Introduction
Leukemia refers to a heterogeneous group of
hematopoietic malignancies that includes four major subtypes: Acute
lymphoblastic leukemia, acute myeloid leukemia (AML), chronic
lymphocytic leukemia and chronic myeloid leukemia (1). Leukemia is considered the most common
childhood cancer; however, it does affect people of all ages,
making it a major cause of morbidity and mortality (2). With >11,000 people diagnosed with
AML each year, 75% of patients succumb to leukemia within 5 years
worldwide (3). In addition, males
are more likely to develop leukemia than females, and individuals
in developed regions are more likely to develop leukemia than
individuals in less developed regions (4). There are numerous risk factors
associated with leukemia, including genetic disorders caused by
aberrant chromosomes, human T cell leukemia virus, previous
long-term exposure to radiation and myelodysplastic syndrome
(5). Leukemia cells are white
blood cells that are not fully developed and exhibit an abnormally
large quantity in leukemia, which are fundamentally necessary to
sustain leukemia (5). Previous
studies have indicated that inducing autophagy and apoptosis of
leukemia cells serves as a feasible therapy for leukemia (6,7). In
recent years, efforts have been made to analyze the basic cellular
and molecular biology of leukemia, and have demonstrated that
complex signaling pathways are involved in leukemia (8); therefore, disrupting these signaling
pathways to induce autophagy and apoptosis of leukemia cells may be
considered an appealing target for the treatment of leukemia.
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT)/mammalian target of rapamycin (mTOR) pathway is a
prototypic survival pathway (9),
which has been reported to serve a crucial role in cell growth,
proliferation and survival, not only under physiological
circumstances but also in numerous tumor cells (10). Previous studies have revealed an
association between leukemia and aberrant activation of the
PI3K/Akt/mTOR signaling pathway (11,12).
The endoplasmic reticulum (ER) is a major site in the cell, which
has a role in protein folding and trafficking, and is responsible
for numerous cellular functions (13). ER stress (ERS) results from
discrepancies between the demand for and the capacities of ER
functions (14). Previous studies
have indicated that ERS is closely associated with the apoptotic
response of human leukemia cells (15,16).
However, the mechanism underlying how ERS suppresses the
progression of disease in an efficient manner remains unknown.
Consequently, the present study explored the effects of ERS on
regulation of the PI3K/AKT/mTOR signaling pathway, and on autophagy
and apoptosis of human leukemia cells, with the aim of introducing
a novel target for the treatment of leukemia.
Materials and methods
Preparation and culture of leukemia
cells
The present study was approved by the ethics
committee of Lanzhou University Second Hospital (Lanzhou, China).
Leukemia cells were derived from routine blood samples collected
from patients at the Third Affiliated Hospital of Xiangya
(Changsha, Hunan, China). All patients provided written informed
consent. The patients all underwent clinical hemograms and
myelograms, and were diagnosed with leukemia (17). Peripheral blood (white blood cells
>2×1010/l; 3–5 ml) or bone marrow fluid (1–1.5 ml)
samples were extracted from the patients. The inner wall of a
needle tube was wetted with 0.2 ml of aseptic heparin sodium
anticoagulant (2 g/l) prior to being used to take bone marrow (or
peripheral blood). Mononuclear cells were isolated using
Hypaque-Ficoll solution. After being washed three times with
serum-free RPMI 1640 (11875093; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), the cells were suspended in Iscove's modified
Dulbecco's media (12440046; Thermo Fisher Scientific, Inc.) to
obtain a 2×107/ml cell suspension. Subsequently T and B
lymphocytes were separated, nylon cotton was used to remove the T
cells and a leukemia cell suspension was obtained. The survival
rate of mononuclear cells was >95%. The obtained cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) (11320082;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum,
100 µg/ml streptomycin and 100 U/ml penicillin in a 37° incubator
containing 5% CO2. Once cell density reached 85%, the
cells were digested with 0.5% trypsin and 0.02% EDTA and were
eluted with PBS. After 3 days, the medium was changed and
subculture was performed at 1:3-1:6. The leukemia cells used in the
subsequent experiments were in the logarithmic growth phase.
Cell drug toxicity test and
grouping
The cells in logarithmic growth phase were
inoculated into a 96-well plate at 2×105 cells/ml and
100 µl fresh DMEM was added to each well for overnight culture.
Following treatment of human leukemia cells with 25, 50, 100, 200
or 500 ng/ml tunicamycin (Sigma-Aldrich; Merck Millipore KGaA,
Darmstadt, Germany) for 24, 48, 72 and 96 h, various indicators
were detected to determine the optimal concentration and duration
of tunicamycin treatment. In the subsequent experiments, leukemia
cells were divided into the following three groups: The ER
activation group, in which cells were treated with the optimal
concentration of tunicamycin (100 ng/ml tunicamycin for 72 h); the
ER activation + TO901317 group, in which cells were treated with
the optimal concentration of tunicamycin + 10 µmol/l PI3K activator
TO901317; and the control group, which consisted of untreated
cells.
MTT assay
The cells in logarithmic growth phase were
inoculated into a 96-well plate at 2×105 cells/ml and
100 µl fresh DMEM was added to each well for overnight culture.
Following the removal of the medium, 100 µl fresh medium and 20 µl
0.5% MTT solution were added to each well for 3 h at 37°C.
Subsequently, the medium was removed and 100 µl dimethyl sulfoxide
was added to each well, and the plate was oscillated at a low speed
for 15 min to fully dissolve the crystals. Finally, an
enzyme-linked immunometric meter was used to measure the optical
density value of each well at 490 nm; three duplicated wells were
set up for each group. The MTT assay was used to measure and
calculate cell proliferation in each group.
Monodansylcadaverine (MDC)
staining
The leukemia cells were inoculated into a 96-well
plate and 100 µl fresh DMEM was added to each well. The cells were
incubated at 37°C in an atmosphere containing 5% CO2 and
saturated humidity for 24 h. The cells were collected and the cell
concentration adjusted to 106 cells/ml. They were washed
twice with PBS. Subsequently, the cells were incubated with 0.05 mM
MDC at 37°C for 1 h and were observed under an inverted
fluorescence microscope (Leica DMI 4000B; Leica Microsystems GmbH,
Wetzlar, Germany). The fluorescence intensity was calculated as
follows: Fluorescence intensity (%)=(fluorescence intensity of
treatment groups-control group)/control group ×100.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining
The leukemia cells were inoculated into a 96-well
plate and 100 µl fresh DMEM was added to each well. The cells were
incubated at 37°C in an atmosphere containing 5% CO2 and
saturated humidity for 24 h. The cells were collected and the cell
concentration adjusted to 106 cells/ml. They were washed
twice with PBS and centrifuged at 1,000 × g for 3 min.
Subsequently, 500 µl binding buffer was added and mixed gently,
following which 5 µl Annexin V-FITC and 5 µl PI were added
(K201-100; BioVision, Inc., Milpitas, CA, USA). The cells were
incubated in the dark for 10 min and flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA) and CellQuest Pro software
version 5.1 (BD Biosciences) were used to detect cell apoptotic
rate. The results were analyzed as follows: The lower left quadrant
consisted of normal cells, the lower right quadrant consisted of
early apoptotic cells, the upper right quadrant consisted of late
apoptotic cells and the upper left quadrant consisted of dead
cells.
Western blotting
Leukemia cells were treated with the optimal
concentration of tunicamycin and were then inoculated into a 6-well
plate at 1.5×106 cells/well for 24 h. Subsequently, the
cells were washed three times with ice-cold PBS and protein was
extracted from the cleaved cells using RIPA cell lysis solution
(BB-3209; BestBio, Co., Shanghai, China). Protein concentration was
measured using a Bradford kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The proteins (20–30 µg)
were separated by 10% SDS-PAGE and were then transferred to a
nitrocellulose membrane. The membrane was washed with Tris-buffered
saline containing 0.1% Tween (TBST) and was blocked with 5% skim
milk powder at room temperature for 1 h. Subsequently, the membrane
was incubated overnight at 4°C with the following primary
antibodies: Rabbit polyclonal antibodies: Anti-caspase-3 (ab13847;
1:500; Abcam, Cambridge, UK), anti-mTOR (ab2732; 1:2,000; Abcam),
anti-AKT (ab8805; 1:500; Abcam), anti-phosphorylated (p)-protein
kinase R-like endoplasmic reticulum kinase (PERK; E19-7579-1;
1:1,000, EnoGene Biotech Co., Ltd., New York, NY, USA), anti-p-α
subunit of eukaryotic initiation factor 2 (elF2α; ab227593;
1:1,000; Abcam), anti-microtubule-associated protein 1A/1B-light
chain 3 (LC3; ab128025; 1:2,000; Abcam) and anti-78-kDa
glucose-regulated protein (GRP78; ab21685; 1:1,000; Abcam), rabbit
antibody Anti-PI3K (ab40776; 1:2,000; Abcam). Following washing
with TBST, the membrane was incubated with a secondary antibody
sheep anti-rabbit IgG (Cell Signaling Technology, Inc., Danvers,
MA, USA) for 1 h at 37°C. Finally, the membrane was visualized
using electrochemiluminescence (ECL) (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) for 3 min. Then the
membrane was put into the Gel imaging system instrument (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and 400 µl ECL
hypersensitive luminescence (Beijing Solarbio Science &
Technology Co., Ltd.) added and developed for 2 min. The relative
expression of the target protein=gray value of target protein
band/gray value of internal reference band (β-actin). The
experiment was repeated 3 times).
Statistical analysis
All experiments were repeated at least three times
under the same conditions. Statistical analyses were conducted
using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Measurement data were
expressed as the mean ± standard deviation, enumeration data were
expressed as a percentage. Differences between two groups were
analyzed using Student's t-test, and differences among multiple
groups were analyzed by one-way analysis of variance (ANOVA) and
LSD analysis. Cell growth conditions following treatment with
various concentrations for various time points were assessed by
repeated measures ANOVA. P<0.05 was considered to indicate a
statistically significant different.
Results
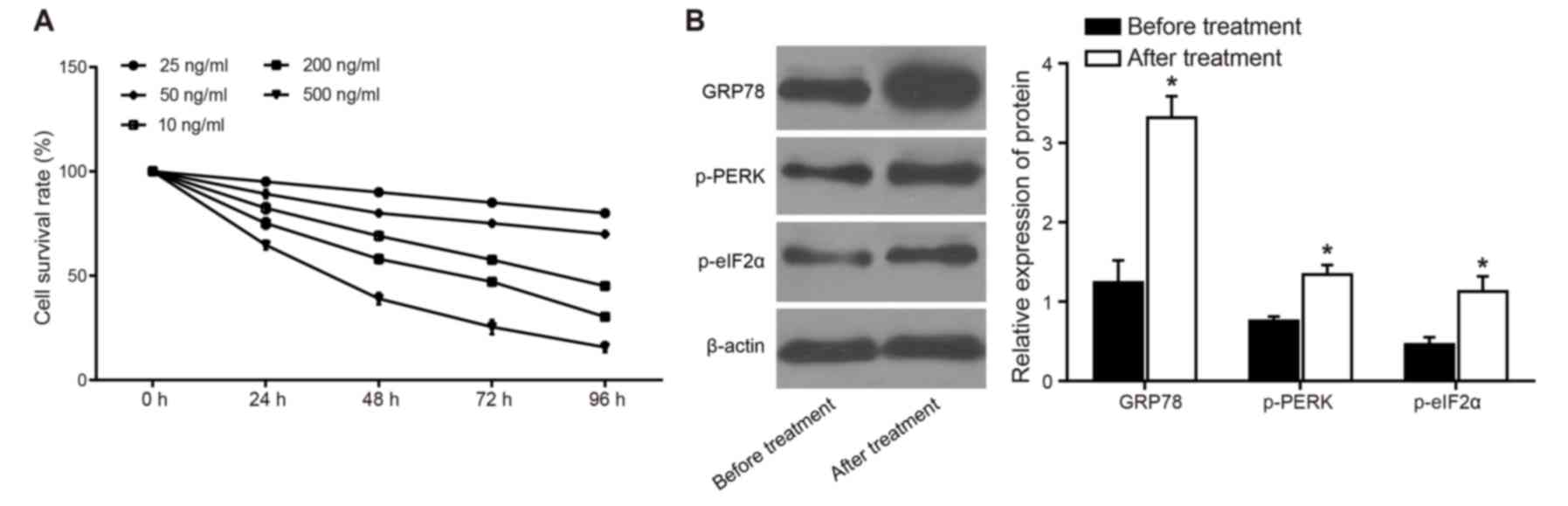
ERS is induced by tunicamycin
The results of an MTT assay and flow cytometry
demonstrated that cell survival rate was decreased as tunicamycin
concentration and treatment duration increased. Specifically, when
cells were treated with 100 ng/ml tunicamycin for 72 h, the
survival rate of human leukemia cells was 57.68±3.12%. This
concentration and duration of tunicamycin treatment was used in
subsequent experiments (Fig.
1A).
After the leukemia cells were treated with 100 ng/ml
tunicamycin for 72 h, the results of a western blot analysis
demonstrated that the expression levels of p-PERK, p-eIF2α and the
ER molecular chaperone GRP78 were significantly increased compared
with in cells prior to treatment (P<0.05; Fig. 1B). These findings indicated that ER
activation was induced in response to treatment with 100 ng/ml
tunicamycin for 72 h.
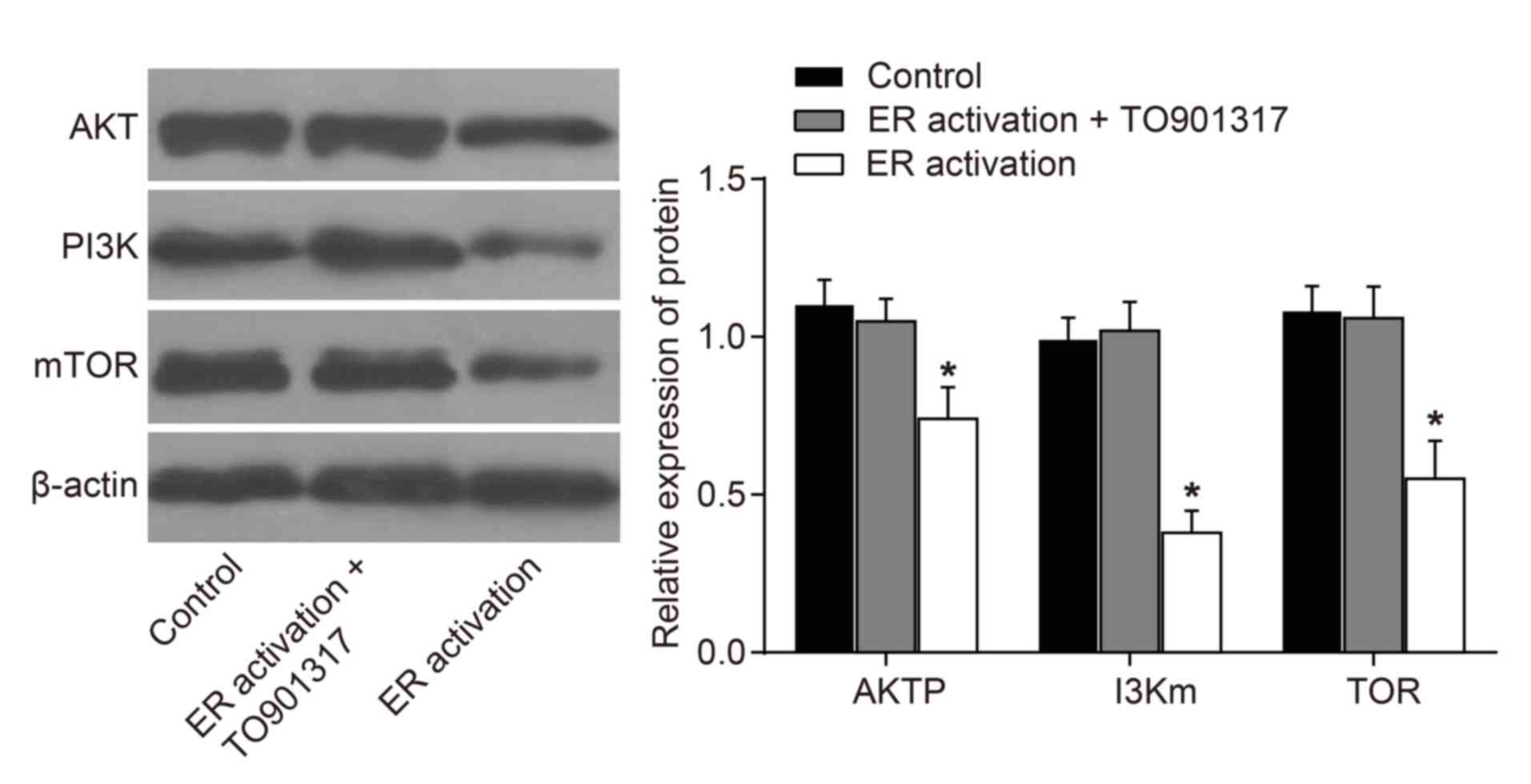
Effects of ERS on PI3K/AKT/mTOR
signaling pathway-associated protein expression
Following treatment of the leukemia cells with 100
ng/ml tunicamycin for 72 h, the results of a western blot analysis
demonstrated that compared with in the control group, the
expression levels of key proteins in the PI3K/AKT/mTOR signaling
pathway exhibited no significant difference in the ER activation +
TO901317 group (P>0.05). However, compared with in the control
and ER activation + TO901317 groups, the expression levels of mTOR,
AKT and PI3K were significantly decreased in the ER activation
group (P<0.05; Fig. 2), which
indicated that ERS may inhibit the PI3K/AKT/mTOR signaling
pathway.
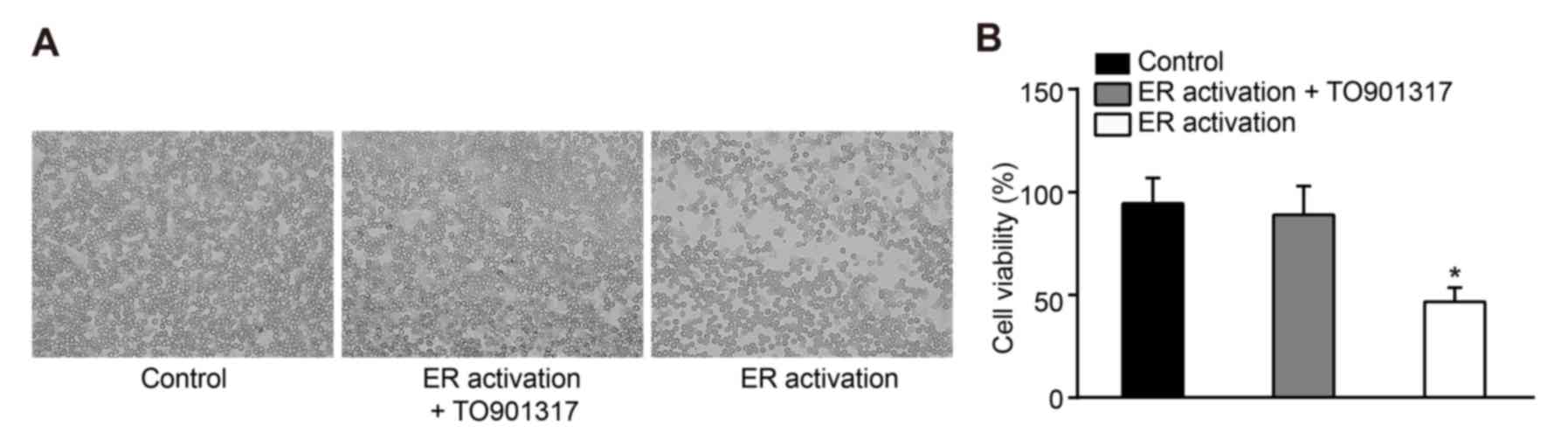
Effects of ERS on cell survival
rates
Observation under a microscope demonstrated that,
following treatment of leukemia cells with 100 ng/ml tunicamycin
for 72 h, cells in the ER activation and ER activation + TO901317
groups exhibited shrinkage and cracking, and the number of cells
markedly decreased while no obvious difference between the ER
activation + TO901317 group and control group was observed
(Fig. 3A). The results of an MTT
assay also revealed that compared with in the control group, cell
viability was significantly inhibited in the ER activation group
(P<0.05). Although cell viability was decreased in the ER
activation + TO901317 group, there was no significant difference
compared with in the control group (P>0.05; Fig. 3B), which suggested that activation
of PI3K may reduce the inhibitory effects of ERS on the viability
of human leukemia cells.
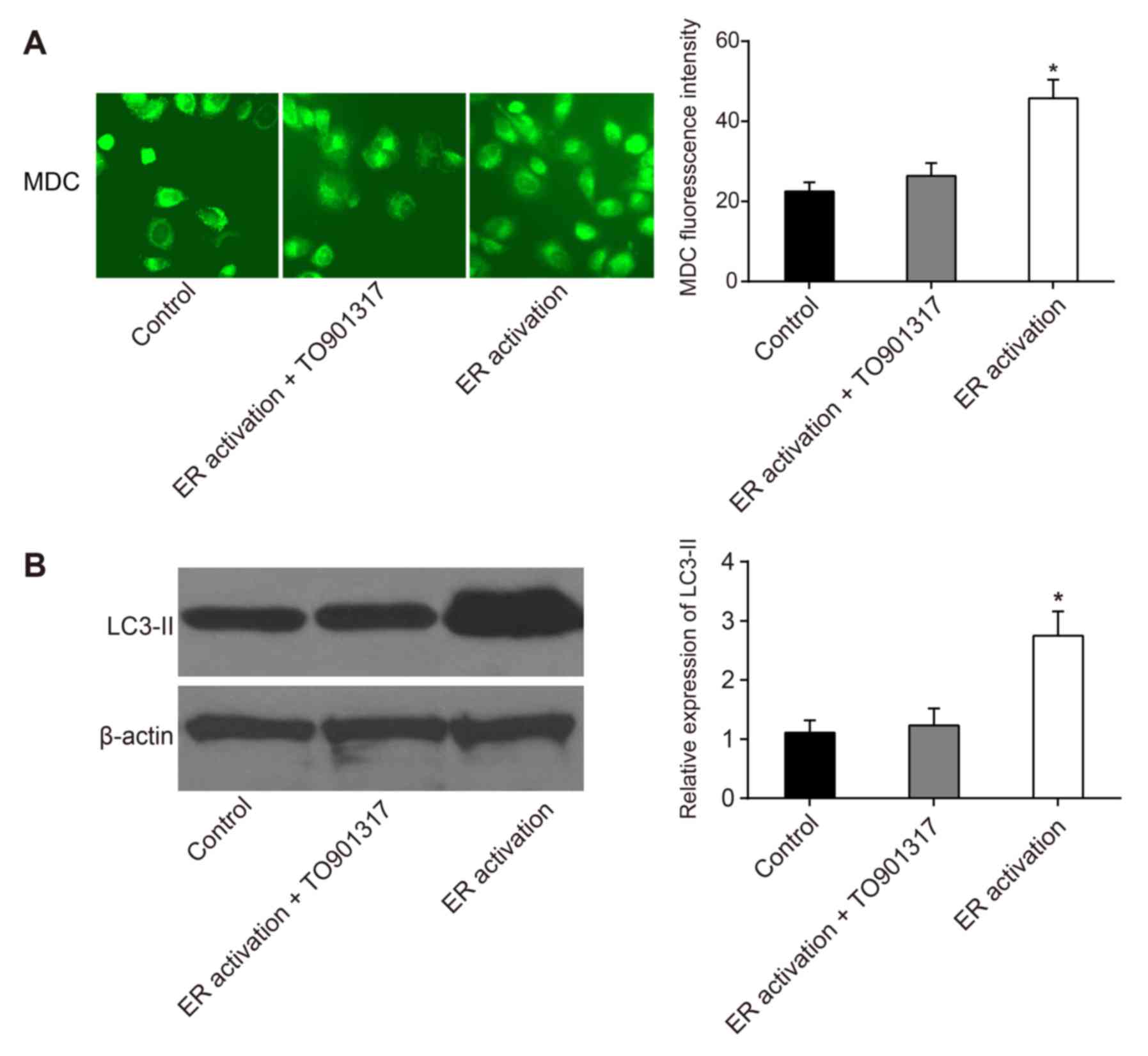
Effects of ERS on autophagy in
leukemia cells
Increased MDC fluorescence intensity indicates that
cell autophagy is increased. Compared with in the control group,
MDC intensity in the ER activation + TO901317 group was slightly
increased; however, there was no significant difference
(P>0.05). MDC fluorescence intensity in was significantly
increased the ER activation group (P<0.05; Fig. 4A). The formation of autophagosomes
is positively associated with the expression of LC3-II. The results
of a western blot analysis demonstrated that compared with in the
control and ER activation + TO901317 groups, the expression levels
of LC3-II were significantly increased in the ER activation group
(P<0.05; Fig. 4B), which
indicated that PI3K activation inhibited the occurrence of
autophagy, suggesting that ERS-induced autophagy of leukemia cells
may be caused by inhibiting the PI3K/AKT/mTOR signaling
pathway.
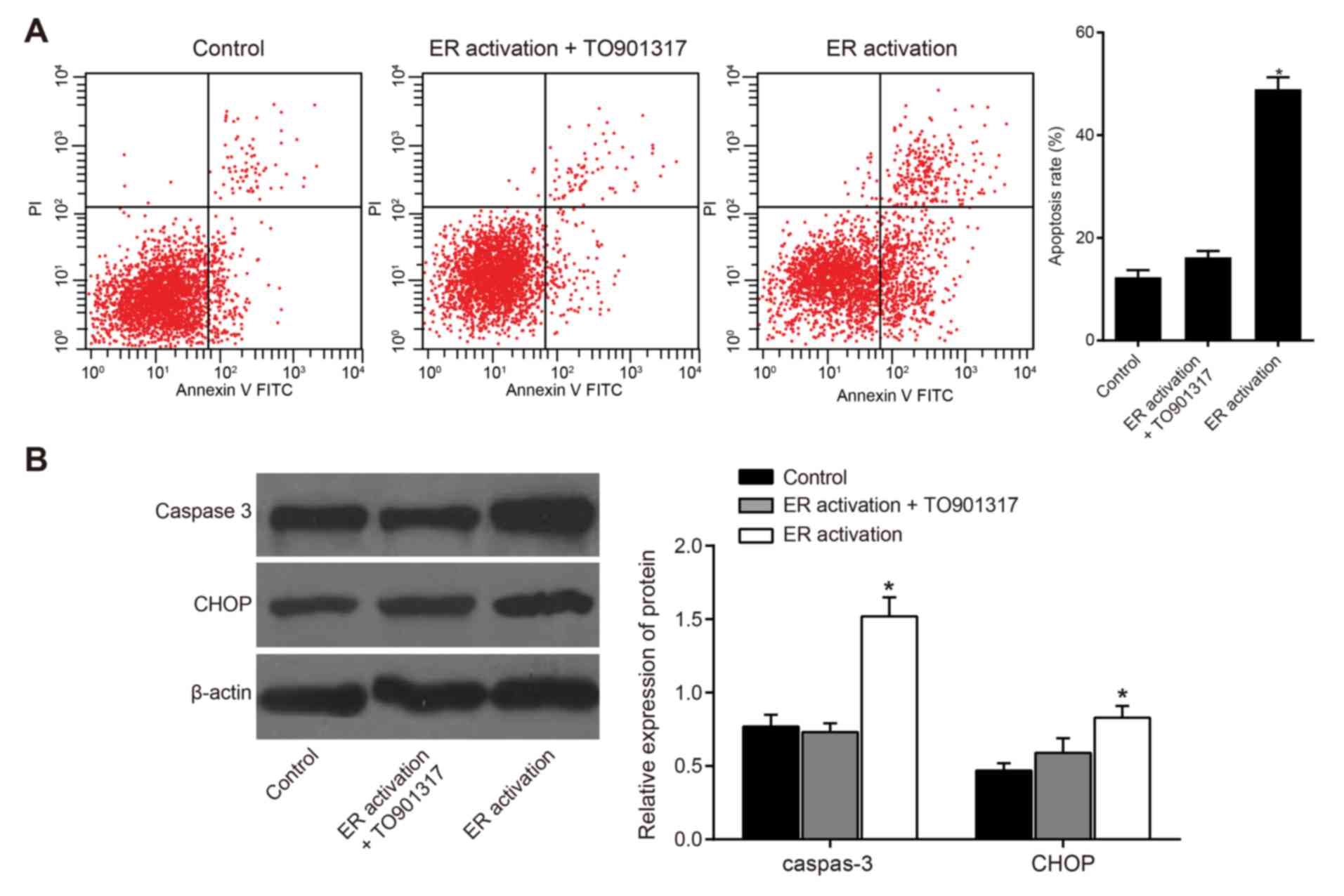
Effects of ERS on apoptosis of
leukemia cells
The results of a flow cytometric analysis
demonstrated that compared with in the control group, the apoptotic
rates of the other two groups were increased; the apoptotic rate in
the ER activation group was significantly increased (P<0.05;
Fig. 5A). The results of a western
blot analysis indicated that in the ER activation group, the
apoptosis-associated key factor caspase-3 was activated and the
expression levels of CHOP were significantly increased compared
with in the control group (P<0.05), thus suggesting that
apoptosis occurred in this cell group. No significant differences
were observed in the expression of caspase-3 and CHOP between the
ER activation + TO901317 group and the control group (P>0.05;
Fig. 5B), thus suggesting that
activation of PI3K could inhibit the apoptosis of human leukemia
cells. In addition, these results indicated that ERS may induce
apoptosis of human leukemia cells by inhibiting the PI3K/AKT/mTOR
signaling pathway.
Discussion
Leukemia affects individuals of all ages and is
therefore a major cause of morbidity and mortality worldwide
(2). Due to the strenuous efforts
made by researchers, understanding the cellular and molecular
biology of leukemia has markedly advanced, and numerous signaling
pathways have been reported to be involved in its pathogenesis,
including the PI3K/AKT/mTOR pathway (8,11).
However, the mechanism underlying how these pathways function
remains to be determined; therefore, studies are required to
explore the specifics behind complex pathways.
The present study revealed that in the presence of
tunicamycin-induced ERS, the expression levels of mTOR, AKT and
PI3K were markedly decreased, thus suggesting that ERS may suppress
the PI3K/AKT/mTOR signaling pathway. Previous studies have reported
that PI3K and its downstream targets AKT and mTOR have an essential
role in physiological processes, including cell growth, survival,
differentiation and proliferation, as well as in the development of
malignant disease (18,19). The PI3K/AKT/mTOR pathway works as
follows: PI3K, which is upstream of AKT, is responsible for
activating AKT, the activated AKT proceeds to phosphorylate target
molecules, including mTOR, which is a master regulator of protein
translation (11). In addition,
phosphatase and tensin homolog (PTEN), which is a
well-characterized human tumor suppressor gene, has been reported
to possess a high frequency of abnormalities in leukemia and has
also been established as an antagonist of the PI3K/AKT/mTOR pathway
(9,20). Therefore, it may be hypothesized
that under ERS, PTEN dephosphorylates and thereby suppresses PI3K,
which further disrupts AKT and mTOR activity, thus inducing
downregulated expression of mTOR, AKT and PI3K (21). Di Nardo et al conducted
experiments on rat hippocampal neurons and revealed that
tunicamycin-induced ERS can downregulate AKT and mTOR activity
(22), which is consistent with
the results of the present study.
The present study also indicated that ERS markedly
inhibits the proliferative capacity of leukemia cells, so as to
induce autophagy and apoptosis via suppression of the PI3K/AKT/mTOR
signaling pathway. As a process that describes the degradation and
recycling of proteins and intracellular components in reaction to
starvation or stress, autophagy can often lead to cell death, which
is most commonly associated with apoptosis (21). It has previously been reported that
ERS causes mitochondrial damage and initiates cell apoptosis, the
process of which may be worsened by oxidative stress when
accompanied by an mTOR inhibitor (23). mTOR is the catalytic subunit of two
biochemically distinct molecular complexes, namely, mTOR complex
(MTORC)1 whose activation increases protein synthesis and inhibits
autophagy, and mTORC2 whose activation causes additional
phosphorylation of AKT and promotes cell survival and proliferation
(24). Therefore, since the mTOR
pathway initiates the translation of mRNAs that encode proteins
required for cell cycle progression, inhibition of the signaling
pathway may arrest the development of disease to some extent
(25). In this sense, ERS may
induce autophagy and apoptosis via the suppression of mTOR
(26). Chen et al reported
similar results to the present study and identified a causal
relationship between ERS and autophagy-mediated apoptosis in human
hepatocellular carcinoma cell lines with the aid of PI3K/AKT/mTOR
signaling pathway inhibition (27).
In conclusion, by inhibiting the PI3K/AKT/mTOR
signaling pathway ERS may induce autophagy and apoptosis of
leukemia cells, and may be a potential target in treating leukemia.
However, although a role has been identified for the PI3K/AKT/mTOR
signaling pathway in autophagy and apoptosis, its specific
mechanism requires verification due to the complicated processes
involved in the pathway. As a result, further studies are required
to investigate the exact mechanism underlying the effects of the
PI3K/AKT/mTOR signaling pathway on autophagy and apoptosis in
various types of leukemia.
Acknowledgements
The authors would like to thank all the reviewers
and editors who gave assistance and helpful discussions for our
manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJL, YC and XJG designed the study. SLC and LSZ
collated the data, designed and developed the database, carried out
data analyses and produced the initial draft of the manuscript. LJL
and YC contributed to drafting and revising the manuscript. All
authors read and approved the final submitted manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Lanzhou University Second Hospital (Lanzhou,
China).
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xie Y, Davies SM, Xiang Y, Robison LL and
Ross JA: Trends in leukemia incidence and survival in the United
States (1973–1998). Cancer. 97:2229–2235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tower RL and Spector LG: The epidemiology
of childhood leukemia with a focus on birth weight and diet. Crit
Rev Clin Lab Sci. 44:203–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ali NA, O'Brien JM Jr, Blum W, Byrd JC,
Klisovic RB, Marcucci G, Phillips G, Marsh CB, Lemeshow S and
Grever MR: Hyperglycemia in patients with acute myeloid leukemia is
associated with increased hospital mortality. Cancer. 110:96–102.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajabli N, Naeimi-Tabeie M, Jahangirrad A,
Sedaghat SM, Semnani S and Roshandel G: Epidemiology of leukemia
and multiple myeloma in Golestan, Iran. Asian Pac J Cancer Prev.
14:2333–2336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rhomberg LR, Bailey LA, Goodman JE, Hamade
AK and Mayfield D: Is exposure to formaldehyde in air causally
associated with leukemia?-A hypothesis-based weight-of-evidence
analysis. Crit Rev Toxicol. 41:555–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama T, Miyazawa K, Naito M, Toyotake
J, Tauchi T, Itoh M, Yuo A, Hayashi Y, Georgescu MM, Kondo Y, et
al: Vitamin K2 induces autophagy and apoptosis simultaneously in
leukemia cells. Autophagy. 4:629–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG and
Zhu YP: Resveratrol induces apoptosis and autophagy in T-cell acute
lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating
p38-MAPK. Biomed Environ Sci. 26:902–911. 2013.PubMed/NCBI
|
|
8
|
Bao T, Smith BD and Karp JE: New agents in
the treatment of acute myeloid leukemia: A snapshot of signal
transduction modulation. Clin Adv Hematol Oncol. 3(287–296):
3022005.
|
|
9
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: Effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martelli AM, Evangelisti C, Chiarini F,
Grimaldi C, Manzoli L and McCubrey JA: Targeting the PI3K/AKT/mTOR
signaling network in acute myelogenous leukemia. Expert Opin
Investig Drugs. 18:1333–1349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikezoe T, Nishioka C, Bandobashi K, Yang
Y, Kuwayama Y, Adachi Y, Takeuchi T, Koeffler HP and Taguchi H:
Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and
rapamycin induces growth arrest of adult T-cell leukemia cells.
Leuk Res. 31:673–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steelman LS, Abrams SL, Whelan J, Bertrand
FE, Ludwig DE, Basecke J, Libra M, Stivala F, Milella M, et al:
Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT
pathways to leukemia. Leukemia. 22:686–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banhegyi G, Baumeister P, Benedetti A,
Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, et al:
Endoplasmic reticulum stress. Ann N Y Acad Sci. 1113:58–71. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahmani M, Davis EM, Crabtree TR, Habibi
JR, Nguyen TK, Dent P and Grant S: The kinase inhibitor sorafenib
induces cell death through a process involving induction of
endoplasmic reticulum stress. Mol Cell Biol. 27:5499–5513. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pae HO, Jeong SO, Jeong GS, Kim KM, Kim
HS, Kim SA, Kim YC, Kang SD, Kim BN and Chung HT: Curcumin induces
pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60
cells. Biochem Biophys Res Commun. 353:1040–1045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haferlach T, Kohlmann A, Schnittger S,
Dugas M, Hiddemann W, Kern W and Schoch C: Global approach to the
diagnosis of leukemia using gene expression profiling. Blood.
106:1189–1198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song Q, Han CC, Xiong XP, He F, Gan W, Wei
SH, Liu HH, Li L and Xu HY: PI3K-Akt-mTOR signal inhibition affects
expression of genes related to endoplasmic reticulum stress. Genet
Mol Res. 15:150378682016. View Article : Google Scholar
|
|
19
|
Kim HS, Kim TJ and Yoo YM: Melatonin
combined with endoplasmic reticulum stress induces cell death via
the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PLoS One.
9:e926272014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gutierrez A, Sanda T, Grebliunaite R,
Carracedo A, Salmena L, Ahn Y, Dahlberg S, Neuberg D, Moreau LA,
Winter SS, et al: High frequency of PTEN, PI3K, and AKT
abnormalities in T-cell acute lymphoblastic leukemia. Blood.
114:647–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Nardo A, Kramvis I, Cho N, Sadowski A,
Meikle L, Kwiatkowski DJ and Sahin M: Tuberous sclerosis complex
activity is required to control neuronal stress responses in an
mTOR-dependent manner. J Neurosci. 29:5926–5937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen MH, Chiang KC, Cheng CT, Huang SC,
Chen YY, Chen TW, Yeh TS, Jan YY, Wang HM, Weng JJ, et al:
Antitumor activity of the combination of an HSP90 inhibitor and a
PI3K/mTOR dual inhibitor against cholangiocarcinoma. Oncotarget.
5:2372–2389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pavlidou A and Vlahos NF: Molecular
alterations of PI3K/Akt/mTOR pathway: A therapeutic target in
endometrial cancer. ScientificWorldJournal. 2014:7097362014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bjornsti MA and Houghton PJ: The TOR
pathway: A target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen XL, Fu JP, Shi J, Wan P, Cao H and
Tang ZM: CXC195 induces apoptosis and endoplastic reticulum stress
in human hepatocellular carcinoma cells by inhibiting the
PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 12:8229–8236. 2015.
View Article : Google Scholar : PubMed/NCBI
|