Introduction
Spondyloarthritis (SpA) includes a group of
immune-mediated inflammatory diseases with similar genetic and
clinical manifestations, including ankylosing spondylitis (AS),
psoriatic arthritis (PsA) and juvenile SpA (JSA) (1,2). In
particular, JSA is a group of chronic inflammatory diseases
associated with human leukocyte antigen B27, affecting children at
≤16 years of age. The prevalence of spondyloarthropathy is ~1.9%
worldwide (3,4). The primary clinical manifestations of
JSA include enthesitis and peripheral arthritis, although symptoms
including acute anterior uveitis, bowel inflammation, psoriasis and
cardiac disease may affect certain patients with JSA (5). An increased incidence is observed
among males compared with females (3). Patients with JSA are likely to
develop AS during their disease course (6). Therefore, research into the
mechanisms underlying JSA is necessary for development of improved
diagnostic and treatment approaches.
A previous study suggested that certain genes,
including Toll-like receptor 4, NLR family pyrin domain containing
3, C-X-C motif chemokine receptor 4 (CXCR4), dual specificity
phosphatase 6, mitogen-activated protein kinase kinase 2 and
protein tyrosine phosphatase, non-receptor type 12 are involved in
the development of JSA (2). CXCR4
was additionally hypothesized to be involved in the pathogenesis of
SpA (7). In one study, CXCR4 and
its ligand CXCL12 were demonstrated to serve a role in retaining
inflammatory cells within the joint (8). Previous studies demonstrated that
CXCR4 and CXCL12 are involved in the development of inflammatory
bowel disease (IBD) (8,9). Furthermore, overexpression of CXCR4
in patients with JSA may lead to mucosal disregulation and IBD
(2). The HOX signaling pathway and
the granule cell survival pathway are additionally associated with
the development of JSA (2).
However, there has been a limited number of genetic studies in
patients with JSA and these studies were limited by small numbers
of patients (6). At present, there
are no effective drugs or therapies available for the treatment of
patients with JSA. As a consequence, it is necessary to study the
mechanism underlying the development of JSA and identify targets
for more effective therapies using bioinformatics analysis.
The present study aimed to screen target genes and
pathways implicated in development of JSA. Differentially expressed
genes (DEGs) in patients with JSA were identified by screening the
dataset GSE58667 (2). Furthermore,
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene
Ontology (GO) term analyses were performed to verify the pathways
associated with JSA. Subsequently, protein-protein interactions
(PPI), transcription factors (TF), microRNA (miRNA) and small
molecule interaction network analyses were performed to screen for
genes and molecules associated with JSA. The results of the present
study may provide information for further investigation of the
mechanisms underlying JSA and for the development of potential
treatment methods.
Materials and methods
Microarray data
mRNA expression profile dataset GSE58667 was
obtained from the National Center of Biotechnology Information
(NCBI) Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo). The data were based on
the GPL570 (HG-U133_Plus_2) Affymetrix Human Genome U133 Plus 2.0
Array platform (Affymetrix; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). A total of 15 human whole blood samples were
collected from 11 patients with JSA and four healthy controls. In
the present study, data from the 11 whole blood samples from
patients with JSA were used as the experimental group. The
expression data obtained from four whole blood samples from healthy
controls were used as the control group.
Data preprocessing and analysis of
DEGs
Raw data were downloaded in CEL format and the
robust multiarray average method (10) in the Affy package (version 1.540)
(11) was used to pre-process the
mRNA expression data by performing background adjustment, quantile
normalization and summarization. The differentially expressed probe
in the experimental group compared with the control group was
screened using the limma (ver.3.32.3) package (12). The cutoff thresholds for
identification of differentially expressed probes were P<0.05
and |log2 fold change (FC) |>0.585, and the probes
were annotated. Ultimately, a heatmap was generated using the
pheatmap package in R (version 3.25; http://www.R-project.org/).
GO and pathway enrichment
analyses
In the present study, GO and KEGG pathway analyses
for DEGs were performed using the Database for Annotation,
Visualization and Integrated Discovery (version 6.8; david.ncifcrf.gov) (13). The number of enriched genes >2
and P<0.05 were selected as cut-off criteria.
PPI network construction and
analysis
The Search Tool for the Retrieval of Interacting
Genes (STRING; version 10.0; string-db.org)
database was used for the construction of the PPI network between
DEGs and other genes (14).
Required confidence (combined score) >0.7 was selected as the
threshold value for PPIs. The PPI networks were constructed using
Cytoscape software (version 3.3.0; www.cytoscape.org) (15) and the network was analyzed to
identify hubs according to the ranking of network connectivity.
In the PPI network, proteins with similar functions
tend to cluster together, and the node function was correlated with
the distance between one node and another node in the network.
Therefore, the bioinformatics analysis in the present study allowed
for the identification of unknown functions of proteins by studying
protein complexes or clusters of functional subnetworks in the PPI
network. The subnetworks were evaluated with the Molecular Complex
Detection (MCODE; version 1.4.2; apps.cytoscape.org/apps/mcode) (16) plugin in Cytoscape. Subsequently,
the functions of subnetworks were evaluated by GO and pathway
enrichment analyses.
Construction and analysis of a
DEG-miRNA-TF regulatory network
TFs and miRNAs associated with DEGs were predicted
based on the Encyclopedia of DNA Elements (http://amp.pharm.mssm.edu/Enrichr/#stats) and ChIP-X
Enrichment Analysis consensus TFs (17) and TargetScan microRNA library
enrichment analysis in the Enrichr database (amp.pharm.mssm.edu/Enrichr) (18). The screening threshold was set as
adjusted P<0.01. Within the predicted TFs, the differentially
expressed TFs in the present study were chosen for the downstream
analysis. Finally, the target gene network of TFs and miRNAs was
established.
Small molecule target network
analysis
The Comparative Toxicogenomics Database (CTD,
ctdbase.org) (19)
includes chemical-gene interactions associated with spondylitis.
The present study identified the chemical small molecules
associated with the DEGs and constructed the chemical-gene
interaction network.
Results
Analysis of DEGs
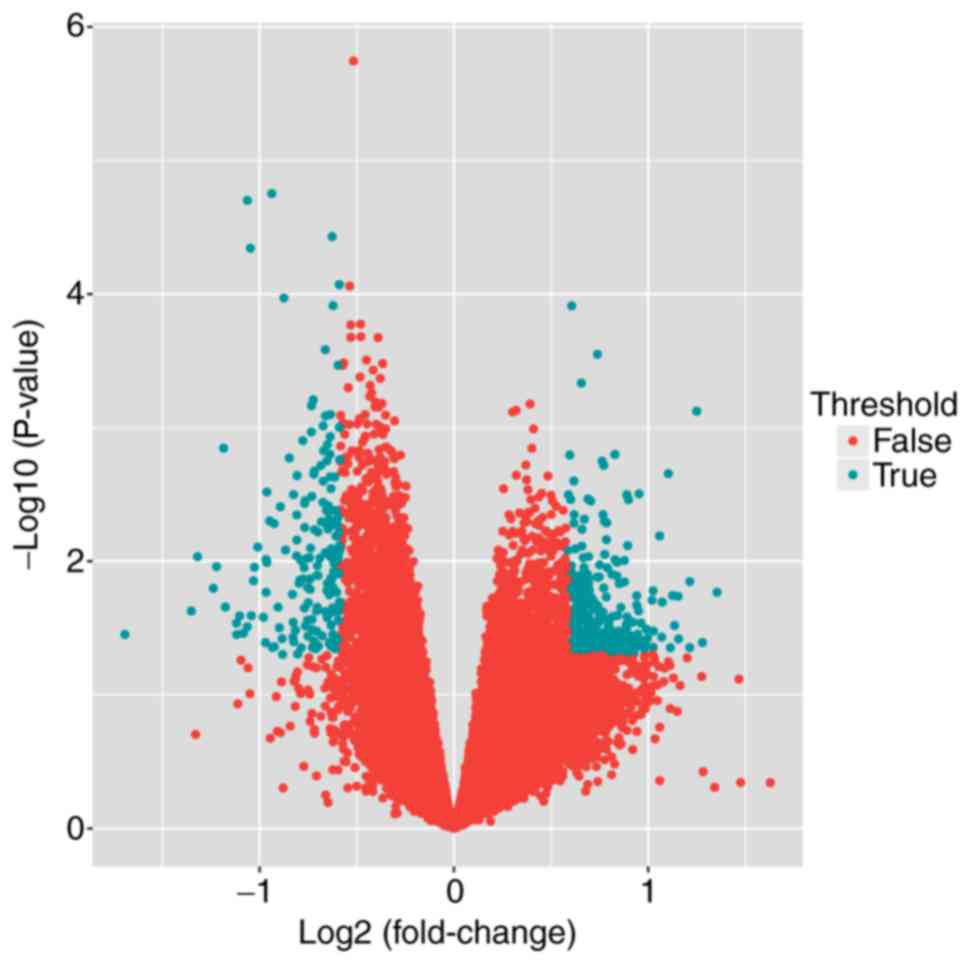
To determine DEGs between patients with JSA and
controls, the publicly available microarray dataset GSE58667 was
accessed from the GEO database and differences in expression were
determined using the limma package. A total of 326 DEGs were
identified using the threshold of P<0.05 and |logFC| >0.585,
including 196 upregulated DEGs and 130 downregulated DEGs (Fig. 1).
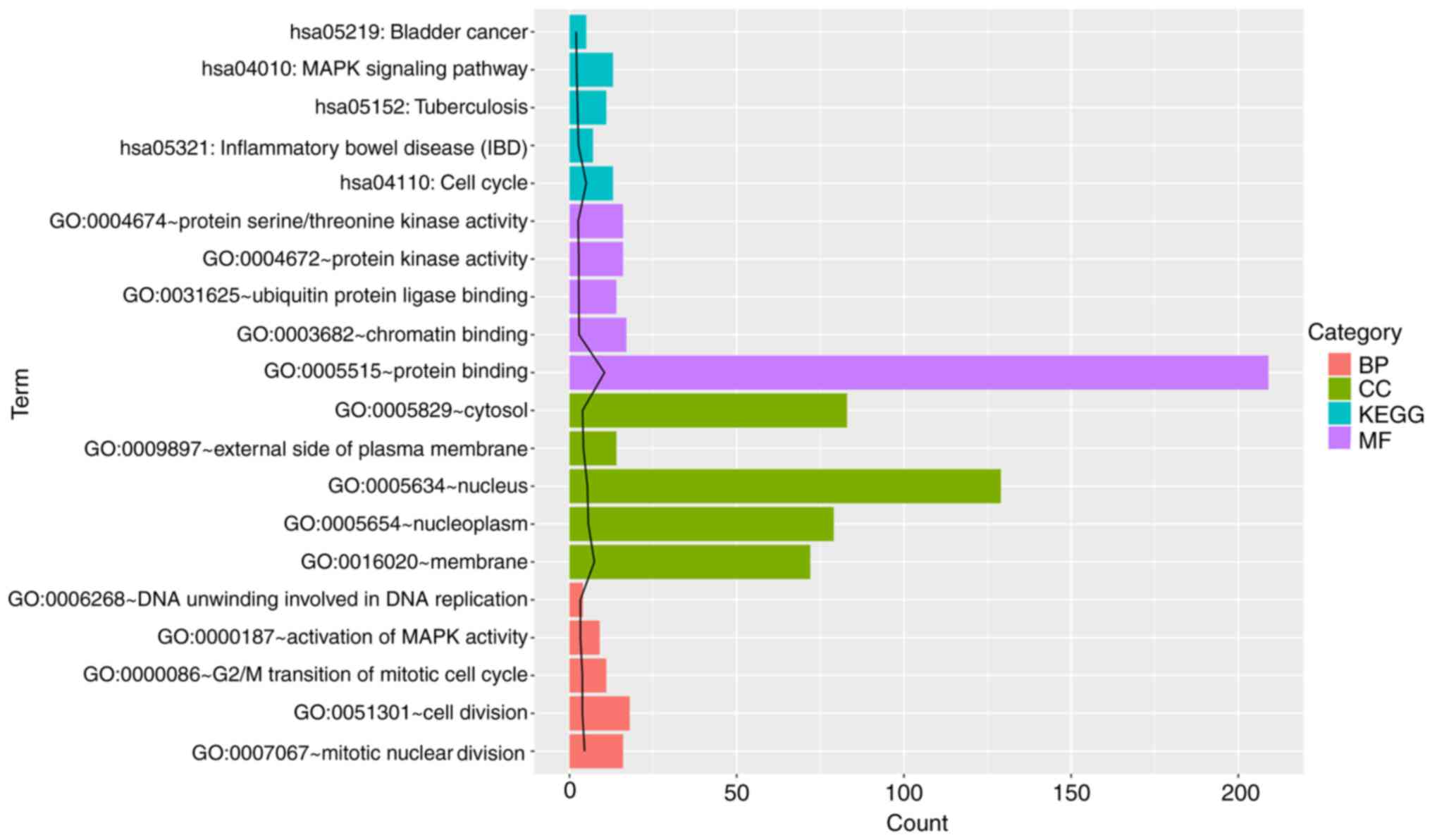
Pathway enrichment analysis
The results demonstrated that a total of 128 GO
terms or KEGG pathways were enriched. Based on the most significant
P-values, the top five GO terms and KEGG pathways were selected.
The DEGs were significantly enriched in five KEGG pathways,
including pathways associated with IBD, the cell cycle and the
mitogen-activated protein kinase (MAPK) signaling pathway (Fig. 2). GO analysis indicated that the
DEGs were significantly enriched in apoptosis-, cell cycle- and
immune response-associated terms. In particular, CXCL5 was revealed
to be enriched in functions associated with immune response as well
as pathways associated with rheumatoid arthritis.
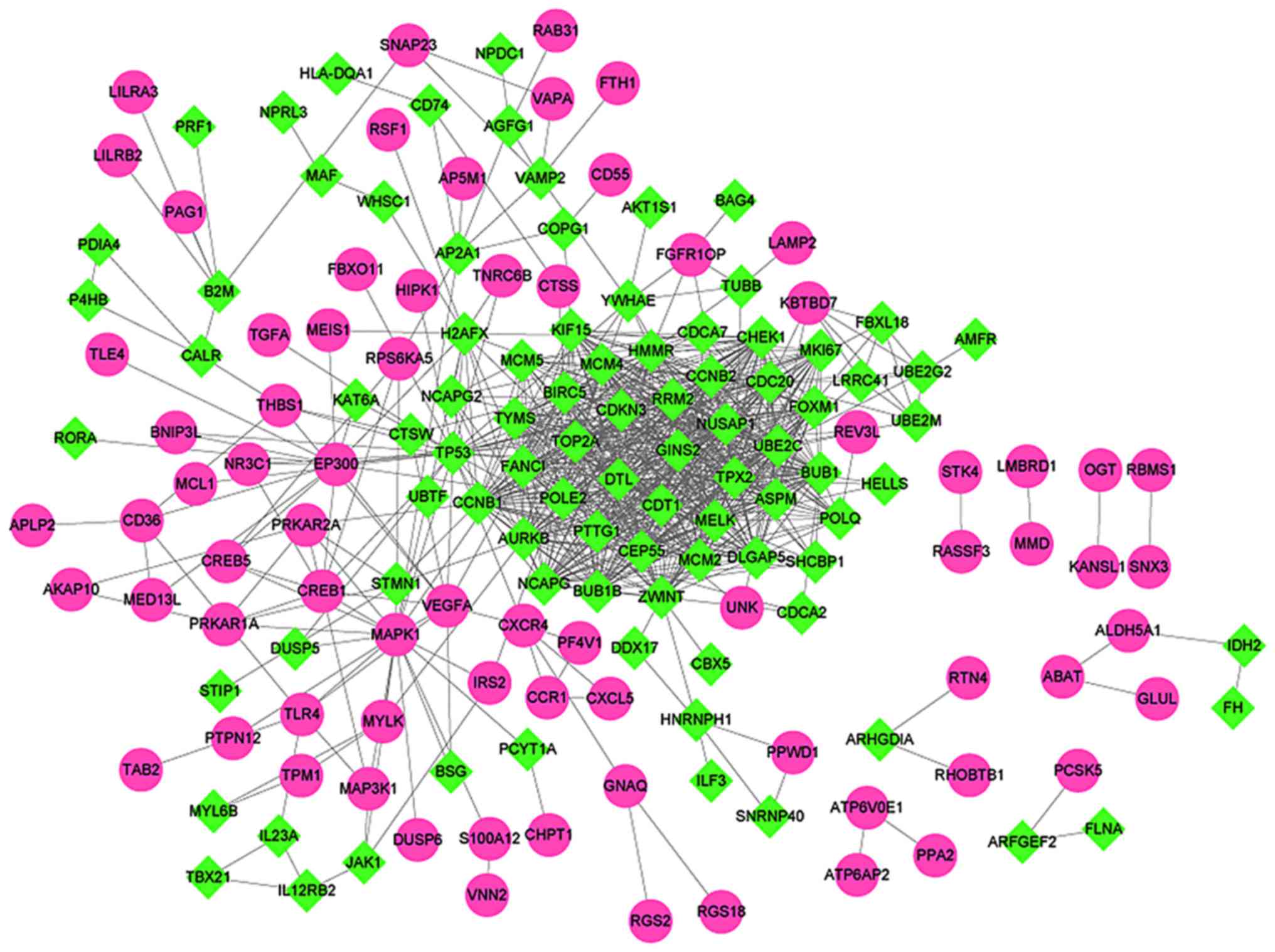
Interaction network analysis of
DEGs
STRING was used to analyze PPIs between DEGs.
Subsequently, the PPI network was constructed, which included a
total of 164 nodes and 762 interaction pairs (Fig. 3). Furthermore, nodes with highest
degrees in the network were analyzed. Table I presents the top 30 target genes
with the highest degrees, all downregulated, including BUB1 mitotic
checkpoint serine/threonine kinase (BUB1), tumor protein p53
(TP53), aurora kinase B (AURKB) and cell division cycle 20
(CDC20).
 | Table I.Top 30 nodes with the highest
degrees. |
Table I.
Top 30 nodes with the highest
degrees.
| Gene | Degree | Differential
expression |
|---|
| BUB1 | 39.0 | Downregulated |
| AURKB | 38.0 | Downregulated |
| CDC20 | 38.0 | Downregulated |
| UBE2C | 38.0 | Downregulated |
| CCNB1 | 38.0 | Downregulated |
| TPX2 | 38.0 | Downregulated |
| TOP2A | 38.0 | Downregulated |
| NCAPG | 37.0 | Downregulated |
| RRM2 | 36.0 | Downregulated |
| BIRC5 | 35.0 | Downregulated |
| MELK | 35.0 | Downregulated |
| CCNB2 | 34.0 | Downregulated |
| CHEK1 | 33.0 | Downregulated |
| HMMR | 33.0 | Downregulated |
| DTL | 33.0 | Downregulated |
| CDKN3 | 33.0 | Downregulated |
| DLGAP5 | 33.0 | Downregulated |
| BUB1B | 32.0 | Downregulated |
| TYMS | 32.0 | Downregulated |
| NUSAP1 | 32.0 | Downregulated |
| ASPM | 32.0 | Downregulated |
| GINS2 | 31.0 | Downregulated |
| ZWINT | 31.0 | Downregulated |
| CEP55 | 31.0 | Downregulated |
| MCM4 | 30.0 | Downregulated |
| FOXM1 | 30.0 | Downregulated |
| TP53 | 30.0 | Downregulated |
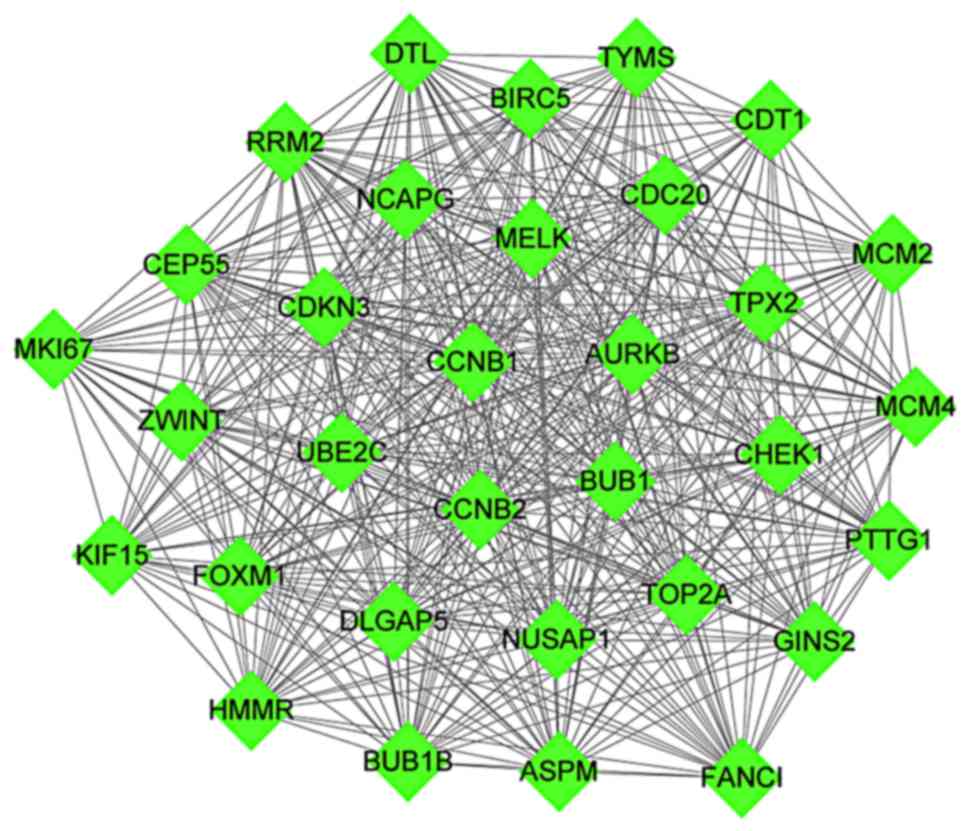
Furthermore, the highest scoring module identified
in the PPI network, which scored equal to 29.806, was acquired
through the analysis of PPI network via the MCODE plug-in. A total
of 32 nodes and 462 interaction pairs were included in this module
(Fig. 4). Enrichment analysis was
conducted for the nodes in the module. A total of 69 GO functions
and KEGG pathways were enriched (data not shown).
Construction and analysis of the
DEG-miRNA-TF regulatory network
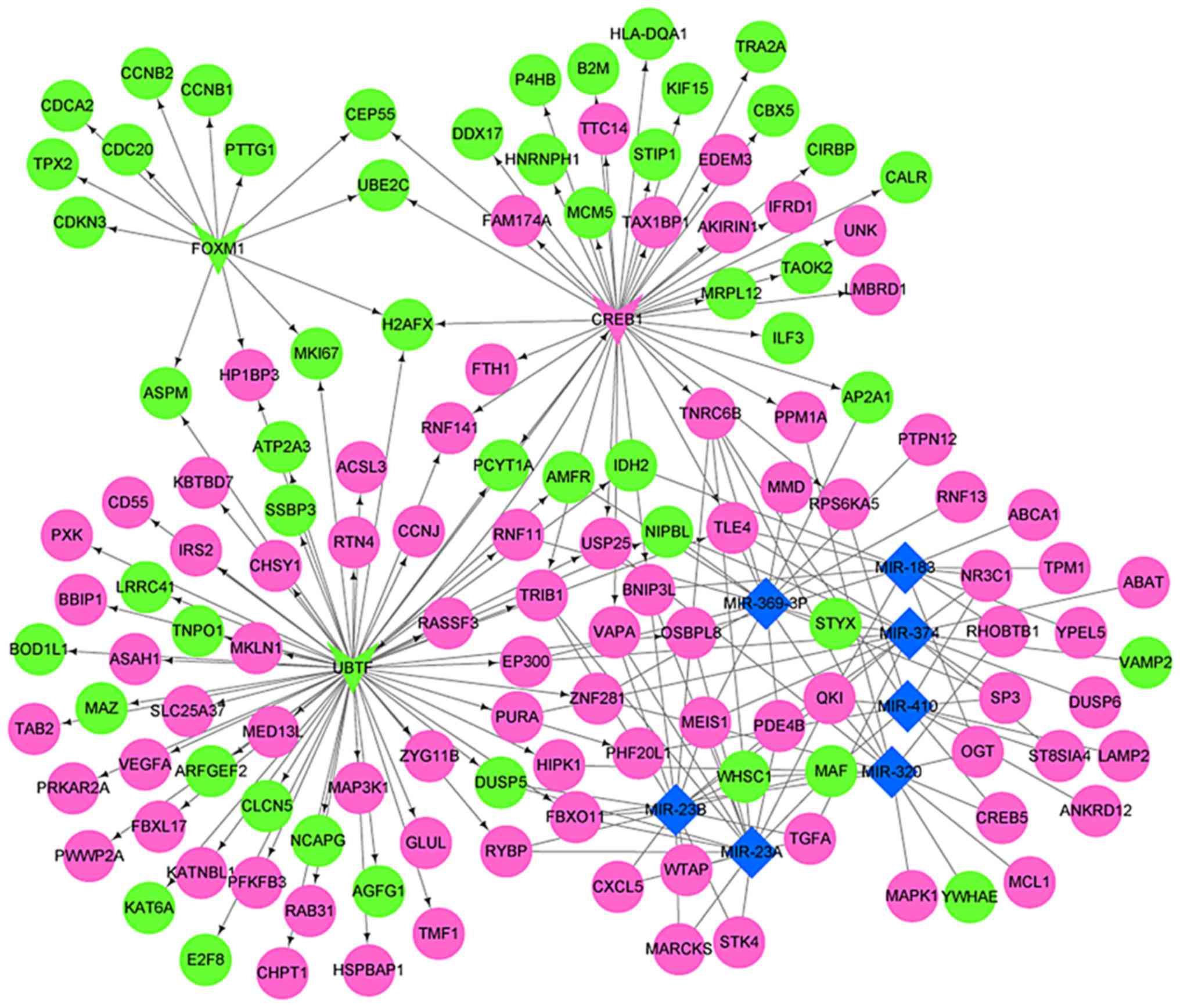
The DEG-miRNA-TF regulatory network was constructed
based on TFs, miRNAs and DEGs from the Enrichr database (Fig. 5). There were three TFs (Table II), seven miRNAs (Table III) and 132 target genes in the
network. The greatest number of genes was regulated by miR-23a
(overlap=18), including BCL2 interacting protein 3 like (BNIP3L),
C-X-C motif chemokine ligand 5 (CXCL5) and dual specificity
phosphatase 5 (DUSP5). The above results indicated that miR-23a may
serve a role in the development of JSA. Furthermore, 22 target
genes were regulated by TFs and miRNAs simultaneously, including
BNIP3L, DUSP5, E1A binding protein p300 (EP300) and F-box protein
11 (FBXO11).
 | Table II.TFs in the microRNA-TF regulatory
network of differentially expressed genes. |
Table II.
TFs in the microRNA-TF regulatory
network of differentially expressed genes.
| TF | Gene count | P-value | Adjusted
P-value |
|---|
| UBTF_ENCODE | 64 |
3.16×10−11 |
1.61×10−9 |
| FOXM1_ENCODE | 13 |
4.75×10−9 |
1.21×10−7 |
| CREB1_CHEA | 40 |
7.04×10−4 |
2.65×10−3 |
 | Table III.miRNAs in the miRNA-transcription
factor regulatory network of differentially expressed genes. |
Table III.
miRNAs in the miRNA-transcription
factor regulatory network of differentially expressed genes.
| miRNA | Overlap | P-value | Adjusted
P-value |
|---|
| miR-369-3P | 16 |
3.32×10−7 |
3.23×10−5 |
| miR-410 | 11 |
3.38×10−7 |
3.23×10−5 |
| miR-374 | 15 |
7.38×10−5 |
4.24×10−3 |
| miR-320 | 14 |
8.88×10−5 |
4.24×10−3 |
| miR-183 | 11 |
1.45×10−4 |
5.56×10−3 |
| miR-23A | 18 |
2.17×10−4 |
6.93×10−3 |
| miR-23B | 18 |
2.17×10−4 |
6.93×10−3 |
Target network analysis of chemical
small molecules
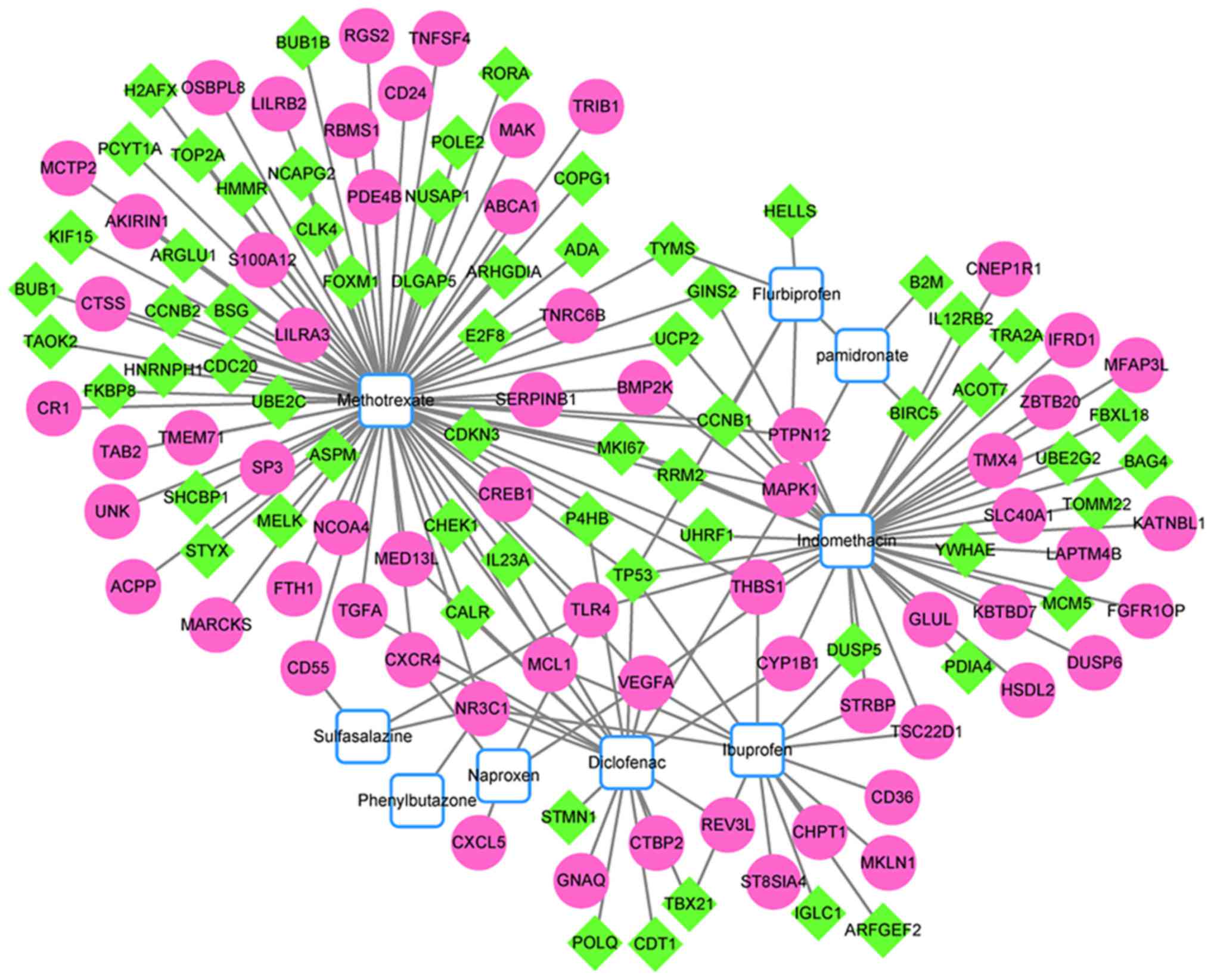
Following construction of the chemical-target gene
interaction network, several small molecules potentially associated
with JSA were identified. A total of nine small molecules may be
associated with JSA, including methotrexate, flurbiprofen,
sulfasalazine and naproxen (Fig.
6).
Discussion
Genetic and environmental factors are involved in
the occurrence of JSA, although the exact etiology and pathogenesis
of JSA remain to be elucidated (5). In the present study, bioinformatics
analyses were performed to determine target genes and pathways
implicated in the development of JSA. The results of the present
study suggested that miR-23a was likely to be involved in the
development of JSA. Additionally, arthritis and MAPK signaling
pathway-associated terms were demonstrated to serve roles in JSA.
Furthermore, the genes BNIP3L, CXCL5, DUSP5 and TP53 were
demonstrated to be involved in the pathogenesis of JSA. In
addition, a total of nine small molecules were screened, which may
either promote or inhibit the development of JSA.
In the present study, the regulatory DEG-miRNA-TF
network was constructed, and it included three TFs, seven miRNAs
and 132 target genes. miR-23a regulated the greatest number of
genes (overlap=18). A previous study provided evidence that the
miR-23a cluster was significantly decreased in the PsA synovium
(20). PsA and JSA are members of
the SpA family of inflammatory diseases (21). Therefore, dysregulation of miR-23a
expression may be involved in the development of JSA.
Three target genes were regulated by miR-23a,
including two upregulated genes, BNIP3L and CXCL5, and a
downregulated gene, DUSP5. The target gene BNIP3L, was regulated by
a TF (CREB1) and miR-23a simultaneously. BNIP3L, a member of the
BCL2/adenovirus E1B 19 kd-interacting protein family, is associated
with apoptosis, autophagy and mitophagy (22). A previous study demonstrated that
BNIP3L induces apoptosis via regulation of the mitochondrial
membrane permeability (23).
Furthermore, BNIP3L has been demonstrated to serve a role in
cardiomyocyte apoptosis (24).
Lamot et al (2) revealed
that blood cells isolated from patients with JSA demonstrated a
higher activity of numerous processes associated with apoptosis,
which indicated broad cellular dysfunction in blood cells. Thus, it
was speculated that BNIP3L may be associated with JSA progression
via involvement in the apoptosis process. However, the exact
underlying mechanisms remain unclear and require further
investigation.
CXCL5, a member of the CXC subfamily of chemokines,
is involved in angiogenesis, tumor growth and metastasis (25). Expression of CXCL5 has been
identified in animal models and patients with gastrointestinal
inflammation (26,27). Mei et al (28) demonstrated that CXCL5 served a role
in innate immunity by regulating neutrophil homeostasis. The
present study demonstrated that CXCL5 was enriched in the functions
of immune response and pathways associated with rheumatoid
arthritis (data not shown). Furthermore, in the present study CXCL5
was associated with the CXCR4 gene in the PPI network, and CXCR4
has been previously demonstrated to be overexpressed in patients
with JSA (2). Therefore, the
results of the present study suggested that inflammatory the
mediator CXCL5 may be implicated in the pathogenesis of JSA, by
mediating inflammation and the innate immune response.
DUSP5 is a nuclear phosphatase protein and its
expression is induced by cytokines, stress and other stimulatory
factors (29). In the present
study, DUSP5 was enriched in the MAPK signaling pathway, which
regulates cell proliferation, apoptosis and the inflammatory
response (30,31). A previous report demonstrated that
activation of the MAPK pathway may be associated with the
inhibition of DUSP5 (32).
Phosphoserine and phosphotyrosine residues of MAPK may be
specifically dephosphorylated by DUSP5 (32). A previous study indicated that the
MAPK signaling pathway is likely to serve a role in the
pathogenesis of SpA (2).
Therefore, the authors of the present study hypothesize that the
MAPK signaling pathway may serve a role in the pathogenesis of JSA,
mediated by DUSP5.
It was previously demonstrated that TP53 is
downregulated in several autoimmune diseases, including rheumatoid
arthritis, systemic lupus erythematosus, multiple sclerosis and
type 1 diabetes (33). A previous
study demonstrated that activation of TP53 led to numerous
responses in cells, including cell cycle arrest (34). TP53 was enriched in four KEGG
pathways in the present study, including ‘cell cycle’, ‘MAPK
signaling pathway’ and ‘p53 signaling pathway’. Lamot et al
(2) demonstrated that patients
with JSA exhibited decreased activity of processes associated with
the cell cycle. In particular, previous data demonstrated that TP53
is able to functionally interact with the MAPK signaling pathway
(34). In the present study, TP53
was associated with the BUB1 gene, which exhibited the highest
degree in the PPI network. These results suggested that TP53 may be
involved in the pathogenesis of JSA by regulating the cell cycle
and MAPK signaling.
The identification of small molecules with potential
therapeutic efficacy for the treatment of JSA was the aim of the
present study. A total of nine small molecules were associated with
JSA, including methotrexate, sulfasalazine, indomethacin and
ibuprofen. Previously, a randomized placebo-controlled study
indicated that sulfasalazine was effective in patients with JSA
(35). Meanwhile, as arthritis in
JSA is predominantly peripheral, methotrexate has also been used in
the treatment for patients with juvenile idiopathic arthritis
(36,37). Multiple small molecules were
identified in the present study; however, their roles in the
treatment of JSA require further investigation.
In conclusion, the results of the present study
indicated that miR-23a may be implicated in the development of JSA.
A total of three target genes (BNIP3L, CXCL5 and DUSP5) regulated
by miR-23a and associated with MAPK signaling were identified in
the present study, and may serve roles in the pathogenesis of JSA.
Furthermore, TP53 was identified, which was implicated in four KEGG
pathways and may be involved in the pathogenesis of JSA. However,
in vitro and in vivo studies are required to confirm
the role of the identified genes and pathways in the pathogenesis
of JSA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Pudong New
Area Scientific Development Fund (grant no. PKJ2013-Y40); Shanghai
Medical Key Specialty Constructive Fund (grant no. ZK2015A14);
Shanghai Pudong New Area Health Planning Committee project (grant
no. PW2016A-21); and the construction of ‘the most important’
discipline of Zhoupu Hospital of Shanghai New Pudong District
(grant no. ZP-XK-2015A-2).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors's contributions
ZW, YH, XW and ZZ designed the study and drafted the
manuscript. CJ, QZ, WS, TC and YZ acquired and interpreted the
data. XW revised the manuscript for important content. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
HarjačEk M, Lamot L, Bukovac LT, Vidović
M and Joos R: Juvenile SpondyloarthritisChallenges in Rheumatology.
INtech; London, UK: pp. 89–128. 2011
|
|
2
|
Lamot L, Borovecki F, Bukovac Tambic L,
Vidovic M, Perica M, Gotovac K and Harjacek M: Aberrant expression
of shared master-key genes contributes to the immunopathogenesis in
patients with juvenile spondyloarthritis. PLoS One. 9:e1154162014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weiß A, Minden K, Listing J, Foeldvari I,
Sieper J and Rudwaleit M: Course of patients with juvenile
spondyloarthritis during 4 years of observation, juvenile part of
GESPIC. RMD Open. 3:e0003662017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoving JL, Lacaille D, Urquhart DM, Hannu
TJ, Sluiter JK and Frings-Dresen MH: Non-pharmacological
interventions for preventing job loss in workers with inflammatory
arthritis. Cochrane Database Syst Rev: CD010208. 2014. View Article : Google Scholar
|
|
5
|
Gmuca S and Weiss PF: Juvenile
spondyloarthritis. Curr Opin Rheumatol. 27:364–372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tse SM and Laxer RM: New advances in
juvenile spondyloarthritis. Nat Rev Rheumatol. 8:269–279. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitaori T, Ito H, Schwarz EM, Tsutsumi R,
Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T and Nakamura T:
Stromal cell-derived factor 1/CXCR4 signaling is critical for the
recruitment of mesenchymal stem cells to the fracture site during
skeletal repair in a mouse model. Arthritis Rheum. 60:813–823.
2014. View Article : Google Scholar
|
|
8
|
Wei F, Moore DC, Wei L, Li Y, Zhang G, Wei
X, Lee JK and Chen Q: Attenuation of osteoarthritis via blockade of
the SDF-1/CXCR4 signaling pathway. Arthritis Res Ther. 14:R1772012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Werner L, Guzner-Gur H and Dotan I:
Involvement of CXCR4/CXCR7/CXCL12 interactions in inflammatory
bowel disease. Theranostics. 3:40–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eschrich SA and Hoerter AM: Libaffy:
Software for processing Affymetrix GeneChip data. Bioinformatics.
23:1562–1564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:(Database Issue). D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lachmann A, Xu H, Krishnan J, Berger SI,
Mazloom AR and Ma'Ayan A: ChEA: Transcription factor regulation
inferred from integrating genome-wide ChIP-X experiments.
Bioinformatics. 26:2438–2444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z,
Meirelles GV, Clark NR and Ma'ayan A: Enrichr: Interactive and
collaborative HTML5 gene list enrichment analysis tool. BMC
Bioinformatics. 14:1282013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davis AP, Grondin CJ, Johnson RJ, Sciaky
D, King BL, McMorran R, Wiegers J, Wiegers TC and Mattingly CJ: The
comparative toxicogenomics database: Update 2017. Nucleic Acids
Res. 45:D972–D978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wade S, Trenkmann M, Mcgarry T, Orr C,
Veale DJ and Fearon U: A8.13 TLR regulated MIR-23A down-regulated
in psoriatic arthritis. Ann Rheum Dis. 75 Suppl 1:(A69): 63–A70.
2016.http://dx.doi.org/10.1136/annrheumdis-2016-209124.166
|
|
21
|
Katsicas MM and Russo R: Biologic agents
in juvenile spondyloarthropathies. Pediatr Rheumatol Online J.
14:172016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Y, Deng X, Chen S, Yang L, Ni J, Wang
R, Lin J, Bai M, Jia Z, Huang S and Zhang A: MicroRNA-30e targets
BNIP3L to protect against aldosterone-induced podocyte apoptosis
and mitochondrial dysfunction. Am J Physiol Renal Physiol.
312:F589–F598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imazu T, Shimizu S, Tagami S, Matsushima
M, Nakamura Y, Miki T, Okuyama A and Tsujimoto Y: Bcl-2/E1B 19
kDa-interacting protein 3-like protein (Bnip3L) interacts with
bcl-2/Bcl-xL and induces apoptosis by altering mitochondrial
membrane permeability. Oncogene. 18:4523–4529. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Wang X, Mei Z, Gong J, Huang L, Gao
X, Zhao Y, Ma J and Qian L: BNIP3L promotes cardiac fibrosis in
cardiac fibroblasts through
[Ca2+]i-TGF-β-Smad2/3 pathway. Sci Rep.
7:19062017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li A, King J, Moro A, Sugi MD, Dawson DW,
Kaplan J, Li G, Lu X, Strieter RM, Burdick M, et al: Overexpression
of CXCL5 is associated with poor survival in patients with
pancreatic cancer. Am J Pathol. 178:1340–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hogan SP, Mishra A, Brandt EB, Royalty MP,
Pope SM, Zimmermann N, Foster PS and Rothenberg ME: A pathological
function for eotaxin and eosinophils in eosinophilic
gastrointestinal inflammation. Nat Immunol. 2:353–360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ajuebor MN and Swain MG: Role of
chemokines and chemokine receptors in the gastrointestinal tract.
Immunology. 105:137–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mei J, Dai N, Liu Y, Hudock K, Liu P,
Deshmukh H, Lee J and Worthen GS: Altered neutrophil homeostasis
and DARC regulate CXCL5-mediated pulmonary immune responses
(INM2P.435). J Immunol. 192 1 suppl:(S56): 182014.
|
|
29
|
Kutty RG, Xin G, Schauder DM, Cossette SM,
Bordas M, Cui W and Ramchandran R: Dual specificity phosphatase 5
is essential for T cell survival. PLoS One. 11:e01672462016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lang R, Hammer M and Mages J: DUSP meet
immunology: Dual specificity MAPK phosphatases in control of the
inflammatory response. J Immunol. 177:7497–7504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moon SJ, Lim MA, Park JS, Byun JK, Kim SM,
Park MK, Kim EK, Moon YM, Min JK, Ahn SM, et al: Dual-specificity
phosphatase 5 attenuates autoimmune arthritis in mice via
reciprocal regulation of the Th17/Treg cell balance and inhibition
of osteoclastogenesis. Arthritis Rheumatol. 66:3083–3095. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Olsen NJ, Moore JH and Aune TM: Gene
expression signatures for autoimmune disease in peripheral blood
mononuclear cells. Arthritis Res Ther. 6:120–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu GS: The functional interactions between
the p53 and MAPK signaling pathways. Cancer Biol Ther. 3:156–161.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Rossum MA, Fiselier TJ, Franssen MJ,
Zwinderman AH, ten Cate R, van Suijlekom-Smit LW, van Luijk WH, van
Soesbergen RM, Wulffraat NM, Oostveen JC, et al: Sulfasalazine in
the treatment of juvenile chronic arthritis: A randomized,
double-blind, placebo-controlled, multicenter study. Dutch Juvenile
Chronic Arthritis Study Group. Arthritis Rheum. 41:808–816. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vilca I, Munitis PG, Pistorio A, Ravelli
A, Buoncompagni A, Bica B, Campos L, Häfner R, Hofer M, Ozen S, et
al: Predictors of poor response to methotrexate in
polyarticular-course juvenile idiopathic arthritis: Analysis of the
PRINTO methotrexate trial. Ann Rheum Dis. 69:1479–1483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giannini EH, Brewer EJ, Kuzmina N, Shaikov
A, Maximov A, Vorontsov I, Fink CW, Newman AJ, Cassidy JT and Zemel
LS: Methotrexate in resistant juvenile rheumatoid arthritis.
Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled
trial. The Pediatric Rheumatology Collaborative Study Group and The
Cooperative Childr. N Engl J Med. 326:1043–1049. 1992. View Article : Google Scholar : PubMed/NCBI
|