Introduction
At present, energy surplus has become the main
factor endangering human health (1). Nonalcoholic fatty liver (NAFLD) and
type 2 diabetes (T2D) are metabolic diseases that are closely
related to energy surplus (1).
With the increasing acceleration of economic development,
metabolism-related diseases, which are characterized by excess
energy, are increasing year by year (2). Statistical analysis showed that in
2010, the prevalence rate of diabetes in China was as high as 11.6%
(3,4). At the same time, the incidence of
NAFLD reached 20.9% in China, and the number of current NAFLD
patients is more than 200 million (2). Therefore, NAFLD and type 2 diabetes
(T2D) have become major obstacles to the health of people around
the world.
The liver is the major organ that promotes the
synthesis and degradation/oxidation of fatty acids (FAs), as well
as the metabolism of cholesterol and phospholipids. In hepatocytes,
excess dietary glucose can be converted into fat, which can be then
stored as triglycerides (TGs) in lipid droplets (5). Once the fatty acid input exceeds the
capacity of β-oxidation, the accumulated acyl-CoA is drained by
triglyceride synthesis, and the oxidation of FFAs is increased
(6). Enhanced FFA β-oxidation
leads to higher levels of free radical formation and more hydrogen
peroxide production in peroxisomes, thereby causing the production
of reactive oxygen species (ROS) (7,8).
Multiple genes are suggested to be involved in ROS
production and glucolipotoxicity (9,10).
Among these genes, nuclear factor-erythroid 2-related factor 2
(Nfe2l2/NRF2) is a basic leucine zipper transcription factor that
regulates the expression of detoxifying and antioxidant genes, such
as heme oxygenase 1 (HO-1), superoxide dismutase (SOD), and
catalase (CAT) (9,10). Under normal conditions, NRF2 is
mainly localized in the cytoplasm via interactions with Kelch-like
ECH-associated protein 1 (Keap1). In response to oxidative stress,
NRF2 translocates into the nucleus and then initiates the
expression of antioxidant enzymes through binding antioxidant
response element (ARE) (11–13).
Thus, NRF2 plays a key role in the antioxidant and cytoprotective
defense system in the liver.
Glucagon-like peptide-1 (GLP-1) receptor agonists
(GLP-1RAs) belong to a novel class of antidiabetic medications that
regulate glucose homeostasis mainly by interacting with GLP-1
receptors (14,15). Liraglutide is a synthetic GLP-1RA
that has high structural similarity to human GLP-1 (16). Recent studies have shown that
liraglutide is characterized by anti-inflammatory and antioxidant
features (17,18). However, whether liraglutide
improves glucolipotoxicity-induced liver cell apoptosis is unclear.
The primary aim of the current study was to evaluate the effects of
liraglutide on glucolipotoxicity-induced liver cell apoptosis and
the underlying mechanisms in Zucker diabetic fatty (ZDF) rats.
Materials and methods
Animals and treatments
Male ZDF rats and Zucker lean (ZL) littermates were
purchased at 5 weeks of age from Vital River Laboratory Animal
Technology Co. Ltd. (Beijing, China). The animals were housed under
controlled temperature (21±2°C), relative humidity (50±10%) and
artificial light (12 h light/dark cycle, lights on at 7 A.M.)
conditions. Littermates from the same mother or a foster mother
were housed in one large cage with ad libitum access to
distilled water and a standard rat diet for ZL rats or a high-fat
diet (Purina5008; LabDiet, St. Louis, MO, USA) for ZDF rats except
on the days when the rats were fasted for 6 h and a blood glucose
level test was performed. The experimental procedure began when the
rats were 8 weeks old. The ZDF rats were further randomly divided
into subgroups, including a liraglutide (Novo Nordisk A/S,
Bagsvaerd, Denmark) group and a saline group (n=8 for each group).
Each rat was given liraglutide (150 ml/kg body weight) or saline
for 6 weeks. The body weight and fasting blood glucose level were
measured every week.
All rat procedures were approved by the Animal
Ethics Committee at the MOH Key Laboratory of Geriatrics, Beijing
Hospital (BJHMOH-2015-1002).
Biochemical Analysis
The serum biochemical profiles, such as aspartate
aminotransferase (AST) and alanine aminotransferase (ALT), were
evaluated with a Biochem-Immuno Autoanalyzer (TBA-40FR; Toshiba,
Tokyo, Japan).
TUNEL staining
Nuclear fragmentation was evaluated using TUNEL
staining with an apoptosis detection kit (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol. The
total number of apoptotic cells were counted in randomly acquired
10 nonoverlapping high-magnification imaging fields (×40) in each
section and an average of apoptotic cell proportion was
calculated.
Western blot analysis
Liver tissue samples were extracted using RIPA
buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). A bicinchoninic protein assay kit (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to determine
the protein concentration. Equal amounts (20 µg each) of proteins
were separated by 10% SDS-PAGE, transferred to PVDF membranes
(Merck KGaA, Darmstadt, Germany), blocked with 8% nonfat dry milk,
and then incubated with specific primary antibodies at 4°C
overnight. Antibodies against NRF2 (ab137550; Abcam, Cambridge,
UK), NAD(P)H quinone dehydrogenase 1 (NQO1) (ab28947; Abcam,
Cambridge, UK), heme oxygenase-1 (HO-1) (ab13248; Abcam), cleaved
caspase 3 (cat. no. 9664; Cell Signaling Technology, Inc., Danvers,
MA, USA), and β-actin (cat. no. 3700; Cell Signaling Technology,
Inc.) were used. Nonspecific binding was blocked using 8% (w/v)
milk in Tris-buffered saline with 1% Tween-20 (TBST; Beijing
Solarbio Science & Technology Co., Ltd.) for 2 h at room
temperature. Following three washes with TBST (5 min/wash), the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit and anti-mouse IgG or
HRP-conjugated mouse anti-goat IgG (all 1:5,000; Zhongshan Gold
Bridge Biological Technology Co., Beijing, China) for 2 h at room
temperature and then washed. β-actin was used as the internal
control. Signals were detected with enhanced chemiluminescence
according to the manufacturer's instructions (EMD Millipore,
Billerica, MA, USA). ImageJ 2.0 software (National Institutes of
Health, Bethesda, MD, USA) was used for density analysis.
Glycogen and triglyceride
measurement
The content of tissue glycogen and TGs was measured
using a Glycogen Assay kit (ab65620; Abcam) and Triglyceride Assay
Quantification kit (ab65336; Abcam).
Hematoxylin and eosin (H&E)
staining
Frozen sections of liver specimens were fixed in
paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.) for H&E staining. In brief, liver tissues were fixed in
4% paraformaldehyde buffer for 1 h at 37°C. Then, the tissues were
embedded in optimal cutting temperature (OCT) solution (Sakura
Finetek, Tokyo, Japan) on dry ice and cut into 5 µm
sections. Then, the slides were first incubated with hematoxylin
(Beijing Solarbio Science & Technology Co., Ltd.) for 5 min and
then washed with 1% ethanol hydrochloride for 3 sec. After washing
with water, the slides were stained with eosin (Beijing Solarbio
Science & Technology Co., Ltd.) for 3 min and dehydrated with
an alcohol gradient. The vacuoles were considered the lipid
droplets (19) and examined under
a light microscope (Olympus BH-2; Olympus Corporation, Tokyo,
Japan) in a blinded manner by a pathologist.
Periodic acid schiff (PAS)
staining
The sections were then stained with PAS reagent
(Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 2 h and then washed with 1% ethanol hydrochloride
for 3 sec. After washing with water, the slides were stained with
eosin (Beijing Solarbio Science & Technology Co., Ltd.) for 3
min and dehydrated with an alcohol gradient. Images were evaluated
using light microscopy (Olympus BH-2; Olympus Corporation).
Immunohistochemistry (IHC)
Liver tissues were fixed with 15% formalin (pH 7.4),
embedded in paraffin, cut into 2 µm sections and mounted on slides.
IHC staining for cleaved caspase 3 (c-caspase 3) was performed
using Histofine Simple Stain MAX-PO MULTI (Nichirei Biosciences
Inc., Tokyo, Japan). After deparaffinization with xylene, sections
were incubated with 0.3% hydrogen peroxide for 15 min to block
endogenous peroxidases prior to c-caspase 3 evaluation. For antigen
retrieval, sections were incubated for 30 min in 0.01 mol/l citrate
buffer (pH 6.0) at 100°C. Proteinase K (P9460, Beijing Solarbio
Science & Technology Co., Ltd.) was used for antigen retrieval
with incubation for 10 min. After blocking with 10% goat serum,
sections were incubated overnight at 4°C with primary antibody
against c-caspase3 at 1:200 dilution (cat. no. 9664, Cell Signaling
Technology, Inc.). After washing sections and incubating them with
secondary antibody for 1 h at room temperature, DAB substrate
(ZLI-9019; Zhongshan Golden Bridge Biological Technology Co.,
Beijing, China) was used to visualize the IHC staining. Images were
evaluated using light microscopy (Olympus BH-2; Olympus
Corporation) in a blinded manner by two pathologists.
Determination of oxidative stress
Blood was collected from the rat hearts in
anticoagulation tubes containing EDTA. The liver tissues were
homogenized. Then, the blood and homogenates were centrifuged at
1,500 × g for 20 min. The supernatants were collected and used for
the determination of antioxidant enzymes using a SOD kit (batch
no.: 20130410; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China); a malondialdehyde (MDA) kit (batch no.: 20130409; Nanjing
Jiancheng Bioengineering Institute); and a glutathione peroxidase
(GSH-PX) kit (batch no.: 201304010; Nanjing Jiancheng
Bioengineering Institute) according to the instructions.
Statistical analysis
The data are presented as the mean ± standard error.
Analysis was performed with GraphPad v7 software (GraphPad, Inc.,
La Jolla, CA, USA). Two-tailed unpaired Student's t-tests were used
for comparisons between two groups. Analysis of variance was
performed for multiple comparison tests followed by Tukey's post
hoc test was used for comparisons of two or more groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Liraglutide improves metabolic
disorders in ZDF rats
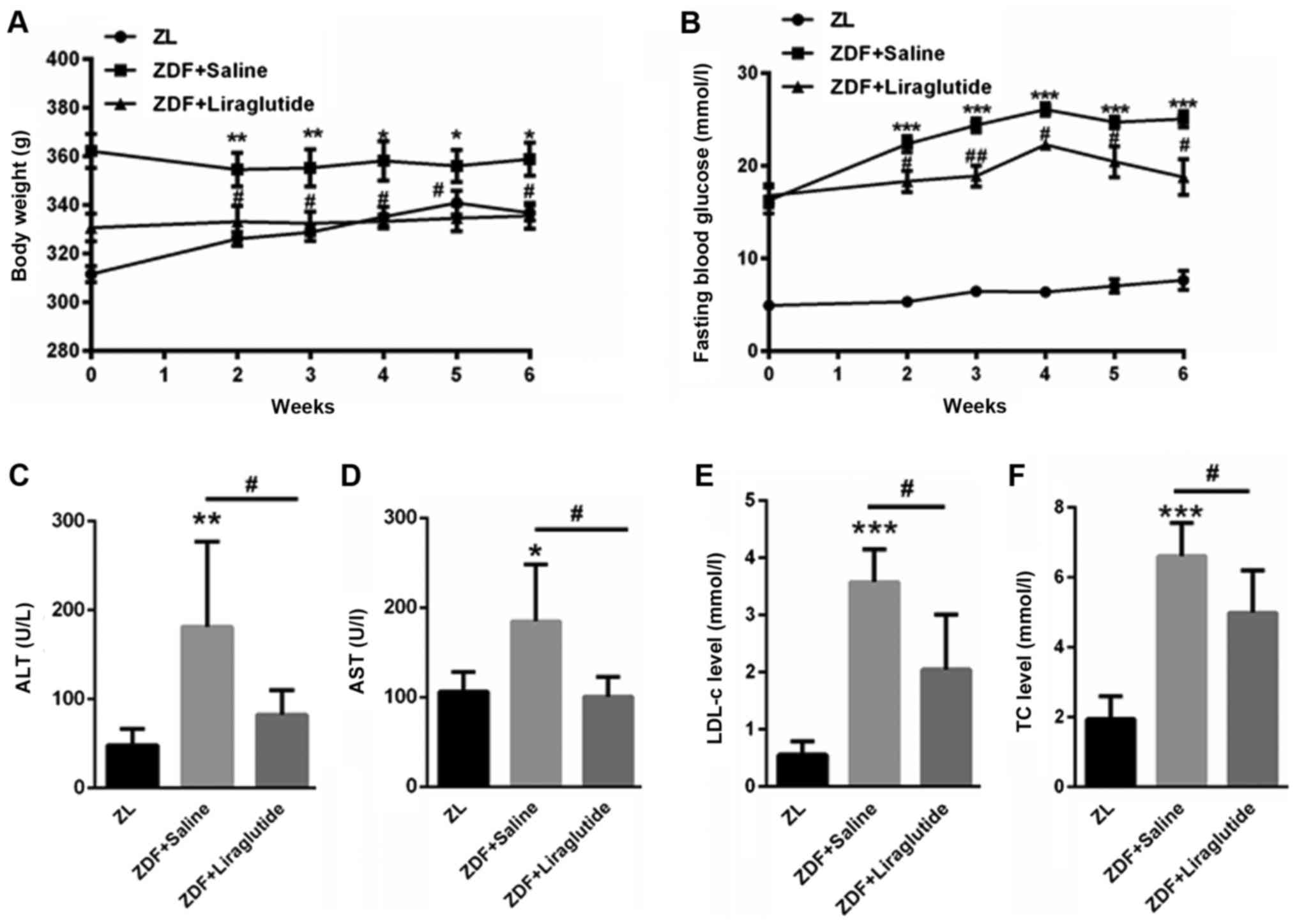
First, we evaluated the body weight of ZL rats and
ZDF rats. Compared with ZL rats (311.5±9.41, 326±8.05, 328.71±9.97,
335.1±11.38, 340.86±13.76, 336.71±8.75 g), ZDF rats had
significantly higher body weight (362.7±17.03 g (P<0.01),
354.4±19.79 g (P<0.01), 355.3±21.62 g (P<0.01), 358.14±21.39
g (P<0.05), 356±18.94 g (P<0.05), 358.88±19.26 g (P<0.05),
at 0, 2, 3, 4, 5, 6 weeks, respectively (Fig. 1A). After liraglutide treatment, the
body weight of ZDF rats decreased to 357.33±13.31 g, 333.12±18.08 g
(P<0.05), 332.2±12.57 g (P<0.05), 333.2±6.22 g (P<0.05),
334.5±13.21 g (P<0.05), 335.5±13.13 g (P<0.05) at each time
point, respectively (Fig. 1A).
Additionally, the fasting blood glucose level was much higher in
ZDF rats (16.28±4.0 mmol/l (P<0.001), 22.36±2.44 mmol/l
(P<0.001), 24.38±1.55 mmol/l (P<0.001), 26.08±1.67 mmol/l
(P<0.001), 24.26±1.49 mmol/l (P<0.001), 24.71±2.19 mmol/l
(P<0.001)) than in ZL rats (4.93±0.67, 5.35±0.24, 6.45±0.97,
6.38±1.11, 7.05±2.09, 7.65±2.90 mmol/l) at 0, 2, 3, 4, 5, 6 weeks,
respectively (Fig. 1B). However,
liraglutide treatment significantly decreased the fasting blood
glucose in ZDF rats (16.9±1.71 mmol/l, 17.91±3.25 mmol/l
(P<0.05), 19.56±2.34 mmol/l (P<0.01), 22.8±1.14 mmol/l
(P<0.05), 21.58±3.18 mmol/l (P<0.05), 19.67±4.20 mmol/l
(P<0.05)) at 0, 2, 3, 4, 5, 6 weeks, respectively (Fig. 1B). The serum levels of ALT
(181.3±95.8 U/l) and AST (178.4±56.7 U/l) were significantly higher
in ZDF rats than in ZL rats (ALT: 47.9±18.6 U/l (P<0.01); AST:
107.9±20.8 U/l (P<0.05)), but the levels decreased after
liraglutide treatment for six weeks (ALT: 86.5±28.5 U/l
(P<0.05); AST: 100.5±22.0 U/l (P<0.05)) (Fig. 1C and D). Furthermore, the serum
LDL-c (3.57±0.57 mmol/l (P<0.001)) and TC levels (6.3±1.1 mmol/l
(P<0.001)) were significantly higher in ZDF rats than in the
control (LDL-c: 0.55±0.24 mmol/l; TC: 1.94±0.66 mmol/l), and
liraglutide decreased the serum LDL-c (2.1±0.99 mmol/l (P<0.05))
and TC levels (4.74±1.24 mmol/l (P<0.05)) in ZDF rats (Fig. 1E and F). These data indicated that
liraglutide treatment improved metabolic disorders in ZDF rats.
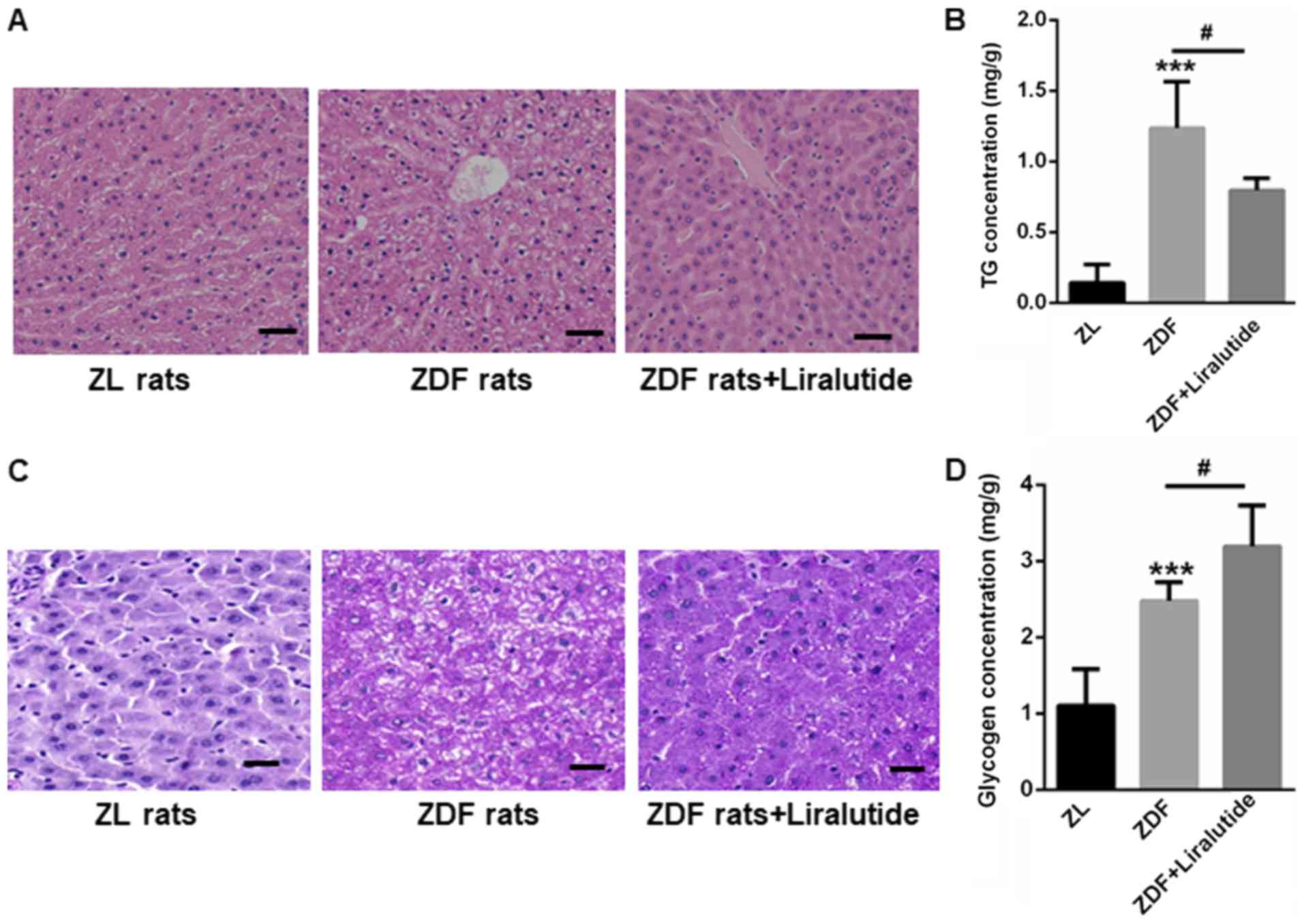
Liraglutide decreases hepatic lipid
and glycogen accumulation
H&E staining showed larger vacuoles in the
livers of ZDF rats than in those of ZL rats, indicating the
accumulation of lipid droplets (Fig.
2A). In contrast, liraglutide treatment reduced the appearance
of large vacuoles in the livers of ZDF rats (Fig. 2A). The hepatic lipid contents were
also higher in the livers of ZDF rats (1.24±0.33 mg/g vs. 0.14±0.13
mg/g, P<0.001), but this increase was significantly reduced
after liraglutide treatment for 6 weeks (0.80±0.09 mg/g, P<0.05)
(Fig. 2B). Interestingly, both PAS
staining and glycogen quantification indicated the accumulation of
glycogen in the livers of ZDF rats (2.48±0.25 mg/g) than those of
ZL rats (1.10±0.48 mg/g) (P<0.001) (Fig. 2C and D). We proposed that although
glycogen synthesis was increased in ZDF rats, the level of glycogen
synthesis was still much less than that needed to handle the input
from blood glucose, thereby leading to a sustained high fasting
blood glucose level. Not surprisingly, liraglutide therapy further
led to an increase in the glycogen content of the livers of ZDF
rats (3.19±0.54 mg/g, P<0.05) (Fig.
2C and D), indicating the protective role of liraglutide in the
livers of diabetic rats.
Liraglutide reduces liver cell
apoptosis in ZDF rats
Glucolipotoxicity-induced liver cell apoptosis has
been widely reported (20,21). Hence, we examined liver cell
apoptosis in ZDF rats in the presence or absence of liraglutide. As
shown in Fig. 3A, more apoptotic
cells were found in the livers of ZDF rats than in those of ZL
rats. However, after liraglutide therapy for 6 weeks, cell
apoptosis was markedly reduced, as indicated by TUNEL staining
(Fig. 3A). IHC staining also
showed increased expression of c-caspase3 in the livers of ZDF
rats, while liraglutide treatment decreased the levels of
c-caspase3 (Fig. 3B). Similarly,
Western blot showed that c-caspase3 expression was significantly
higher in the livers of ZDF rats (2.29±0.36) than in those of ZL
rats (1±0.17) (P<0.01) but that this increase could largely be
reversed by liraglutide treatment (1.43±0.20) (P<0.05; Fig. 3C).
Liraglutide increases the activity of
antioxidant enzymes
MDA, a lipid peroxidation product, was quantified in
the serum and livers of ZL and ZDF rats. Compared with ZL rats
(3.3±0.7 ZD ng/ml), ZDF rats tended to have higher MDA content
(3.8±1.2 ng/ml), but the increase was not significant (Fig. 4A). In the livers of ZDF rats,
enhancement of MDA was found (2.26±0.34 ng/mg protein vs. 1.65±0.29
ng/mg protein), but no statistical significance (Fig. 4D). After liraglutide treatment for
6 weeks, the serum and hepatic MDA contents both decreased to
1.2±0.5 ng/ml (P<0.05) and 1.92±0.12 ng/mg protein (P<0.05)
(Fig. 4A and D). Moreover, the
activity of GSH and SOD was lower in the serum (GSH: 2.2±0.1 U/ml
(P>0.05); SOD: 125.3±6.2 U/ml (P<0.05)) and livers (GSH:
84.3±4.9 ng/mg protein (P>0.05); SOD: 374.64±0.48 ng/mg protein
(P<0.05)) of ZDF rats than those in the serum (GSH: 2.4±0.1
U/ml; SOD: 137.4±5.2 U/ml) and livers (GSH: 137.7±46.1 ng/mg
protein; SOD: 433.1±15.1 ng/mg protein) of ZL rats (Fig. 4B, C, E, and F). In addition,
liraglutide treatment significantly increased the activity of these
antioxidant enzymes in both the serum (GSH: 2.5±0.1 U/ml
(P<0.05); SOD: 142.1±6.2 U/ml (P<0.05)) and livers (GSH:
98.3±5.8 ng/mg protein (P<0.05); SOD: 414.8±17.0 ng/mg protein
(P<0.05)) of ZDF rats (Fig. 4B, C,
E, and F). These data showed that liraglutide could enhance the
antioxidant capacity of ZDF rats.
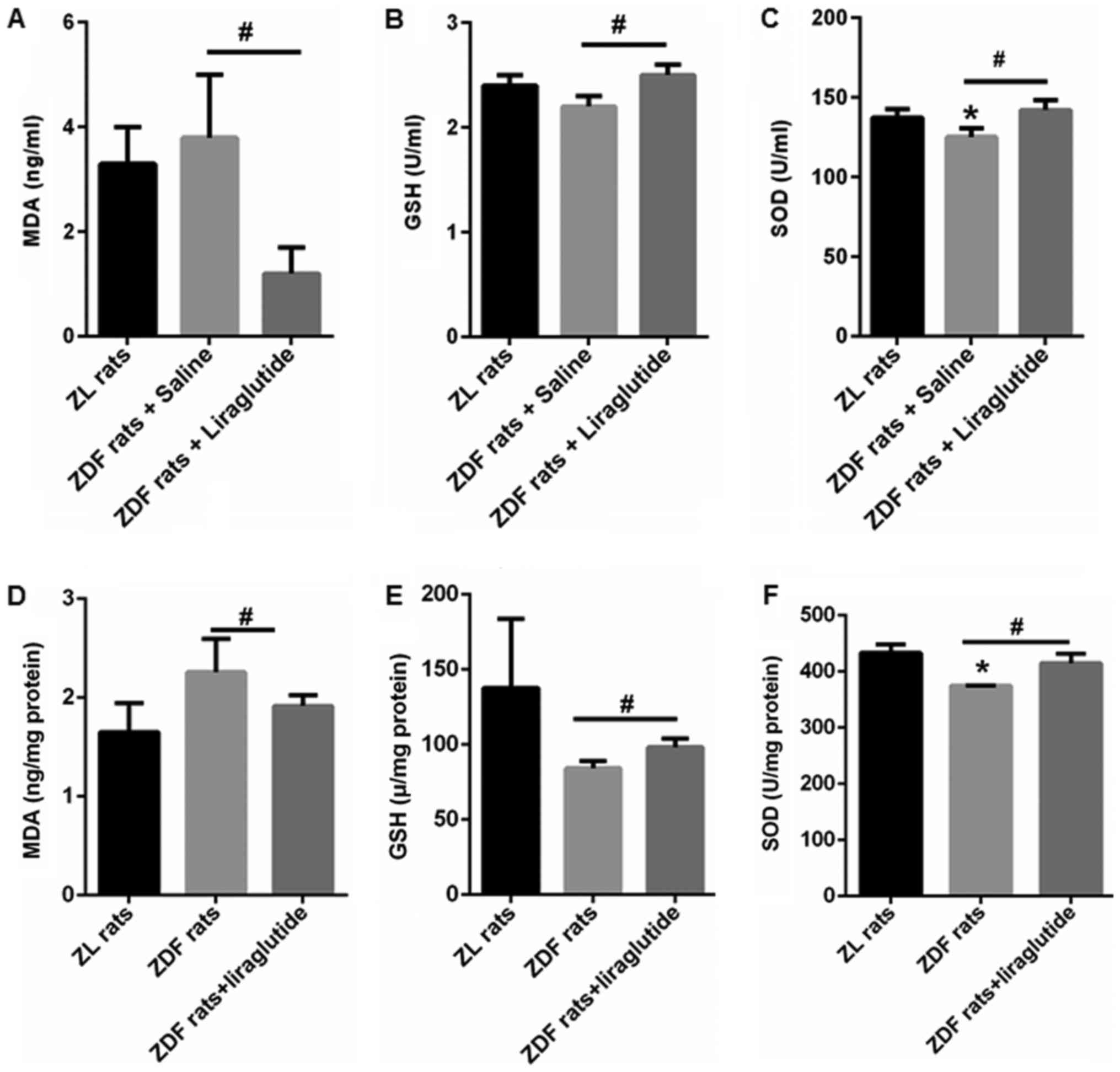 | Figure 4.Liraglutide increases the activity of
antioxidant enzymes. The (A) contents of serum MDA, (B) activity of
serum GSH, (C) activity of serum SOD, (D) contents of hepatic MDA,
(E) activity of hepatic GSH and (F) activity of hepatic SOD was
determined in the ZL and ZDF rats with or without liraglutide
treatment. Data are presented as the mean ± standard error (n=5
rats for each group). *P<0.05 vs. ZL rats;
#P<0.05, as indicated. ZDF, Zucker diabetic fatty;
ZL, Zucker lean; MDA, malondialdehyde; SOD, superoxide dismutase;
GSH, glutathione peroxidase. |
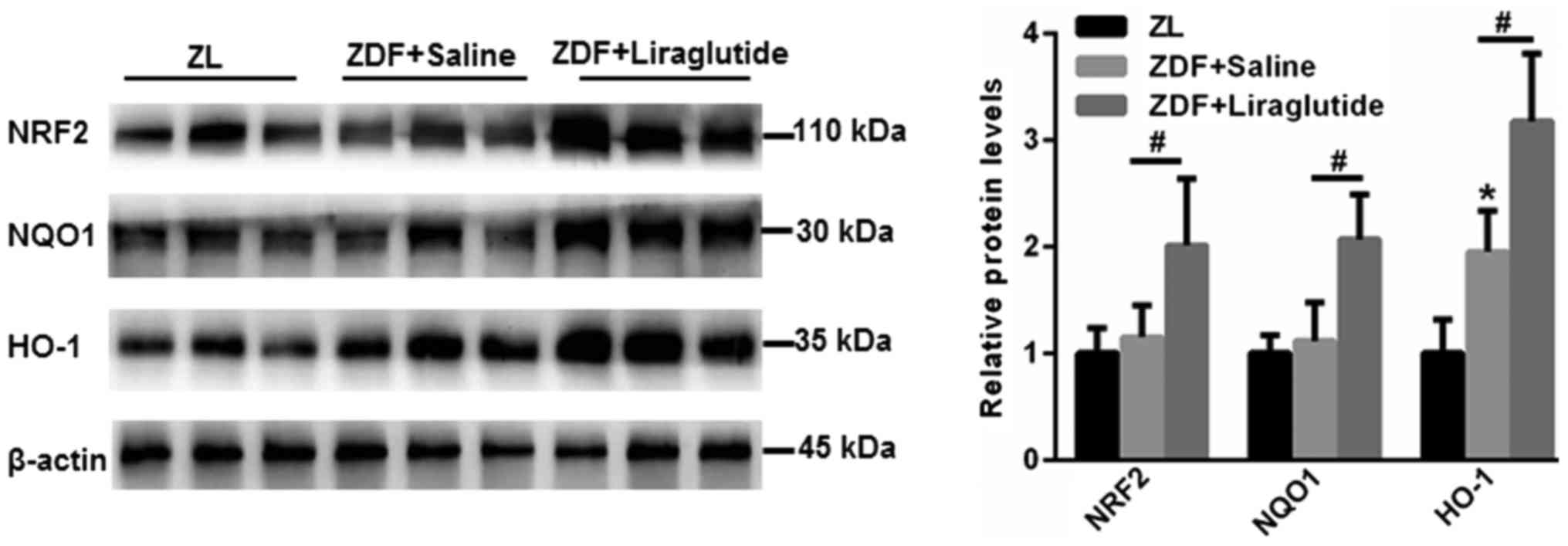
Liraglutide enhances the activation of
the NRF2 signaling pathway
NRF2/HO-1 is involved in an important antioxidant
signaling pathway that participates in the lipid peroxidation found
in hepatic metabolic disorders. Hence, the expression of NRF2 and
downstream antioxidant enzymes, including NQO1 and HO-1, was
explored. As shown in Fig. 5, no
significant changes in NRF2 and NQO1 were found in the livers of
ZDF rats compared with those of ZL rats (P>0.05). However, the
expression of HO-1 was higher in the livers of ZDF rats
(P<0.05), which may be due to a stress-induced self-regulatory
mechanism. Strikingly, the expression of NRF2 (P<0.05), NQO1
(P<0.05) and HO-1 (P<0.05) was significantly upregulated in
the livers of ZDF rats after liraglutide treatment (Fig. 5). These data indicated that the
activation of NRF2 signaling contributed to the improved
antioxidant effects of liraglutide in the livers of ZDF rats.
Discussion
Metabolic disorders have become an alarming public
health trend around the world, and they result in an increased risk
of developing cardiovascular disease, type 2 diabetes and NAFLD,
which are referred to as metabolic syndromes (22–24).
Liraglutide is a GLP-1 analog that shares 97% sequence identity
with human GLP-1 (16). In January
2010, liraglutide was approved by the FDA for the treatment of
hyperglycemia in patients with T2DM. Subsequently, an increasing
number of studies have shown the protective role of liraglutide in
the livers of diabetic and obesity patients (25,26).
High glucose and lipid accumulation can trigger ROS
generation and cell apoptosis, induce inflammation, and finally
results in the pathogenesis of liver disease (27). Here, we found that the fasting
blood glucose, as well as lipid contents, AST, ALT, LDL-c, TC and
the apoptosis index were elevated in the livers of ZDF rats than
those of ZL rats. Therefore, we investigated whether liraglutide
could protect liver cells against oxidative stress in ZDF rats. In
line with previous studies (28,29),
our data showed that liraglutide significantly decreased the body
weight, hyperglycemia and hyperlipidemia in ZDF rats compared with
those in ZL rats. Furthermore, reduced liver cell apoptosis was
observed in ZDF rats after liraglutide therapy for 6 weeks. These
data validated the beneficial results of liraglutide in diabetic
and obese ZDF rats.
The liver is characterized by high metabolic
activity, and it is the major organ responsible for the
biotransformation and subsequent detoxification of xenobiotics
(30). Hence, the liver is at high
risk of increased ROS and electrophile production, especially
during the progression of diabetes, NAFLD, and other chronic liver
diseases (5,30,31).
In hepatocytes, multiple antioxidant enzymes are dedicated to
eliminating the endogenous and exogenous oxidants that result from
lipid peroxidation (32). Among
them, many enzymes are transcribed from genes, including
antioxidant response elements (AREs) in their promoter regions
(33). NRF2 is well known to
induce the expression of ARE-containing genes encoding antioxidant
enzymes in response to cellular stresses, including ROS (34). Compared with wild-type mice,
NRF2−/− mice are more susceptible to chemical-induced
oxidative/electrophilic stress in the liver (35,36).
Additionally, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced
oxidative damage can be largely improved by overexpression of NRF2
in mice (37). Furthermore,
knockout of NRF2 in hepatocytes leads to increased liver cell
injury under the conditions of excessive iron accumulation
(38). Hence, NRF2 activity plays
a key role in the protection against oxidative stress-induced liver
cell injury.
Liraglutide was shown to alleviate oxidative stress
in β-islet cells or in the brains of diabetic mice subjected to
middle cerebral artery occlusion (39,40).
However, whether liraglutide protects livers from lipid
peroxidation via the NRF2 signaling pathway is unclear. For the
first time, we show novel data indicating that the expression of
the antioxidant transcription factor NRF2, as well as the
transcription of downstream target genes, including NQO1 and HO-1,
was increased by liraglutide treatment. Additionally, both serum
and hepatic GSH and SOD levels were enhanced after liraglutide
therapy. This change ultimately leads to a lower state of oxidative
stress in liver cells, thereby improving oxidative stress-induced
liver cell injury. Multiple studies have suggested that ROS
markedly enhance the transformation of fatty liver into
non-alcoholic steatohepatitis (NASH) (41,42).
Excessive ROS generated by the overconsumption of lipid and glucose
results in burden on quenching by hepatic antioxidants (42). NRF2 plays a key role in regulating
multiple antioxidant-related genes that are associated in the
cellular defense against oxidative stress (43). It is well suggested that enhanced
antioxidant activity improves liver cell apoptosis via NRF2
signaling (44,45). For instance, safflower yellow B is
demonstrated to inhibit HepG2 cell apoptosis induced by oxidative
stress through activating the AKT/Nrf2 pathway (44). In the present study, we showed
liraglutide protects liver cells from hepatic
glucolipotoxicity-induced oxidative stress and liver cell apoptosis
in ZDF rats through NRF2-dependent mechanism.
Even though, whether the effects of liraglutide on
hepatocytes apoptosis are direct effects or not via NRF2 signaling
remains to be further elucidated. GLP-1 is demonstrated to be
beneficial against cell apoptosis in various circumstances.
However, the presence of GLP-1 receptor in hepatocytes is
controversial. Recently, it is indicated that the metabolic
products of GLP-1 retain important antioxidant and anti-apoptotic
activities that are GLP-1 R independent (46). For instance, GLP-1 has been shown
to protect endothelial cells from advanced glycation end products
(AGEs)-induced apoptosis by inhibiting the release of mitochondrion
cytochrome c (47). And GLP-1
analogue, liraglutide, may prevent high glucose induced
mitochondrial fragmentation and apoptosis in human endothelial
cells via inducing mitochondrial fusion processes (48). Additionally, liraglutide is also
suggested to protect cardiomyocyte from oxidative stress and
apoptosis via activating AMPK-Sirt1 pathway (49). In neural tissues, the beneficial
effects of GLP-1 on cell apoptosis is indicated to be due to the
activation of the Akt pathway (50). Based on these findings, we propose
that the anti-apoptotic effects of GLP-1 may be NRF-2 dependent and
independent of GLP-1R in liver tissues.
In summary, liraglutide can enhance the antioxidant
activity of liver cells by activating the NRF2 signaling pathway,
which then results in a decrease in liver cell apoptosis induced by
glucolipotoxicity in ZDF rats.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants (grant
nos. 81570789 and 81700765) from National Natural Science
Foundation of China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG and CL performed the experiments and analyzed the
data. CY and BL performed the animal experiments. JW, YL and PY
performed part of the animal experiments. GH and JL designed the
experiments, analyzed the data and gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee at the MOH Key Laboratory of Geriatrics, Beijing Hospital
(no. BJHMOH-2015-1002).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castro AV, Kolka CM, Kim SP and Bergman
RN: Obesity, insulin resistance and comorbidities? Mechanisms of
association. Arq Bras Endocrinol Metabol. 58:600–609. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Xue J, Chen P, Chen L, Yan S and Liu
L: Prevalence of nonalcoholic fatty liver disease in mainland of
China: A meta-analysis of published studies. J Gastroenterol
Hepatol. 29:42–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serviddio G, Bellanti F and Vendemiale G:
Free radical biology for medicine: Learning from nonalcoholic fatty
liver disease. Free Radic Biol Med. 65:952–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stein Y and Shapiro B: Uptake and
metabolism of triglycerides by the rat liver. J Lipid Res.
1:326–331. 1960.PubMed/NCBI
|
|
7
|
Mota M, Banini BA, Cazanave SC and Sanyal
AJ: Molecular mechanisms of lipotoxicity and glucotoxicity in
nonalcoholic fatty liver disease. Metabolism. 65:1049–1061. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zámbó V, Simon-Szabó L, Szelényi P,
Kereszturi E, Bánhegyi G and Csala M: Lipotoxicity in the liver.
World J Hepatol. 5:550–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hine CM and Mitchell JR: NRF2 and the
phase II response in acute stress resistance induced by dietary
restriction. J Clin Exp Pathol. S4:pii: 7329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schaedler S, Krause J, Himmelsbach K,
Carvajal-Yepes M, Lieder F, Klingel K, Nassal M, Weiss TS, Werner S
and Hildt E: Hepatitis B virus induces expression of antioxidant
response element-regulated genes by activation of Nrf2. J Biol
Chem. 285:41074–41086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holland R and Fishbein JC: Chemistry of
the cysteine sensors in Kelch-like ECH-associated protein 1.
Antioxid Redox Signal. 13:1749–1761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Volonte D, Liu Z, Musille PM, Stoppani E,
Wakabayashi N, Di YP, Lisanti MP, Kensler TW and Galbiati F:
Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by
caveolin-1 promotes stress-induced premature senescence. Mol Biol
Cell. 24:1852–1862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nauck MA: Incretin-based therapies for
type 2 diabetes mellitus: Properties, functions, and clinical
implications. Am J Med. 124 1 Suppl:S3–S18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kazafeos K: Incretin effect: GLP-1, GIP,
DPP4. Diabetes Res Clin Pract. 93 Suppl 1:S32–S36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zueger PM, Schultz NM and Lee TA: Cost
effectiveness of liraglutide in type II diabetes: A systematic
review. Pharmacoeconomics. 32:1079–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Mesquita FC, Guixé-Muntet S,
Fernández-Iglesias A, Maeso-Díaz R, Vila S, Hide D, Ortega-Ribera
M, Rosa JL, García-Pagán JC, Bosch J, et al: Liraglutide improves
liver microvascular dysfunction in cirrhosis: Evidence from
translational studies. Sci Rep. 7:32552017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Y, Li Z, Liang S, Li Y, Yang L, Lu
M, Gu HF and Xia N: Hepatic adenylate cyclase 3 is upregulated by
Liraglutide and subsequently plays a protective role in insulin
resistance and obesity. Nutr Diabetes. 6:e1912016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Design and validation of a histological
scoring system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kowluru A and Matti A: Hyperactivation of
protein phosphatase 2A in models of glucolipotoxicity and diabetes:
Potential mechanisms and functional consequences. Biochem
Pharmacol. 84:591–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Purwana I, Liu JJ, Portha B and Buteau J:
HSF1 acetylation decreases its transcriptional activity and
enhances glucolipotoxicity-induced apoptosis in rat and human beta
cells. Diabetologia. 60:1432–1441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ford ES, Giles WH and Dietz WH: Prevalence
of the metabolic syndrome among US adults: Findings from the third
National Health and Nutrition Examination Survey. JAMA.
287:356–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boudreau DM, Malone DC, Raebel MA, Fishman
PA, Nichols GA, Feldstein AC, Boscoe AN, Ben-Joseph RH, Magid DJ
and Okamoto LJ: Health care utilization and costs by metabolic
syndrome risk factors. Metab Syndr Relat Disord. 7:305–314. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alberti KG, Zimmet P and Shaw J: Metabolic
syndrome-a new world-wide definition. A consensus statement from
the International diabetes federation. Diabet Med. 23:469–480.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polyzos SA, Kountouras J and Mantzoros CS:
Adipose tissue, obesity and non-alcoholic fatty liver disease.
Minerva Endocrinol. 42:92–108. 2017.PubMed/NCBI
|
|
26
|
Cuthbertson DJ, Irwin A, Gardner CJ,
Daousi C, Purewal T, Furlong N, Goenka N, Thomas EL, Adams VL,
Pushpakom SP, et al: Improved glycaemia correlates with liver fat
reduction in obese, type 2 diabetes, patients given glucagon-like
peptide-1 (GLP-1) receptor agonists. PLoS One. 7:e501172012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Lin Y, Wang S, Zhang L and Guo L:
GLP-1 inhibits high-glucose-induced oxidative injury of vascular
endothelial cells. Sci Rep. 7:80082017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brand CL, Galsgaard ED, Tornehave D, Rømer
J, Gotfredsen CF, Wassermann K, Knudsen LB, Vølund A and Sturis J:
Synergistic effect of the human GLP-1 analogue liraglutide and a
dual PPARalpha/gamma agonist on glycaemic control in Zucker
diabetic fatty rats. Diabetes Obes Metab. 11:795–803. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larsen PJ, Wulff EM, Gotfredsen CF, Brand
CL, Sturis J, Vrang N, Knudsen LB and Lykkegaard K: Combination of
the insulin sensitizer, pioglitazone, and the long-acting GLP-1
human analog, liraglutide, exerts potent synergistic
glucose-lowering efficacy in severely diabetic ZDF rats. Diabetes
Obes Metab. 10:301–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng J, Deng S, Wang Y, Li P, Tang L and
Pang Y: Specific inhibition of Acyl-CoA oxidase-1 by an acetylenic
acid improves hepatic lipid and reactive oxygen species (ROS)
metabolism in rats fed a high fat diet. J Biol Chem. 292:3800–3809.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levonen AL, Hill BG, Kansanen E, Zhang J
and Darley-Usmar VM: Redox regulation of antioxidants, autophagy,
and the response to stress: Implications for electrophile
therapeutics. Free Radic Biol Med. 71:196–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rushmore TH, Morton MR and Pickett CB: The
antioxidant responsive element. Activation by oxidative stress and
identification of the DNA consensus sequence required for
functional activity. J Biol Chem. 266:11632–11639. 1991.PubMed/NCBI
|
|
33
|
Lee JM, Moehlenkamp JD, Hanson JM and
Johnson JA: Nrf2-dependent activation of the antioxidant responsive
element by tert-butylhydroquinone is independent of oxidative
stress in IMR-32 human neuroblastoma cells. Biochem Biophys Res
Commun. 280:286–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JM, Calkins MJ, Chan K, Kan YW and
Johnson JA: Identification of the NF-E2-related factor-2-dependent
genes conferring protection against oxidative stress in primary
cortical astrocytes using oligonucleotide microarray analysis. J
Biol Chem. 278:12029–12038. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klaassen CD and Reisman SA: Nrf2 the
rescue: Effects of the antioxidative/electrophilic response on the
liver. Toxicol Appl Pharmacol. 244:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Wu KC, Lu YF, Ekuase E and Klaassen
CD: Nrf2 protection against liver injury produced by various
hepatotoxicants. Oxid Med Cell Longev. 2013:3058612013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu H, Cui W and Klaassen CD: Nrf2 protects
against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced
oxidative injury and steatohepatitis. Toxicol Appl Pharmacol.
256:122–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Silva-Gomes S, Santos AG, Caldas C, Silva
CM, Neves JV, Lopes J, Carneiro F, Rodrigues PN and Duarte TL:
Transcription factor NRF2 protects mice against dietary
iron-induced liver injury by preventing hepatocytic cell death. J
Hepatol. 60:354–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li PC, Liu LF, Jou MJ and Wang HK: The
GLP-1 receptor agonists exendin-4 and liraglutide alleviate
oxidative stress and cognitive and micturition deficits induced by
middle cerebral artery occlusion in diabetic mice. BMC Neurosci.
17:372016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimoda M, Kanda Y, Hamamoto S, Tawaramoto
K, Hashiramoto M, Matsuki M and Kaku K: The human glucagon-like
peptide-1 analogue liraglutide preserves pancreatic beta cells via
regulation of cell kinetics and suppression of oxidative and
endoplasmic reticulum stress in a mouse model of diabetes.
Diabetologia. 54:1098–1108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He W, Xu Y, Zhang C, Lu J, Li J, Xiang D,
Yang J, Chang M and Liu D: Hepatoprotective effect of calculus
bovis sativus on nonalcoholic fatty liver disease in mice by
inhibiting oxidative stress and apoptosis of hepatocytes. Drug Des
Devel Ther. 11:3449–3460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lim JS, Mietus-Snyder M, Valente A,
Schwarz JM and Lustig RH: The role of fructose in the pathogenesis
of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol.
7:251–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Musso G, Cassader M and Gambino R:
Non-alcoholic steatohepatitis: Emerging molecular targets and
therapeutic strategies. Nat Rev Drug Discov. 15:249–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma Z, Li C, Qiao Y, Lu C, Li J, Song W,
Sun J, Zhai X, Niu J, Ren Q and Wen A: Safflower yellow B
suppresses HepG2 cell injury induced by oxidative stress through
the AKT/Nrf2 pathway. Int J Mol Med. 37:603–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vani Gokila M, Kumar KJ, Liao JW, Chien
SC, Mau JL, Chiang SS, Lin CC, Kuo YH and Wang SY: Antcin C from
antrodia cinnamomea protects liver cells against free
radical-induced oxidative stress and apoptosis in vitro and in vivo
through Nrf2-dependent mechanism. Evid Based Complement Alternat
Med. 2013:2960822013.PubMed/NCBI
|
|
46
|
Thomas MC: The potential and pitfalls of
GLP-1 receptor agonists for renal protection in type 2 diabetes.
Diabetes Metab. 43 Suppl 1:2S20–2S27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhan Y, Sun HL, Chen H, Zhang H, Sun J,
Zhang Z and Cai DH: Glucagon-like peptide-1 (GLP-1) protects
vascular endothelial cells against advanced glycation end products
(AGEs)-induced apoptosis. Med Sci Monit. 18:BR286–BR291. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schisano B, Harte AL, Lois K, Saravanan P,
Al-Daghri N, Al-Attas O, Knudsen LB, McTernan PG, Ceriello A and
Tripathi G: GLP-1 analogue, Liraglutide protects human umbilical
vein endothelial cells against high glucose induced endoplasmic
reticulum stress. Regul Pept. 174:46–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inoue T, Inoguchi T, Sonoda N, Hendarto H,
Makimura H, Sasaki S, Yokomizo H, Fujimura Y, Miura D and
Takayanagi R: GLP-1 analog liraglutide protects against cardiac
steatosis, oxidative stress and apoptosis in streptozotocin-induced
diabetic rats. Atherosclerosis. 240:250–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kimura R, Okouchi M, Fujioka H, Ichiyanagi
A, Ryuge F, Mizuno T, Imaeda K, Okayama N, Kamiya Y, Asai K and Joh
T: Glucagon-like peptide-1 (GLP-1) protects against
methylglyoxal-induced PC12 cell apoptosis through the
PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience.
162:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|