
caspase-3 and caspase-7. Oncotarget. 6:21802–21815. 2015.
View Article : Google Scholar : PubMed/NCBI
|