Introduction
Gastric cancer is one of the most common malignant
diseases and is the second leading cause of cancer-related death
worldwide. The prognosis for advanced gastric cancer remains poor
(1). Early stage gastric cancer
patients are asymptomatic or experience minor symptoms (2). Most patients with symptoms that can
be noticed clinically have reached an advanced stage (2). Surgery is the only potentially
curative treatment for gastric cancer. To improve upon low survival
outcomes one of major obstacles in cancer therapy, the development
of tumor chemoresistance, needs to be overcome. Therefore, it is
necessary to investigate the mechanisms involved in
chemoresistance, to develop strategies that sensitize cancer cells
to chemotherapeutic agents.
5-Fluorouracil (5-FU) is one of the most commonly
used chemotherapeutic agents for gastric cancer. Despite its many
advantages, clinical applications of 5-FU have been limited by drug
resistance that arises due to several factors, including altered
drug influx and efflux, enhancement of drug inactivation, and
mutations to the drug target (3).
Several intracellular enzymes, thymidine synthase (TS),
dihydropyrimidine dehydrogenase (DPD), methylenetetrahydrofolate
reductase (MTHFR) and thymidine phosphorylase (TP), are considered
important predictors for 5-FU sensitivity or resistance (4). However, mechanisms underlying 5-FU
anti-tumor activity and drug resistance have not been fully
revealed yet.
TS expression and activity are increased in several
tumor tissues, including lung, cervical, breast, and gastric
cancers. They are considered to be indicators of cell proliferation
and are associated with poor prognosis. TS has been used as an
important target in chemotherapy (5,6).
Several studies have shown that TS expression is a key regulator of
5-FU resistance/sensitivity (7,8).
Adenosine monophosphate-activated protein kinase
(AMPK) is a heterodimeric enzyme consisting of a catalytic
α-subunit and two non-catalytic β and γ subunits. AMPK functions in
the cellular metabolism of glucose, lipid, and protein (9). The role of metformin, an AMPK
activator, as a chemosensitizer has been investigated in
Bel-7402/5-FU cells (hepatocellular carcinoma) and MCF7/5-FU cells
(breast cancer) (10,11). It has been reported that AICAR,
another AMPK activator, enhanced the pro-apoptotic effect of 5-FU
in 5-FU-resistant SGC-7901 cells (gastric cancer) (12). Additionally, it has been reported
that phosphorylated AMPK level is reduced in 5-FU-resistant gastric
cancer cells while glucose metabolism is increased in
5-FU-resistant HepG2 cells (13).
However, it remains unknown as to whether AMPK can increase
chemosensitization in gastric cancer cells.
Corosolic acid (2a-hydroxyursolic acid), one of the
main triterpenoids, has been discovered in many medicinal plants
such as Lagerstroemia speciosa (banaba) and Weigela
subsessilis (14,15). Corosolic acid not only displays
remarkable hypoglycemic effects in some animal experiments and
clinical trials (16,17), but has also been shown to possess
antitumor effects against several cancers, including liver, colon,
lung, and gastric cancer (18–21).
Previous studies have reported that corosolic acid can enhance the
anticancer effect of 5-FU in SNU-620 and NCI-N87 gastric cancer
cells, suggesting that it might act as an AMPK activator (21–25).
Among natural chemicals, curcumin, epigallocatechin gallate (EGCG),
and sinomenine have been found to be able to sensitize 5-FU
resistance in gastric cancers (26–28).
However, whether corosolic acid can do the same for 5-FU resistance
in cancers remains unclear.
Therefore, the objective of this study was to
determine the effect of corosolic acid on the response of gastric
cancer to 5-FU. We used 5-FU resistant human gastric cancer cells
(SNU-620/5-FUR) and treated them with corosolic acid in
the presence or absence of 5-FU to investigate the effect of
corosolic acid on 5-FU resensitization, and determine the mechanism
of action.
Materials and methods
Materials
RPMI-1640, fetal bovine serum (FBS) and
penicillin/streptomycin were obtained from HyClone (GE Healthcare
Life Sciences, Logan, UT, USA). Trypsin/EDTA was purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
following primary antibodies were used: Rabbit polyclonal
anti-human thymidylate synthase (1:1,000; no. 3766), rabbit
polyclonal anti-human caspase-3 (1:1,000; no. 9662), rabbit
polyclonal anti-human poly-(ADP-ribose) polymerase (PARP) (1:1,000;
no. 9542), rabbit polyclonal anti-human AMPK (1:1,000; no. 2532),
rabbit monoclonal anti-human phospho-AMPK (Thr172) (1:1,000; no.
2535), rabbit polyclonal anti-human mTOR (1:1,000; no. 2972),
rabbit polyclonal anti-human phospho-mTOR (Ser2448) (1:1,000; no.
2971), rabbit polyclonal anti-human 4E-binding protein 1 (4EBP1)
(1:1,000; no. 9452) and rabbit polyclonal anti-human phospho-4EBP1
(Thr70) (1:1,000; no. 9455) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA), and rabbit polyclonal
anti-human GAPDH (1:1,000; sc-25778) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase-conjugated anti-mouse and anti-rabbit antibodies were
obtained from Transduction Lab (Lexington, KY, USA).
SuperSignal® West Pico Chemiluminescent Substrate was
purchased from Pierce (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 5-FU was provided by Choongwae Pharmaceutical Co., Ltd.
(Seoul, Korea). Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Laboratories (Kumamoto, Japan) and the EzWay Annexin-V-FITC
Apoptosis Detection kit was purchased from KomaBiotech, Inc.
(Seoul, Korea). A Mitochondrial Apoptosis Staining kit was
purchased from PromoKine® (PromoCell GmbH, Heidelberg,
Germany). Corosolic acid, compound c, AICAR and all other reagents
were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany).
Cell culture
Human gastric carcinoma SNU-620 cells were purchased
from Korean Cell Line Bank (Seoul, Korea). Cells were grown in
RPMI-1640 media supplemented with 10% (v/v) FBS, penicillin (100
U/ml)/streptomycin (100 µg/ml) at 37°C in a humidified
CO2 (5%)-controlled incubator. 5-FU-resistant
SNU-620/5-FUR cells were established by repeated
cultures of SNU-620 with constant treatment with 7.5 µM 5-FU.
Cell growth inhibition assay
Cells were seeded at 5×103 cells/ml in
96-well microplates and allowed to attach for 24 h. 5-FU (~750 µM)
or corosolic acid (~25 µM) were added to the medium at various
concentrations. Following treatment, the cell cytotoxicity and/or
proliferation was assessed using the CCK-8 assay. Briefly, highly
water-soluble tetrazolium salt
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt], produced an orange-colored water-soluble product,
formazan. The quantity of formazan dye generated by dehydrogenases
in the cells was directly proportional to the number of living
cells. CCK-8 (10 µl) was added to each well and incubated for 3 h
at 37°C; cell proliferation and cytotoxicity were assessed by
measuring the absorbance at 450 nm using a microplate reader
(Corning Incorporated, Corning, NY, USA). Three replicated wells
were used per experimental condition.
Annexin V/Propidium iodide
staining
Cells were cultured at a 106 density and
treated with corosolic acid and/or compound c for 24 h. Cells were
centrifuged and washed three times with phosphate-buffered saline
(PBS), and centrifuged. The supernatant was discarded and
resuspended in 0.5 ml of cold PBS. The cells were processed and
labeled according to the EzWay Annexin V-FITC Apoptosis Detection
Kit that was used for this assay. The labeled cells were analyzed
in a flow cytometer (BD FACSCanto™II; BD Biosciences, Franklin
Lakes, NJ, USA).
Western blotting analysis
Cells were harvested using Trypsin-EDTA, washed
twice with cold PBS, lysed with lysis buffer (10 mM Tris, pH 7.4,
150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% NP-40, 1 mM PI, 1 mM
DTT, 1 mM PMSF), and placed on ice for 1 h with occasional
vortexing. Centrifugation followed at 13,000 × g for 10 min at 4°C
to collect the supernatant. A Pierce BCA Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.) was used to determine the protein
concentration. The cell lysate (50 µg) was subjected to
SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to
polyvinylidene difluoride (PVDF) membrane. Blots were blocked with
5% skim milk in PBS containing 0.05% Tween-20 for 1 h at 25°C, then
incubated with primary antibodies (1:1,000) overnight at 4°C,
followed by incubation with anti-rabbit horseradish
peroxidase-conjugated IgG (1:3,000) for 2 h at room temperature and
visualized with enhanced chemiluminescence.
Statistical analysis
All results presented were confirmed in at least
three independent experiments. Data were presented as the mean ±
standard deviation. Statistical differences were analyzed by
one-way analysis of variance followed by Tukey's post hoc test
using SPSS version 24.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
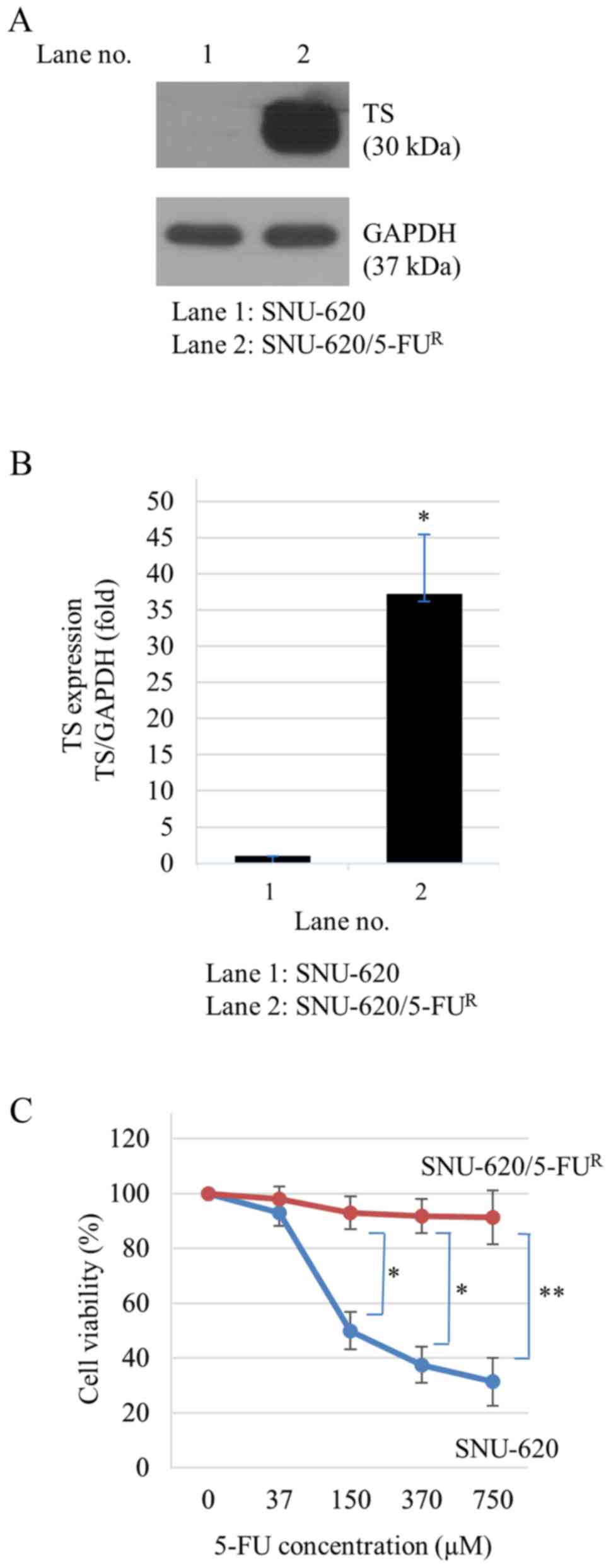
Establishment of 5-FU resistant
SNU-620 gastric cancer cells
We established a 5-FU-resistant gastric cancer cell
line (SNU-620/5-FUR) by continuous exposure of the
parental cells SNU-620 to 7.5 µM 5-FU for approximately 6 months.
TS protein level was markedly increased in SNU-620/5-FUR
cells compared to that in the 5-FU-sensitive parental cells based
on Western blot analysis, indicating that SNU-620/5-FUR
cells were resistant to 5-FU (Fig. 1A
and B). To confirm that SNU-620/5-FUR cells were
resistant to 5-FU, 5-FU was added at various concentrations (~750
µM). Cell viability was then determined by CCK-8 assay. 5-FU
decreased cell viabilities of SNU-620 cells in a dose-dependent
manner. However, it was not cytotoxic to SNU-620/5-FUR
cells (Fig. 1C).
AMPK phosphorylation level was reduced
in 5-FU-resistant SNU-620/5-FUR gastric cancer
cells
AMPK phosphorylation was found to be reduced by
32.7% in 5-FU-resistant SNU-620/5-FUR gastric cancer
cells compared to the SNU-620 cells (Fig. 2A and B). To confirm the role of
AMPK in cell resensitization to 5-FU, cells were treated with AMPK
activator AICAR and the AMPK inhibitor compound c. TS protein
expression and cell viability were then determined by western
blotting analysis and CCK-8 assay. AICAR dramatically decreased TS
expression (Fig. 2C and D) while
AMPK inhibition by compound c treatment increased TS protein
expression in SNU-620/5-FUR cells (Fig. 2C and D). Activation of AMPK by
AICAR decreased viability of SNU-620/5-FUR cells.
However, inhibition of AMPK by compound c did not decrease the
viability of SNU-620/5-FUR cells (Fig. 2E). These results suggest that 5-FU
resistance is strongly regulated by AMPK. Therefore, AMPK
phosphorylation might be a therapeutic target for overcoming
5-FU-resistance in gastric cancers.
Corosolic acid activates AMPK and
suppresses mTOR/4EBP1 phosphorylation in SNU-620/5-FUR
cells
Previous studies have reported that pharmacological
activators of AMPK such as AICAR and metformin can induce apoptosis
of gastric cancers (12,29). Moreover, corosolic acid,
(2α,3β)-2,3-dihydroxyurs-12-en-28-oic acid (Fig. 3A) can activate AMPK and induce
apoptosis in gastric cancers (25). Corosolic acid can also inhibit
inflammation in adipose tissues (23). To evaluate the effect of corosolic
acid on AMPK activation in SNU-620/5-FUR cells, western
blot analysis was performed. Results revealed that treatment with
10 and 25 µM corosolic acid dramatically increased AMPK
phosphorylation (Fig. 3B).
However, corosolic acid failed to activate AMPK in 5-FU sensitive
SNU-620 gastric cancer cells (data not shown). We also tested the
status of mTOR/4EBP1, a downstream molecular marker of AMPK
signaling. Activated AMPK inhibited the activation of mTOR/4EBP1 in
corosolic acid treated SNU-620/5-FUR cells (Fig. 3B). Corosolic acid decreased the
level of TS expression in a dose-dependent manner, with a pattern
similar to that of AMPK activation (Fig. 3B). This suggests possible
cross-talk between AMPK and 5-FU resistance after corosolic acid
treatment. To determine whether corosolic acid-induced AMPK
activation was associated with enhanced growth rate of
SNU-620/5-FUR cells after treatment with compound c (40
µM) and/or corosolic acid (1, 10, 25 and 50 µM), cell viability was
measured by CCK-8 assay. Results are shown in Fig. 3C. Compared to solo treatment with
10 or 25 µM corosolic acid, additional compound c treatment
increased cell viabilities by 27.1 and 40%, respectively. These
results suggest that corosolic acid-induced AMPK activation might
be a mechanism involved in 5-FU resistance.
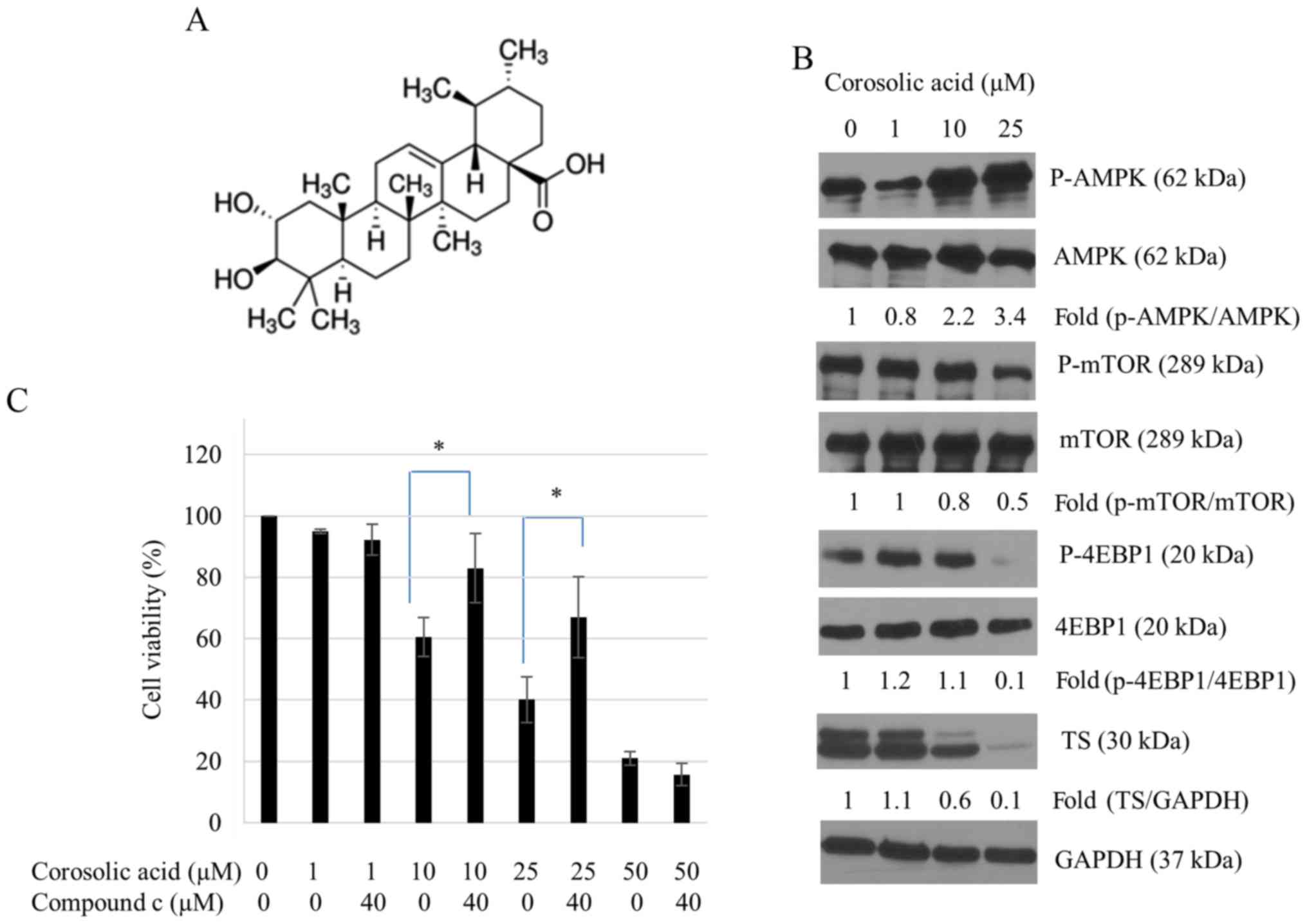 | Figure 3.Corosolic acid activates AMPK
signaling in SNU-620/5-FUR cells. (A) Structure of
corosolic acid [(2α,3β)-2,3-Dihydroxyurs-12-en-28-oic acid,
2α-Hydroxyursolic acid]. (B) Cells were incubated with corosolic
acid for 24 h and equal amounts of protein samples were resolved by
SDS-PAGE followed by immunoblotting using antibodies against AMPK,
p-AMPK, mTOR, p-mTOR, 4EBP1, and P-4EBP1. GAPDH expression served
as an internal control. (C) Cell growth inhibition was determined
by Cell Counting Kit-8 assay. Values are presented as the mean ±
standard deviation of three independent experiments. *P<0.05, as
indicated. AMPK, adenosine monophosphate-activated protein kinase;
5-FU, 5-fluorouracil; SNU-620/5-FUR, 5-FU-resistant
gastric cancer cell line; TS, thymidine synthase; p-,
phosphorylated; mTOR, mammalian target of rapamycin; 4EBP1,
4E-binding protein 1. |
Corosolic acid resensitizes
SNU-620/5-FUR gastric cancer cells to 5-FU
To investigate the sensitization effect of corosolic
acid on 5-FU-resistant gastric cancer cells,
SNU-620/5-FUR cells were treated with 5-FU (150 µM, 50%
inhibitory concentration in 5-FU sensitive SNU-620 cells) and/or
corosolic acid and growth rates were measured by CCK-8 assay.
Results showed that single treatment with 150 µM of 5-FU or 25 µM
of corosolic acid decreased the growth rate
SNU-620/5-FUR cells by 93.4 and 42.7%, respectively.
However, combination treatment significantly enhanced sensitivity
of SNU-620/5-FUR cells to 5-FU (Fig. 4A). To estimate the sensitization
effect of corosolic acid, 5-FU-sensitive cells were also exposed to
5-FU (150 µM) with or without corosolic acid. However, the
combination effect observed in SNU-620/5-FUR cells was
not evident in SNU-620 cells (Fig.
4A). In addition, TS expression in SNU-620/5-FUR
cells was diminished by treatment with corosolic acid alone. It was
drastically reduced following treatment with corosolic acid and
5-FU in combination (Fig. 4B and
C). These results suggest that corosolic acid can probably
sensitize 5-FU resistance.
Corosolic acid enhances sensitivity of
SNU-620/5-FUR gastric cancer cells to 5-FU by
upregulating AMPK
The effect of corosolic acid on AMPK activation in
the presence or absence of 5-FU was tested in
SNU-620/5-FUR cells. 5-FU (150 µM) moderately increased
AMPK activation. A combination of corosolic acid (25 µM) with 5-FU
was more effective. However, compound c significantly blocked AMPK
phosphorylation (Fig. 5A and B).
Compared to treatment with corosolic acid or 5-FU alone, the
combination of corosolic acid and 5-FU resulted in increased
caspase-3 and PARP cleavage and cytochrome c translocation, while
compound c partly blocked 5-FU+corosolic acid-induced apoptotic
activities (Fig. 5C). Apoptotic
cell percentages were examined after treated with corosolic acid,
5-FU, or compound c, by flow cytometric analysis. A significant
increase in the percentage of apoptotic cells was observed in
SNU-620/5-FUR gastric cancer cells after treatment with
corosolic acid in the presence of 5-FU. However, compound c
significantly blocked apoptosis compared to a combination of
corosolic acid and 5-FU (Fig. 5D).
Percentages of early and late apoptotic/necrotic cells were
increased from 0.5±0.1 to 21.7±2.0% in corosolic acid (25 µM)
treated cells, 68.6±13.7% in cells treated with a combination of
corosolic acid (25 µM) and 5-FU (150 µM), and 39.7±2.8% in cells
treated with combination of corosolic acid (25 µM), 5-FU (150 µM),
and compound c (40 µM) (Fig. 5E).
Compound c significantly decreased the percentage of apoptotic
cells compared to corosolic acid or 5-FU (Fig. 5D and E). These results suggest that
corosolic acid-induced AMPK activation plays a key role in
enhancing sensitivity of 5-FU-resistant gastric cancer cells to
5-FU.
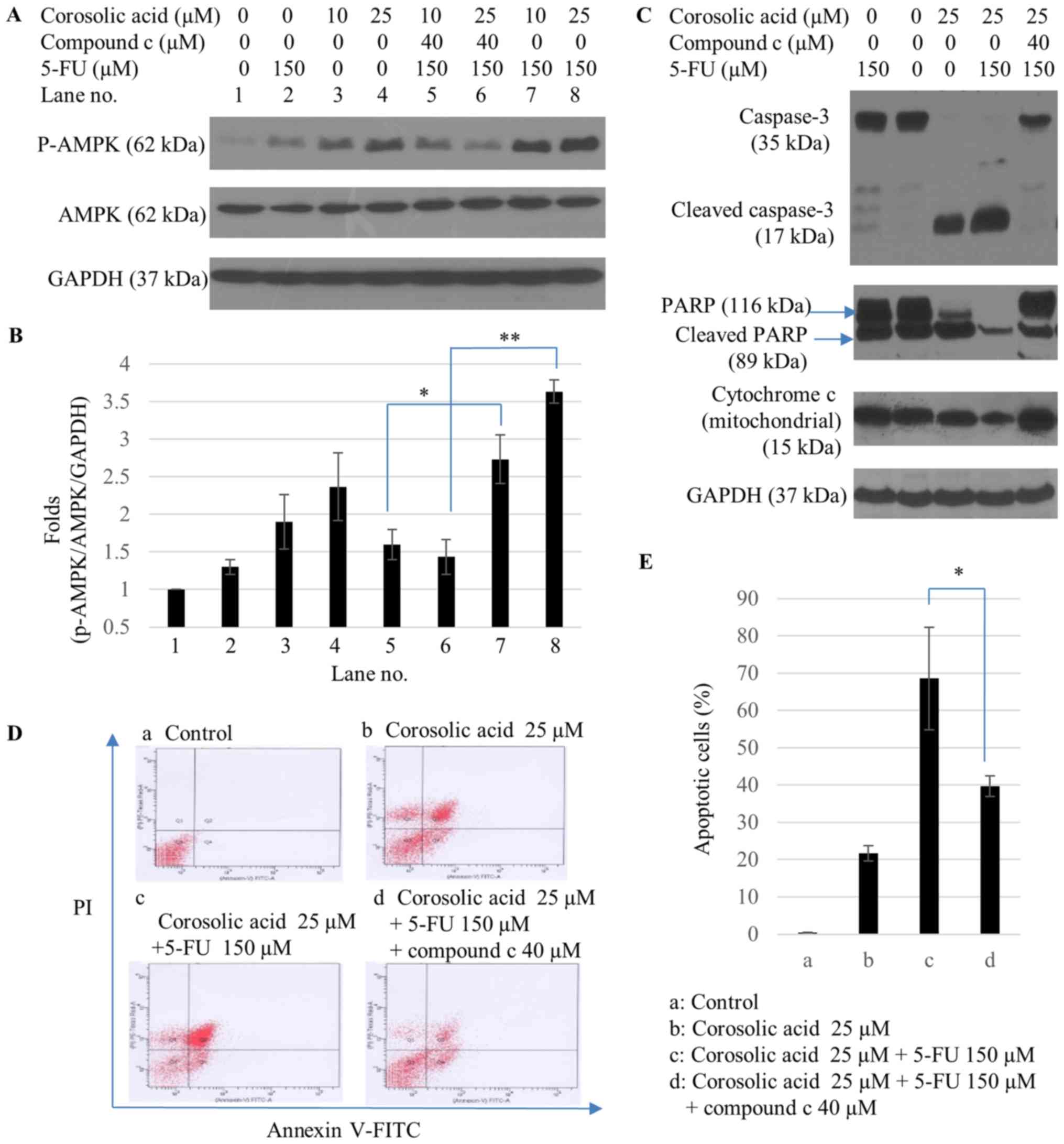 | Figure 5.Corosolic acid-induced AMPK
activation enhances 5-FU sensitivity in SNU-620/5-FUR
gastric cancer cells (A) Following treatment with corosolic acid
(10 and 25 µM), 5-FU (150 µM), or compound c (40 µM), AMPK and
p-AMPK protein expression levels were determined by western blot
analysis. (B) Values are presented as the mean ± standard deviation
of three independent experiments. (C) Activation of apoptosis in
cells treated with 5-FU (150 µM), corosolic acid (25 µM), or
compound c (40 µM) was measured by caspase-3 and PARP cleavages and
cytochrome c located in mitochondria based on western blot
analysis. GAPDH was used as internal protein loading control. (D)
Apoptotic cell population was evaluated by flow cytometry analysis
following double staining with Annexin V and PI. (E) Percentages of
early apoptosis plus late apoptosis/necrosis are shown in the bar
graph. *P<0.05 and **P<0.01, as indicated. AMPK, adenosine
monophosphate-activated protein kinase; 5-FU, 5-fluorouracil;
SNU-620/5-FUR, 5-FU-resistant gastric cancer cell line;
TS, thymidine synthase; p-, phosphorylated; PARP, poly-(adenosine
diphosphate-ribose) polymerase; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Discussion
5-FU is a heterocyclic aromatic organic compound
with a structure similar to that of pyrimidine molecules of DNA and
RNA. It is an analogue of uracil with a fluorine atom at the C-5
position in place of hydrogen (3).
5-FU is used for treating gastric cancer. It can increase overall
survival by 6% and reduce the risk of mortality by 18% (30). However, the occurrence of
resistance to 5-FU treatment is a major problem for most gastric
cancer patients, resulting in limited overall efficacy (31). High-level expression of TS,
increased activity of deoxyuridine triphosphatase, methylation of
MLH1 gene, and overexpression of Bcl-2, Bcl-XL, and Mcl-1 proteins
have been reported to be associated with cancer resistance to 5-FU
(3). Although the precise
mechanism involved in gastric cancer resistance to 5-FU remains
unknown, several reports have suggested that AMPK might be a
biological predictor and beneficial target for cancer treatment
through metabolic alteration (32). Previous clinical studies have shown
that increases in phosphorylated AMPK is associated with tumor
grade and prognosis for several solid tumors (33). More recently, phosphorylated AMPK
levels have been reported as being significantly reduced in
5-FU-resistant gastric cancer cells (AGS cells) compared to
5-FU-sensitive cells (SGC-7901 cells) (12). In this study, we demonstrated that
AMPK phosphorylation level was significantly decreased in 5-FU
resistant gastric cancer cells (SNU-620/5-FUR) compared
to sensitive gastric cancer cells (SNU-620). The present study
aimed to identify alterative therapeutic approaches for enhancing
5-FU sensitivity by activating AMPK. TS expression level was
decreased by AICAR, an AMPK activator. Consistent with this
finding, treatment with the AMPK inhibitor, compound c, increased
TS expression. AICAR significantly decreased viability of 5-FU
resistant cells without altering viability of 5-FU sensitive cells
(data not shown). Even though compound c is widely known as an AMPK
inhibitor, this compound is involved in killing cancer cells by
multiple mechanisms (Calpain/Cathepsin pathway; AKT; mTORC1/C2;
cell cycle block; necroptosis; autophagy) (34). Liu et al (34) suggests that compound c kills cancer
cells by an AMPK-independent mechanism. Therefore, we need to
perform further research to find out why compound c did not
increase cell viability in gastric cancer cells. These data suggest
that AMPK might be a potent regulator of 5-FU resistance in gastric
cancers.
Several studies have demonstrated that corosolic
acid can activate AMPK in adipose tissue, endothelial cells, and
gastric cancer cells (23–25). A recent clinical study revealed
that metformin, an AMPK activator, can reduce gastric cancer risk
in patients with type 2 diabetes mellitus (35). In addition, metformin can reverse
multidrug resistance in breast cancer cells and hepatocellular
carcinoma Bel-7402/5-fluorourscil cells (10,11).
In the present study, we found that corosolic acid activated AMPK
phosphorylation in 5-FU resistant SNU-620/5-FUR cells
followed by decreased phosphorylation levels of mTOR/4EBP1.
Corosolic acid-induced AMPK activation down-regulated cell
viability in a dose-dependent manner. However, AMPK activity was
significantly inhibited by compound c in 5-FU resistant
SNU-620/5-FUR cells. Therefore, corosolic acid-induced
AMPK activation plays an important role in overcoming
5-FU-resistance in gastric cancer. In Fig. 3C, 1 µM corosolic acid + compound c
(Lane 3) and 50 µM corosolic acid + compound c (Lane 9) did not
preserve cell viability. 1 µM corosolic acid and 50 µM corosolic
acid did not activate AMPK in our experiments, which might be a
possible reason corosolic acid-treated cells could not be reversed
by compound c. To investigate the sensitization effect of corosolic
acid to 5-FU, resistant or sensitive cells were treated with
corosolic acid (25 µM) in the presence or absence of 5-FU (150 µM).
Viability of SNU-620/5-FUR cells treated with 5-FU in
the presence of corosolic acid was significantly inhibited.
However, no difference in viability of SNU-620 cells was found
after such treatment. Corosolic acid in combination with 5-FU
significantly decreased TS expression level in
SNU-620/5-FUR cells compared to treatment with corosolic
acid or 5-FU alone. Because TS is a major marker of 5-FU resistance
status, these results indicate that corosolic acid might be able to
reverse and/or sensitize 5-FU resistance of
SNU-620/5-FUR cells.
AMPK activation is known to play an important role
in enhancing chemosensitivity to certain chemotherapeutic agents
such as 5-FU in breast cancer, intrahepatic cholangiocarcinoma, and
gall bladder cancer (10,11,36).
To investigate corosolic acid-induced AMPK activation involved in
the resensitization effect of 5-FU, we determined AMPK
phosphorylation level in the presence or absence of 5-FU. Our
results showed that corosolic acid (10 and 25 µM) synergistically
enhanced p-AMPK in the presence of 5-FU. Sensitivity of cells to
chemotherapy might be estimated by examining apoptosis. The
apoptotic rate was analyzed by western blot to detect caspase-3 and
PARP cleavage, as well as cytochrome c translocation, and Annexin
V-PI staining. The expression of cleaved caspase-3 and PARP and
cytochrome c translocation in SNU-620/5-FUR cells were
increased by 5-FU. The apoptotic rate in SNU-620/5-FUR
cells treated with a combination of corosolic acid and 5-FU was
significantly higher than when treated with corosolic acid alone.
Chemically inhibited AMPK activation by compound c abolished AMPK
phosphorylation and apoptotic activities in the presence of 5-FU.
These findings suggest that corosolic acid might be able to enhance
chemosensitivity of gastric cancer to 5-FU.
We agree with that gastric cancer is a heterogenous
disease. To investigate the effect of corosolic acid on 5-FU
chemoresistance in various gastric cancer cell types, several
gastric cancer cell lines (SNU-1, SNU-5, AGS, SNU-484, SNU-601, and
NCI-N87), have being established that are 5-FU resistant.
Investigations of these are planned.
In conclusion, our study revealed that 5-FU
resistant gastric cancer cells (SNU-620/5-FUR) had lower
phosphorylated AMPK expression than 5-FU sensitive parental cells
(SNU-620). Therefore, AMPK expression might be a possible treatment
target for 5-FU resistant gastric cancers. We also found that
corosolic acid could sensitize 5-FU resistance and inhibit
viability of 5-FU resistant gastric cancer cells by activating the
AMPK pathway. Therefore, corosolic acid could be used as an
effective complimentary medicine to restore chemosensitivity of
drug resistant gastric cancer cells to 5-FU. Further studies need
to focus on chemotherapeutic sensitization by corosolic acid in
combination with other chemotherapeutics, and to investigate the
detailed molecular mechanisms involved.
Acknowledgements
The present study is based on a doctoral thesis
(Chungnam National University, 2017).
Funding
The present study was supported by the Research Fund
of Chungnam National University (grant no. 2015109701).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JBP designed the study and prepared the manuscript.
JSL contributed to the conception of the study, analyzed the data
and drafted the manuscript. MSL, EYC and SK performed the
experiments. JYS was involved in the study conception and design,
and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith JK, McPhee JT, Hill JS, Whalen GF,
Sullivan ME, Litwin DE, Anderson FA and Tseng JF: National outcomes
after gastric resection for neoplasm. Arch Surg. 142:387–393. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai MM, Wang CS, Tsai CY, Chi HC, Tseng
YH and Lin KH: Potential prognostic, diagnostic and therapeutic
markers for human gastric cancer. World J Gastroenterol.
20:13791–13803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scartozzi M, Maccaroni E, Giampieri R,
Pistelli M, Bittoni A, Del Prete M, Berardi R and Cascinu S:
5-Fluorouracil pharmacogenomics: Still rocking after all these
years? Pharmacogenomics. 12:251–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnston PG, Lenz HJ, Leichman CG,
Danenberg KD, Allegra CJ, Danenberg PV and Leichman L: Thymidylate
synthase gene and protein expression correlate and are associated
with response to 5-fluorouracil in human colorectal and gastric
tumors. Cancer Res. 55:1407–1412. 1995.PubMed/NCBI
|
|
6
|
Saga Y, Suzuki M, Mizukami H, Urabe M,
Fukushima M, Ozawa K and Sato I: Enhanced expression of thymidylate
synthase mediates resistance of uterine cervical cancer cells to
radiation. Oncology. 63:185–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Copur S, Aiba K, Drake JC, Allegra CJ and
Chu E: Thymidylate synthase gene amplification in human colon
cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol.
49:1419–1426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fakhrejahani E, Miyamoto A and Tanigawa N:
Correlation between thymidylate synthase and dihydropyrimidine
dehydrogenase mRNA level and in vitro chemosensitivity to
5-fluorouracil, in relation to differentiation in gastric cancer.
Cancer Chemother Pharmacol. 60:437–446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YH, Liang H, Liu X, Lee JS, Cho JY,
Cheong JH, Kim H, Li M, Downey TJ, Dyer MD, et al: AMPKα modulation
in cancer progression: Multilayer integrative analysis of the whole
transcriptome in Asian gastric cancer. Cancer Res. 72:2512–2521.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling S, Tian Y, Zhang H, Jia K, Feng T,
Sun D, Gao Z, Xu F, Hou Z, Li Y and Wang L: Metformin reverses
multidrug resistance in human hepatocellular carcinoma
Bel-7402/5-fluorouracil cells. Mol Med Rep. 10:2891–2897. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu C, Zhang W, Zheng G, Zhang Z, Yin J and
He Z: Metformin reverses multidrug resistance and
epithelial-mesenchymal transition (EMT) via activating
AMP-activated protein kinase (AMPK) in human breast cancer cells.
Mol Cell Biochem. 386:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Qi Y, Liu H, Wang X, Zhu H and Wang
Z: AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric
cancer cells. Mol Cell Biochem. 411:299–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hua HW, Jiang F, Huang Q, Liao ZJ and Ding
G: Re-sensitization of 5-FU resistance by SPARC through negative
regulation of glucose metabolism in hepatocellular carcinoma.
Tumour Biol. 36:303–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou W, Li Y, Zhang Q, Wei X, Peng A, Chen
L and Wei Y: Triterpene acids isolated from Lagerstroemia
speciosa leaves as alpha-glucosidase inhibitors. Phytother Res.
23:614–618. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thuong PT, Min BS, Jin W, Na M, Lee J,
Seong R, Lee YM, Song K, Seong Y, Lee HK, et al: Anti-complementary
activity of ursane-type triterpenoids from Weigela
subsessilis. Biol Pharm Bull. 29:830–833. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukushima M, Matsuyama F, Ueda N, Egawa K,
Takemoto J, Kajimoto Y, Yonaha N, Miura T, Kaneko T, Nishi Y, et
al: Effect of corosolic acid on postchallenge plasma glucose
levels. Diabetes Res Clin Pract. 73:174–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miura T, Ueda N, Yamada K, Fukushima M,
Ishida T, Kaneko T, Matsuyama F and Seino Y: Antidiabetic effects
of corosolic acid in KK-Ay diabetic mice. Biol Pharm Bull.
29:585–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Zhao Y, Xu Y, Guan Y, Zhang X, Chen
Y, Wu Q, Zhu G, Chen Y, Sun F, et al: Blocking inhibition to YAP by
ActinomycinD enhances anti-tumor efficacy of Corosolic acid in
treating liver cancer. Cell Signal. 29:209–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sung B, Kang YJ, Kim DH, Hwang SY, Lee Y,
Kim M, Yoon JH, Kim CM, Chung HY and Kim ND: Corosolic acid induces
apoptotic cell death in HCT116 human colon cancer cells through a
caspase-dependent pathway. Int J Mol Med. 33:943–949. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nho KJ, Chun JM and Kim HK: Corosolic acid
induces apoptotic cell death in human lung adenocarcinoma A549
cells in vitro. Food Chem Toxicol. 56:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HS, Park JB, Lee MS, Cha EY, Kim JY
and Sul JY: Corosolic acid enhances 5-fluorouracil-induced
apoptosis against SNU-620 human gastric carcinoma cells by
inhibition of mammalian target of rapamycin. Mol Med Rep.
12:4782–4788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MS, Cha EY, Thuong PT, Kim JY, Ahn MS
and Sul JY: Down-regulation of human epidermal growth factor
receptor 2/neu oncogene by corosolic acid induces cell cycle arrest
and apoptosis in NCI-N87 human gastric cancer cells. Biol Pharm
Bull. 33:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Leng J, Li JJ, Tang JF, Li Y, Liu
BL and Wen XD: Corosolic acid inhibits adipose tissue inflammation
and ameliorates insulin resistance via AMPK activation in high-fat
fed mice. Phytomedicine. 23:181–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Zhou ZH, Chen MH, Yang J, Leng J,
Cao GS, Xin GZ, Liu LF, Kou JP, Liu BL, et al: Inhibition of
mitochondrial fission and NOX2 expression prevent NLRP3
inflammasome activation in the endothelium: The role of corosolic
acid action in the amelioration of endothelial dysfunction.
Antioxid Redox Signal. 24:893–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee MS, Lee CM, Cha EY, Thuong PT, Bae K,
Song IS, Noh SM and Sul JY: Activation of AMP-activated protein
kinase on human gastric cancer cells by apoptosis induced by
corosolic acid isolated from Weigela subsessilis. Phytother
Res. 24:1857–1861. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang H, Zhang X, Cui S, Wang J, Ruan Q,
Huang Y and Yang D: Role and mechanism research on reversal of
5-fluorouracil resistance by epigallocatechin gallate in gastric
cancer drug-resistance cells lines SGC-7901/5-FU. Zhonghua Wei
Chang Wai Ke Za Zhi. 19:1170–1175. 2016.(In Chinese). PubMed/NCBI
|
|
28
|
Liao F, Yang Z, Lu X, Guo X and Dong W:
Sinomenine sensitizes gastric cancer cells to 5-fluorouracil in
vitro and in vivo. Oncol Lett. 6:1604–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The antidiabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim DC, Park KR, Jeong YJ, Yoon H, Ahn MJ,
Rho GJ, Lee J, Gong YD and Han SY: Resistance to the c-Met
inhibitor KRC-108 induces the epithelial transition of gastric
cancer cells. Oncol Lett. 11:991–997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W and Guan KL: AMP-activated protein
kinase and cancer. Acta Physiol (Oxf). 196:55–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hadad SM, Baker L, Quinlan PR, Robertson
KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG,
et al: Histological evaluation of AMPK signaling in primary breast
cancer. BMC Cancer. 9:3072009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Chhipa RR, Nakano I and Dasgupta B:
The AMPK inhibitor compound C is a potent AMPK-independent
antiglioma agent. Mol Cancer Ther. 13:596–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tseng CH: Metformin reduces gastric cancer
risk in patients with type 2 diabetes mellitus. Aging (Albany NY).
8:1636–1649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao H, Xie J, Peng J, Han Y, Jiang Q, Han
M and Wang C: Hispidulin inhibits proliferation and enhances
chemosensitivity of gallbladder cancer cells by targeting HIF-1α.
Exp Cell Res. 332:236–246. 2015. View Article : Google Scholar : PubMed/NCBI
|