Introduction
Acute lung injury (ALI) is a common disease with a
high morbidity and mortality rate worldwide (1–2).
More than 2 million ALI cases caused by chemical poisoning
including paraquat are reported annually in China, resulting in
over 150,000 deaths (1). Numerous
factors may induce ALI, including pathogens, toxins, infections and
autoimmune factors (1). A number
of previous studies have revealed that acute inflammation and
oxidative stress are involved in the development of ALI (2–4).
Numerous animal models of ALI have been established to investigate
the pathogenesis of this disease and to develop novel therapeutic
treatments (1–4).
Paraquat (PQ) is a nonselective bipyridyl herbicide,
which is widely used for crop management globally, however, its
usage is particularly prevalent in developing countries (5). PQ is highly toxic to humans and
animals when absorbed into the body via ingestion, skin contact or
inhalation (5,6). When PQ accumulates in the lung, it
can induce a systemic inflammatory response (5,6),
which can subsequently result in severe lung injury, which is
predominantly marked by edema, hemorrhage, interstitial
inflammation and progressive fibrosis (7,8). At
present, numerous drugs have been used for the attenuation of the
adverse side effects exhibited by patients with PQ-induced lung
injury; however, an effective and specific treatment strategy has
not yet been established (9). To
develop novel treatments for PQ poisoning, the underlying
mechanisms of PQ-induced ALI require further investigation.
It has been well established that the
renin-angiotensin system (RAS) serves a role in maintaining the
homeostasis of blood pressure and electrolyte balance. However,
previous studies have demonstrated that RAS is involved in a number
of inflammatory diseases, including cardiovascular, renal and lung
diseases (10–12). Angiotensin converting enzyme 2
(ACE2), a member of the RAS, suppresses the production of
Angiotensin II (Ang-II) by catalyzing the conversion of Ang-II to
Angiotensin 1–7 [Ang-(1–7)], and serves an important role in
regulation of Ang-II (13). In
addition, previous studies have revealed that Ang-II serves a role
in the development of ALI and Ang-II has been demonstrated to be
involved in the development of lung fibrosis (14,15).
Furthermore, studies have also revealed that ACE2-deficient mice
suffer from increased aggravation of lung injury compared with
wild-type mice, whereas treatment with recombinant ACE2 and
Ang-(1–7) was revealed to attenuate associated
lung injury (16). All of these
results suggest that ACE2/Ang-(1–7) may
have a protective effect against lung injury and may serve as a
therapeutic agent for the treatment of lung injury.
TIIA, an active compound in Salvia
miltiorrhizae Bunge, also known as danshen, exhibits
anti-inflammatory effects and has been used to treat inflammatory
diseases such as bleomycin-induced pulmonary fibrosis in rats and
LPS-treated acute lung injury in mice (15,17).
Our previous studies have revealed that treatment with TIIA
attenuates lung injury induced by lipopolysaccharide (LPS) and
bleomycin (15,17,18),
and that ACE2/Ang-(1–7) is involved in the underlying
mechanisms associated with the therapeutic effect of TIIA on
bleomycin-induced pulmonary fibrosis in rats (15,17,18).
The present study aimed to determine whether TIIA exhibits
therapeutic effects regarding PQ-induced ALI, and to determine its
underlying mechanisms. The results of the present study
demonstrated that TIIA attenuated PQ-induced lung injury and
indicated that its effects were associated with ACE2/Ang-(1–7).
Materials and methods
Experimental groups
All experiments involving animals were approved by
the Animal Care and Use Committee of the Fourth Military Medical
University (Xi'an, China) in accordance with the Declaration of the
National Institutes of Health Guide for Care and Use of Laboratory
Animals (19). Male Sprague-Dawley
rats (n=30; body weight, 250±15 g, aged 9 weeks) obtained from the
Animal Center of the Fourth Military Medical University were used
in the present study. Rats were housed at a temperature of 22±2°C
with a 12-h light-dark cycle and 21% O2, and were fed
regular laboratory chow and water ad libitum. A total of 30
rats were randomly divided into 3 groups: Control group
(saline-treated group; n=10), PQ group (paraquat-treated group;
n=10), and PQ + TIIA group (paraquat and TIIA-treated group; n=10).
The rats in the control and PQ groups were intratracheally
administered 0.9% saline or PQ (35 mg/kg) on the first day. The
rats in PQ + TIIA group were intratracheally administered PQ (35
mg/kg) on the first day, and then administered TIIA (25 mg/kg)
daily for a further 3 days (7,15).
The dose of TIIA administrated was determined in accordance with a
previous study (15). At the
termination of drug treatments, all rats were fasted overnight (12
h), anesthetized using 20% urethane (1,000 mg/kg) via
intraperitoneal injection and then sacrificed via exsanguination.
Lung tissue was then collected for subsequent experiments.
Reagents
Tanshinone IIA (sulfonate; purity of 99%) was
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). Kits for
determining myeloperoxidase (MPO) activity and LDH were obtained
from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
An ELISA kit for the detection of Ang-(1–7)
(cat. no. E-33102) was purchased from Beijing Chenglin
Biotechnology Co., Ltd. (Beijing, China). ACE2 mouse monoclonal
antibodies (cat. no. ab108252) were purchased from Abcam
(Cambridge, UK). Ang-(1–7) rabbit polyclonal antibodies (cat. no.
P3638Rb-r) were purchased from Wuhan EIAab Science Co., Ltd.
(Wuhan, China). β-actin (cat. no. YM1110) was purchased from
ImmunoWay Biotechnology Co. (Plano, TX, USA). Goat anti-mouse
antibody (cat. no. K175622C) and Goat anti-rabbit antibody (cat.
no. K166616H) were purchased from OriGene Technologies, Inc.
(Beijing, China). The BCA kit was purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Goat serum (cat. no.
ZLI-9022) was purchased from OriGene Technologies, Inc. Mouse serum
was made in our laboratory.
Wet/dry weight (W/D) ratio
The W/D ratio was used to determine lung water
content as well as the severity of ALI. At the end of all
experiments, the rats were sacrificed and the lungs were isolated
from the thoracic cavity. The middle lobe of the right lung was
immediately weighed. Following this, the lobes were incubated in a
drying oven at 50°C for 96 h and weighed again. Following this, the
W/D ratio was determined.
MPO activity analysis
MPO is predominantly secreted by neutrophils and can
be used to determine neutrophil infiltration as an index of
inflammation (20). Left lungs
were obtained from rats, and the MPO activity was determined.
Briefly, lung tissues were frozen and homogenized and then treated
in accordance with the protocol of the MPO detection kit. Finally,
MPO of lung tissues were detected using a spectrophotometer at a
wavelength of 460 nm.
Collection of bronchoalveolar lavage
fluid (BALF) and determination of total protein levels
BALF in the left lungs of the rats were collected
using 5 ml phosphate-balanced saline solution (18). Collected BALF was centrifuged at
800 × g for 10 min at 4°C, and the supernatant was collected and
stored at −70°C. Total protein content was determined in accordance
with the manufacturer's instructions of the BCA kit (21).
Determination of total cell count,
total protein levels and lactate dehydrogenase (LDH) activity in
BALF
Total cell count in BALF was determined using a
hemocytometer. Total protein content in BALF was determined using
the bicinchoninic acid protein assay (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The activity of LDH, an
indicator of cell/tissue damage (22), was determined at a wavelength of
460 nm using an LDH determination kit according to the
manufacturer's protocol.
Lung histological analysis
Lung tissues were fixed in 10% formaldehyde for 72 h
at 37°C, embedded in paraffin and then cut into 5-µm-thick
sections. Following this, sections were subjected to hematoxylin
and eosin (H&E) staining for 30 min and Trichrome Masson's
staining for 1 h at room temperature. All sections were
investigated using an Olympus BX50 bright field microscope
(magnification, ×20) equipped with an image analysis program (Image
ProPlus, version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Immunohistochemical staining
analysis
Lung sections (5 µm) were prepared as described
above and then deparaffinized, rehydrated and blocked via
incubation with 0.3% H2O2 for 30 min at room
temperature. Following antigen retrieval performed via treatment
with citrate buffer in a microwave for 10 min, the sections were
blocked for 1 h with normal goat serum at 37°C. Following this, the
sections were incubated with the ACE2 mouse monoclonal antibodies
(1:200) and Ang-(1–7) rabbit polyclonal antibodies (1:200) at
4°C overnight. Sections were washed using PBS and then incubated
goat anti-mouse antibody and goat anti-mouse antibody for 60 min at
37°C. Chromagen detection was performed for 8 min at room
temperature using the 3′3′-diaminobenzidine signal detection
method. Negative controls were performed using mouse serum as the
primary antibody. Staining was revealed to be positive when deep
brown color was observed. All sections were investigated using an
Olympus BX50 bright field microscope equipped with an image
analysis program (Image ProPlus, version 6.0; Media Cybernetics,
Inc.) and the morphological semiquantitative analysis was performed
on microscopic images. The integrated optical density was
determined for arbitrary areas, and 10 fields of view were
investigated per sample (magnification, ×20).
Western blot analysis
Rat lung tissues were homogenized in liquid nitrogen
and total lysates were then isolated from rat lung tissues using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Following protein quantitation using
a coomassie brilliant blue assay, protein samples (50 µg) were
separated via 12% SDS-PAGE and then transferred onto nitrocellulose
membranes. Membranes were then blocked using 5% non-fat milk for 1
h at 37°C and then incubated with mouse monoclonal antibodies
against ACE2 (1:400) and β-actin (1:1,000) overnight at 4°C.
β-actin was used as a loading control. The blots were visualized
using the ECL Plus reagent (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and then densitometrically analyzed using ImageJ software
version 1.37 (National Institutes of Health, Bethesda, MD,
USA).
ELISA analysis
Concentrations of Ang-(1–7) in
lung tissue were determined using ELISA kits according to the
manufacturer's instructions.
Statistical analysis
All data are presented as the mean ± standard
deviation of three independent experiments. Statistical analysis
was performed using one-way analysis of variance following by the
Bonferroni post hoc test, using GraphPad Prism 5.0 software
(GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
TIIA attenuates adverse
histopathological effects in lung tissue with PQ-induced ALI
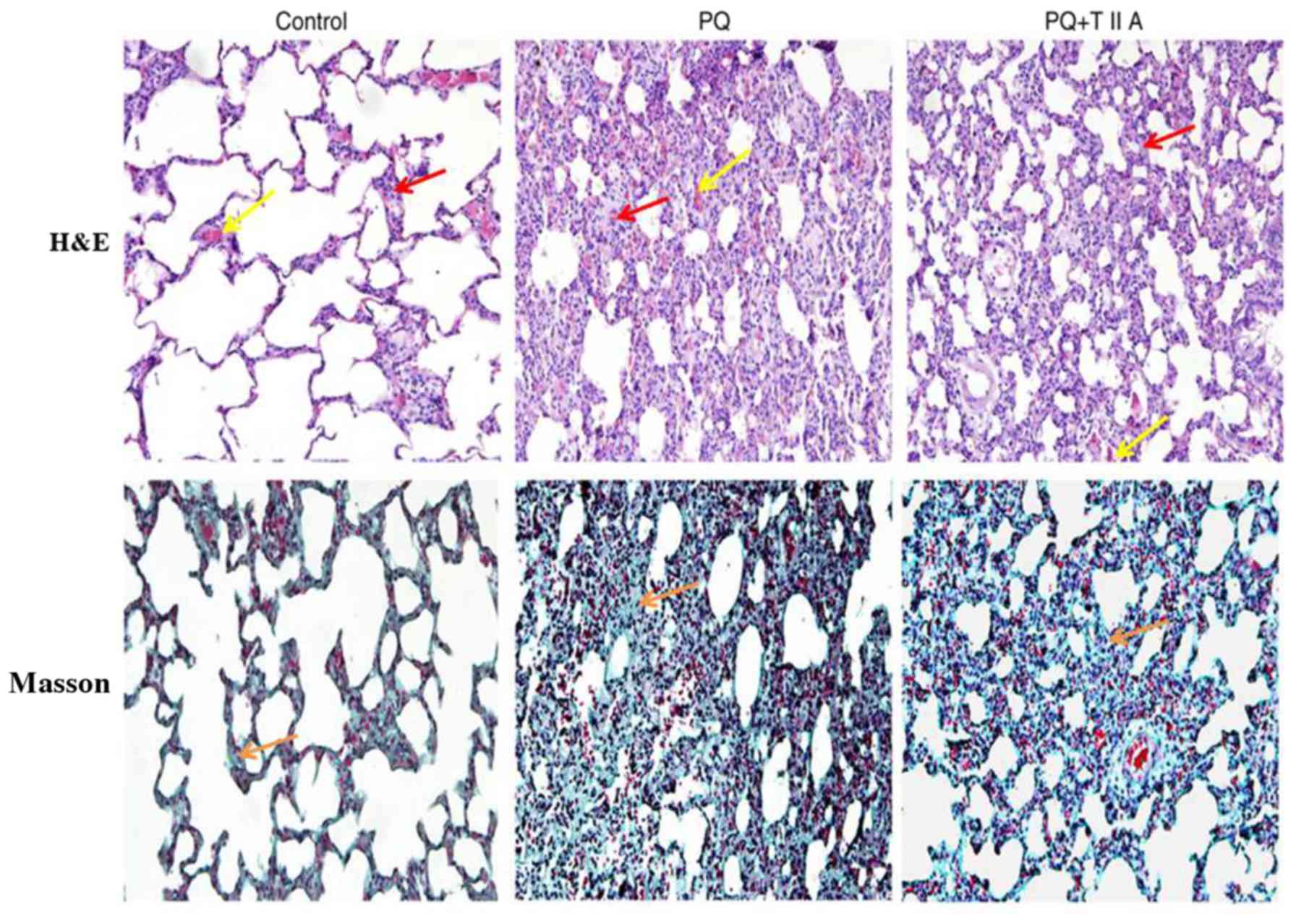
Results of the histopathological analyses performed
via H&E and Masson's staining, using lung sections obtained
from the three different experimental groups, are presented in
Fig. 1. The results revealed
normal lung tissue structure and clear pulmonary alveoli in the
control group. Marked differences in lung histology were
demonstrated between the PQ group and PQ + TIIA group. In the PQ
group, lung edema, hemorrhage, alveolar septal thickening, influx
of inflammatory cells and fibrin deposition were observed. By
contrast, similar effects were exhibited by the PQ + TIIA group but
to a lesser extent compared with those exhibited by the PQ group,
which suggests that TIIA attenuated lung injury.
TIIA attenuates the W/D ratio and
neutrophil infiltration in lung tissues following administration of
PQ
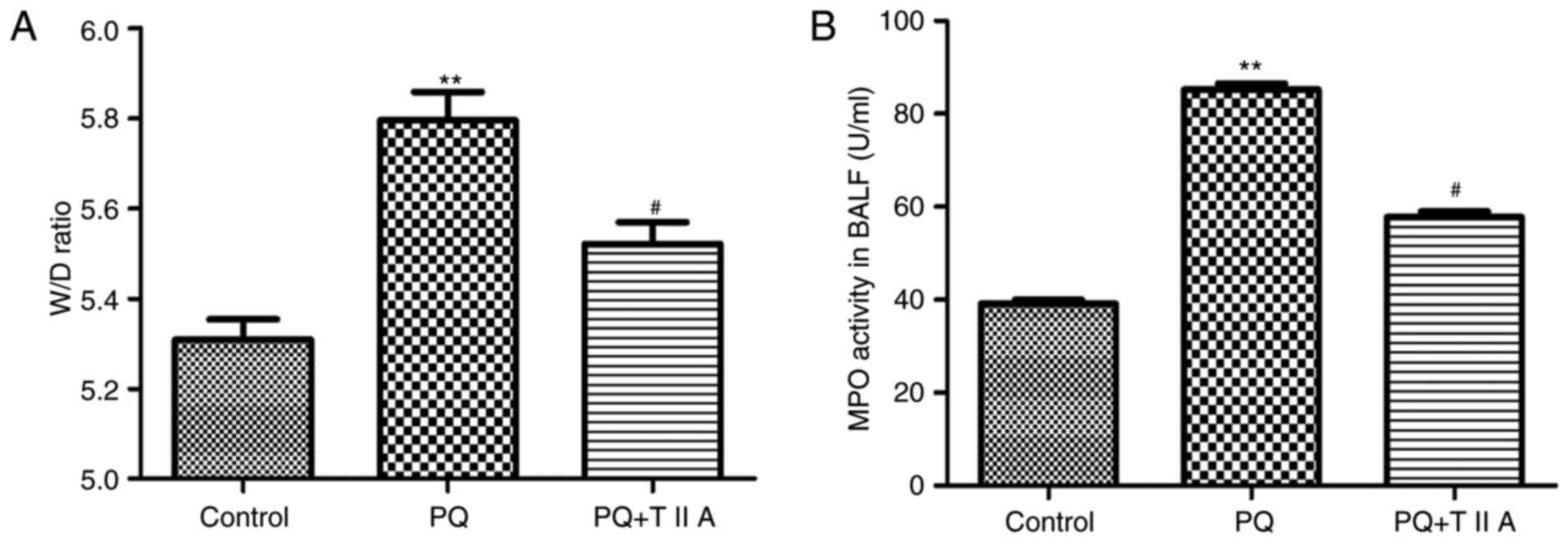
To further investigate the effect of TIIA on
PQ-induced lung edema, alterations in the lung W/D ratio were
determined. The results demonstrated that the W/D ratio was
significantly increased in the PQ group compared with the control
group (Fig. 2A). However, the W/D
ratio exhibited by the PQ group was significantly attenuated
following treatment with TIIA (Fig.
2A). In addition, to investigate neutrophil infiltration in the
lungs, MPO activity was determined. PQ administration significantly
increased MPO activity in BALF compared with the control group
(Fig. 2B); however, MPO activity
was significantly suppressed in the PQ + TIIA group compared with
the PQ group (Fig. 2B).
TIIA attenuates increased LDH and
protein levels, as well as the total cell count, in the BALF of
rats with PQ-induced ALI
Rats treated with PQ exhibited a significant
increase in BALF LDH levels compared with the control group
(Table I), whereas this effect was
significantly attenuated following treatment with TIIA (Table I). In addition, the total cell
count and total protein levels in BALF were significantly enhanced
in the PQ group compared with control group (Table I); however, this effect was
significantly attenuated following treatment with TIIA (Table I).
 | Table I.Effects of Tanshinone IIA on LDH
concentration, total cell count and protein concentration in
bronchoalveolar lavage fluid. |
Table I.
Effects of Tanshinone IIA on LDH
concentration, total cell count and protein concentration in
bronchoalveolar lavage fluid.
| Groups | Protein
concentration (µg/ml) | Total cells
(1×104/ml) | LDH (U/ml) |
|---|
| Control | 30.51±3.23 | 18.65±1.09 | 9.53±0.99 |
| PQ |
75.36±5.76a |
63.17±4.89a |
75.56±2.14a |
| PQ + TIIA |
41.47±3.56b |
35.43±5.30b |
39.03±3.18b |
TIIA attenuates the suppressed ACE2
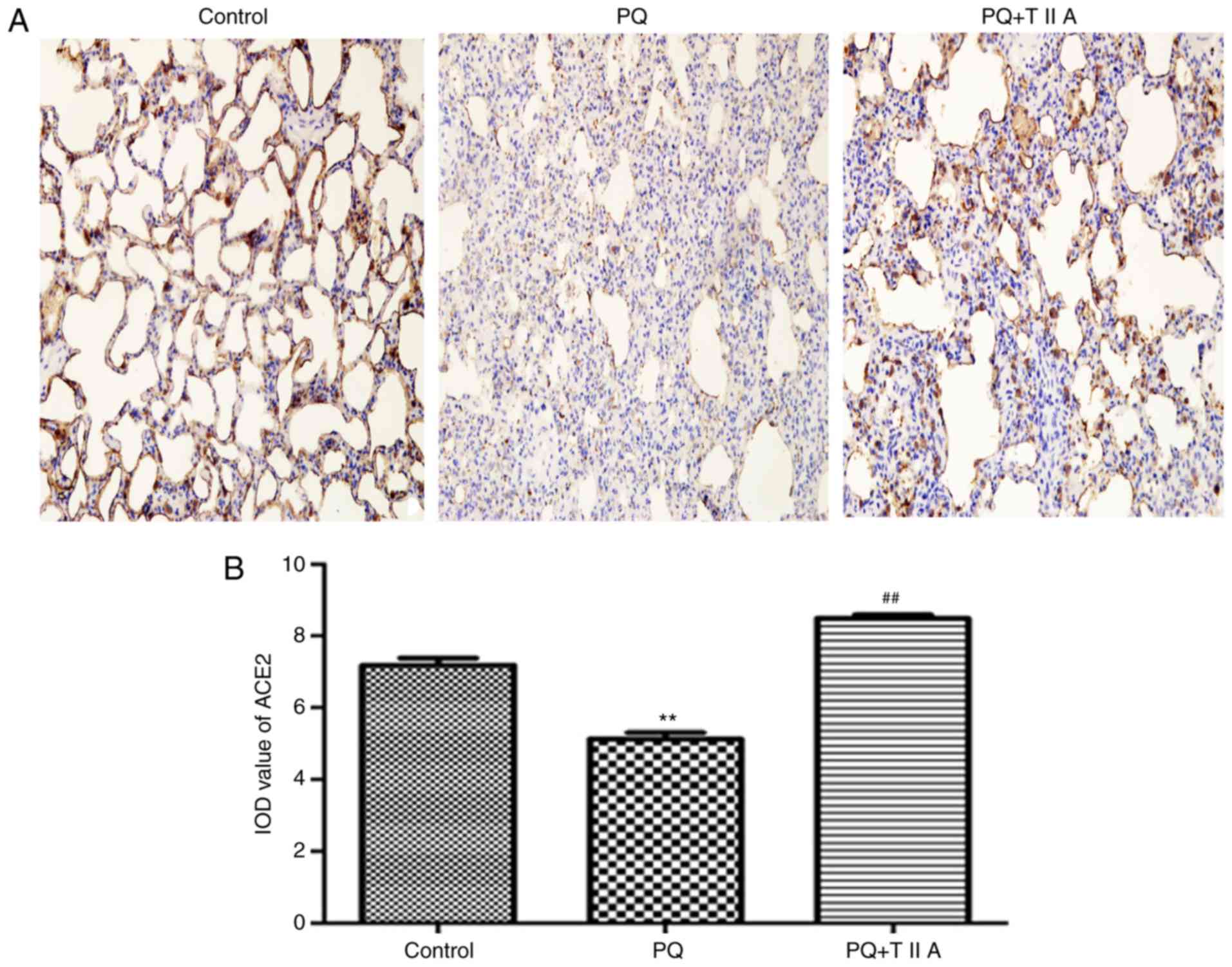
expression in the lung tissues of rats with PQ-induced ALI
Immunohistochemical staining revealed that the
expression of ACE2 was significantly decreased in the PQ group
compared with the control group, whereas the expression of ACE2 was
significantly attenuated following treatment with TIIA compared
with the PQ group (Fig. 3A and B).
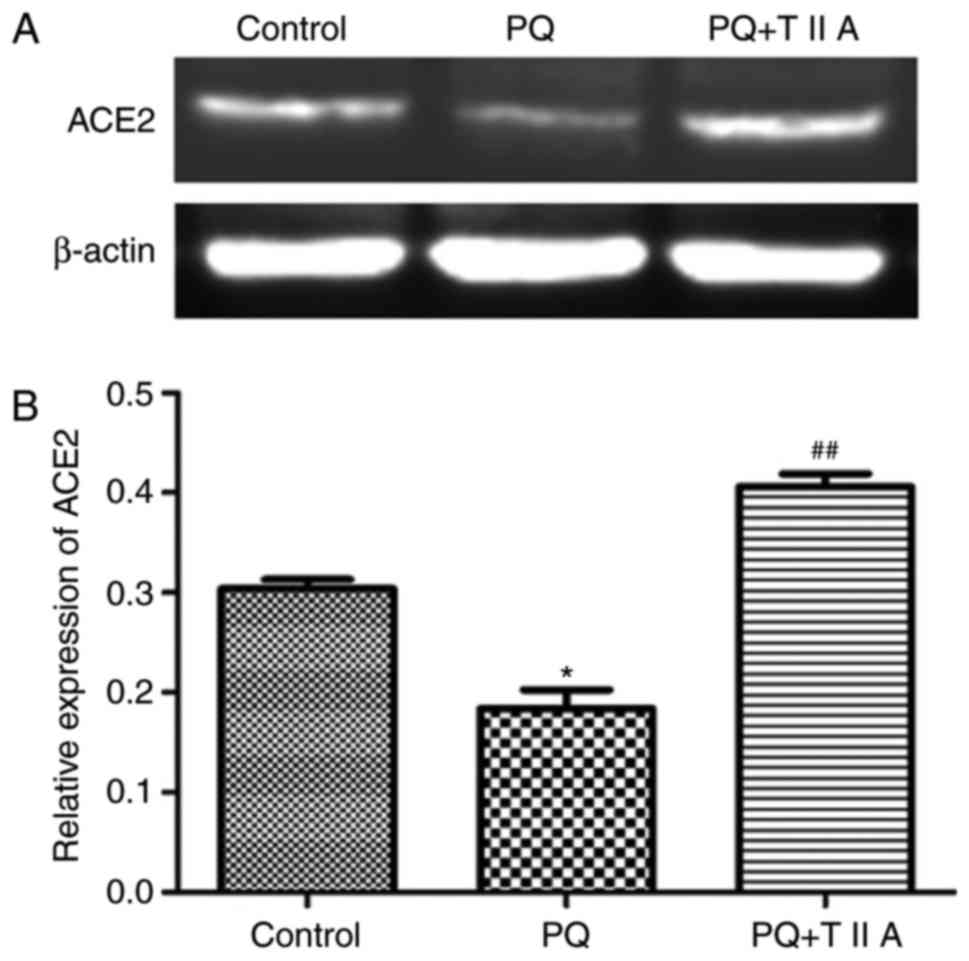
Furthermore, the protein level of ACE2 was investigated by western
blot analysis, the results of which demonstrated that the protein
expression levels of ACE2 were significantly suppressed following
administration of PQ compared with the control group; however,
treatment with TIIA significantly attenuated this effect (Fig. 4A and B).
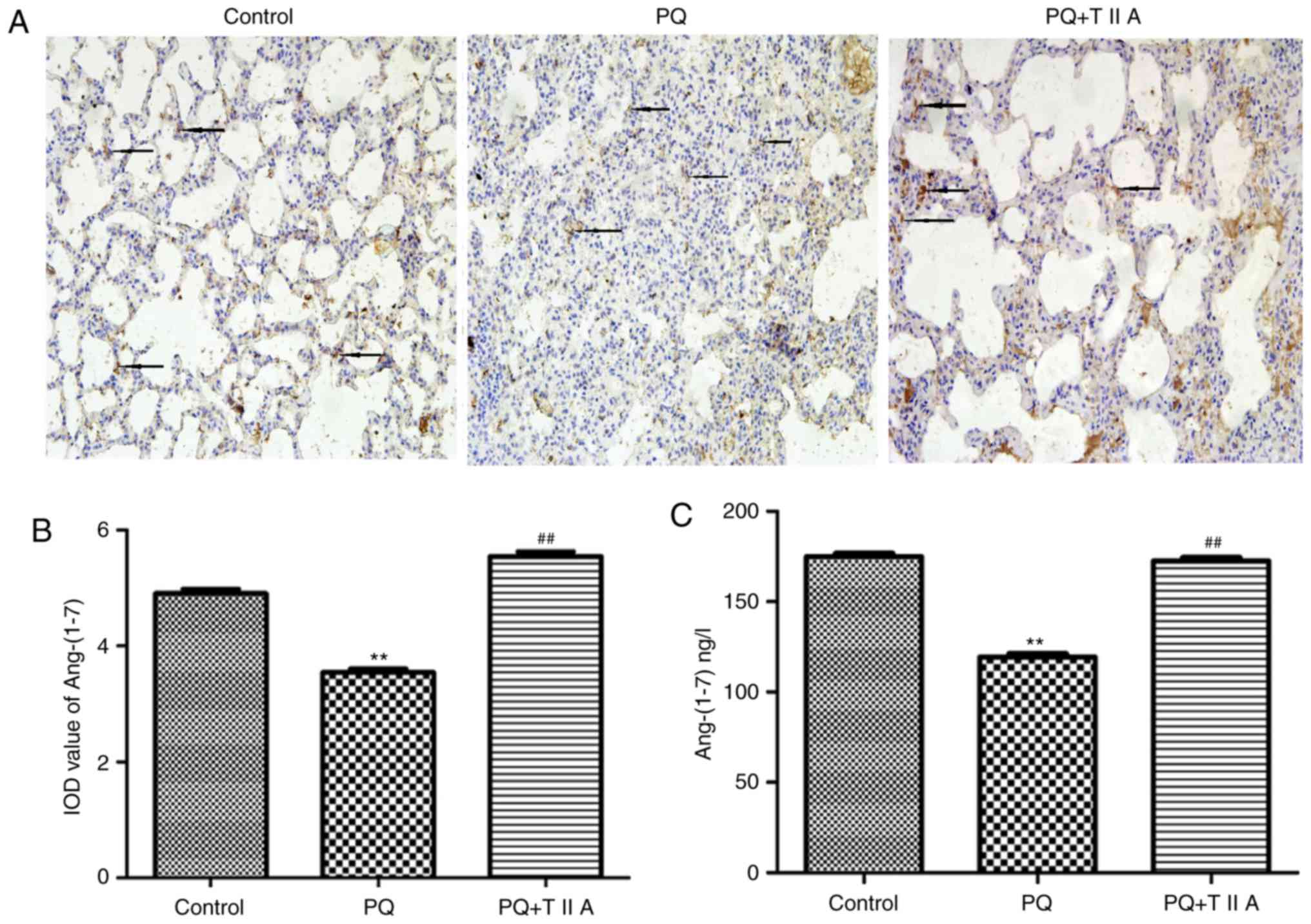
TIIA attenuates decreased expression
levels of Ang-(1–7) in in the lung tissues of rats with
PQ-induced ALI
Expression levels of Ang-(1–7) were
demonstrated to be significantly suppressed in the PQ group
compared with the control group; however, the expression of
Ang-(1–7) was significantly increased in the PQ +
TIIA group compared with the PQ group (Fig. 5A and B). Furthermore, the protein
expression levels of Ang-(1–7) were
determined by ELISA. The results revealed that Ang-(1–7)
expression was significantly decreased following administration of
PQ compared with the control group; however, treatment with TIIA
significantly attenuated this effect (Fig. 5C).
Discussion
Small quantities of PQ can be rapidly distributed
among numerous organs in the body and cause multiple organ damage
(23). PQ is a strong
pneumotoxicant, particularly due to its ability to accumulate in
the lung, which is facilitated by alveolar epithelial cells via the
polyamine uptake pathway, resulting in ALI (23). Despite the mechanism underlying the
development of lung injury by PQ remaining undetermined, previous
studies have demonstrated that oxidative and inflammatory mediators
induced by PQ may result in tissue injury (24). Current treatments for PQ poisoning
primarily consist of anti-inflammatory and anti-oxidative
treatments for the attenuation of PQ-induced ALI (25,26).
However, the effectiveness of such treatments has been contested
(12,27). Therefore, further investigation for
a suitable and effective treatment is required to improve
therapeutic outcomes for PQ-induced ALI.
Danshen is a herbal drug that can be isolated from
the dried root or rhizome of Salvia miltiorrhizae Bunge, and
has been used clinically for the treatment of cardiovascular
disease by improving microcirculation as well as promoting tissue
repair and regeneration (15).
TIIA has been demonstrated to be responsible for the majority of
therapeutic properties exhibited by Danshen (17). TIIA can exert a number of
biochemical effects, such as anti-oxidant and anti-inflammatory
effects. Our previous studies have demonstrated that TIIA can
attenuate lung injury induced by LPS and seawater (28–31),
pulmonary fibrosis induced by bleomycin (15) and hypoxic pulmonary hypertension
(32). As a result, the present
study aimed to investigate whether TIIA can attenuate PQ-induced
ALI. The pathological results demonstrated that PQ induced alveolar
epithelial cell disruption, hemorrhaging, edema, hypoxemia,
infiltration of inflammatory cells into the interstitial and
alveoli spaces and diffuse alveolar collapse, which is consistent
with the results of previous studies (22,32).
However, TIIA was revealed to markedly attenuate such pathological
alterations. Furthermore, PQ was revealed to significantly increase
the W/D ratio, protein levels and LDH and MPO activity in BALF. The
pathological results suggested that TIIA significantly attenuated
these biochemical parameters.
At present, RAS is considered to represent an
important factor in the inflammatory response, and Ang-II has been
revealed to represent a growth factor involved in the regulation of
cell growth and fibrosis, and may also be involved in the
regulation of lung injury progression via numerous mechanisms
(33,34). In addition, ACE2, a member of RAS,
functions as a counter-regulator of ACE in the regulation of Ang-II
and Ang-(1–7) production (35). Numerous studies have demonstrated
that the ACE2/Ang-(1–7) axis exhibits protective effects in
numerous organs by attenuating the pathological effects induced by
the overactivation of the ACE/Ang-II axis, including hypertensive
cardiac remodeling, liver fibrosis and LPS-induced lung fibrosis
(36–39). A further study also revealed that
ACE2 has a negative regulatory role regarding the severity of lung
injury (40). In our previous
study, it was demonstrated that TIIA attenuates bleomycin induced
pulmonary fibrosis via modulation of the ACE2/Ang-(1–7) axis
in rats (15). Therefore, a
further aim of the present study was to investigate whether the
therapeutic effect exhibited by TIIA against PQ-induced ALI is
associated with the ACE2/Ang-(1–7)
axis. In the present study, the results revealed that PQ
significantly suppressed the expression of ACE2/Ang-(1–7);
however, treatment with TIIA significantly attenuated the
expression levels of ACE2/Ang-(1–7)
post-treatment with PQ. Therefore, the results suggest that
treatment with TIIA exhibits a therapeutic effect against
PQ-induced ALI.
In conclusion, the present study investigated the
protective effects, and underlying mechanisms of, TIIA associated
with PQ-induced ALI using a rat animal model. The results suggest
that TIIA serves an important role in PQ-induced ALI, and that the
ACE-2/Ang-(1–7) axis is associated with the therapeutic
effects exhibited by TIIA. The results of the present study may
provide further insight into the underlying mechanisms regarding
the therapeutic effect of TIIA on PQ-induced ALI, and improve
clinical treatment strategies for patients with PQ-induced ALI.
However, the present study did not perform knockdown of ACE2 and
Ang-(1–7)/loss of function experiments. The
absence of such experiments is a limitation of the present study,
and thus should be investigated by future studies.
Acknowledgements
Not applicable.
Funding
This present study was funded by the National Nature
Science Foundation of China (grant nos. 31671186, 81270328 and
81471816).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL and XS conceived and designed the study, YW and
HW performed the experiments and wrote the paper, and WN, JC and ML
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Animal Care and Use Committee of the Fourth Military Medical
University (Xi'an, China) in accordance with the Declaration of the
National Institutes of Health Guide for Care and Use of Laboratory
Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun S, Jiang Y, Wang R, Liu C, Liu X, Song
N, Guo Y, Guo R, Du L, Jiang S, et al: Treatment of
paraquat-induced lung injury with an anti-c5a antibody: Potential
clinical application. Crit Care Med. 46:e419–e425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han J, Ma D, Zhang M, Yang X and Tan D:
Natural antioxidant betanin protects rats from paraquat-induced
acute lung injury interstitial pneumonia. Biomed Res Int.
2015:6081742015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai L, Yi F, Dai Z, Huang X, Zhao YD,
Mirza MK, Xu J, Vogel SM and Zhao YY: Loss of caveolin-1 and
adiponectin induces severe inflammatory lung injury following LPS
challenge through excessive oxidative/nitrative stress. Am J
Physiol Lung Cell Mol Physiol. 306:L566–L573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sunil VR, Vayas KN, Cervelli JA, Malaviya
R, Hall L, Massa CB, Gow AJ, Laskin JD and Laskin DL:
Pentoxifylline attenuates nitrogen mustard-induced acute lung
injury, oxidative stress and inflammation. Exp Mol Pathol.
97:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amirshahrokhi K: Anti-inflammatory effect
of thalidomide in paraquat-induced pulmonary injury in mice. Int
Immunopharmacol. 17:210–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin Y, Guo X, Zhang SL and Sun CY:
Analysis of paraquat intoxication epidemic (2002–2011) within
China. Biomed Environ Sci. 26:509–512. 2013.PubMed/NCBI
|
|
7
|
Choi JS, Jou SS, Oh MH, Kim YH, Park MJ,
Gil HW, Song HY and Hong SY: The dose of cyclophosphamide for
treating paraquat-induced rat lung injury. Korean J Intern Med.
28:420–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu MW, Su MX, Zhang W, Wang YQ, Chen M,
Wang L and Qian CY: Protective effect of Xuebijing injection on
paraquat-induced pulmonary injury via down-regulating the
expression of p38 MAPK in rats. BMC Complement Altern Med.
14:4982014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Cao Y, Zeng Z, Liang M, Xue Y, Xi C,
Zhou M and Jiang W: Angiotensin-converting enzyme
2/angiotensin-(1–7)/Mas axis prevents lipopolysaccharide-induced
apoptosis of pulmonary microvascular endothelial cells by
inhibiting JNK/NF-κB pathways. Sci Rep. 5:82092015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou CH, Chuang LY, Lu CY and Guh JY:
Interaction between TGF-β and ACE2-Ang-(1–7)-Mas pathway in high
glucose-cultured NRK-52E cells. Mol Cell Endocrinol. 366:21–30.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Man FS, Tu L, Handoko ML, Rain S,
Ruiter G, François C, Schalij I, Dorfmüller P, Simonneau G, Fadel
E, et al: Dysregulated renin-angiotensin-aldosterone system
contributes to pulmonary arterial hypertension. Am J Respir Crit
Care Med. 186:780–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song B, Zhang ZZ, Zhong JC, Yu XY, Oudit
GY, Jin HY, Lu L, Xu YL, Kassiri Z, Shen WF, et al: Loss of
angiotensin-converting enzyme 2 exacerbates myocardial injury via
activation of the CTGF-fractalkine signaling pathway. Circ J.
77:2997–3006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tipnis SR, Hooper NM, Hyde R, Karran E,
Christie G and Turner AJ: A human homolog of angiotensin-converting
enzyme. Cloning and functional expression as a
captopril-insensitive carboxypeptidase. J Biol Chem.
275:33238–33243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li G, Yuzhen L, Yi C, Xiaoxiang C, Wei Z,
Changqing Z and Shuang Y: DNaseI protects against Paraquat-induced
acute lung injury and pulmonary fibrosis mediated by mitochondrial
DNA. Biomed Res Int. 2015:3869522015.PubMed/NCBI
|
|
15
|
Wu H, Li Y, Wang Y, Xu D, Li C, Liu M, Sun
X and Li Z: Tanshinone IIA attenuates bleomycin-induced pulmonary
fibrosis via modulating angiotensin-converting enzyme
2/angiotensin-(1–7) axis in rats. Int J Med Sci. 11:578–586. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan
B, Yang P, Sarao R, Wada T, Leong-Poi H, et al:
Angiotensin-converting enzyme 2 protects from severe acute lung
failure. Nature. 436:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Cao F, Liu L, Zhang B, Wang Y, Dong
H, Cui Y, Dong M, Xu D, Liu Y, et al: Tanshinone IIA-induced
attenuation of lung injury in endotoxemic mice is associated with
reduction of hypoxia-inducible factor 1α expression. Am J Respir
Cell Mol Biol. 45:1028–1035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu M, Dong MQ, Cao FL, Liu ML, Wang YX,
Dong HY, Huang YF, Liu Y, Wang XB, Zhang B, et al: Tanshinone IIA
reduces lethality and acute lung injury in LPS-treated mice by
inhibition of PLA2 activity. Eur J Pharmacol. 607:194–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Council for Science and
Technology: Regulations on the Management of Experimental Animals.
State Council Bulletin. 2017.1 Supplement:(Chinese).
|
|
20
|
Adam M, Meyer S, Knors H, Klinke A,
Radunski UK, Rudolph TK, Rudolph V, Spin JM, Tsao PS,
Costard-Jäckle A and Baldus S: Levosimendan displays
anti-inflammatory effects and decreases MPO bioavailability in
patients with severe heart failure. Sci Rep. 5:97042015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ollivett TL, Caswell JL, Nydam DV,
Duffield T, Leslie KE, Hewson J and Kelton D: Thoracic
ultrasonography and bronchoalveolar lavage fluid analysis in
holstein calves with subclinical lung lesions. J Vet Intern Med.
29:1728–1734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ede LC, O'Brien J, Chonmaitree T, Han Y
and Patel JA: Lactate dehydrogenase as a marker of nasopharyngeal
inflammatory injury during viral upper respiratory infection:
Implications for acute otitis media. Pediatr Res. 73:349–354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomita M, Okuyama T, Katsuyama H, Miura Y,
Nishimura Y, Hidaka K, Otsuki T and Ishikawa T: Mouse model of
paraquat-poisoned lungs and its gene expression profile.
Toxicology. 231:200–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Zhao H, Liu W, Li T, Wang Y and
Zhao M: NLRP3 inflammasome activation is essential for
paraquat-induced acute lung injury. Inflammation. 38:433–444. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu S, Liu K, Sun Q, Liu W, Xu W, Denoble
P, Tao H and Sun X: Consumption of hydrogen water reduces
paraquat-induced acute lung injury in rats. J Biomed Biotechnol.
2011:3050862011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Novaes RD, Gonçalves RV, Cupertino MC,
Marques DC, Rosa DD, Mdo Peluzio C, Neves CA and Leite JP: Bark
extract of Bathysa cuspidata attenuates extra-pulmonary acute lung
injury induced by paraquat and reduces mortality in rats. Int J Exp
Pathol. 93:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He F, Xu P, Zhang J, Zhang Q, Gu S, Liu Y
and Wang J: Efficacy and safety of pulse immunosuppressive therapy
with glucocorticoid and cyclophosphamide in patients with paraquat
poisoning: A meta-analysis. Int Immunopharmacol. 27:1–7. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D,
Zhao P, Zhang B, Cao F, Wang Y, et al: Tanshinone IIA ameliorates
seawater exposure-induced lung injury by inhibiting aquaporins
(AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol.
176:39–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li JH, Xu M, Xie XY, Fan QX, Mu DG, Zhang
Y, Cao FL, Wang YX, Zhao PT, Zhang B, et al: Tanshinone IIA
suppresses lung injury and apoptosis, and modulates protein kinase
B and extracellular signal-regulated protein kinase pathways in
rats challenged with seawater exposure. Clin Exp Pharmacol Physiol.
38:269–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie XY, Zhang B, Li JH, Fan QX, Zhang Y,
Mu DG, Li WP, Xu M, Zhao PT, Jin FG and Li ZC: Sodium tanshinone
iia sulfonate attenuates seawater aspiration-induced acute
pulmonary edema by up-regulating Na(+),K(+)-ATPase activity. Exp
Lung Res. 37:482–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li
JH, Xie XY, Fan QX, Liu W, Mu DG, et al: Tanshinone IIA attenuates
seawater aspiration-induced lung injury by inhibiting macrophage
migration inhibitory factor. Biol Pharm Bull. 34:1052–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Dong MQ, Liu ML, Xu DQ, Luo Y,
Zhang B, Liu LL, Xu M, Zhao PT, Gao YQ and Li ZC: Tanshinone IIA
modulates pulmonary vascular response to agonist and hypoxia
primarily via inhibiting Ca2+ influx and release in normal and
hypoxic pulmonary hypertension rats. Eur J Pharmacol. 640:129–138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JL, Lin-Tan DT, Chen KH and Huang WH:
Repeated pulse of methylprednisolone and cyclophosphamide with
continuous dexamethasone therapy for patients with severe paraquat
poisoning. Crit Care Med. 34:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Yang J, Yu X, Cheng S, Gan H and
Xia Y: Angiotensin II-induced early and late inflammatory responses
through NOXs and MAPK pathways. Inflammation. 40:154–165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong J, Basu R, Guo D, Chow FL, Byrns S,
Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z and Oudit
GY: Angiotensin-converting enzyme 2 suppresses pathological
hypertrophy, myocardial fibrosis, and cardiac dysfunction.
Circulation. 122:717–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q, Yang Y, Huang Y, Pan C, Liu L and
Qiu H: Angiotensin-(1–7) attenuates lung fibrosis by way of Mas
receptor in acute lung injury. J Surg Res. 185:740–747. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lubel JS, Herath CB, Tchongue J, Grace J,
Jia Z, Spencer K, Casley D, Crowley P, Sievert W, Burrell LM and
Angus PW: Angiotensin-(1–7), an alternative metabolite of the
renin-angiotensin system, is up-regulated in human liver disease
and has antifibrotic activity in the bile-duct-ligated rat. Clin
Sci (Lond). 117:375–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mercure C, Yogi A, Callera GE, Aranha AB,
Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM and
Reudelhuber TL: Angiotensin(1–7) blunts hypertensive cardiac
remodeling by a direct effect on the heart. Circ Res.
103:1319–1326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Passos-Silva DG, Verano-Braga T and Santos
RA: Angiotensin-(1–7): Beyond the cardio-renal actions. Clin Sci
(Lond). 124:443–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan
B, Huan Y, Yang P, Zhang Y, Deng W, et al: A crucial role of
angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced
lung injury. Nat Med. 11:875–879. 2005. View Article : Google Scholar : PubMed/NCBI
|